Abstract
We reviewed the literature that is the basis for our proposal that (2→8)-α-Neu5Ac conjugates will be safe and effective vaccines for Group B meningococci (GBMs), Escherichia coli K1, and Pasteurella haemolytica A2. Although (2→8)-α-Neu5Ac is a virulence factor and a protective antigen of these three pathogens, it is also a component of normal tissues (neural cell adhesion molecule). Natural, anti–(2→8)-α-Neu5Ac present in most adults, vaccine-induced antibodies, and even high levels of spontaneously appearing monoclonal anti–(2→8)-α-Neu5Ac did not cause autoimmunity. Although it is not possible to prove a null hypothesis, there are no epidemiologic, serologic, immunologic, or clinical data to indicate that (2→8)-α-Neu5Ac antibodies will induce pathology or an autoimmune disease. No increased pathology caused by these antibodies was found, even in neonates and infants of mothers recovered from GBM meningitis. The lack of pathology mediated by anti–(2→8)-α-Neu5Ac may be explained by different presentations of (2→8)-α-Neu5Ac on bacterial and mammalian cells and by the unusual physicochemical properties of anti–(2→8)-α-Neu5Ac. Based on clinical and experimental data collected over 30 y and because (2→8)-α-Neu5Ac is an essential virulence factor and a protective antigen for GBM, E. coli K1, and P. haemolytica A2, protein conjugates of it are easy to prepare using inexpensive and plentiful ingredients, and they would be compatible with routinely administered infant vaccines, clinical studies of these conjugates should proceed.
Keywords: polysialic acid, bacterial meningitis
Now that groups A, C, W135, and Y capsular polysaccharide (CP) conjugate vaccines are used routinely, Group B meningococci (GBM) are the most common cause of meningococcal disease in developed countries (1). Not commonly appreciated is that the GBM CP (2→8)-α-Neu5Ac is almost identical to the CP of Escherichia coli K1* (2), which is a major cause of neonatal meningitis, urinary tract infections in young girls, and systemic infections in elderly people (3–5) and a major virulence factor for Pasteurella haemolytica A2, a cause of shipping fever of goats and sheep (6). It is also the CP of Moraxella nonliquefaciens (7). Furthermore, this polysaccharide is found on the surface of many tissues, including those tissues of the mammalian fetus—it may be one of the most common surface structures of mammals (8–11). The view of (2→8)-α-Neu5Ac as a self antigen of humans and a potential cause of immunopathology has hindered its development as a vaccine (12, 13). It seems improbable that such a common surface structure could be the target of autoimmune serum antibodies. In this essay, we review the data that allow us to propose that (2→8)-α-Neu5Ac conjugates will be as safe and effective as the polysaccharide protein conjugate vaccines for the other four meningococcal serogroups, Haemophilus influenzae type b, pneumococci, and Salmonella typhi (Vi) (1, 14, 15).
CP
CPs are both essential virulence factors and protective antigens of bacterial pathogens that cause disease by their ability to invade the blood stream. CPs confer the property of invasiveness by shielding the bacterium from the actions of complement on deeper structures such as the LPS of Gram-negative and the cell wall teichoic acid of Gram-positive pathogens (16, 17). CPs are bound to the outer membrane of Gram-negative pathogens by a glycolipid at their reducing end and to the mucopeptides of Gram-positive pathogens by a diphosphate bond (18, 19). It is not clear why only 1 of 6 H. influenzae, 6 of 19 meningocooccal, 1 of 93 E. coli, and about 23 of 90 pneumococcal CP are associated with virulence (14).
Are There Safety Issues for (2→8)-α-Neu5Ac–Based Vaccines?
Because of the similarity between the CP of GBM, E. coli K1, and fixed animal tissues, the works by Finne et al. (13) and Saukkonen et al. (20) commented on the possibility of autoimmunity caused by vaccine-induced serum anti–(2→8)-α-Neu5Ac. This notion has received general acceptance. Our reasons to dispute this notion are narrated below.
