Abstract
Long-term humoral immunity is maintained by the formation of high-affinity class-switched memory B cells and long-lived antibody-secreting plasma cells. In healthy humans, a substantial fraction of IgG-positive memory B cells express self-reactive and polyreactive IgG antibodies that frequently develop by somatic mutations. Whether self- and polyreactive IgG-secreting B cells are also tolerated in the long-lived plasma cell pool is not known. To address this question, we cloned and expressed the Ig genes from 177 IgG-producing bone marrow plasma cells of four healthy donors. All antibodies were highly mutated but the frequency of self- and polyreactive IgG antibodies was significantly lower than that found in circulating memory B cells. The data suggest that in contrast to the development of memory B cells, entry into the bone marrow plasma cell compartment requires previously unappreciated selective regulation by mechanisms that limit the production of self- and polyreactive serum IgG antibodies.
Newly developing self-reactive B cells are efficiently counterselected at two tolerance checkpoints before becoming circulating naive B cells (1, 2). Antigen-mediated activation of naive B cells in the presence of T-cell help induces germinal centers and the development of memory B cells and long-lived bone marrow plasma cells that express high affinity, isotype class-switched antibodies (3, 4). Surprisingly, compared with naive B cells, circulating IgG-positive memory B cells are significantly enriched for self-reactive and polyreactive antibodies (5). These antibodies develop in the germinal center from non–self-reactive or polyreactive precursors by somatic mutations and appear in the serum of healthy humans in low amounts (6). Whether cells producing self-reactive and polyreactive IgG can enter the bone marrow plasma cell compartment has not been determined.
To address this question, we produced recombinant monoclonal antibodies from isolated IgG-secreting plasma cells from bone marrow of four healthy donors (HDs) and compared the Ig gene features and antibody reactivity profiles to historic data obtained from IgG-positive memory B cells (5, 7). Ig gene sequence analysis showed that bone marrow plasma cells carry significantly higher numbers of somatic mutations than circulating memory B cells and that the two compartments differ in their Ig light (IgL) chain variable (V) gene use and IgG isotype subclass distribution. Further, the frequency of polyreactive and self-reactive IgG-positive antibodies was significantly lower in bone marrow plasma cells than in the memory B-cell compartment. In summary, the data suggest that entry into the bone marrow plasma cell compartment is selective for B cells producing highly mutated antibodies that are not self- or polyreactive.
Results
Ig Gene Features of Human IgG-Positive Bone Marrow Plasma Cells.
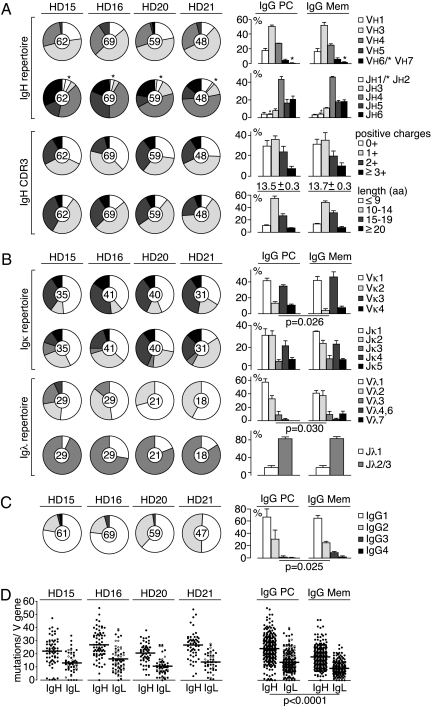
To characterize the antibody gene repertoire of IgG-expressing bone marrow plasma cells, we cloned the Igγ and IgL chain genes of 238 purified single CD138+CD27+CD38+ bone marrow mononuclear cells from four HDs (Fig. S1 and Table S1) (7, 8). The data were compared with historic data obtained from the analysis of IgG antibodies expressed by circulating memory B cells from four independent donors (5, 7). Ig gene sequence analysis showed no significant differences between plasma cells and memory B cells in Ig heavy (IgH) chain V and joining (J) gene use and IgH complementarity determining region 3 (CDR3) features, such as length and number of positive charges (Fig. 1A). Only small differences were observed for IgL chain V