Abstract
Interleukin-1α (IL-1α) and -β both bind to the same IL-1 receptor (IL-1R) and are potent proinflammatory cytokines. Production of proinflammatory (pro)–IL-1α and pro–IL-1β is induced by Toll-like receptor (TLR)-mediated NF-κB activation. Additional stimulus involving activation of the inflammasome and caspase-1 is required for proteolytic cleavage and secretion of mature IL-1β. The regulation of IL-1α maturation and secretion, however, remains elusive. IL-1α exists as a cell surface-associated form and as a mature secreted form. Here we show that both forms of IL-1α, the surface and secreted form, are differentially regulated. Surface IL-1α requires NF-κB activation only, whereas secretion of mature IL-1α requires additional activation of the inflammasome and caspase-1. Surprisingly, secretion of IL-1α also required the presence of IL-1β, as demonstrated in IL-1β–deficient mice. We further demonstrate that IL-1β directly binds IL-1α, thus identifying IL-1β as a shuttle for another proinflammatory cytokine. These results have direct impact on selective treatment modalities of inflammatory diseases.
Keywords: inflammation, innate immunity, pattern recognition
Pathogens activate the innate immune system and inflammatory responses via pattern-recognition receptors (PRRs), which include Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-like helicases (RLHs), and C-type lectin receptors (CLRs) (1, 2). Stimulation of TLRs leads to MyD88-dependent NF-κB activation and induces expression of the 31-kDa precursors of both IL-1α and IL-1β. Both cytokines bind to the IL-1 receptor (IL-1R) and are potent proinflammatory cytokines, mainly produced by myeloid cells such as macrophages, monocytes, and dendritic cells. IL-1α and IL-1β are processed into smaller biologically active forms, which are then secreted via the ER/Golgi-independent, but poorly understood, unconventional protein secretion pathway (3, 4). The cleavage mechanism of proinflammatory (pro)–IL-1β into mature IL-1β is well characterized. Upon assembly of different types of inflammasomes, caspase-1 is activated and cleaves pro–IL-1β into an N-terminal 14-kDa inactive propiece and a C-terminal 17-kDa mature and biologically active form of IL-1β. In contrast to IL-1β, IL-1α exists not only as a mature soluble form, but also as a cell-surface protein. Pro–IL-1α is thought to be myristoylated and translocated to the cell membrane where it associates with cell-surface components by an unknown mechanism (5, 6). It has been speculated that phosphorylation of pro–IL-1α induces a conformational change leading to surface anchoring via lectin binding (7, 8). Except for lectin-binding sites, IL-1α contains no transmembrane regions, GPI anchors, or other hydrophobic regions allowing surface anchoring. Processing of IL-1α induces the cleavage of pro–IL-1α into a propiece and a mature soluble form. This cleavage depends on calpains, Ca2+-dependent proteases present at the cytosolic side of the cell membrane (6, 9, 10). Though pro–IL-1α does not contain any predicted cleavage site for caspase-1, there is evidence that it can bind to caspase-1, and that caspase-1 may be involved in the secretion of IL-1α (11, 12). Therefore, caspase-1 may act as a regulator of unconventional protein secretion (4). Here, we investigated the role of the inflammasome and caspase-1 on the expression of secreted and surface IL-1α and found that surface expression was caspase-1 independent, whereas secretion of mature IL-1α required activation of both inflammasome and caspase-1. Surprisingly, we also found a role for IL-1β in the secretion of IL-1α.
Results
IL-1α Expressed on the Cell Surface Is Biologically Functional and Depends on TLR Stimulation Only, Whereas Secretion of Mature IL-1α Requires Additional Stimuli.