Unique Immunologic Properties of (2→8)-α-Neu5Ac
The observation that purified poly-(2→8)-α-Neu5Ac induces low or no serum antibodies in adult humans or laboratory animals was the signal for most investigators that antibodies to this CP would induce autoimmunity (21). However, additional findings by those scientists are generally overlooked. (i) Systemic GBM infections in humans induce antibody to (2→8)-α-Neu5Ac, (ii) injection of killed GBM into laboratory animals induced serum anti–(2→8)-α-Neu5Ac, and (iii) cross-reactivity occurs between (2→8)-α-Neu5Ac and the closely related Group C meningococcal capsular polysaccharide [GCMP; (2→9)-α-Neu5Ac] (22–24).
Why Is Purified (2→8)-Neu5Ac Nonimmunogenic in Human Adults?
Two experimental approaches may help our understanding of this property. The first approach examined oligosaccharides or peptides as inhibitors of the precipitin reaction of the homologous antigen antibody. The work by Kabat (25) showed that maximal inhibition of antidextran is demonstrable with oligoglucans composed of four to seven sugars. The work by Schechter (26) using poly-l-alanines as inhibitors of anti–poly-l-alanine showed that maximal inhibition was observed with homopeptides of 6 or 7 aa. GCMP [(2→9)-α-Neu5Ac] antibodies showed similar results. However, different results were obtained with inhibition of (2→8)-α-Neu5Ac antibodies (prepared by multiple i.v. injections of formalin-fixed GBM or E. coli K1) (27); ∼17 saccharides were required to give maximal inhibition of (2→8)-α-Neu5Ac antibodies (28, 29). Based on physicochemical measurements, it was hypothesized that (2→8)-α-Neu5Ac has a less ordered structure in solution than other CPs (30). Second, the work by Mandrell and Zollinger (31) reported that the antigen-specific binding of (2→8)-α-Neu5Ac antibodies at 37 °C was approximately one logarithm lower than at 4 °C; (2→9)-α-Neu5Ac antibody binding was almost the same at 4 °C and 37 °C. These observations suggest that (2→8)-α-Neu5Ac in solution may not stimulate B-lymphocytes to produce serum IgG antibodies (32, 33).
(2→8)-α-Neu5Ac Antibodies Are Not Harmful
Natural Antibodies.
Most adults have serum IgG anti–(2→8)-α-Neu5Ac that is induced without signs or symptoms (34, 35). A critical level of these so-called natural (2→8)-α-Neu5Ac antibodies has been shown to confer immunity to both E. coli K1 and GBM in laboratory animals and GBM in humans (36). Their development is an age-related phenomenon as recorded for many CP antibodies (37). In one study of matched normal maternal-term newborn sera, the cord (2→8)-α-Neu5Ac antibody levels were equal to or higher than the antibody levels of their mothers, which was observed for other placentally derived CP antibodies; most term fetuses are exposed in utero to (2→8)-α-Neu5Ac antibodies (38).
(2→8)-α-Neu5Ac Antibodies Develop in Response to Infection.
The (2→8)-α-Neu5Ac antibodies, resulting from systemic GBM infections, have secondary biologic activities such as bactericidal and opsonophagocytic activities (39). Some of these antibodies had little or no bactericidal activity but conferred immunity in laboratory animals by inducing opsonophagocytosis (40, 41).
High-Level Monoclonal Anti–(2→8)-α-Neu5Ac Antibodies Occur Without Ill Effects.
An IgM antibody (IgMNOV) from a patient with macroglobulinemia (23 mg/mL) did not elicit adverse effects in the patient. IgMNOV did not bind GCMP or E. coli K92 CP [alternating poly-α(2→8)-Neu5Ac and poly-α(2→9)-Neu5Ac] (28, 29). This monoclonal antibody reacted with seemingly unrelated polynucleotides and denatured DNA, supporting the hypothesis that charged groups with a given spacing may determine the specificity of antigen–antibody interactions on otherwise dissimilar molecular structures (27). IgMNOV protected newborn rats against E. coli K1 infection equally well as horse 46 (2→8)-α-Neu5Ac antiserum (42). Similar monoclonal antibodies were identified from a collection of human paraproteins from patients with Waldenstrom macroglobulinemia or multiple myeloma (43). Seven IgM anti–(2→8)-α-Neu5Ac proteins from this collection showed similar temperature-dependent binding and mouse-protective activities as described.
