Abstract
Measles virus is a highly infectious, enveloped, pleomorphic virus. We combined electron cryotomography with subvolume averaging and immunosorbent electron microscopy to characterize the 3D ultrastructure of the virion. We show that the matrix protein forms helices coating the helical ribonucleocapsid rather than coating the inner leaflet of the membrane, as previously thought. The ribonucleocapsid is folded into tight bundles through matrix–matrix interactions. The implications for virus assembly are that the matrix already tightly interacts with the ribonucleocapsid in the cytoplasm, providing a structural basis for the previously observed regulation of RNA transcription by the matrix protein. Next, the matrix-covered ribonucleocapsids are transported to the plasma membrane, where the matrix interacts with the envelope glycoproteins during budding. These results are relevant to the nucleocapsid organization and budding of other paramyxoviruses, where isolated matrix has been observed to form helices.
Keywords: image reconstruction, subtomogram averaging
Measles is a common, acute disease caused by measles virus (MV), which is one of the most infectious viruses known. The symptoms include fever, conjunctivitis, respiratory infection, and maculopapular rash, and the disease is severe, particularly among children and in immune-compromised patients (1). The high mortality of measles is often associated with secondary bacterial infections that take place during the period of virus-induced immunosuppression. There has been an effective vaccine against the virus since the 1960s, but because of poor vaccination coverage in many developing countries the virus still remains a major problem. In 2008, measles caused 164,000 deaths worldwide, being one of the leading causes of death among young children (2).
MV belongs to the genus Morbillivirus in the family of Paramyxoviridae that also includes other major human pathogens, such as mumps virus, respiratory syncytial virus (RSV), and the parainfluenza viruses. All of the paramyxoviruses are enveloped viruses that enclose a helical nucleocapsid composed of the negative-stranded ssRNA genome and nucleoprotein (N), along with the matrix protein (M), the phosphoprotein (P), and the large polymerase protein (L). In addition, MV has two membrane-spanning glycoproteins, fusion (F), and an attachment protein, hemagglutinin (H), on the surface. These two proteins are responsible for the binding and entry of the virus into host cells (3). The M protein of paramyxoviruses is thought to coordinate assembly of the virion and to form a thin layer bound to the inner leaflet of the virion membrane. For MV, interactions of the M protein with the cellular membrane (4–6), with the N protein (6–9), and with the tails of the two surface glycoproteins (10–13) have been described. The interaction with the N protein has been shown to be mediated by two leucine residues at the C terminus of the intrinsically unstructured C-terminal domain of N (NTAIL). This interaction was shown to be crucial for both recruiting the nucleocapsid to the plasma membrane and regulating viral RNA synthesis (8).
Ultrastructural studies on paramyxoviruses using electron cryomicroscopy (cryo-EM) combined with image processing have been limited mainly by the substantial pleomorphism and large size of the viral particles. To date, Sendai virus is the only member of the Paramyxoviridae for which a 3D structure of the virion has been described (14). For MV, such studies have not been performed, but two differing ultrastructural models can be proposed (Fig. 1). In the first, commonly used model (15), M remains bound to the membrane after budding, leaving the nucleocapsid largely free inside the particle (Fig. 1A), similar to the Sendai virus structure (14). In the second model, M mainly covers the nucleocapsid, creating a regularly packed form efficient for genome packaging and budding that could influence viral RNA synthesis (Fig. 1B). This model is based on observations that M is a common contaminant of nucleocapsid preparations (16) and early electron microscopy studies, which have described two distinct types of tubular structures with different diameters inside MV-infected cells (17–21), where the larger of the two tubes has been reported to contain M (20). Although the structure of recombinant helical nucleocapsids (∼20 nm in diameter) has been well characterized (22–24), the in situ organization of the different structural components in measles virions is unresolved, as illustrated by the two hypotheses presented in Fig. 1. The resolution of the structural organization will have a direct impact on our understanding of MV assembly.
Fig. 1.
Schematic diagram illustrating two possible ultrastructural models for MV. (A) M coats the viral membrane and the nucleocapsid is free in the interior. (B) M coats the helical nucleocapsid. Color key: nucleocapsid, brown; M, light blue; membrane, red; H, dark blue; F, yellow.
Here we have used cryo-EM to characterize the 3D ultrastructure of purified MV particles. We observed both bare and covered nucleocapsids in the virion, supporting the model presented in Fig. 1B. Tomographic subvolume alignment and averaging allowed us to describe the 3D organization of these two concentric helical structures inside the virions. In addition, by using immunosorbent electron microscopy (EM), we show that the inner helix contains N and the outer helix contains M. The matrix-covered nucleocapsids were seen to form tight bundles. Taken together, these data reveal MV organization and further deepen our understanding of MV maturation.
Results
Measles Virions Are Highly Pleomorphic, Covered by the Surface Glycoproteins to a Varying Extent, and Contain Two Types of Tubular Structures Inside.