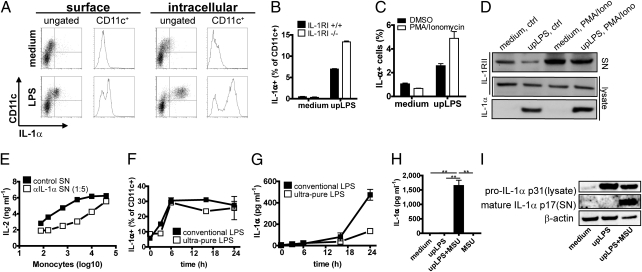
To study the kinetics of IL-1α surface expression, we stimulated human monocytes with LPS. Maximal levels of the cell-surface–bound IL-1α were detected after 6 h, whereas intracellular IL-1α levels continuously increased even at later time points (Fig. 1A and Fig. S1). As it remains unknown how IL-1α is anchored to the cell membrane, it has been debated whether surface-associated IL-1α is biologically active, or if it merely represents mature secreted IL-1α that is bound to its receptor IL-1RI (13). However, IL-1α surface expression on murine bone marrow-derived dendritic cells (bmDCs) from IL-1R−/− mice was comparable to that of C57BL/6 WT mice, indicating that IL-1R is not required for cell-surface expression of IL-1α (Fig. 1B). For unknown reasons, IL-1α surface expression was even consistently higher in IL-1RI−/− than in WT cells. Furthermore, to exclude that the IL-1α detected by surface staining was not IL-1α bound to the decoy IL-1RII, we shedded the IL-1RII in IL-1RI−/− bmDCs using phorbol myristate acetate (PMA)/ionomycin (14) and compared the amount of surface IL-1α by flow cytometry. Shedding of IL-1RII did not decrease levels of surface IL-1α (Fig. 1C), whereas shedding was confirmed by increased amounts of IL-1RII in the supernatant by Western blotting (Fig. 1D).
Fig. 1.
IL-1α expressed on the cell surface is biologically functional and depends only on TLR stimulation, whereas secretion of mature IL-1α requires additional stimuli. (A) Cell-surface and intracellular expression of IL-1α in human monocytes after LPS stimulation at different time points was measured by flow cytometry. Surface expression (maximal after 6 h) and intracellular expression (maximal after 24 h). Representative stainings of three independent experiments. (B) bmDCs derived from IL-1RI−/− mice (open bars) and WT mice (closed bars) were stimulated with upLPS for 24 h, and surface-associated IL-1α was measured by flow cytometry. Data are mean and SD of duplicates. One representative of three independent experiments is shown. (C and D) bmDCs derived from IL-1RI−/− mice were stimulated with medium or upLPS in presence (open bars) or absence (closed bars) of PMA/Ionomycin for surface IL-1RII shedding. Amounts of surface IL-1α were compared by flow cytometry (C). Shedding of IL-1RII was confirmed by an IL-1RII Western blot of precipitated supernatant. Lysates of cells were tested for intracellular IL-1RII and IL-1α expression by Western blot (D). Data shows mean and SD of one representative of two independent experiments. (E) EL4-6.1 cells were incubated with paraformaldehyde-fixed human monocytes expressing surface IL-1α after LPS stimulation in the presence (open squares) or absence (closed squares) of anti–IL-1α antibody. IL-2 production was quantified by ELISA. Mean and SD of triplicates. (F and G) Human monocytes were stimulated with upLPS (open squares) or conventional LPS (closed squares) over the indicated time period. Surface IL-1α expression was measured by flow cytometry (F), and secreted IL-1α was quantified by ELISA (G). (H and I) IC-21 macrophages were treated with upLPS with or without MSU during the last 6 h of a 24-h stimulation period. IL-1α secretion was measured by ELISA (H). Mean and SD of six replicates. *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired, two-tailed Mann–Whitney U test). Pro–IL-1α and mature IL-1α were assessed in cell lysate and supernatant, respectively, by Western blot (I). As control actin, Western blot was performed.
To assess whether cell-surface–associated IL-1α was biologically active, human monocytes were stimulated with LPS, washed to eliminate secreted IL-1α, and fixed with paraformaldehyde to block further secretion. Serial dilutions of these monocytes were then coincubated with an IL-1 reporter cell line secreting IL-2 upon IL-1RI signaling. Monocytes were able to induce IL1-RI–dependent IL-2 secretion in a cell number-dependent manner, and IL-2 secretion could be suppressed by an anti–IL-1α antibody (Fig. 1E).
