Abstract
Tetherin (Bst-2 CD317) is a cell-surface protein whose expression is induced by IFNα. Although tetherin expression causes the retention of retrovirus particles on the surface of infected cells, it is not known whether tetherin inhibits retroviral replication or pathogenesis in vivo. Mutation of tetherin antagonists often has little effect on retroviral replication in vitro, and, although tetherin can reduce the yield of extracellular viral particles, some studies suggest that tetherin actually enhances direct cell-to-cell viral transmission. We generated tetherin-deficient mice to determine the effect of this protein on murine retrovirus replication and pathogenesis. We find that tetherin markedly inhibits the replication of Moloney murine leukemia virus (Mo-MLV) and is required for the antiretroviral activity of IFNα to be fully manifested in vitro. Surprisingly, Mo-MLV replication and disease progression was not significantly different in WT and tetherin-deficient mice, but this finding was explained by the fact that Mo-MLV infection did not induce detectable tetherin expression on candidate target cells in vivo. Indeed, IFNα induction was required to reveal the anti–Mo-MLV activity of tetherin in vivo. Moreover, LP-BM5, an MLV strain that has been demonstrated to induce immune activation and IFNα expression, achieved higher levels of viremia and induced exaggerated pathology in tetherin-deficient mice. These data indicate that tetherin is a bona fide antiviral protein and can reduce retroviral replication and disease in vivo.
Mammals encode an array of molecules that can be constitutively expressed or induced by IFNs and have been demonstrated or suspected to have direct antiretroviral activity. One such molecule is tetherin, an unusual type I IFN-induced membrane protein that has both transmembrane and glycophosphatidylinositol membrane anchors (1, 2). Tetherin was first demonstrated to cause the retention of HIV-1 and Moloney murine leukemia virus (Mo-MLV) particles on the surface of infected cells (3, 4), but subsequent studies have shown that it can induce the retention of a variety of enveloped virus particles, including widely divergent representatives of the retrovirus, filovirus, rhabdovirus, arenavirus, and herpesvirus families (5–8). Mechanistic studies have shown that virion retention occurs after the infiltration of their lipid envelopes by the tetherin protein itself, which leads to the tethering of virions on the surface of infected cells (9–11).
There is as yet no evidence that tetherin influences viral replication and pathogenesis in vivo. Indeed, some studies suggest that tetherin does not inhibit, and can even enhance, the transmission of HIV-1 from infected cells to neighboring uninfected cells by concentrating virions at the cell surface and enhancing the formation of so-called “virological synapses” (12). Because a significant proportion of cell-to-cell retroviral transmission in vitro and in vivo may occur via direct cell contact (13–16) and because deletion of the tetherin antagonist, Vpu, from the HIV-1 genome has little effect on replication in some cell-culture assays (17), the role of tetherin as an antiviral factor in vivo is uncertain (18, 19). Moreover, some studies suggest that tetherin has immunomodulatory rather than direct antiviral activity (20, 21).
In this study, we use tetherin-deficient cells and animals to examine the role of tetherin in inhibiting retroviral replication in vitro and in mediating the antiretroviral activity of IFNα. We demonstrate that tetherin has potent antiretroviral activity in vitro and is required for the full antiretroviral activity of IFNα both in vitro and in vivo. Moreover, although tetherin in not required for the development of a normal murine immune system, its absence can exacerbate the replication and pathogenesis of a murine retrovirus.
Results
Tetherin Potently Inhibits Retroviral Replication in Vitro.