Because conventional EM methods do not standardly preserve high-resolution information for the 3D reconstruction of pleomorphic enveloped particles, we used cryo-EM to look at measles virions in their native hydrated state (25, 26). We had two strains available, a WT and an Edmonston vaccine strain. The WT virus had better budding efficiency under the conditions used (88% for WT, 0.1% for Edmonston) and a 100-fold better specific infectivity after purification (WT 2 × 109 PFU⋅mg−1 vs. Edmonston 2 × 107 PFU⋅mg−1) (SI Materials and Methods; see Virus Growth and Purification) and was thus used for most of the detailed image analysis (Fig. 2 A–D and Movies S1, S2, S3, S4, and S5). Similar features were seen in tomograms of the Edmonston preparations (Fig. S1 and Movie S6). The reason for the difference in budding efficiency is not clear; it could be because of the cell types used or the differences in the genomes of the two strains, although the matrix and nucleoprotein sequences are very similar. The WT particles varied in diameter from about 50 to 510 nm and exhibited a multitude of different shapes (Fig. 2 A–D and Movies S1, S2, S3, S4, and S5). Some of the particles, from both strains, using different gradient materials for purification (sucrose versus the isotonic OptiPrep), had clear vesicles inside (Fig. 2C, Fig. S1A, and Movie S3).
Fig. 2.
MV ultrastructure and nucleocapsid organization. (A–D) Tomographic slices of MV showing the general morphology of the virions. In the virions, two types of tubular structures with 20-nm (white arrow) and 30-nm (black arrows) diameters can be identified. See corresponding Movies S1, S2, S3, and S4. (C) A vesicle inside a virion is indicated with an asterisk. (E–G) Different types of nucleocapsid structures from broken virions are shown: a 30-nm structure in E, a partially-covered 20-nm structure in F, and a completely bare 20-nm structure in G. Tomographic slices are 7.7-nm thick. (Scale bar, 100 nm.)
It has been postulated that the glycoproteins on the virion surface would interact with a layer of M protein adjacent to the inner leaflet of the viral membrane. The tomograms showed a membrane with apparently randomly distributed glycoproteins on the surface (Fig. 2, Fig. S1A, Fig. S2, and Movies S1, S2, S3, S4, and S5). We analyzed the density distribution across the membrane to investigate whether or not there was significant protein density directly underneath the membrane for five particles of similar size containing nucleocapsids (Fig. 3, and SI Materials and Methods; see Image Processing). This analysis revealed strong density for the membrane and for the glycoprotein ectodomains (∼12 nm in length) (Fig. 3B) but only very low density proximal to the inner surface of the membrane. The average thickness of the membrane was 7 nm, which is similar to the value described earlier for the thinnest regions in the Sendai envelope and much less than the 12 nm reported for areas in Sendai where a matrix layer is possibly lining the membrane (14). Thus, MV seems to lack an ordered, continuous M protein layer next to the membrane. The average picture does not rule out that small membrane patches are coated with M.
Fig. 3.
Membrane density profile. (A) A slab of density is shown for a tomographic reconstruction of one virion. Part of the membrane density defined for the analysis is indicated as a gray surface. Some surface normals used for extracting and orienting subvolumes are shown as sticks. (B) Plot of density distribution calculated from the extracted subvolumes as a function of distance from the center of the membrane. The extent of the membrane and glycoprotein layer (F/H) is indicated with bars.
Two types of tubular structures could be detected in both Edmonston and WT virion preparations. The tubes were ∼20 and 30 nm in diameter (Fig. 2 and Movies S1, S2, S3, S4, S5, and S6). In a total of 88 WT particles observed, 30-nm tubes could be visually detected in 19 particles and 20-nm tubes in 12 particles, both usually present in the same particle. The 20-nm tubes have the typical appearance of a MV herringbone-like ribonucleocapsid. The 30-nm tubes also contain the same 20-nm tube but have an additional surrounding layer of protein density. Occasionally, a partially uncovered nucleocapsid could be seen free in the sample, most likely originating from a disrupted virion (Fig. 2F). The 30-nm tubes appeared relatively straight and rigid compared with the bare nucleocapsids that were often curved. Both ends and sides of some tubes were found touching the viral envelope.
Layer Covering the Nucleocapsid Is Composed of the Matrix Protein.
Based on earlier observations of the Sendai virus M protein's ability to self-assemble into helices (27, 28) and the MV M protein to bind the nucleocapsid (6–9), we hypothesized that the outer layer in the 30-nm tube could be composed of the M protein. Therefore, we carried out immunosorbent EM of infected cell lysates using anti-M and anti-N–coated grids (Fig. 4). A constant time (30 min) was used to observe the EM sample grids and images were collected whenever tubular structures were recognized. The anti-N grids were relatively densely covered by structures assigned almost solely to the 20-nm tubes (Table 1). The structures were of varying length and clearly of two different forms, one resembling the untreated recombinant nucleocapsid and the other resembling the trypsin-treated recombinant nucleocapsid (Fig. 4A) that have been reported previously (22–24). Anti-M grids had both the 20-nm tubes and the 30-nm tubes, approximately one-third being of the 30-nm type (Table 1). In these pull-down experiments, both the 20-nm and the 30-nm tubes were found as singular tubes and as aggregates of tubes reflecting M-to-M and M-to-N interactions, rather than cross-reactivity of the antibodies. Anti-P grids were used as controls to rule out the possibility that the P protein could form the outer helix, as it is known that P also interacts with N (29–31). On anti-P grids, fewer than 10 tubular structures in total could be observed and they were all of the 20-nm type. Hence, the 30-nm diameter tubes are matrix-covered nucleocapsids (MCNC).