To compare the kinetics of IL-1α surface expression with IL-1α secretion, monocytes were stimulated with LPS for different time periods. Though surface expression of IL-1α was already detectable 3 h after LPS stimulation (Fig. 1F and Fig. S1), and reached its maximum after only 6 h, IL-1α secretion was low during the first 16 h and continuously increased up to 24 h (Fig. 1G). Interestingly, we observed that stimulation of monocytes using a conventional (i.e., not highly purified LPS preparation) stimulated both IL-1α surface expression and secretion, whereas an ultrapure (up)LPS preparation induced only surface expression but not secretion of IL-1α (Fig. 1 F and G), suggesting that IL-1α secretion was induced by contaminations in the conventional LPS preparation, which may stimulate additional pathways other than TLR4 and NF-κB. It is known that muramyldipeptid (MPD)-contaminated LPS activates the NALP3 inflammasome (15). To investigate whether such contaminants induced IL-1α secretion through inflammasome activation, a macrophage cell line was stimulated with upLPS or upLPS in combination with the NALP3 inflammasome activator monosodium urate (MSU) (15). Indeed, cells stimulated with upLPS secreted the mature form of IL-1α only in the presence of MSU (Fig. 1 H and I).
Inflammasome Activation Is Crucial for IL-1α Secretion, but Not Required for Surface Expression.
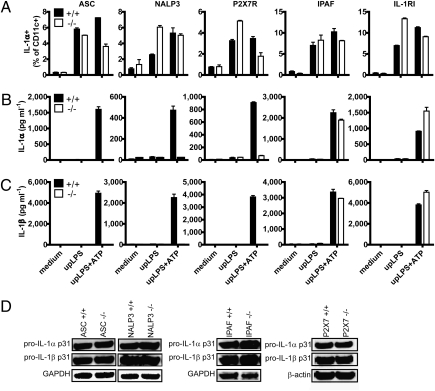
To further confirm the role of inflammasome activation in IL-1α secretion, bmDCs from apoptosis-associated Speck-like protein containing a caspase recruitment domain (ASC)-, NALP3-, P2X7R-, and IPAF-deficient mice were stimulated using upLPS alone or upLPS plus ATP. ATP activates the ASC-dependent NALP3 (also known as cryopyrin, CIAS1, or NLRP3) inflammasome via P2X7 receptor (purinoceptor 7), which leads to pannexin pore formation and efflux of K+ (16, 17). ATP does not activate the ICE-protease activating factor (IPAF) (also known as NLRC4) inflammasome (18–20). Several inflammasomes share the adapter protein ASC, which is able to link the pyrin (PYD) domain of an inflammasome sensor to the caspase recruitment domain (CARD) of caspase-1 (1, 2). As expected, secretion of IL-1β was impaired in ASC-deficient bmDCs when treated with upLPS and ATP. In line with the above data, the same was observed for IL-1α secretion, which was also impaired in ASC−/− bmDCs. In contrast, IL-1α surface expression was not affected in ASC−/− bmDCs (Fig. 2 A–C). Consistent with our observations in the ASC−/− bmDCs, cells from NALP3−/− and P2X7R−/− mice expressed normal levels of cell surface IL-1α (Fig. 2A), but were not able to secrete IL-1α and IL-1β upon stimulation with upLPS and ATP (Fig. 2 B and C). Furthermore, the reduction of IL-1α secretion in the ASC−/−, NALP3−/−, and P2X7R−/− cells was not due to a missing feedback loop of secreted IL-1β signaling via IL-1RI, because we found comparable levels of IL-1α secretion in bmDCs from IL-1RI−/− and WT mice (Fig. 2 A–C). Expectedly, IPAF deficiency did not have an impact on IL-1α and IL-1β secretion using upLPS and ATP stimulation (Fig. 2 B and C). Induction of intracellular pro–IL-1α and pro–IL-1β by upLPS was comparable in all of the above-tested bmDCs and therefore to excluded transcriptional differences (Fig. 2D).
Fig. 2.