gene use with increased use of Vκ2, Vκ4, and Vλ1 by plasma cells (Fig. 1B). The IgG subclass distribution of bone marrow plasma cells reflected that of memory B cells and serum IgG antibodies with IgG1 > IgG2 > IgG3 > IgG4, but IgG3 and IgG4 antibodies were rare and were not detected in all samples (Fig. 1C). As expected from cells that have undergone affinity maturation in the germinal center, bone marrow plasma cells showed clear signs of antigen-mediated selection with higher ratios of replacement (R) to silent (S) somatic mutations in V gene CDRs than in framework regions (R = 6.0 vs. S = 3.6 for VH, R = 2.9 vs. S = 1.9 for Vκ, and R = 3.0 vs. S = 1.8 for Vλ; Fig. S2). Overall, the frequency of somatic mutations in IgH and IgL V genes of bone marrow plasma cells was significantly higher than in circulating IgG-positive memory B cells (Fig. 1D; P < 0.0001). In summary, the Ig gene repertoire and Ig gene features of antibodies produced by IgG-expressing bone marrow plasma cells are similar to circulating memory B cells but plasma cell antibodies show higher overall levels of somatic hypermutation.
Fig. 1.
Ig gene features of IgG-positive plasma cells. Ig gene repertoire and Ig gene features of IgG plasma cell antibodies from four HDs (HD15, HD16, HD20, and HD21) are shown in comparison with historical data from IgG memory B cell antibodies (5, 7). VH/JH gene family use and IgH CDR3 amino acid-positive charges and length (A) and Vκ/Jκ and Vλ/Jλ gene family use (B) and IgG subclass distribution (C) are shown. Pie charts show the data for individual HDs with the absolute number of sequences analyzed indicated in the pie chart center. Bar graphs compare the data obtained from IgG plasma cell antibodies (n = 238; κ, n = 147; λ, n = 97) to historical data from IgG memory B cell antibodies (n = 254; κ, n = 172; λ, n = 83) (5, 7). Error bars indicate SD. (D) Dots represent the absolute number of somatic mutations (nucleotide exchanges compared with the nearest germline gene segment) in individual IgH and IgL Vκ and Vλ genes in IgG plasma cell antibodies from all four HDs. Horizontal lines indicate averages.
Low Frequency of Self-Reactive IgG-Positive Bone Marrow Plasma Cells.
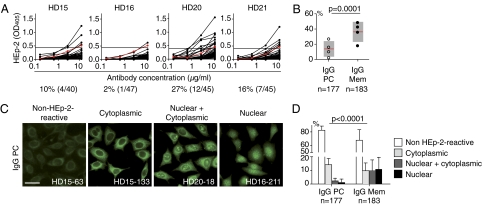
IgG-positive memory B cells are generated in response to foreign antigens including microbes and vaccines (5, 9, 10). However, a substantial number of circulating memory B cells express self-reactive IgG antibodies that react with the human larynx carcinoma cell line HEp-2 by ELISA, which is used as a clinical diagnostic assay for the detection of serum IgG autoantibodies (5, 11). These antibodies are also detectable at low levels in serum of HDs (5, 6). To determine the frequency of self-reactive antibodies expressed by IgG-secreting bone marrow plasma cells, we cloned and expressed the Ig genes of 177 bone marrow plasma cells in vitro and measured their reactivity (8). The frequency of HEp-2 cell self-reactive IgG-positive bone marrow plasma cells ranged from 2% to 27% in the ELISA (Fig. 2A). Despite this high variation among individuals, the mean frequency of cells expressing self-reactive IgG antibodies was significantly lower in plasma cells than in circulating IgG-positive memory B cells (13.6 ± 10.3% vs. 36.3 ± 13.3%, Fisher’s exact P = 0.0001; median 13% vs. 41.9%, Mann–Whitney P = 0.057; Fig. 2B and Table S1) (5, 7). No obvious age- or sex-associated differences in the frequency of self-reactive antibodies were observed between plasma cells and memory B cells. Antibodies specific for vaccine antigens or common pathogens were identified in both compartments (Fig. S3 and Table S1) (5).