Fig. 4.
Electron micrographs of MV-infected and m and n cotransfected cell lysates prepared by immunosorbent EM. (A and B) Three different tubular forms can be observed from infected cells: (A, anti-N grid) 20-nm nucleocapsids with two different packing modes marked with a black arrow [similar to intact recombinant nucleocapsids (22–24)] and a white arrow [similar to trypsin-treated recombinant nucleocapsids (22–24)] and (B, anti-M grid) 30-nm tubes where matrix covers the nucleocapsids. In C a hollow 30-nm tube from an m and n cotransfected cell lysate (anti-M grid) is shown together with a 20-nm nucleocapsid. (Scale bar, 50 nm.)
Table 1.
Immunosorbent EM of lysates from virus-infected cells
*All nucleocapsids with partial or complete outer layer coverage were included.
†SD from three individual experiments.
When a similar immunosorbent-EM experiment was conducted on lysates of m and n cotransfected HEK293E cells, no M-covered nucleocapsids were observed. Instead, 20-nm nucleocapsids of both types were prevalent on the anti-N grids, along with some hollow 30-nm matrix tubes on the anti-M grids (Fig. 4C). This finding indicates that M is capable of assembling into helical structures without a nucleocapsid scaffold, as shown for the Sendai and RSV matrix (27, 28, 32), and that some additional viral factor not present in cotransfected cell lysates is needed for MCNC assembly.
Matrix Layer Has a Helical Symmetry, Which Is Different from the Helical Symmetry of the Nucleocapsid.
To analyze the structure of the MCNC, we carried out subvolume alignment and averaging of MCNC segments extracted from the tomograms. The inner nucleocapsid and outer matrix layer were treated separately from one another in the refinement, as initial attempts to refine both layers simultaneously were unsuccessful (SI Materials and Methods; see Image Processing). The averaged structure revealed two concentric helices, indicating that the matrix layer also has helical symmetry (Fig. 5). Strikingly, this symmetry is different from the symmetry of the helical nucleocapsid: First, the inner nucleocapsid is a left-handed helix with a pitch (i.e., ridge-to-ridge distance) of 6.4 nm, as determined by autocorrelation analysis (Fig. 5E). This value is in line with previously reported values (5.0–6.6 nm) for recombinant nucleocapsids (22, 24). The outer M protein layer is also a left-handed helix, but has a larger pitch of 7.2 nm (Fig. 5E). This value is identical to the one reported for purified Sendai M helices (27). Second, rotational self-correlation plots clearly demonstrated that the nucleocapsid is a one-start helix but the M protein layer is a five-start helix (Fig. 5F). Third, the almost perfect correlation of the M helix with its 180° rotated (around the axis perpendicular to the helical axis) copy indicated that the M helix lacks directionality: that is, it has a so-called “dyad” axis (Fig. 5F). This finding would further suggest that the repeating structural units of the M helix are dimers, which have their twofold symmetry axis perpendicular to the helical axis. Although the limited resolution in our reconstruction (4.4 nm, based on Fourier shell correlation 0.5 criterion) did not allow us to demarcate individual subunits, dimers of M would be consistent with data on Sendai M protein (28, 33). In contrast, the nucleocapsid helix correlated poorly with its 180° rotated copy and thus has clear directionality, as expected based on visual inspection of the tomograms (Fig. 2 and Movies S1, S2, S3, S4, and S5) and earlier reconstructions of MV recombinant nucleocapsids (22–24).
Fig. 5.
Averaged structure of the MCNC. (A–D) Isosurface representations of the MCNC structure. The structure is seen from the side in A to C, and a slice taken along the axis is shown in D. Both the outer (blue) and inner (orange) parts show a clear helical twist. The transparent surfaces were rendered at a low threshold and the opaque surfaces at a high threshold (0.5 σ and 1.0 σ above the mean density, respectively). (Scale bar, 10 nm.) The stars in D represent the five-start helical arrangement. (E) Translational self-correlation plot shows the correlation coefficient plotted as a function of shift along the helical axis. (F) Rotational self-correlation plot shows the correlation coefficient plotted as a function of rotation around the helical axis (solid line). The same function was plotted for a map correlated against its copy, which had been rotated 180° to turn it upside down (dotted line). The coloring in E and F corresponds to that in A to D.
Matrix-Covered Nucleocapsids Form Tightly Packed Bundles Inside the Virions.
We carried out two independent sets of template matching by correlating first the averaged matrix helix and then the averaged nucleocapsid helix against the tomograms to position them segment by segment back into their original context (Fig. 6). The 3D search found most of the M-covered nucleocapsids that had been defined manually, some additional ones, and several stretches of bare nucleocapsids. The directionality of the nucleocapsid segments was in agreement with the directionality of the other segments in the same tube. The template matches to the M helices were often found pointing in two opposing directions within the same tube, consistent with the fact that the M helix lacked directionality.