Inflammasome activation is crucial for IL-1α secretion, but not required for surface IL-1α expression. (A–C) bmDCs derived from ASC-, NALP3-, P2X7R-, IPAF-, and IL-RI–deficient (open bars) or WT mice (closed bars) were stimulated with upLPS in presence or absence of ATP as indicated during the last 30 min of a 24-h stimulation. Surface IL-1α expression was analyzed by flow cytometry (A). IL-1α and -β secretion in the supernatant was quantified by ELISA (B and C). Mean and SD of duplicates and one representative of three independent experiments are shown. (D) Intracellular pro–IL-1α and -β were measured by Western blot in lysates prepared from bmDCs stimulated with upLPS. One representative of two independent experiments is shown.
In Vitro and in Vivo Secretion of IL-1α Is Caspase-1 Dependent.
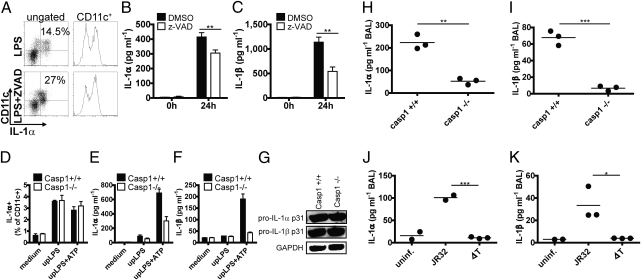
The importance of inflammasome activation for IL-1α secretion was confirmed by inhibiting caspases with the pan-caspase inhibitor carbobenzoxy-Val-Ala-Asp (OMe) fluoromethylketone (z-VAD fmk). This resulted in decreased IL-1α and -β secretion in LPS-pulsed human monocytes. In contrast, IL-1α surface expression was enhanced (Fig. 3 A–C). The role of caspase-1 in IL-1α and -β secretion was confirmed by stimulation of caspase-1−/− bmDCs with upLPS and ATP, which resulted in decreased IL-1α and IL-1β secretion (Fig. 3 E and F), whereas the intracellular proforms of both cytokines were induced at normal levels (Fig. 3G). In line with the above findings, caspase-1−/− bmDCs were able to express cell-surface IL-1α under all experimental conditions (Fig. 3D), confirming that IL-1α surface expression is independent of the inflammasome and caspase-1. The validity of the above in vitro observations was tested in vivo in an airway infection model. Caspase-1−/− and WT mice were infected intranasally (i.n.) with the Gram-negative bacterium Legionella pneumophila (Lpn), and the secretion of IL-1α and IL-1β was measured in bronchoalveolar lavage (BAL). Indeed, caspase-1−/− mice exhibited both impaired IL-1α and IL-1β secretion (Fig. 3 H and I). Similar results were obtained in WT mice infected with either WT Lpn or ΔT Lpn, the latter lacking the type IV secretion system (T4SS) and therefore being unable to activate caspase-1 (21, 22) (Fig. 3 J and K).
Fig. 3.
In vitro and in vivo secretion of IL-1α is caspase-1 dependent. (A–C) Human monocytes were stimulated with LPS in presence or absence of the pan-caspase inhibitor z-VAD fmk. IL-1α surface expression was analyzed by flow cytometry after 6 h of stimulation (A), and IL-1α and -β secretion was quantified by ELISA after 24 h of stimulation (B and C). Control samples were treated with the DMSO containing solvent (closed bars). Mean and SD of six replicates. *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired, two-tailed Mann–Whitney U test). One representative of two independent experiments is shown. (D–F) bmDCs derived from caspase-1–deficient (open bars) and WT control mice (closed bars) were stimulated with upLPS in the presence or absence of ATP during the last 30 min of a 24-h stimulation period. Surface IL-1α expression was analyzed by flow cytometry (D). IL-1α (E) and -β (F) secretion were quantified by ELISA. Data shows mean and SD of duplicates and one representative of three independent experiments. (G) Pro–IL-1α and -β were measured by Western blot in lysates prepared from bmDCs stimulated with upLPS. (H and I) IL-1α (H) and -β (I) levels in the BAL of Lpn-infected caspase-1–deficient and C57BL/6 control mice were quantified by ELISA. Mean and SD of n = 3, and one representative of three independent experiments is shown. *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired, two-tailed t test). (J and K) IL-1α (J) and IL-1β (K) levels were measured in the BAL of WT mice infected with WT JR32 Lpn or ΔT Lpn. Mean and SD of n = 2 or n = 3 and one representative of three independent experiments is shown. *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired, two-tailed t test).