Fig. 2.
Low frequency of self-reactive IgG-positive plasma cells compared with memory B cells. IgG plasma cell antibodies from four HDs were tested for self-reactivity with HEp-2 cells. (A) HEp-2 cell lysate ELISA. Black lines represent individual plasma cell antibodies. Horizontal lines indicate cutoff OD405 for positive reactivity determined by comparison with low-positive control serum (red line). Data are representative of at least two independent experiments. (B) Dot plots compare the frequency of HEp-2 lysate-reactive plasma cell antibodies determined in A to the frequency of HEp-2 lysate-reactive IgG-positive memory B cells in individual HDs (5, 7). Horizontal lines represent mean values of reactivity for all HDs; gray boxes indicate SD; n indicates the number of tested antibodies. (C) Representative HEp-2 cell IFA staining patterns of antibodies cloned from IgG-positive plasma cells. (Scale bar, 50 μm.) (D) Bar graphs compare the frequency of nonreactive (white) and HEp-2 self-reactive IgG plasma cell antibodies and memory B-cell antibodies with nuclear (black), nuclear plus cytoplasmic (dark gray), and cytoplasmic (light gray) IFA staining patterns (5, 7). Error bars indicate the SD; n indicates the number of tested antibodies. P values were calculated by Fisher’s exact test.
Antibodies with HEp-2 cell ELISA reactivity may include antinuclear antibodies (ANAs) that are expressed by ∼10% of circulating IgG-positive memory B cells and if detected in serum serve as diagnostic markers in autoimmunity (5, 7). To discriminate between ANAs and antibodies binding to cytosolic or nuclear and cytosolic antigens, we performed indirect immunofluorescence assay (IFA) on fixed HEp-2 cells (Fig. 2 C and D and Table S1). In agreement with the HEp-2 ELISA results, the frequency of IFA-positive antibodies was significantly lower in plasma cells than in the circulating IgG-positive memory B cell compartment (Fisher’s exact P < 0.0001). Further, the few IFA-positive antibodies from bone marrow plasma cells reacted mostly with extranuclear self-antigens or nuclear plus cytoplasmic antigens and were nearly devoid of true ANAs (Fig. 2D, median frequency of ANA-positive cells: 0% for plasma cells and 8.9% for memory B cells, Mann–Whitney P = 0.026). Thus, B cells producing HEp-2 cell-reactive antibodies including ANAs are selected against in the bone marrow plasma cell compartment.
Low Frequency of Polyreactive IgG-Positive Bone Marrow Plasma Cells.
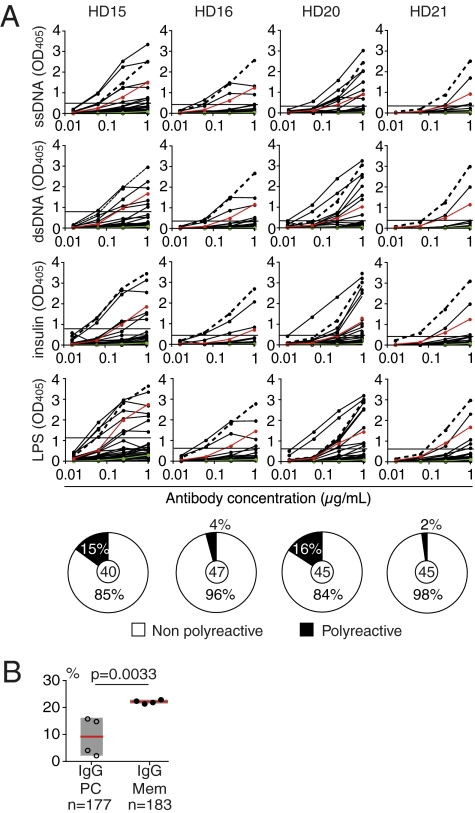
Production of antigen-specific IgG antibodies in response to infection can be associated with the development of self- and polyreactive antibodies (12–15). In addition, 23% of circulating IgG-positive memory B cells normally express polyreactive antibodies, most of which arise by somatic mutations (5). To determine the frequency of polyreactivity in the IgG-secreting bone marrow plasma cell compartment, we tested all antibodies by ELISA for binding to a panel of self- and foreign antigens comprising single-stranded and double-stranded DNA (ssDNA and dsDNA), LPS, and insulin (Fig. 3 and Table S1). On average 9.3% of bone marrow plasma cells reacted with at least two structurally diverse antigens in these assays and were therefore polyreactive (range 2–16%; Fig. 3A). Despite the high degree of variation among the individuals, this frequency was significantly lower than that observed for IgG-positive memory B cell antibodies (Fig. 3B; Fisher’s exact P = 0.0033; average 23% and range 22–23% for IgG memory B cell antibodies; median 9.7% for IgG plasma cell antibodies vs. 22.5% for IgG memory B cell antibodies; Mann–Whitney P = 0.0286). Again, no obvious association between age and frequency of polyreactive plasma cells was observed. Thus, we conclude that self- and polyreactivity is a less common feature of the IgG antibodies produced by bone marrow plasma cells than by circulating memory B cells.