Experiments conducted by Raff et al. (44) are of interest. EBV-transformed lymphoblastoid cells producing antibodies to E. coli K1 O-acetyl–negative and -positive cells were isolated and cultivated in vitro. The resultant monoclonal anti–(2→8)-α-Neu5Ac antibodies were isolated and shown to have protective in vitro and in vivo activities against both E. coli K1 and GBM; all protective activities required complement.
Noncovalently Bound Complexes of (2→8)-α-Neu5Ac and Proteins
Workers studied the immunologic properties of complexes of (2→8)-α-Neu5Ac and proteins. Complexes are readily formed between poly-(2→8)-α-Neu5Ac and polyanions such as methylated BSA (45, 46). Injected into male volunteers, these complexes were safe and elicited IgM anti–(2→8)-α-Neu5Ac that was bactericidal and protected mice by passive immunization (46, 47). Their T cell-independent properties, predicting their inability to elicit booster responses in infants, terminated their development.
Covalently Bound (2→8)-α-Neu5Ac Conjugates
As observed with the CPs of H. influenzae type b, the other CP types of meningococci and pneumococci, protein conjugates of (2→8)-α-Neu5Ac, and E. coli K1 CP administered to young outbred mice by a clinically acceptable route, schedule, and dosage, all had T cell-dependent properties and elicited antibodies with bactericidal and in vivo protective activities (38). These results were soon confirmed and extended to rhesus monkeys (P = 0.0001) (48, 49). On the basis of these data, clinical studies of poly-(2→8)-α-Neu5Ac protein conjugates were suggested as vaccine candidates (38, 45).
Cross-Reactivity Between GBM and GCM CP
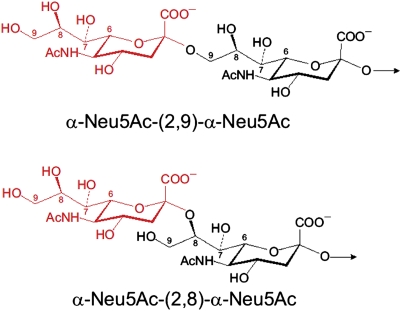
The basis for this cross-reactivity may be deduced from their structures (Fig. 1). Both are linear homopolymers of Neu5Ac differing only in their linkage: GBM capsular polysaccharide with an α-(2→8) bond and GCMP with an α-(2→9) bond (23). Both share an identical propyl terminus (C7–C9) at their nonreducing end when exposed to the host. Several findings are related to this structural identity. Horse 46, injected repeatedly with formalin-fixed GBM for ∼10 y, produced low levels of α-(2→9)-Neu5Ac antibodies (42). The work by Brandt et al. (22) showed that α-(2→8)-Neu5Ac antibodies were induced with multiple i.v. injections of formalin-treated Group C meningococci (GCM) and after systemic infection with GCM. The work by Shin et al. (50) reported that 1 of 15 murine monoclonals, induced by injection of E. coli K1 strains, reacted with both groups B and C CPs. Some infants injected with α-(2→9)-Neu5Ac conjugates also responded with serum IgG antibody to α-(2→8)Neu5Ac. Although the numbers are too small for statistical significance, none of these responders exhibited adverse reactions. These data suggest that we have been inducing (2→8)-α-Neu5Ac antibodies with (2→9)-α-Neu5Ac conjugates without obvious neurologic consequences for at least one decade. This cross-reactivity may provide an explanation for the epidemiological data found by surveillance of meningococcal disease in the United States from 1998 to 2007, which was designed to evaluate the effect of (2→9)-α-Neu5Ac vaccine alone in 1977 and then, the vaccine with the quadrivalent meningococcal A, C, Y, and W135 conjugates. Both meningococcal vaccines were administered mostly to college students and recruits in the US Armed Forces. The incidence of groups C, Y, and W135 meningitis in the United States declined about fivefold during 1998–2007. However, about a twofold decrease in GBM disease was also observed during this period, consistent with a low level of cross-immunogenicity induced by the (2→9)-α-Neu5Ac vaccines (1).