IL-1α Secretion Is IL-1β Mediated and Uses a Distinct Pathway from IL-1α Surface Expression.
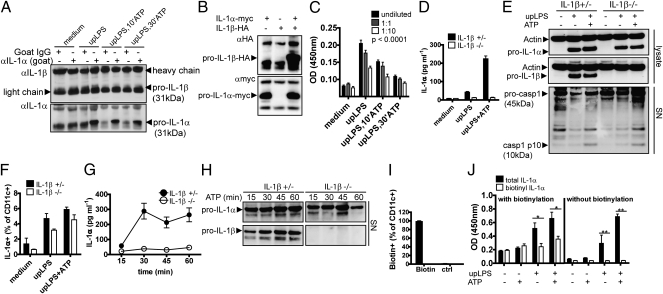
The reason why IL-1α secretion is caspase-1 dependent, even though it is not a direct substrate for caspase-1, was previously attributed to the binding of IL-1α to caspase-1, thereby facilitating unconventional protein secretion (4). Surprisingly, coimmunoprecipitation (co-IP) experiments using lysates of upLPS-primed murine bmDCs revealed a weak band for IL-1β after an anti–IL-1α pull-down, suggesting an intracellular interaction between IL-1α and IL-1β (Fig. 4A). To confirm this interaction between IL-1α and IL-1β, we overexpressed human pro–IL-1α (myc-tagged) and pro–IL-1β (HA-tagged) in Cos cells. Precipitation of pro–IL-1α using an anti-myc antibody led to coprecipiation of pro–IL-1β, detected using an anti-HA antibody (Fig. 4B). Furthermore, the endogenous association of IL-1α and IL-1β could be demonstrated in lysates of differently stimulated bmDCs using a modified sandwich ELISA with an IL-1α capture antibody and a non–cross-reactive IL-1β detection antibody (Fig. 4C). Though upLPS-stimulated cells showed a strong signal for IL-1α–bound IL-1β, this signal was reduced when cells were additionally treated with ATP, most likely due to reduced intracellular availability as a result of secretion, and thereby confirming the co-IP data of the endogenous protein. The requirement of IL-1β for secretion of IL-1α was confirmed in a functional assay showing that IL-1β–deficient cells were unable to secrete IL-1α (Fig. 4D), whereas the level of intracellular pro–IL-1α were comparable in IL-1β+/− and IL-1β−/− bmDCs (Fig. 4E). Therefore, the IL-1β dependence of IL-1α secretion was not explained by differences in transcription, as previously reported (23). In contrast to that, IL-1β secretion was independent of IL-1α secretion shown in IL-1α–deficient mice (Fig. S2). Furthermore, the inflammasome activity, measured by the unconventionally secreted caspase-1 p10 subunit in the supernatant, was not impaired in IL-1β–deficient cells when stimulating with upLPS and ATP (Fig. 4E). Consistent with the inflammasome-independent presentation of IL-1α on the cell surface, cell-bound IL-1α was readily detected in IL-1β–deficient bmDCs upon stimulation (Fig. 4F).
Fig. 4.