Fig. 3.
Low frequency of polyreactive IgG-positive plasma cells compared with memory B cells. (A) IgG plasma cell antibodies (black lines) from HDs were tested for polyreactivity by ELISA with ss/dsDNA, insulin, and LPS. Dotted lines represent the high-positive control antibody ED38 (35); horizontal lines show cutoff OD405 for positive reactivity determined by comparison with the negative control antibody mGO53 (green line) (2) and low-positive control eiJB40 (red line) (2). Pie charts summarize the frequency of polyreactive clones for individual HDs. The numbers of tested antibodies are indicated in the pie chart centers. Data are representative of at least two independent experiments. (B) Dot plots compare the frequency of polyreactive plasma cell antibodies to the frequency of IgG-positive memory B cells in individual HDs (5, 7). Horizontal lines represent mean values of reactivity for all HDs; gray boxes indicate SD; n indicates the number of tested antibodies.
Discussion
In summary, our findings suggest that entry into the IgG-positive bone marrow plasma cell compartment in healthy humans is selective against self-reactivity compared with circulating IgG-positive memory B cells. Memory B cells and plasma cells develop in response to environmental factors but also depend on the individual genetic background. Therefore, heterogeneity in the antibody repertoire among the individuals likely reflects differences in these two factors.
Long-lived bone marrow plasma cells are terminally differentiated and protect from infection through the production of high-affinity antigen-specific serum antibodies (16–18). Low levels of poly- and self-reactivity in this compartment may be essential to avoid the production of self-reactive and polyreactive serum IgG antibodies that might lead to the induction of inflammatory autoimmune reactions and the development of autoimmune disease (19).
Bone marrow plasma cells develop in germinal centers, where they accumulate somatic mutations (20). Selection in the germinal center is based on high affinity of the B cell antigen receptor that is necessary to capture and present sufficient amounts of antigen to T follicular helper cells, presumably the limiting factor in the selection process (21–23). The low level of self- and polyreactivity that we measured in IgG-positive bone marrow plasma cells may be a by-product of somatic mutations and does not exclude the possibility that the antibodies show high affinity for foreign antigens that were not tested (24). Antibody self- and polyreactivity may be tolerated during germinal center reactions if affinity for the activating antigen is high enough and antigen presentation is sufficient to receive proper help from cognate T follicular helper cells. Further, access to cross-reacting self- and nonself antigens is likely limited under normal circumstances and thus may not have strong impact on antigen presentation and the positive selection process during germinal center reactions. Alternatively and not mutually exclusive, self-tolerance of bone marrow plasma cells may be ensured by negative selection of autoreactive antigen-experienced B cells, for example, during their differentiation in the germinal center or before homing to the bone marrow and occupying niches that promote long-term plasma cell survival. Activation of the inhibitory IgG Fc receptor IIb (FcγRIIb) by autoantigen-containing immune complexes may provide a mechanism to regulate specifically autoreactive germinal center B cells and memory B cells in mice and humans (25, 26). FcγRIIb also regulates plasma cell survival in both species (27, 28). Thus, FcγRIIB is of major importance for the regulation of antigen-experienced autoreactive B cells and may influence the relative representation of autoreactive B cells in different compartments.