Fig. 6.
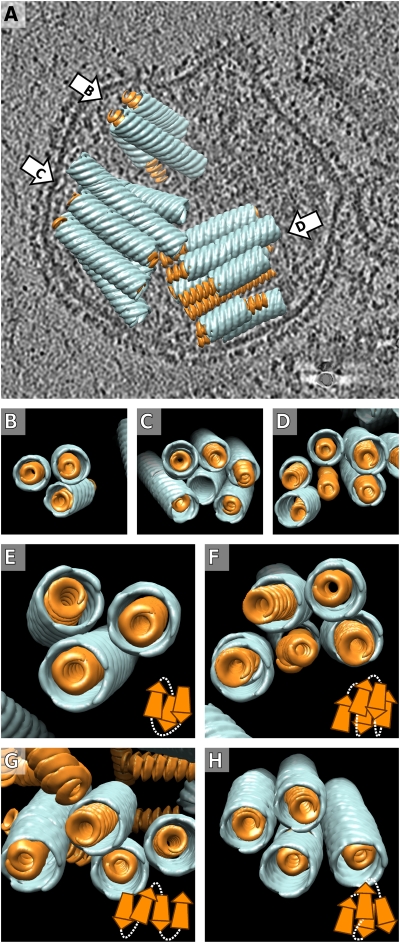
Organization of the MCNC in virions. (A) The averaged structures for the matrix (blue) and nucleocapsid (orange) filaments were placed back into the density map of a virion (one section is shown in gray scale, positive density is black) (Movie S5). (B–D) End-on views of the bundles in A are shown from the directions indicated with arrows in A. One M-helix was hollow in C and one NC helix was bare in D, reflecting either inaccuracies in the computational analysis or biological variation in the MCNC structure. (E–H) Different examples of MCNC packing in virions are shown. The embedded schematic diagrams illustrate possible connectivity between the MCNC filaments, consistent with an antiparallel arrangement of neighboring filaments.
Detection of the MCNC in the tomograms allowed us to study if the MCNC pack randomly or in a specific way. The MCNC were often detected in tightly-packed bundles. The average center-to-center distance in 20 pairs of nearly parallel MCNC was ∼30 nm, similar to the diameter of the MCNC, suggesting very close interactions within the bundles. The adjacent MCNC within the bundles were often antiparallel (Fig. 6 E–H). The number of MCNC per virion in the eight virions analyzed varied from 3 to 17 and the length of individual tubes from 46 to 160 nm. The total length of the detected MCNC in a virion varied from 230 to 1,400 nm (Table 2).
Table 2.
Number and length of MCNC in virions
| Virion | No. of MCNC | Average length of MCNC* (nm) | Total length (nm) |
| 1 | 3 | 84 (11) | 250 |
| 2 | 3 | 77 (10) | 230 |
| 3 | 4 | 97 (20) | 390 |
| 4 | 5 | 120 (19) | 580 |
| 5 | 10 | 72 (16) | 650 |
| 6 | 11 | 90 (31) | 900 |
| 7 | 13 | 120 (30) | 1,400 |
| 8 | 17 | 74 (8) | 1,200 |
*SD in parentheses.
Discussion
The morphology of MV and other paramyxoviruses has been characterized extensively in the past using negative-stain EM. Except for the observations of filamentous nucleocapsids in these viruses, the interior of the virion has remained largely uncharacterized because of limitations of the negative-stain technique. These limitations are overcome by cryo-EM, which allows 3D structural investigations of samples in their native hydrated state. A recent cryo-EM study showed that Sendai virions are highly pleomorphic (14), agreeing with the results of the present study. The MV membrane contains an apparently random distribution of surface glycoproteins that were so densely packed that we could not discern if, for example, H and F proteins were bound to each other on the surface, as biochemical data suggest (Fig. 2 A–D and Movies S1, S2, S3, S4, and S5), or partition in to different areas of the membrane based on curvature, as has been reported in influenza virus (34–38). Some of the MV particles analyzed in this study contained vesicles, similarly to those reported in Sendai virus (14). Thus, such vesicles may be a common feature of the paramyxoviruses.
Our cryo-EM data give insight on the MV M protein organization. The M protein has been generally thought to line the inner leaflet of the membrane in budded virions, interacting with the viral glycoprotein tails (Fig. 1A). This hypothesis has been based on the observations that the M protein is translocated to cell membranes, has a tendency to bind membranes, and is capable of driving virus-like particle formation when expressed without other viral proteins (5, 39). However, an alternative hypothesis presented in Fig. 1B, driven by the data we present in this article, and still explaining these observations, suggests that the majority of the M protein in virions is in fact not lining the viral membrane, but rather forms a layer covering the nucleocapsids in a helical fashion. Bundles of the MCNC are then found inside the virions. This organization in turn casts doubt on models for MV assembly that assume M interacts with N only at the cytoplasmic membrane during budding. Several observations support the hypothesis that the MCNC forms already in the cytosol before transport to the cytoplasmic membrane: First, early electron microscopy data indicate that there are both thinner “smooth filaments” and wider “granular filaments” in the cytosol of infected cells (18, 19). The “granular filaments” probably correspond to the MCNC and concentrate toward the cell periphery, close to where particles are observed to bud (18, 19). Second, in our immunosorbent EM analysis, MCNC could be detected in cell lysates of infected cells. Third, efficient nucleocapsid transport to the plasma membrane requires accumulation of matrix in intracellular membranes (6). Taking these observations together, we suggest that the formation of the viral RNA-containing nucleocapsid, covered with M protein, is required for efficient transport to the budding sites. The assembly of the MCNC could also explain mechanistically how the M protein can act as a repressor of transcription and viral RNA synthesis (8, 9), as the matrix coat could prevent the polymerase from binding to the viral RNA, wound on the outer edge of the nucleocapsid (24). This repression could also be important during entry where M uncoating would be required for transcription to start.