IL-1α interacts with IL-1β for secretion. (A and B) Co-IP of bmDCs stimulated with medium or upLPS in the presence or absence of ATP as indicated during the last 10 or 30 min of a 24-h stimulation using an anti–IL-1α pull-down or an isotype control antibody (A) and co-IP of Cos cells transfected with pro–IL-1α-myc or pro–IL-1β-HA alone or in combination of both using an anti-myc pull-down antibody (B). (C) Sandwich ELISA of lysed bmDCs stimulated with upLPS in the absence or presence of ATP during the last 10 or 30 min of a 24-h stimulation using IL-1α capture antibody and IL-1β detection antibody of undiluted (closed bars), 1:1 (gray bars), and 1:10 (open bars) diluted bmDCs lysates stimulated as indicated. Mean and SD of n = 4, and one representative of two independent experiments is shown (two-way ANOVA). (D–F) bmDCs derived from IL-1β–deficient (open bars) or WT mice (closed bars) were stimulated with upLPS in the presence or absence of ATP as indicated during the last 30 min of a 24-h stimulation period. IL-1α secretion in the supernatant was quantified by ELISA (D), intracellular pro–IL-1α and -β and actin control in lysates as well as secreted caspase-1 p10 subunit in the supernatant were analyzed by Western blot (E), and surface IL-1α expression was analyzed by flow cytometry (F). Mean and SD of n = 2, and one representative of two independent experiments are shown. (G and H) upLPS-pulsed bmDCs derived from IL-1β+/− and IL-1β−/− mice were stimulated with ATP for different time periods. Secreted IL-1α quantified by ELISA (G) and the corresponding release of pro–IL-1α and pro–IL-1β in the precipitated supernatants shown by Western blot (H). Mean and SD of n = 4, and one representative of two independent experiments is shown. One representative of two independent experiments is shown. (I and J) upLPS-stimulated bmDCs were either biotinylated or left untreated. Biotinylation of bmDCs was measured by flow cytometry (I). After biotinylation, cells were stimulated with the second stimulus ATP or left untreated. Comparison of total vs. biotinylated IL-1α in the supernatant by using a sandwich ELISA with the detection antibody for quantification of total IL-1α (closed bars) and by a modified ELISA without the detection antibody to quantify the amount of biotinylated IL-1α (open bars) in the supernatant (J). Mean and SD of n = 3, n = 4, or n = 5, and one representative of two independent experiments is shown. *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired, two-tailed Mann–Whitney U test).
Note that upon stimulation of bmDCs with upLPS and ATP, with time, no secretion of IL-1α was detected by ELISA in IL-1β−/− mice (Fig. 4G). However, a release of significant amounts of pro–IL-1α was detectable in the supernatant by Western blotting (Fig. 4H). This finding indicates that the ELISA only detected the mature form of IL-1α. IL-1β–deficient bmDCs release pro–IL-1α, but were not able to actively secrete intracellularly processed mature IL-1α (Fig. 4 G and H). Pro–IL-1α has lower biological activity than mature IL-1α, but could be further cleaved by other extracellular proteases, i.e., cathepsin G, elastase, proteinase-3, and chymase in vivo.
This and the previous experiments suggest the presence of two distinct pathways for IL-1α surface expression and IL-1α secretion. This interpretation was further supported by cell-surface protein biotinylation of upLPS–pulsed, and therefore surface IL-1α–expressing, bmDCs. Following biotinylation of surface proteins, including IL-1α (Fig. 4I), cells were treated with the inflammasome activator ATP to induce IL-1α secretion. Quantitative ELISA demonstrate that the secreted IL-1α was almost entirely composed of nonbiotinylated IL-1α, indicating that secreted IL-1α was not cleaved off from the surface but rather released from intracellular compartments (Fig. 4J).
Discussion
Our data show that IL-1α surface expression and secretion can be dissected into two independent pathways. Though TLR stimulation alone is sufficient for the production of IL-1α determined for the cell surface, additional activation of the inflammasome is required for the production of secreted and soluble IL-1α. This study further demonstrates that, against former notion, secretion of IL-1α is not the result of cleavage of cell-surface–bound IL-1α, but occurs via a distinct pathway dependent on inflammasome, caspase-1, and IL-1β. Because IL-1α does not have a caspase-1 cleavage site, the observed inflammasome and caspase-1 dependency appears to be explained by binding of IL-1α to IL-1β and piggy-backing on IL-1β during transfer from the cytoplasm to the extracellular space via the caspase-1, and probably also via calpains, which are relevant for cleavage of IL-1α. The finding that bmDCs from IL-1β−/− mice are also deficient in secretion of mature IL-1α suggests that certain findings in IL-1β−/− mice should be revisited. Susceptibility to Mycobacterium tuberculosis (Mtb) infection may be one such example. IL-1β−/− mice were found to be more susceptible to M. tuberculosis infection than WT mice (24), but when WT mice were depleted with IL-1α or IL-1β antibodies, it was IL-1α but not IL-1β that determined susceptibility to infection (25). Recently, Mtb susceptibility was also confirmed in IL-1α−/− mice (26). Moreover, our data suggests a unique role for IL-1β in unconventional protein secretion. It remains to be resolved whether interaction with IL-1β is also a prerequisite for the secretion of other unconventionally secreted proteins, such as FGF-2 and Bid.