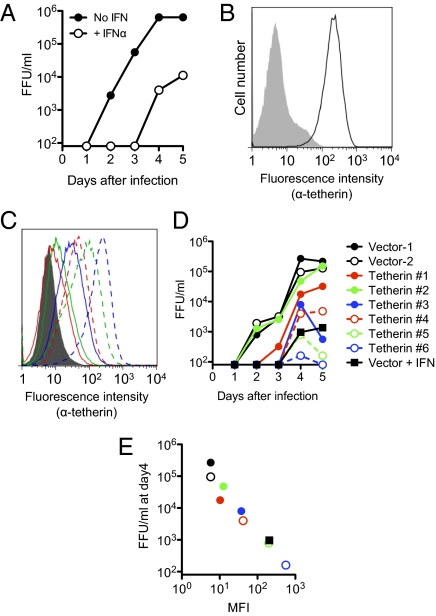
Tetherin is constitutively expressed on a few cell types (e.g., plasmacytoid dendritic cells, plasma B cells) but is absent from many others in mice (20). However, its expression is induced by type I IFN in a wide range of cells (20). Because tetherin is one of many IFN-stimulated genes (ISGs) that are possible effectors of IFN's antiretroviral activity, we first determined whether and how IFNα inhibits the replication of a murine retrovirus (Mo-MLV) in cell culture and what role, if any, tetherin plays in the in vitro antiretroviral activity of IFNα. Mo-MLV replication in NIH/3T3 cells was potently inhibited by IFNα, with yields of virus reduced by 10- to 100-fold over 5 d of replication (Fig. 1A). Notably, tetherin was not constitutively expressed on NIH/3T3 cells but was strongly induced by treatment with IFNα (Fig. 1B). We generated a panel of NIH/3T3-derived cell lines, each expressing a different level of murine tetherin, some of which closely matched those on IFNα-treated cells (Fig. 1C). Mo-MLV replication in these cell lines, as measured by the levels of infectious virus in culture supernatants, correlated inversely with the level of tetherin expression (Fig. 1 D and E). Strikingly, Mo-MLV replication in IFNα-treated NIH/3T3 cells was similar in magnitude to that in cells that were not treated with IFNα but expressed similar levels of tetherin (Fig. 1 D and E), suggesting that tetherin could be a major contributor to the antiretroviral activity of IFNα in vitro.
Fig. 1.
Tetherin potently inhibits Mo-MLV replication in vitro. (A) Accumulation of Mo-MLV, in focus-forming units/milliliter (FFU/mL), in the supernatant of NIH/3T3 cells infected at a multiplicity of infection of 0.005 in the presence or absence of 100 U/mL IFNα. (B) Flow cytometric analysis of tetherin expression on untreated (shaded histogram) or 100 U/mL IFNα-treated (unshaded histogram) NIH/3T3 cells. (C) Tetherin levels on a panel of six NIH/3T3 cell clones stably expressing tetherin (colored histograms) or empty vector (shaded histogram). (D) Mo-MLV replication in cell lines from C and on IFNα-treated cells (Vector + IFN). (E) Mo-MLV accumulation at day 4 plotted against tetherin expression [mean fluorescence intensity (MFI)] for the cell lines in C and D. Note that the colors, symbols, and line styles in C–E are matched.
Generation and Characterization of Tetherin-Deficient Mice.
To determine whether tetherin is indeed a key effector of the antiretroviral action of type I IFN, we generated tetherin-deficient mice (Fig. S1). To accommodate the possibility that tetherin might have some essential function in mice, a conditional knockout (CKO) strategy was adopted, whereby sequences comprising the majority of exon 1 (which encodes the transmembrane and the bulk of the extracellular domain) were flanked by loxP sites (Fig. S1A). However, tetherin-null mice, resulting from breeding tetherin CKO mice to mice expressing Cre under the control of a promoter expressed in the germ line (Fig. 1 A and B), were fertile, had no apparent physical or behavioral deficits, and were born at the expected frequency (Fig. S1 D and E). Tetherin-deficient mice had numbers of total splenocytes, thymocytes, and bone marrow cells that were similar to those of WT animals and had normal proportions of splenic CD3+, B220+, CD11b+, and CD11c+ cells (Fig. S2A) as well as normal thymocyte subsets (Fig. S2B). Tetherin-deficient mice also had normal percentages of bone marrow erythromyeloid progenitor and hematopoietic progenitor cell populations (MP and LSK, respectively, in Fig. S2C). Plasmacytoid dendritic (Siglec-H+) cells, which normally express high levels of tetherin, were also found at normal frequencies (Fig. S2A) but, importantly, were devoid of cell-surface tetherin when they were harvested from tetherin-deficient donors (Fig. S2D). Thus, the absence of tetherin appeared to be fully compatible with the development of the major organ systems of mice, and, in particular, no apparent perturbations of the immune system occurred as a consequence of tetherin deletion. Importantly, tetherin-deficient cells responded similarly to WT cells when stimulated with Toll-like receptor (TLR) 3, 7, and 9 agonists in vitro (Fig. S3), suggesting that there was no functional impairment in the activation of these cells in the absence of tetherin.