Fig. 1.
The identical N-terminal regions of GBM CP [(2→8)-α-Neu-5Ac] and GCM CP [(2→9)-α-Neu-5Ac; C7–C9].
This cross-reactivity between GBM and GCM is not an unexpected effect, because several references describe the superior immunogenicity of the terminal nonreducing end of polysaccharides and the similar region of polypeptides (51–54).
Long-Term Follow-Up of GBM and GCM Patients
Extensive evidence from epidemiologic studies showed no immunopathology in patients mediated by (2→8)-α-Neu5Ac antibodies, including infants born to mothers recovered from GBM meningitis (the controls were patients with GCM). The work by Stein et al. (55) examined five studies published in the referenced literature that compared GBM cases with at least one other meningococcal serogroup or another control group. There were 1,284 cases of GBM and 823 cases of GCM infections. All cases were reviewed retrospectively, and the nature and rates of sequelae were compared. There were equal or slightly lower rates of mortality and sequelae in patients convalescent from GBM than from GCM or other meningococcal serogroup infections. There was no mention of Guillan–Barré, multiple sclerosis, or other autoimmune diseases in any of the patients. The works by Howitz et al. (56, 57) from the Statens Seruminstitut in Copenhagen evaluated autoimmunity in patients convalescent from GBM (n = 2,894) and GCM (n = 914) diseases in Denmark from 1977 to 2004. They did not find an association of GBM infection and autoimmune disease for up to 31 y after meningococcal disease (56, 57). GBM meningitis posed no risk of stillbirth, preterm birth, small for age, birth defects, or diseases of the nervous system or autoimmune disease within the first 3 y of life. Similar studies in Iceland were confirmatory; a retrospective population-based study of all invasive meningococcal disease was cross-referenced to autoimmune disorders and other diseases (58). Subsequently, a subset of survivors of GBM (n = 70) and GCM (n = 50) was recruited for detailed clinical examination, standardized questionnaires, and measurement of serum anti-CP. There was no increased risk of autoimmunity among the GBM compared with the GCM patients. Disease severity during the acute phase was higher among the GCM meningitis patients compared with the GBM patients (P = 0.02). During convalescence, the incidence of seizure disorders, hearing impairment, cognitive problems, psychiatric disorders, and readmissions to the hospital were similar between the two groups; survivors of GCM had a higher incidence of reported arthritis and migraine headaches (P = 0.01). Levels of (2→8)-α-Neu5Ac IgG antibodies were similar in both groups. In summary, there was no difference in the sequelae of systemic infections in both groups.
Poly-(2→8)-α-Neu5Ac Was Used as a Vaccine
A similar conclusion to ours regarding the safety of (2→8)-α-Neu5Ac as a vaccine component was published in 1984 (59). GBM capsular polysaccharide–GBM serotype 2 protein complexes adsorbed onto alum administered to 500 individuals of various ages in the United States, 30 Norwegian adults, and 2,000 South African children did not induce adverse reactions. IgM anti-Neu5Ac was elicited.
Why Does Anti–(2→8)-Neu5Ac Not Induce Tissue Reactions, Although It Is Bactericidal?
We have no direct evidence to answer this important question. After the initial observations in 1983, there have been many reports of in vitro binding of (2→8)-α-Neu5Ac antibodies to human and animal tissues (9–11, 55, 59–63). (2→8)-α-Neu5Ac antibodies have been shown to bind to purified components such as neural cell adhesion molecules (N-CAMs) from many tissues of mammals and birds, including human fetal brain cells (9). However, this binding could only be shown on fixed tissues, such as frozen sections, or treated with formalin or similar reagents. No in vivo binding has been shown, but (2→8)-α-Neu5Ac antibodies have been described as autoimmune (61).