Our observation that IL-1β–deficient mice do not secrete mature IL-1α, but may still release significant amounts of pro–IL-1α, is likely due to cell damage upon massive stimulation. Cell damage and inflammasome activation may explain why another group detected IL-1α in the serum of LPS-infected IL-1β–deficient mice (27). This may be either pro–IL-1α, which is less biologically active, or pro–IL-1α matured by extracellular proteases, i.e., cathepsin G, elastase, proteinase-3, and chymase. Alternatively, cells other than macrophages and dendritic cells studied by us may have been the source of IL-1α after LPS injection.
We have tested three cell types—bmDCs, macrophages, and monocytes—with regard to the signaling requirements for the secretion of mature IL-1α. Though others have shown that monocytes do not require an additional inflammasome activation to secrete mature IL-1 due to the fact that monocytes express a constitutively active caspase-1 (28), in our hands, two separate stimuli were required in monocytes.
The identification of distinct pathways, i.e., for IL-1α surface expression and IL-1α secretion, suggests that the two forms of IL-1α may exert different biological functions. Indeed, a differential role of surface-bound and secreted IL-1α in different inflammatory situations has recently been suggested. Surface-bound but not secreted IL-1α triggering senescence-associated secretion of IL-6/IL-8 (29) and surface-bound IL-1α caused inflammatory destruction of cartilage during arthritis (30, 31). Thus, the presented data on the regulation of cell-surface and secreted IL-1α may offer the possibility to selectively modulate the expression of the two forms of IL-1α. Such regulation may furthermore allow us to dissect protection against pathogens from inflammatory diseases. Insight into such regulation of IL-1α processing may furthermore allow investigations to better understand the cytokine’s role in protection against pathogens and its role in mediating inflammatory diseases, and consequently, to design new and improved treatment options against such diseases.
Methods
Mice.
All knockout mouse strains used have been described elsewhere. Mice were treated according to guidelines of Swiss veterinary authorities.
In Vitro Stimulation Experiments.
Cells were stimulated with upLPS from E. coli 0111:B4 (InvivoGen) or LPS from E. coli 0111:B4 (Sigma-Aldrich) at 1 μg/mL. upLPS-stimulated cells were stimulated additionally using either 5 mM ATP (Sigma-Aldrich) for 30 min or 150 μg/mL MSU (Alexis) for 6 h. Cells were treated with 2.5 μM caspase inhibitor z-VAD fmk (Alexis) or solvent only (DMSO) with a final DMSO content of 0.1% 1 h before stimulation with upLPS or LPS. IC-21 macrophages were plated at a density of 5 × 105 cells in a six-well plate, and bmDCs in a density of 2 × 106 cells in a six-well plate or 7.5 × 104 cells in a 96-well plate. Human monocytes were plated at a density of 2 × 106 cells per 3 mL in a six-well plate.
Bacteria and Infection.
Legionella pneumophilia (Lpn) strains used in this study were JR32 (WT Philadelphia-1) (32) and GS3011 (ΔT, icmT deletion lacking a functional Icm/Dot T4SS) (33). Lpn was grown for 3 d on charcoal yeast extract agar plates. Mice were infected intranasally (i.n.) with 5 × 106 cfu Lpn in 20 μL PBS. At 4 h postinfection, the BAL was collected by flushing the airways with 1 mL PBS after sublethal anesthesia. Cells were recovered from the BAL by centrifugation and the cell-cleared BAL fluid was stored at −20 °C for further analysis.
Additional details regarding methods can be found in SI Methods.