Tetherin Is Required for the Full Antiretroviral Activity of IFNα in Vitro.
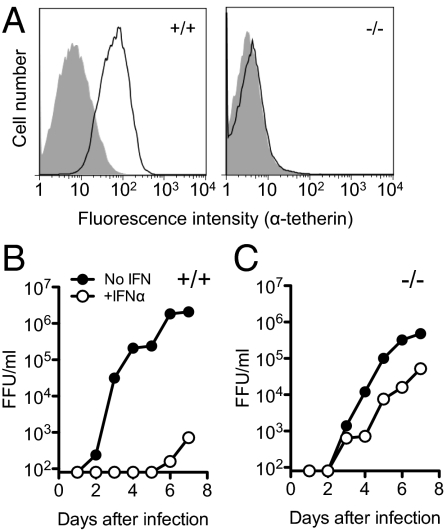
Tetherin was not expressed at detectable levels on murine embryonic fibroblasts (MEFs) derived from WT or tetherin-deficient mice (Fig. 2A), which each supported similar levels of Mo-MLV replication in vitro (Fig. 2 B and C). Conversely, tetherin was strongly induced in WT (but not tetherin-deficient) MEFs when they were treated with IFNα (Fig. 2A). Notably, IFNα potently inhibited the replication of Mo-MLV in WT MEFs, but its antiviral efficacy was greatly reduced in tetherin-deficient MEFs, although there was some residual antiviral activity of IFNα in the absence of tetherin (Fig. 2 B and C). Thus, tetherin expression was sufficient to recapitulate the effect of IFNα on Mo-MLV replication and necessary for the full effect of IFNα on Mo-MLV replication to be manifested in cultured cells.
Fig. 2.
Tetherin is required for the full antiretroviral activity of IFNα in vitro. (A) Tetherin expression on WT (+/+) and tetherin-deficient (−/−) MEFs that were untreated (shaded histogram) or treated with 100 U/mL IFNα for 24 h (unshaded histogram). (B and C) Mo-MLV replication in WT (B) or tetherin-deficient (C) MEFs, in the presence (+IFNα) and absence (No IFN) of 100 U/mL IFNα assayed as in Fig. 1.
Tetherin Is Constitutively Expressed on Few Cells in Vivo and Is Not Induced by Mo-MLV Infection.
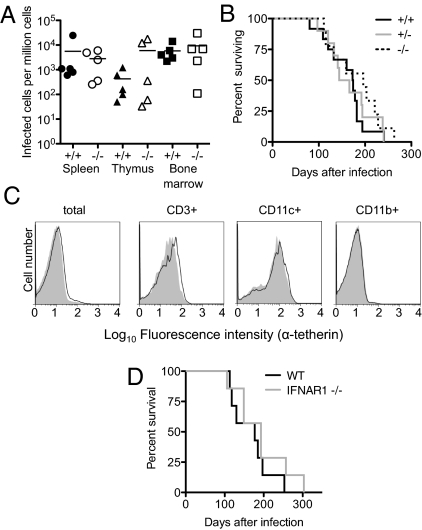
Mo-MLV infection of neonatal C57BL/6 mice gives rise to large numbers of infected cells in bone marrow, spleen, and thymus at 8 d after infection (Fig. 3A), with tumors (mostly of T-cell origin) becoming obvious and eventually fatal at 100–300 d after challenge (Fig. 3B). Surprisingly, upon Mo-MLV infection of neonates, the numbers of Mo-MLV–infected cells did not differ in WT or tetherin-deficient littermates at 8 d after infection (Fig. 3A). Moreover, mice succumbed to Mo-MLV–induced disease at approximately the same rate, irrespective of tetherin genotype (Fig. 3B). This finding at first suggested that tetherin had little effect on Mo-MLV replication in vivo, despite being an effective inhibitor in vitro (Fig. 1).
Fig. 3.
No effect of tetherin deficiency on Mo-MLV infection and pathogenesis in vivo. (A) Shown are numbers of infected cells, determined by using infectious center assays, performed with cells harvested from Mo-MLV–infected mouse tissues at 8 d after infection. (B) Kaplan–Meier survival curves of Mo-MLV–infected mice: +/+, n = 12; +/−, n = 10; −/−, n = 9. (C) Flow cytometric analysis of tetherin expression on bone marrow-derived cells from mock-infected (shaded histograms) or Mo-MLV–infected (unshaded histograms) mice at 12 d after infection. (D) Kaplan–Meier survival curves of Mo-MLV–infected WT (n = 7) or IFNAR1-deficient (n = 7) mice.