The signals that drive differentiation into memory B cells vs. plasma cells are not well understood (25). In mice, only few antigen-specific plasma cells home to the bone marrow early after primary immunization, suggesting that the majority of them develop from memory B cells upon secondary antigen encounter and that selection into the plasma cell compartment requires higher affinity than differentiation into memory B cells (26, 27). In support of this idea, antigen reexposure drives preexisting IgG memory B cells primarily toward differentiation into antibody secreting cells, whereas IgM memory B cells, which are likely to be of lower affinity than IgG memory B cells, initiate secondary germinal centers, undergo class switch recombination, and may thereby help to replenish the IgG memory B cell pool (28). In humans, indirect evidence suggests that memory B cells may participate in germinal center reactions (29). The higher frequency of somatic V gene mutations in bone marrow plasma cells compared with memory B cells may support the idea that bone marrow plasma cells have gone through multiple rounds of germinal center selection. High specificity may be the end product of iterative affinity maturation processes. Competition for survival niches may further limit access of plasma cells to the bone marrow compartment (30).
In contrast to plasma cells, memory B cells are quiescent in the absence of antigen and require reactivation before differentiation into antibody-secreting cells (31, 32). Cross- or polyreactivity of IgG memory B cell antibodies may be beneficial in cases where the antigen load is low, as it is for example in the case of HIV, or when antigenic variants are encountered, e.g., in viral infection (13, 33). Thus, memory B cells may play an important role in maintaining flexibility of memory responses to infectious agents whereas terminally differentiated bone marrow plasma cells secrete high-affinity antigen-specific serum IgG antibodies.
Materials and Methods
Healthy Donors.
All samples were collected after patients signed informed consent in accordance with protocols reviewed by the Institutional Review Board of The Rockefeller University and in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki. Mononuclear cells from one male [age 37 (HD15)] and three female [ages 23 (HD21), 28 (HD20), and 51 (HD16)] HDs were purified from bone marrow by Ficoll-Paque (GE Healthcare) density gradient centrifugation. CD138+ mononuclear cells were enriched by magnetic beads (Miltenyi). Enriched CD138+ bone marrow cells were stained with anti-CD27-FITC, anti-CD38-APC, and anti-CD138-PE antibodies (Becton Dickinson). Single CD138+CD27+CD38+ B cells were sorted as previously described (5). The data obtained from the analysis of bone marrow plasma cell antibodies were compared with previously published data from IgG-positive memory B cells from two male (ages 17 and 25) and two female (ages 26 and 31) HDs (5).
cDNA, PCRs, and Antibody Production and Purification.
Single-cell Ig gene RT-PCR was performed as previously described (5). All PCR products were sequenced and analyzed for Ig V and J gene use and CDR3 analysis, number of V gene mutations by IgBLAST comparison (http://www.ncbi.nlm.nih.gov/igblast/) (Table S1), and IgG isotype subclass using the international ImMunoGeneTics information system (http://imgt.cines.fr).
Antibody Production and Purification.
HEK293T (ATCC; CRL-11268) cells were transiently transfected with equal amounts (10–15 μg each) of IgH and corresponding IgL chain encoding plasmid DNA by calcium–phosphate precipitation and antibodies were purified from supernatants using protein-G beads as previously described (5).
ELISAs and IFAs.