The MCNC were often found in tightly packed bundles, suggesting that the M protein in different MCNC could interact with each other. As the length of individual MCNC were too short to accommodate the whole genome, and the bundles seem to contain antiparallel segments, it is likely that the individual MCNC in one bundle are linked together by bare regions of nucleocapsid, difficult to resolve in the tomograms because of various artifacts, such as the missing wedge. Supporting this finding, both bare nucleocapsids and MCNC were observed in the same virion. In addition, the total length of MCNC in any individual bundle was smaller than that expected for a full-length genome (around 1 μm), so different bundles need to be connected. Some of the particles could be polyploid (Table 2, viruses 7 and 8) as described earlier (40). Because of the nature of transmission cryo-EM, very large particles, (greater than ∼600 nm in diameter and likely to be polyploid), through which the electron beam does not penetrate, were excluded from our detailed analysis.
M may have a role in compact packing of the genome, effectively reducing the total surface area of the virion, thereby aiding budding. If the majority of the M protein in virions is organized around the nucleocapsids before reaching the plasma membrane, as our results suggest, the driving force in curving the cell membrane for budding seems unlikely to be exerted by the multimerization of the M protein on the inner leaflet of the plasma membrane but is more likely to be driven by actin polymerization, as suggested by Bohn et al. (41). It is also possible that the M protein has at least two roles, one responsible for budding, the other for recruiting the nucleocapsid to the budding sites. A postranslational modification responsible for M separation into two bands on SDS/PAGE gels (39) would be the most likely nominator of these two roles. In contrast, Sendai virus particles do have matrix lining the envelope, so the budding and entry of these two viruses may well have diverged (14). Indeed, it has been shown that Sendai matrix does not control the transcription and replication of the genome, as expected if matrix and nuclocapsid interact only at the plasma membrane (42). Interestingly, Sendai M protein can assemble into helices on its own, and has been shown to coat nucleocapsids in preparations isolated from virions (28). In influenza virus, the position of the matrix is dynamic. In budded virus it is found next to the membrane as a layer, but after exposure to the low pH of the endosome it dissociates from the membrane (38).
How does the matrix layer assemble on the nucleocapsids? Image processing of the MCNC structure revealed first, that native nucleocapsids, containing viral RNA in the presence of additional proteins, such as M, L, and P, are similar in pitch to those reported from untrypsinized recombinant nucleocapsids (22, 24), indicating that no major structural changes occur in the nucleocapsid upon matrix binding. Our data indicate that where M assembles as a helix on to the outside of the nucleocapsid, the resulting MCNC is rather straight, but flexible bare regions allow bundling of the nucleocapsid (Fig. 6 and Movie S5). Second, there is an unexpected symmetry mismatch between the outer matrix helix and the inner nucleocapsid helix. This finding suggests some flexibility is required in the interaction between these proteins. There is abundant evidence from sequence predictions, protease digestion, NMR, and CD spectroscopy, that the intrinsically disordered NTAIL is exposed on the outside of assembled nucleocapsids (22, 23, 43) and folds cooperatively on interaction with P (44). Additionally, interaction and mutational studies have shown that the NTAIL residues L523 and L524 are critical for binding M (8). Thus, we propose that the ribonucleocapsid could help in nucleating the assembly of the M helix, but M–M interactions promote the further growth of the M helix and define its organization. Supporting the hypothesis that M can self-assemble, we observed single-shelled, 30-nm diameter helices similar to the MCNC outer layer in both immunosorbent EM of transfected cell lysate (Fig. 4C) and in a virion tomogram (Fig. S2).
How is the formation of segmental MCNC promoted and controlled? Some possibilities that could be tested with an in vitro assembly system are: (i) the tertiary structure of the RNA or active transcription causes flaws or bends in the ribonucleocapsid; or (ii) other viral proteins, such as P, prevent M assembly (44).
In conclusion, we have characterized the ultrastructure of MV and described a previously undescribed matrix-nucleocapsid complex existing in the virions. We suggest a detailed model for the organization of the matrix protein inside the virions in which M and nucleocapsid form a bundled two-layer helical structure for efficient packaging of the genome in the virions. Although the MCNC may not be a common feature of the paramyxoviruses, matrix proteins from other paramyxoviruses, and the more distantly related vesicular stomatitis virus (42) and influenza virus (36), also have a strong tendency to form helical structures, which reflects the protein's propensity to self-assemble (27, 28, 32). Our revised model of measles virus, founded on direct tomographic evidence, will direct future studies to investigate the importance of matrix assembly on to the ribonucleoprotein in paramyxovirus budding and cell entry.