Because tetherin exhibited a clear antiretroviral activity in vitro but not, apparently, in vivo, we next asked whether tetherin was expressed on cells that are infected with Mo-MLV in mice. In fact, the majority of cells harvested from bone marrow of mice did not express tetherin (Fig. S4), and 12 d of Mo-MLV infection did not induce up-regulation of tetherin expression (Fig. 3C), despite the fact that infectious center assays suggested that a large fraction (∼10–100%) of splenocytes, thymocytes, and bone marrow cells were infected with Mo-MLV at this time point (Fig. S5). Altogether, these data suggested the possibility that Mo-MLV replication and pathogenesis in vivo is not affected by tetherin, at least in part because Mo-MLV replication does not induce a robust type I IFN response and, perhaps as a consequence, the bulk of Mo-MLV replication occurs in cells that do not express tetherin. This hypothesis was supported by the fact that IFNα receptor-deficient mice developed lethal Mo-MLV–induced disease with similar kinetics to WT mice (Fig. 3D).
Induction of Tetherin Expression Reveals Its Antiretroviral Activity in Vivo.
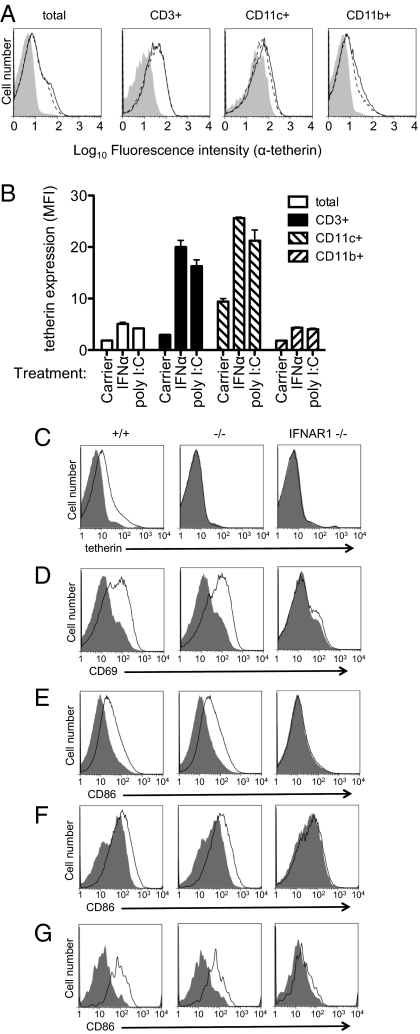
To determine whether tetherin could exert an antiretroviral effect in vivo if its expression was induced by stimulating a type I IFN response, we treated Mo-MLV–infected mice with polyinosinic:polycytidylic acid [poly(I:C)], a type I IFN inducer, and measured release of virus into plasma a short time (24 h) later. Poly(I:C) caused up-regulation of tetherin on various cell types (Fig. 4 A and B), including T cells, which are thought to be the preferred target for Mo-MLV replication. Importantly, both WT and tetherin-deficient mice responded similarly to poly(I:C) treatment (Fig. 4 D–G) by up-regulating several activation markers, with the obvious exception of tetherin (Fig. 4C). Additionally, the poly(I:C)-induced activation, including tetherin up-regulation, depended on type I IFN signaling, as demonstrated by the absence of cell activation in IFN (α, β, and ω) receptor 1 (IFNAR1)-deficient animals (Fig. 4 C–G). These data suggested that, with the exception of tetherin up-regulation, the response to poly(I:C) was unaffected in tetherin-deficient mice.
Fig. 4.
Endogenous tetherin is induced by type I IFN in vivo. (A) One-week-old mice were mock-treated (shaded histograms), IFNα-treated (solid line), or poly(I:C)-treated (dashed line), and bone marrow was harvested at 24 h later for flow cytometric analysis of tetherin levels on various cell types. (B) Quantitation of tetherin expression as in A from three donors. Error bars indicate 1 SD. (C–G) WT (+/+), tetherin-deficient (−/−), and IFNAR1-deficient (IFNAR1−/−) mice were treated with poly(I:C), and FACS analysis was performed on splenocytes 24 h later. Shaded histograms represent control PBS-treated animals, and unshaded histograms represent poly(I:C)-treated animals. Tetherin expression was assessed on total splenocytes (C), and expression of the indicated activation markers was assessed on CD3+ (D), B220+ (E), and CD11c+ (F) cells.