Several factors may account for the resistance of living tissue to anti–(2→8)-α-Neu5Ac. The first factor requires an understanding of the N-CAM structure. N-CAM and related molecules are the major carrier for (2→8)-α-Neu5Ac (10). N-CAM is a membrane-associated glycoprotein composed of a long polypeptide chain that mediates cellular and developmental processes (61–63); (2→8)-α-Neu5Ac is covalently bound to N-CAM approximately in the middle of the peptide chain (62, 63). The carboxyl terminus inserts into the cell membrane (Fig. 2). This insertion site would be the cell membrane site vulnerable to injury by anti–N-CAM, but the poly-(2→8)-α-Neu5Ac of N-CAM is separated from the cell membrane by an ∼65-kDa portion of the N-CAM peptide. This finding is in contrast to the demonstrable injury produced by anti–(2→8)-α-Neu5Ac and complement to the bacterial surface of viable and actively growing bacteria (64). The second factor is that both IgM and IgG (2→8)-α-Neu5Ac antibodies have low avidity, with considerable reduction of binding at 37 °C compared with the binding at 4 °C (31). The presentation of capsules on bacterial surfaces fosters antibody and complement action and provides a site for the antibody-initiated, complement-mediated cytolysis to form a hole on the bacterial surface, leading to lysis and cell death (64).
Fig. 2.
The insertion of (2→8)-α-(Neu-5Ac) into the peptide of N-CAM, proposed in the work by Cunningham et al. (62), is shown. (A) Schematic drawing of the linear topography of N-CAM. (B) Peptides of N-CAM released by V-8 protease after treatment with neuraminidase. Reprinted from ref. 62 with permission from the authors.
Use of Non-CP Antigens
The use of non-CP antigens as vaccines for GBM has two important limitations. The first limitation is that these noncapsular polysaccharide vaccines do not induce high levels of GBM antibodies, and the second and most important limitation is that these vaccines will not induce immunity to E. coli K1 (65). Although quantitative epidemiologic data are lacking, E. coli K1 causes more systemic infections than GBM.†
Acknowledgments
We are grateful for helpful comments and suggestions from Willie Vann, Vince Pozsgay, Joanna Kubler-Kielb, and Irwin Arias. This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*E. coli K1 has a form variation in the CP (2→8)-α-Neu5Ac. About 1 in 30 E. coli K1 colonies from fresh cultures have the C7 or C9 of their CP O-acetylated.
†We were unable to cite all of the pertinent references because of space constraints.
References
- 1.Cohn AC, et al. Changes in Neisseria meningitidis in the United States, 1998-2007; implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50:184–191. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 2.Orskov F, et al. Form variation in Escherichia coli K1: Determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979;149:669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins JB, et al. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974;290:1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- 4.Kaijser B, Hanson LÅ, Jodal U, Lidin-Janson G, Robbins JB. Frequency of E. coli K antigens in urinary-tract infections in children. Lancet. 1977;1:663–666. doi: 10.1016/s0140-6736(77)92111-0. [DOI] [PubMed] [Google Scholar]
- 5.Orskov I, Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985;95:551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adlam C, Knights JM, Mugridge A, Williams JM, Lindon JC. Production of colominic acid by Pasteurella haemolytica serotype A2 organisms. FEMS Microbiol Lett. 1987;42:23–25. [Google Scholar]
- 7.Bǿvre K, Bryn K, Closs O, Hagen N, Frǿholm LO. Surface polysaccharide of Moraxella non-liquifaciens identical to Neisseria meningitidis group B polysaccharide. A chemical and immunological investigation. Nat Inst Publ Hlth Ann. 1983;6:65–73. [PubMed] [Google Scholar]
- 8.Hoffman S, et al. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982;257:7720–7729. [PubMed] [Google Scholar]