Materials and Methods
Virus Growth and Purification.
WT virus (a gift from I. Davidkin, Helsinki, Finland) was grown in Vero-SLAM cells (a gift from Y. Yanagi, Fukuoka, Japan) and Edmonston vaccine strain (ATCC VR 24) in B-Vero cells. The viruses were purified using ultracentrifugation in Optiprep and sucrose gradients (SI Materials and Methods; see Virus Growth and Purification).
Cloning of m and n and Expression in HEK293E Cells.
Full-length m and n were amplified by RT-PCR from TRIzol extracted MV WT RNA and cloned into plasmid pTT5SH8Q2 (a gift from Y. Durocher, Montreal, Canada) using NotI and HindIII restriction sites. HEK293E (45) cells were transfected with polyethyleneimine and a 1:1 molar ratio of m- and n-containing vectors. Cells were harvested 3 d after transfection and lysed in 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% Nonidet P-40, and 0.5 mM Pefabloc SC (Sigma-Aldrich) protease inhibitor and transferred to –80 °C until use. Expression of both proteins was verified with Western blots using anti-M (MAB8910; Millipore) and anti-N (2F3, Santa Cruz Biotechnology) antibodies.
Cryo-EM.
Samples of purified virus were mixed with 10-nm colloidal gold and vitrified on holey carbon-coated grids (C-flat 200 or 400 mesh; Electron Microscopy Sciences). Cryo-EM was conducted at liquid nitrogen temperature and low-dose conditions using a 200-kV transmission electron microscope (F20; FEI). Images were recorded with a CCD camera (Ultrascan 4000; Gatan). In total, 27 tilt series (typically ±60° at 2° increments) were collected in SerialEM (46) at underfocii ranging from 3 to 6 μm and at a nominal magnification of 39,400×, resulting in a sampling of 0.38 nm per pixel.
Image Processing.
Image processing details are described in SI Materials and Methods; see Image Processing. Briefly, from tomographic reconstructions, individual MCNC segments were extracted as subvolumes that were then subjected to subvolume alignment and averaging using Jsubtomo (47). The inner and outer helices were refined separately. The density distribution across the membrane was calculated from a subvolume average obtained from tomograms of the virus surface.
Immunosorbent EM.
Infected cells were lysed in PBS with 5% Triton X-100 and 1 mM Pefabloc SC to release subviral complexes. Transfected cell lysates were supplemented with 5% Triton X-100 to enhance solubilisation of M. For immunosorbent EM analysis of the released complexes, carbon-coated copper grids (Electron Microscopy Sciences) were glow-discharged and coated with anti-M, anti-N or anti-P (9H4; Santa Cruz Biotechnology) for 10 min. The grids were then blocked with 3% BSA in PBS for 60 to 90 min, washed with PBS, and incubated for 40 min on cell lysate drops from virus-infected or plasmid-transfected cells. The grids were then washed and negatively stained with 1% potassium phosphotungstate (pH 7.0). All steps were carried out at room temperature. The stained grids were observed with an F20 electron microscope at 68,000× magnification.
Supplementary Material
Acknowledgments
We thank Ritva Kajander and Pasi Laurinmäki for excellent technical assistance; Jyrki Hokkanen for graphics; the Biocenter Finland National Cryo-Electron Microscopy Unit, Institute of Biotechnology, Helsinki University, and the CSC-IT Center for Science Ltd. for providing facilities; Dr. Ilkka Julkunen and Dr. Irja Davidkin for wild-type virus and facilities; Dr. Yves Durocher for plasmid pTT5SH8Q2; Prof. Yusuke Yanagi for the Vero-SLAM cell line; and Prof. Timo Hyypiä for useful discussions and comments on the manuscript. This work was supported by the Academy of Finland Centre of Excellence Programme in Virus Research 2006–2011, Grant 129684 (to S.J.B.); Academy of Finland Grants 130750 (to J.T.H.), 128539 (to P.S.), and 139178 (to S.J.B.); the Sigrid Juselius Foundation (S.J.B.); and Viikki Graduate School in Molecular Biosciences and European Molecular Biology Organization Short-Term Fellowship ASTF 171-2009 (to L.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The average models reported in this paper have been deposited with the Unified Data Resource for 3D Electron Microscopy, EMDatabank.org (accession codes EMD-1973 and EMD-1974).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105770108/-/DCSupplemental.