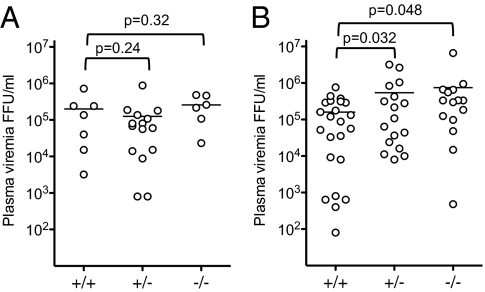
Although plasma viremia was highly variable in cohorts of Mo-MLV–infected animals, there was no significant difference in plasma viremia in tetherin-deficient mice compared with WT or heterozygous littermates at 12 d after infection (Fig. 5A). However, when animals were treated with a single dose of poly(I:C) at 12 d after infection and then measured for plasma viremia the following day, the level of plasma viremia was significantly higher (∼fivefold, P < 0.05) in tetherin-deficient mice compared with WT littermates (Fig. 5B).
Fig. 5.
Tetherin induction reduces Mo-MLV plasma viremia in vivo. Mo-MLV–infected mice were left untreated and plasma viremia was measured at 12 d after infection (A) or were treated with poly(I:C) at 12 d after infection and plasma viremia was assayed 24 h later (B). Horizontal lines indicate the mean of each data set.
Tetherin-Deficient Mice Exhibit Exacerbated Murine AIDS Virus-Induced Disease.
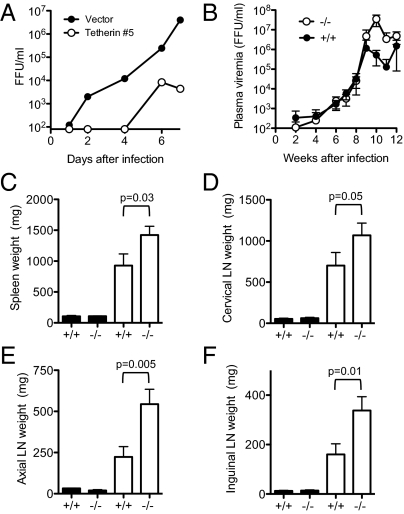
These accumulated data indicated that tetherin is not required for the development and function of the immune system, but it can indeed inhibit the release and replication of Mo-MLV in vitro and in vivo, provided that its expression is induced in cells in which the virus is replicating. We next determined whether tetherin could influence replication and the course of disease during retroviral infection without the need for an intervention [e.g., poly(I:C) treatment] to stimulate its expression. To do so, we used a different MLV, termed LP-BM5, that induces immune activation, polyclonal B-cell proliferation, and impaired immune responses and is sometimes referred to as the murine AIDS virus because of superficial similarities in the aforementioned pathogenesis to that of HIV infection in humans (22, 23). Moreover, previous studies have shown that LP-BM5 induces the expression of type I IFN in infected mouse tissues at later time points (∼8 wk after infection) (24) Consistent with this finding, tetherin expression was found to be up-regulated in the splenocytes of LP-BM5–infected WT mice at 14 wk after infection compared with uninfected WT controls (Fig. S6). In vitro experiments revealed that LP-BM5 replication was inhibited by tetherin to a similar extent as Mo-MLV replication, as expected (Fig. 6A). Although infection of WT and tetherin-deficient mice with LP-BM5 resulted in similar levels of plasma viremia at early time points, measurements at later times (>8 wk) after infection, when type I IFN is induced (24) and tetherin up-regulation is evident (Fig. S6), showed a clear effect of tetherin on LP-BM5 replication. Specifically, viremia peaked at 9 to10 wk after infection and was ∼10- to 100-fold higher in tetherin-deficient mice compared with WT controls (Fig. 6B). Shortly thereafter, tetherin-deficient mice became moribund and they, along with WT controls, were killed at 14 wk after infection. Inspection of lymphoid tissues from age-matched uninfected WT and tetherin-deficient mice revealed no changes in uninfected mice as a result of the presence or absence of tetherin (Fig. 6 C–F). However, the splenomegaly and lymphadenopathy that accompanies LP-BM5 infection was significantly exaggerated in the tetherin-deficient mice, with up to twofold greater mean spleen and lymph node weights compared with infected WT controls (Fig. 6 C–F). Overall, assessment of both virological and pathologic parameters revealed clearly greater viral replication and more aggressive LP-BM5–induced disease progression in the absence of tetherin.