- 9.Drake PM, et al. Polysialic acid, a glycan with highly restricted expression, is found on human and murine leukocytes and modulates immune responses. J Immunol. 2008;181:6850–6858. doi: 10.4049/jimmunol.181.10.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chazal G, Durbec P, Jankovski A, Rougon G, Cremer H. Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse. J Neurosci. 2000;20:1446–1457. doi: 10.1523/JNEUROSCI.20-04-01446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston BD, Jacobs JL, Glick MC, Troy FA. Extended polysialic acid chains (n greater than 55) in glycoproteins from human neuroblastoma cells. J Biol Chem. 1988;263:9443–9448. [PubMed] [Google Scholar]
- 12.Edelman GM. Cell adhesion molecules. Science. 1983;219:450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- 13.Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 14.Robbins JB, Schneerson R, Anderson P, Smith DH. The 1996 Albert Lasker Medical Research Awards. Prevention of systemic infections, especially meningitis, caused by Haemophilus influenzae type b. Impact on public health and implications for other polysaccharide-based vaccines. JAMA. 1996;276:1181–1185. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 15.Thiem VD, et al. The Vi conjugate typhoid vaccine is safe, elicits protective levels of IgG anti-Vi, and is compatible with routine infant vaccines. Clin Vaccine Immunol. 2011;18:730–735. doi: 10.1128/CVI.00532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood WB, Jr, Smith MR. The inhibition of surface phagocytosis by the capsular slime layer of pneumococcus type III. J Exp Med. 1949;90:85–96. doi: 10.1084/jem.90.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looney RJ, Steigbigel RT. Role of the Vi antigen of Salmonella typhi in resistance to host defense in vitro. J Lab Clin Med. 1986;108:506–516. [PubMed] [Google Scholar]
- 18.Gotschlich EC, Fraser BA, Nishimura O, Robbins JB, Liu TY. Lipid on capsular polysaccharides of gram-negative bacteria. J Biol Chem. 1981;256:8915–8921. [PubMed] [Google Scholar]
- 19.Sǿrensen UB, Henrichsen J, Chen HC, Szu SC. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 20.Saukkonen K, Haltia M, Frosch M, Bitter-Süerman D, Leinonen M. Antibodies to the capsular polysaccharide of Neisseria meningitidis group B or E. coli K1 bind to the brains of infant rats in vitro but not in vivo. Microb Pathog. 1986;1:101–105. doi: 10.1016/0882-4010(86)90036-7. [DOI] [PubMed] [Google Scholar]
- 21.Wyle FA, et al. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 22.Brandt BL, Wyle FA, Artenstein MS. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972;108:913–920. [PubMed] [Google Scholar]
- 23.Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, Smith IC. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975;250:1926–1932. [PubMed] [Google Scholar]
- 24.Yamasaki R. Conformations of group B and C polysaccharides of Neisseria meningitidis and their epitope expression. In: Roth J, Rutishauser U, Troy FA, editors. Polysialic Acid. Basel: Birkhauser; 1993. pp. 1–9. [Google Scholar]
- 25.Kabat EA. The upper limit for the size of the human antidextran combining site. J Immunol. 1960;84:82–85. [PubMed] [Google Scholar]
- 26.Schechter I. Mapping of the combining sites of antibodies specific to polyalanine chains. Ann N Y Acad Sci. 1971;190:394–419. doi: 10.1111/j.1749-6632.1971.tb13551.x. [DOI] [PubMed] [Google Scholar]
- 27.Jennings HJ, Roy R, Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol. 1985;134:2651–2657. [PubMed] [Google Scholar]
- 28.Kabat EA, et al. A human monoclonal macroglobulin with specificity for alpha(2→8)-linked poly-N-acetyl neuraminic acid, the capsular polysaccharide of group B meningococci and Escherichia coli K1, which cross-reacts with polynucleotides and with denatured DNA. J Exp Med. 1986;164:6641–6652. doi: 10.1084/jem.164.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabat EA, et al. The epitope associated with the binding of the capsular polysaccharide of the group B meningococcus and of Escherichia coli K1 to a human monoclonal macroglobulin, IgMNOV. J Exp Med. 1988;168:699–711. doi: 10.1084/jem.168.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson TJ, Venable RM, Egan W. Conformational flexibility of the group B meningococcal polysaccharide in solution. J Am Chem Soc. 2003;125:2930–2939. doi: 10.1021/ja0210087. [DOI] [PubMed] [Google Scholar]