References
- 1.Rima BK, Duprex WP. Morbilliviruses and human disease. J Pathol. 2006;208:199–214. doi: 10.1002/path.1873. [DOI] [PubMed] [Google Scholar]
- 2.WHO Global reductions in measles mortality 2000–2008 and the risk of measles resurgence. Wkly Epidemiol Rec. 2009;84:509–516. [PubMed] [Google Scholar]
- 3.Yanagi Y, Takeda M, Ohno S. Measles virus: Cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87:2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- 4.Hirano A, Wang AH, Gombart AF, Wong TC. The matrix proteins of neurovirulent subacute sclerosing panencephalitis virus and its acute measles virus progenitor are functionally different. Proc Natl Acad Sci USA. 1992;89:8745–8749. doi: 10.1073/pnas.89.18.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riedl P, Moll M, Klenk HD, Maisner A. Measles virus matrix protein is not cotransported with the viral glycoproteins but requires virus infection for efficient surface targeting. Virus Res. 2002;83:1–12. doi: 10.1016/s0168-1702(01)00379-3. [DOI] [PubMed] [Google Scholar]
- 6.Runkler N, Pohl C, Schneider-Schaulies S, Klenk HD, Maisner A. Measles virus nucleocapsid transport to the plasma membrane requires stable expression and surface accumulation of the viral matrix protein. Cell Microbiol. 2007;9:1203–1214. doi: 10.1111/j.1462-5822.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirano A, Ayata M, Wang AH, Wong TC. Functional analysis of matrix proteins expressed from cloned genes of measles virus variants that cause subacute sclerosing panencephalitis reveals a common defect in nucleocapsid binding. J Virol. 1993;67:1848–1853. doi: 10.1128/jvi.67.4.1848-1853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki M, et al. The matrix protein of measles virus regulates viral RNA synthesis and assembly by interacting with the nucleocapsid protein. J Virol. 2009;83:10374–10383. doi: 10.1128/JVI.01056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suryanarayana K, Baczko K, ter Meulen V, Wagner RR. Transcription inhibition and other properties of matrix proteins expressed by M genes cloned from measles viruses and diseased human brain tissue. J Virol. 1994;68:1532–1543. doi: 10.1128/jvi.68.3.1532-1543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cathomen T, et al. A matrix-less measles virus is infectious and elicits extensive cell fusion: Consequences for propagation in the brain. EMBO J. 1998;17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cathomen T, Naim HY, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998;72:1224–1234. doi: 10.1128/jvi.72.2.1224-1234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spielhofer P, et al. Chimeric measles viruses with a foreign envelope. J Virol. 1998;72:2150–2159. doi: 10.1128/jvi.72.3.2150-2159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tahara M, Takeda M, Yanagi Y. Altered interaction of the matrix protein with the cytoplasmic tail of hemagglutinin modulates measles virus growth by affecting virus assembly and cell-cell fusion. J Virol. 2007;81:6827–6836. doi: 10.1128/JVI.00248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loney C, Mottet-Osman G, Roux L, Bhella D. Paramyxovirus ultrastructure and genome packaging: Cryo-electron tomography of sendai virus. J Virol. 2009;83:8191–8197. doi: 10.1128/JVI.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin D. In: Fields Virology. Knipe D, et al., editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1401–1441. [Google Scholar]
- 16.Stallcup KC, Wechsler SL, Fields BN. Purification of measles virus and characterization of subviral components. J Virol. 1979;30:166–176. doi: 10.1128/jvi.30.1.166-176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida JD, Howatson AF. A negative staining method for cell-associated virus. J Cell Biol. 1963;16:616–620. doi: 10.1083/jcb.16.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubois-Dalcq M, Barbosa LH. Immunoperoxidase stain of measles antigen in tissue culture. J Virol. 1973;12:909–918. doi: 10.1128/jvi.12.4.909-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyanagi S, ter Meulen V, Katz M, Koprowski H. Comparison of subacute sclerosing panencephalitis and measles viruses: an electron microscope study. J Virol. 1971;7:176–187. doi: 10.1128/jvi.7.1.176-187.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown HR, Goller N, Thormar H, Norrby E. Fuzzy material surrounding measles virus nucleocapsids identified as matrix protein. Brief report. Arch Virol. 1987;94:163–168. doi: 10.1007/BF01313735. [DOI] [PubMed] [Google Scholar]
- 21.Robbins SJ, Bussell RH, Rapp F. Isolation and partial characterization of two forms of cytoplasmic nucleocapsids from measles virus-infected cells. J Gen Virol. 1980;47:301–310. doi: 10.1099/0022-1317-47-2-301. [DOI] [PubMed] [Google Scholar]
- 22.Bhella D, Ralph A, Yeo RP. Conformational flexibility in recombinant measles virus nucleocapsids visualised by cryo-negative stain electron microscopy and real-space helical reconstruction. J Mol Biol. 2004;340:319–331. doi: 10.1016/j.jmb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Schoehn G, et al. The 12 A structure of trypsin-treated measles virus N-RNA. J Mol Biol. 2004;339:301–312. doi: 10.1016/j.jmb.2004.03.073. [DOI] [PubMed] [Google Scholar]