Fig. 6.
Tetherin restricts LP-BM5 MLV replication and pathogenesis in vivo. (A) LP-BM5 replication in NIH/3T3 cells stably expressing tetherin or vector alone, assayed as in Fig. 1A. (B) Plasma viremia, measured by focal immunoassay, in LP-BM5–infected mice over the course of 12 wk of infection. (C–F) Weights of lymphoid tissues from LP-BM5–infected animals (white bars; +/+, n = 8; −/−, n = 8) harvested at 14 wk after infection or age-matched, uninfected animals (black bars; +/+, n = 3; −/−, n = 2). Error bars indicate 1 SE.
Discussion
These data demonstrate that tetherin inhibits the replication of murine retroviruses both in vitro and in vivo. However, it appears that some retroviruses (e.g., Mo-MLV) encounter tetherin rather infrequently in vivo because its presence or absence did not affect Mo-MLV replication or pathogenesis in vivo. Because previous work has indicated that the in vivo replication of murine retroviruses is limited by adaptive immune responses (25), this finding, along with extensive immunophenotyping, strongly suggests that tetherin is not required for the development of a functional adaptive immune system. Furthermore, our findings suggest that Mo-MLV replicates in vivo without inducing a robust IFN response and evades a molecule that would otherwise profoundly limit its replication simply by not inducing its expression.
Many other enveloped viruses, including HIV-1 and simian immunodeficiency virus, induce a robust type I IFN response (26, 27). As such, these viruses are likely to frequently encounter tetherin (28, 29), and their replication is consequently inhibited. Indeed, this appears to be the situation for LP-BM5, whose replication and pathogenesis are attenuated by tetherin (Fig. 6). The elevated replication of LP-BM5 in tetherin-deficient mice could be attributable to both induction of type I IFN and infection of plasma B cells (22, 30), one of the few cell types that constitutively expresses tetherin in mice. Several viruses have evolved to antagonize tetherin function (in the case of HIV-1 and simian immunodeficiency virus with their Vpu, Nef, and envelope proteins) (3, 31–34), underscoring its potential role in limiting viral replication in vivo. Overall, it appears that tetherin has arisen in mammals primarily, and likely exclusively, as a directly acting innate protection against viral disease.
Materials and Methods
Generation of Tetherin-Deficient Mice.
A targeting construct was generated containing a Neo cassette flanked by 2 FRT sites and loxP sites flanking the first exon of the tetherin gene (Fig. S1A). Founder mice were crossed to FLP1 transgenic mice, creating the tetherin CKO allele (Bst2tm1Bsz), then crossed to Zp3-cre mice to delete the floxed sequence in the germ line. Mice heterozygous for the deleted allele were intercrossed to generate WT (+/+), heterozygous (+/−), and homozygous (−/−) knockout littermates that were used for experiments. See SI Materials and Methods for further details.
Viruses.
Mo-MLV was produced by transfecting NIH/3T3 cells with the infectious clone pNCS. LP-BM5 virus was harvested from infected SC-1 cells (AIDS Research and Reference Reagent Program, National Institutes of Health, Bethesda, MD). Viral stocks were titered by focal immunoassay. See SI Materials and Methods for further details.
Cells and In Vitro Virus Replication Assays.
NIH/3T3 cell clones stably expressing various levels of murine tetherin were generated by retroviral transduction. MEFs were generated by using standard procedures. For in vitro replication assays, NIH/3T3 cells or MEFs were infected with virus in the presence or absence of IFNα, and replication was monitored by focal immunoassay. See SI Materials and Methods for further details.
Mouse Infections.
Mice were infected by i.p. injection with Mo-MLV at 24–36 h after birth or with LP-BM5 at 6–10 wk old. Infected cells in mouse tissues and plasma viremia were measured by focal immunoassay on NIH/3T3 (Mo-MLV) or SC-1 (LP-BM5) cells. See SI Materials and Methods for further details.