- 31.Mandrell RE, Zollinger WD. Measurement of antibodies to meningococcal group B polysaccharide: Low avidity binding and equilibrium binding constants. J Immunol. 1982;129:2172–2178. [PubMed] [Google Scholar]
- 32.Sulitzeanu D. Antibody-like receptors on immunocompetent cells. Curr Top Microbiol Immunol. 1971;54:1–18. doi: 10.1007/978-3-642-65123-6_1. [DOI] [PubMed] [Google Scholar]
- 33.Vitetta ES, Baur S, Uhr JW. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971;134:242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toropainen M, et al. Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis. Infect Immun. 2005;73:4694–4703. doi: 10.1128/IAI.73.8.4694-4703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins JB. Acquisition of natural and immunization-induced immunity to Haemophilus influenzae type b diseases. In: Schlessinger D, editor. Microbiology. Washington, DC: ASM; 1975. pp. 400–405. [Google Scholar]
- 38.Devi SJN, Robbins JB, Schneerson R. Antibodies to poly[(2----8)-alpha-N-acetylneuraminic acid] and poly[(2----9)-alpha-N-acetylneuraminic acid] are elicited by immunization of mice with Escherichia coli K92 conjugates: Potential vaccines for groups B and C meningococci and E. coli K1. Proc Natl Acad Sci USA. 1991;88:7175–7179. doi: 10.1073/pnas.88.16.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leinonen M, Frasch CE. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: Use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect Immun. 1982;38:1203–1207. doi: 10.1128/iai.38.3.1203-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lifely MR, Esdaile J. Specificity of the immune response to the group B polysaccharide of Neisseria meningitidis. Immunology. 1991;74:490–496. [PMC free article] [PubMed] [Google Scholar]
- 41.Artenstein MS, et al. Serologic studies of meningococcal infection and polysaccharide vaccination. J Infect Dis. 1971;124:277–288. doi: 10.1093/infdis/124.3.277. [DOI] [PubMed] [Google Scholar]
- 42.Allen PZ, Glode M, Schneerson R, Robbins JB. Identification of immunoglobulin heavy-chain isotypes of specific antibodies of horse 46 group B meningococcal antiserum. J Clin Microbiol. 1982;15:324–329. doi: 10.1128/jcm.15.2.324-329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandrell RE, Azmi FH, Granoff DM. Complement-mediated bactericidal activity of human antibodies to poly alpha 2—>8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- 44.Raff HV, Devereux D, Shuford W, Abbott-Brown D, Maloney G. Human monoclonal antibody with protective activity for Escherichia coli K1 and Neisseria meningitidis group B infections. J Infect Dis. 1988;157:118–126. doi: 10.1093/infdis/157.1.118. [DOI] [PubMed] [Google Scholar]
- 45.Zollinger WD, Mandrell RE, Griffiss JM, Altieri P, Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979;63:836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lifely MR, et al. Immunogenicity in adult males of a Neisseria meningitidis group B vaccine composed of polysaccharide complexed with outer membrane proteins. Vaccine. 1991;9:60–66. doi: 10.1016/0264-410x(91)90318-z. [DOI] [PubMed] [Google Scholar]
- 47.Borrow R, et al. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24:5093–5107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 48.Bartoloni A, Norelli F, Ceccarini C, Rappuoli R, Costantino P. Immunogenicity of meningococcal B polysaccharide conjugated to tetanus toxoid or CRM197 via adipic acid dihydrazide. Vaccine. 1995;13:463–470. doi: 10.1016/0264-410x(94)00007-a. [DOI] [PubMed] [Google Scholar]