Supplementary Material
Acknowledgments
We thank Manfred Kopf, Stefan Dreher, and members of the T.M.K., A.O., and L.E.F. laboratories for helpful discussion. We thank Gabriel Sollberger, Wolf-Dietrich Hardt, Andreas Müller, Patrick Kaiser, and Rina Käppli for caspase-1−/− mice, and Wolf-Dietrich Hardt and Médéric Didard for IL-1β−/− mice. This work was supported by Swiss National Science Foundation Grant 310000-113947 (to A.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109176108/-/DCSupplemental.
References
- 1.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 2.Stutz A, Golenbock DT, Latz E. Inflammasomes: Too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 4.Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson FT, Bursten SL, Fanton C, Locksley RM, Lovett DH. The 31-kDa precursor of interleukin 1 alpha is myristoylated on specific lysines within the 16-kDa N-terminal propiece. Proc Natl Acad Sci USA. 1993;90:7245–7249. doi: 10.1073/pnas.90.15.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 7.Beuscher HU, Nickells MW, Colten HR. The precursor of interleukin-1 alpha is phosphorylated at residue serine 90. J Biol Chem. 1988;263:4023–4028. [PubMed] [Google Scholar]
- 8.Brody DT, Durum SK. Membrane IL-1: IL-1 alpha precursor binds to the plasma membrane via a lectin-like interaction. J Immunol. 1989;143:1183–1187. [PubMed] [Google Scholar]
- 9.Carruth LM, Demczuk S, Mizel SB. Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J Biol Chem. 1991;266:12162–12167. [PubMed] [Google Scholar]
- 10.Kobayashi Y, et al. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc Natl Acad Sci USA. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 12.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 13.Suttles J, Carruth LM, Mizel SB. Detection of IL-1 alpha and IL-1 beta in the supernatants of paraformaldehyde-treated human monocytes. Evidence against a membrane form of IL-1. J Immunol. 1990;144:170–174. [PubMed] [Google Scholar]
- 14.Cui X, Rouhani FN, Hawari F, Levine SJ. Shedding of the type II IL-1 decoy receptor requires a multifunctional aminopeptidase, aminopeptidase regulator of TNF receptor type 1 shedding. J Immunol. 2003;171:6814–6819. doi: 10.4049/jimmunol.171.12.6814. [DOI] [PubMed] [Google Scholar]
- 15.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Pétrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari D, et al. The P2X7 receptor: A key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 18.Sutterwala FS, Flavell RA. NLRC4/IPAF: A CARD carrying member of the NLR family. Clin Immunol. 2009;130:2–6. doi: 10.1016/j.clim.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassel SL, Sutterwala FS. Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol. 2010;40:607–611. doi: 10.1002/eji.200940207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latz E. The inflammasomes: Mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeibundGut-Landmann S, Weidner K, Hilbi H, Oxenius A. Nonhematopoietic cells are key players in innate control of bacterial airway infection. J Immunol. 2011;186:3130–3137. doi: 10.4049/jimmunol.1003565. [DOI] [PubMed] [Google Scholar]
- 22.Zamboni DS, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 23.Horai R, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer-Barber KD, et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guler R, et al. Blocking IL-1α but not IL-1β increases susceptibility to chronic Mycobacterium tuberculosis infection in mice. Vaccine. 2011;29:1339–1346. doi: 10.1016/j.vaccine.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 26.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4:252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fantuzzi G, et al. Effect of endotoxin in IL-1 beta-deficient mice. J Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- 28.Netea MG, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niki Y, et al. Macrophage- and neutrophil-dominant arthritis in human IL-1 alpha transgenic mice. J Clin Invest. 2001;107:1127–1135. doi: 10.1172/JCI11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niki Y, et al. Membrane-associated IL-1 contributes to chronic synovitis and cartilage destruction in human IL-1 alpha transgenic mice. J Immunol. 2004;172:577–584. doi: 10.4049/jimmunol.172.1.577. [DOI] [PubMed] [Google Scholar]
- 32.Sadosky AB, Wiater LA, Shuman HA. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segal G, Purcell M, Shuman HA. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.