- 24.Desfosses A, Goret G, Farias Estrozi L, Ruigrok RWH, Gutsche I. Nucleoprotein-RNA orientation in the measles virus nucleocapsid by three-dimensional electron microscopy. J Virol. 2011;85:1391–1395. doi: 10.1128/JVI.01459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grünewald K, Cyrklaff M. Structure of complex viruses and virus-infected cells by electron cryo tomography. Curr Opin Microbiol. 2006;9:437–442. doi: 10.1016/j.mib.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Subramaniam S, Bartesaghi A, Liu J, Bennett AE, Sougrat R. Electron tomography of viruses. Curr Opin Struct Biol. 2007;17:596–602. doi: 10.1016/j.sbi.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heggeness MH, Smith PR, Choppin PW. In vitro assembly of the nonglycosylated membrane protein (M) of Sendai virus. Proc Natl Acad Sci USA. 1982;79:6232–6236. doi: 10.1073/pnas.79.20.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewitt JA, Nermut MV. A morphological study of the M-protein of Sendai virus. J Gen Virol. 1977;34:127–136. doi: 10.1099/0022-1317-34-1-127. [DOI] [PubMed] [Google Scholar]
- 29.Bernard C, et al. Interaction between the C-terminal domains of N and P proteins of measles virus investigated by NMR. FEBS Lett. 2009;583:1084–1089. doi: 10.1016/j.febslet.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Gely S, et al. Solution structure of the C-terminal X domain of the measles virus phosphoprotein and interaction with the intrinsically disordered C-terminal domain of the nucleoprotein. J Mol Recognit. 2010;23:435–447. doi: 10.1002/jmr.1010. [DOI] [PubMed] [Google Scholar]
- 31.Kingston RL, Hamel DJ, Gay LS, Dahlquist FW, Matthews BW. Structural basis for the attachment of a paramyxoviral polymerase to its template. Proc Natl Acad Sci USA. 2004;101:8301–8306. doi: 10.1073/pnas.0402690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McPhee HK, et al. Influence of lipids on the interfacial disposition of respiratory syncytical virus matrix protein. Langmuir. 2011;27:304–311. doi: 10.1021/la104041n. [DOI] [PubMed] [Google Scholar]
- 33.Hewitt JA. Studies on the subunit composition of the M-protein of Sendai virus. FEBS Lett. 1977;81:395–397. doi: 10.1016/0014-5793(77)80562-0. [DOI] [PubMed] [Google Scholar]
- 34.Corey EA, Iorio RM. Mutations in the stalk of the measles virus hemagglutinin protein decrease fusion but do not interfere with virus-specific interaction with the homologous fusion protein. J Virol. 2007;81:9900–9910. doi: 10.1128/JVI.00909-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corey EA, Iorio RM. Measles virus attachment proteins with impaired ability to bind CD46 interact more efficiently with the homologous fusion protein. Virology. 2009;383:1–5. doi: 10.1016/j.virol.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plemper RK, Hammond AL, Cattaneo R. Measles virus envelope glycoproteins hetero-oligomerize in the endoplasmic reticulum. J Biol Chem. 2001;276:44239–44246. doi: 10.1074/jbc.M105967200. [DOI] [PubMed] [Google Scholar]
- 37.Plemper RK, Hammond AL, Gerlier D, Fielding AK, Cattaneo R. Strength of envelope protein interaction modulates cytopathicity of measles virus. J Virol. 2002;76:5051–5061. doi: 10.1128/JVI.76.10.5051-5061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calder LJ, Wasilewski S, Berriman JA, Rosenthal PB. Structural organization of a filamentous influenza A virus. Proc Natl Acad Sci USA. 2010;107:10685–10690. doi: 10.1073/pnas.1002123107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohl C, Duprex WP, Krohne G, Rima BK, Schneider-Schaulies S. Measles virus M and F proteins associate with detergent-resistant membrane fractions and promote formation of virus-like particles. J Gen Virol. 2007;88:1243–1250. doi: 10.1099/vir.0.82578-0. [DOI] [PubMed] [Google Scholar]
- 40.Rager M, Vongpunsawad S, Duprex WP, Cattaneo R. Polyploid measles virus with hexameric genome length. EMBO J. 2002;21:2364–2372. doi: 10.1093/emboj/21.10.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohn W, Rutter G, Hohenberg H, Mannweiler K, Nobis P. Involvement of actin filaments in budding of measles virus: studies on cytoskeletons of infected cells. Virology. 1986;149:91–106. doi: 10.1016/0042-6822(86)90090-5. [DOI] [PubMed] [Google Scholar]
- 42.Mottet-Osman G, et al. Suppression of the Sendai virus M protein through a novel short interfering RNA approach inhibits viral particle production but does not affect viral RNA synthesis. J Virol. 2007;81:2861–2868. doi: 10.1128/JVI.02291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen MR, et al. Intrinsic disorder in measles virus nucleocapsids. Proc Natl Acad Sci USA. 2011;108:9839–9844. doi: 10.1073/pnas.1103270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bourhis J-M, Canard B, Longhi S. Structural disorder within the replicative complex of measles virus: Functional implications. Virology. 2006;344:94–110. doi: 10.1016/j.virol.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 45.Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30(2):E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Huiskonen JT, et al. Electron cryotomography of Tula hantavirus suggests a unique assembly paradigm for enveloped viruses. J Virol. 2010;84:4889–4897. doi: 10.1128/JVI.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.