Focal Immunoassay for Quantitation of Mo-MLV and LP-BM5.
NIH/3T3 or SC-1 monolayers were inoculated with serially diluted cell-culture supernatants, plasma, or mouse cell suspensions. After 48 h, cells were fixed, and foci of infection were detected and counted after staining with an antibody to MLV Gag and an HRP-conjugated secondary antibody. See SI Materials and Methods for further details.
Flow Cytometry.
Adherent cells or single-cell suspensions were made from tissues, harvested and stained with various antibodies, and analyzed with an LSR II flow cytometer (Becton Dickinson) and FlowJo software (Tree Star). See SI Materials and Methods for further details.
Supplementary Material
Acknowledgments
We thank Stephen Goff for pNCS and the Rockefeller University Gene Targeting Resource Center for assistance in the generation of tetherin-deficient mice. This work was supported by National Institutes of Health Grant R01AI501111 (to P.D.B.) and by the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113694108/-/DCSupplemental.
References
- 1.Ishikawa J, et al. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics. 1995;26:527–534. doi: 10.1016/0888-7543(95)80171-h. [DOI] [PubMed] [Google Scholar]
- 2.Kupzig S, et al. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 3.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 4.Van Damme N, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jouvenet N, et al. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci USA. 2009;106:2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J Virol. 2009;83:2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidner JM, et al. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J Virol. 2010;84:12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick K, et al. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 2010;6:e1000701. doi: 10.1371/journal.ppat.1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammonds J, Wang JJ, Yi H, Spearman P. Immunoelectron microscopic evidence for Tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 2010;6:e1000749. doi: 10.1371/journal.ppat.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Caballero D, et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jolly C, Booth NJ, Neil SJ. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J Virol. 2010;84:12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr JM, Hocking H, Li P, Burrell CJ. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology. 1999;265:319–329. doi: 10.1006/viro.1999.0047. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrov DS, et al. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993;67:2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol. 2010;84:8360–8368. doi: 10.1128/JVI.00443-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherer NM, et al. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyagi E, Andrew AJ, Kao S, Strebel K. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc Natl Acad Sci USA. 2009;106:2868–2873. doi: 10.1073/pnas.0813223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrew A, Strebel K. The interferon-inducible host factor bone marrow stromal antigen 2/tetherin restricts virion release, but is it actually a viral restriction factor? J Interferon Cytokine Res. 2011;31:137–144. doi: 10.1089/jir.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrew AJ, Miyagi E, Kao S, Strebel K. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology. 2009;6:80. doi: 10.1186/1742-4690-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasius AL, et al. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 21.Cao W, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolicoeur P. Murine acquired immunodeficiency syndrome (MAIDS): An animal model to study the AIDS pathogenesis. FASEB J. 1991;5:2398–2405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- 23.Mosier DE, Yetter RA, Morse HC., 3rd Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985;161:766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshi M, et al. The absence of IDO upregulates type I IFN production, resulting in suppression of viral replication in the retrovirus-infected mouse. J Immunol. 2010;185:3305–3312. doi: 10.4049/jimmunol.0901150. [DOI] [PubMed] [Google Scholar]
- 25.Hasenkrug KJ, Dittmer U. The role of CD4 and CD8 T cells in recovery and protection from retroviral infection: Lessons from the Friend virus model. Virology. 2000;272:244–249. doi: 10.1006/viro.2000.0387. [DOI] [PubMed] [Google Scholar]
- 26.Malleret B, et al. Primary infection with simian immunodeficiency virus: Plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 27.Stacey AR, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erikson E, et al. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc Natl Acad Sci USA. 2011;108:13688–13693. doi: 10.1073/pnas.1101684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homann S, Smith D, Little S, Richman D, Guatelli J. Upregulation of BST-2/tetherin by HIV infection in vivo. J Virol. 2011;85:10659–10668. doi: 10.1128/JVI.05524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pattengale PK, et al. Immunopathology of B-cell lymphomas induced in C57BL/6 mice by dualtropic murine leukemia virus (MuLV) Am J Pathol. 1982;107:362–377. [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta RK, et al. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc Natl Acad Sci USA. 2009;106:20889–20894. doi: 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia B, et al. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5:e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Tortorec A, Neil SJ. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J Virol. 2009;83:11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, et al. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6:54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.