- 49.Zollinger WD, Moran EE, Devi SJ, Frasch CE. Bactericidal antibody responses of juvenile rhesus monkeys immunized with group B Neisseria meningitidis capsular polysaccharide-protein conjugate vaccines. Infect Immun. 1997;65:1053–1060. doi: 10.1128/iai.65.3.1053-1060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin JS, Lin JS, Anderson PW, Insel RA, Nahm MH. Monoclonal antibodies specific for Neisseria meningitidis group B polysaccharide and their peptide mimotopes. Infect Immun. 2001;69:3335–3342. doi: 10.1128/IAI.69.5.3335-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cisar J, Kabat EA, Dorner MM, Liao J. Binding properties of immunoglobulin combining sites specific for terminal or nonterminal antigenic determinants in dextran. J Exp Med. 1975;142:435–459. doi: 10.1084/jem.142.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez C, Sverremark E. Immune responses to bacterial polysaccharides: Terminal epitopes are more immunogenic than internal structures. Cell Immunol. 1994;153:67–78. doi: 10.1006/cimm.1994.1006. [DOI] [PubMed] [Google Scholar]
- 53.Pozsgay V, Kubler-Kielb J, Schneerson R, Robbins JB. Effect of the nonreducing end of Shigella dysenteriae type 1 O-specific oligosaccharides on their immunogenicity as conjugates in mice. Proc Natl Acad Sci USA. 2007;104:14478–14482. doi: 10.1073/pnas.0706969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubler-Kielb J, et al. A bicomponent Plasmodium falciparum investigational vaccine composed of protein-peptide conjugates. Proc Natl Acad Sci USA. 2010;107:1172–1177. doi: 10.1073/pnas.0913374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein DM, Robbins J, Miller MA, Lin FY, Schneerson R. Are antibodies to the capsular polysaccharide of Neisseria meningitidis group B and Escherichia coli K1 associated with immunopathology? Vaccine. 2006;24:221–228. doi: 10.1016/j.vaccine.2005.07.084. [DOI] [PubMed] [Google Scholar]
- 56.Howitz MF, et al. Risk of adverse birth outcome after group B meningococcal disease: Results from a Danish national cohort. Pediatr Infect Dis J. 2009;28:199–203. doi: 10.1097/INF.0b013e31818c9049. [DOI] [PubMed] [Google Scholar]
- 57.Howitz M, et al. Lack of association between group B meningococcal disease and autoimmune disease. Clin Infect Dis. 2007;45:1327–1334. doi: 10.1086/522190. [DOI] [PubMed] [Google Scholar]
- 58.Gottfredsson M, et al. Comparative long-term adverse effects elicited by systemic Group B and C meningococcal infections. Clin Infect Dis. 2011 doi: 10.1093/cid/cir500. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zollinger WD, Boslego JE, Frasch CE, Froholm LO. Safety of vaccines containing meningococcal group B polysaccharide. Lancet. 1984;2:166. doi: 10.1016/s0140-6736(84)91083-3. [DOI] [PubMed] [Google Scholar]
- 60.Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissue. J Immunol. 1967;138:4402–4407. [PubMed] [Google Scholar]
- 61.Nedelec J, Boucraut J, Garnier JM, Bernard D, Rougon G. Evidence for autoimmune antibodies directed against embryonic neural cell adhesion molecules (N-CAM) in patients with group B meningitis. J Neuroimmunol. 1990;29:49–56. doi: 10.1016/0165-5728(90)90146-e. [DOI] [PubMed] [Google Scholar]
- 62.Cunningham BA, Hoffman S, Rutishauser U, Hemperly JJ, Edelman GM. Molecular topography of the neural cell adhesion molecule N-CAM: Surface orientation and location of sialic acid-rich and binding regions. Proc Natl Acad Sci USA. 1983;80:3116–3120. doi: 10.1073/pnas.80.10.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crossin KL, Edelman GM, Cunningham BA. Mapping of three carbohydrate attachment sites in embryonic and adult forms of the neural cell adhesion molecule. J Cell Biol. 1984;99:1848–1855. doi: 10.1083/jcb.99.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joiner KA, Brown EJ, Frank MM. Complement and bacteria: Chemistry and biology in host defense. Annu Rev Immunol. 1984;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- 65.Zollinger WD, Poolman JT, Maiden MC. Meningococcal serogroup B vaccines: Will they live up to expectations? Expert Rev Vaccines. 2011;10:559–561. doi: 10.1586/erv.11.41. [DOI] [PMC free article] [PubMed] [Google Scholar]