Antibodies were tested for reactivity with dsDNA, ssDNA, LPS, and insulin (Sigma); for seasonal influenza and pneumococcal polysaccharide vaccines (Fluzone; Sanofi Pasteur) (Pneumovax23; Merck) by ELISA; and for HEp-2 cell reactivity by ELISA (INOVA Diagnostics) and indirect IFA (BION Enterprises) as described (2, 34). Threshold values for reactivity are indicated in the graphs and were set by using the published control antibodies mGO53 (negative), eiJB40 (low positive), and ED38 (high positive) for polyreactivity ELISAs and additional positive and negative control sera for HEp-2 reactivity (5, 8, 35).
Statistical Analysis.
P values for Ig gene repertoire analysis, analysis of positive charges in IgH CDR3, and antibody reactivity were calculated by Fisher’s exact test or chi-square test. P values for IgH CDR3 length and V gene mutations were calculated by nonpaired two-tailed Student’s t test. Additionally, a Mann–Whitney test was performed for antibody reactivity.
Supplementary Material
Acknowledgments
We thank Arlene Hurley, Nurse Specialist at The Rockefeller University Hospital, for help with the recruitment of healthy volunteers, and Klara Velinzon, The Rockefeller University, for single-cell sorting. This work was supported by German Research Foundation Grant WA-2590/1-1 (to H.W.). H.M. was supported by the Fondation Recherche Médicale. M.C.N. is a Howard Hughes Medical Institute investigator. S.Y. was a Charles H. Revson Fellow in Biomedical Research and received support from the Charles A. Dana Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113395108/-/DCSupplemental.
References
- 1.von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 2.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 3.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 4.Tarlinton D, Radbruch A, Hiepe F, Dörner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008;20:162–169. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Tiller T, et al. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berneman A, Guilbert B, Eschrich S, Avrameas S. IgG auto- and polyreactivities of normal human sera. Mol Immunol. 1993;30:1499–1510. doi: 10.1016/0161-5890(93)90458-n. [DOI] [PubMed] [Google Scholar]
- 7.Mietzner B, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiller T, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egner W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol. 2000;53:424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 13.Mouquet H, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pender MP. The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis. Neuroscientist. 2010;17:351–367. doi: 10.1177/1073858410381531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sansonno L, et al. B cells and HCV: An infection model of autoimmunity. Autoimmun Rev. 2009;9:93–94. doi: 10.1016/j.autrev.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Dörner T, Radbruch A. Selecting B cells and plasma cells to memory. J Exp Med. 2005;201:497–499. doi: 10.1084/jem.20050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 18.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiepe F, et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7:170–178. doi: 10.1038/nrrheum.2011.1. [DOI] [PubMed] [Google Scholar]
- 20.Dilosa RM, Maeda K, Masuda A, Szakal AK, Tew JG. Germinal center B cells and antibody production in the bone marrow. J Immunol. 1991;146:4071–4077. [PubMed] [Google Scholar]
- 21.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 22.Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237:72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 23.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casson LP, Manser T. Random mutagenesis of two complementarity determining region amino acids yields an unexpectedly high frequency of antibodies with increased affinity for both cognate antigen and autoantigen. J Exp Med. 1995;182:743–750. doi: 10.1084/jem.182.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 26.Benner R, Hijmans W, Haaijman JJ. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981;46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Phan TG, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dogan I, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 29.Bende RJ, et al. Germinal centers in human lymph nodes contain reactivated memory B cells. J Exp Med. 2007;204:2655–2665. doi: 10.1084/jem.20071006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu VT, Beller A, Nguyen TT, Steinhauser G, Berek C. The long-term survival of plasma cells. Scand J Immunol. 2011;73:508–511. doi: 10.1111/j.1365-3083.2011.02544.x. [DOI] [PubMed] [Google Scholar]
- 31.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 32.Lanzavecchia A, et al. Understanding and making use of human memory B cells. Immunol Rev. 2006;211:303–309. doi: 10.1111/j.0105-2896.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrammert J, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuiji M, et al. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meffre E, et al. Surrogate light chain expressing human peripheral B cells produce self-reactive antibodies. J Exp Med. 2004;199:145–150. doi: 10.1084/jem.20031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.