Abstract
Bipolar disorder is a debilitating psychopathology with unknown etiology. Accumulating evidence suggests the possible involvement of Na+,K+-ATPase dysfunction in the pathophysiology of bipolar disorder. Here we show that Myshkin mice carrying an inactivating mutation in the neuron-specific Na+,K+-ATPase α3 subunit display a behavioral profile remarkably similar to bipolar patients in the manic state. Myshkin mice show increased Ca2+ signaling in cultured cortical neurons and phospho-activation of extracellular signal regulated kinase (ERK) and Akt in the hippocampus. The mood-stabilizing drugs lithium and valproic acid, specific ERK inhibitor SL327, rostafuroxin, and transgenic expression of a functional Na+,K+-ATPase α3 protein rescue the mania-like phenotype of Myshkin mice. These findings establish Myshkin mice as a unique model of mania, reveal an important role for Na+,K+-ATPase α3 in the control of mania-like behavior, and identify Na+,K+-ATPase α3, its physiological regulators and downstream signal transduction pathways as putative targets for the design of new antimanic therapies.
Keywords: Atp1a3, mouse model, sodium potassium adenosine triphosphate alpha 3
Bipolar disorder is a genetically heterogeneous, heritable, and highly debilitating mood disorder defined by the presence of one or more manic episodes of abnormally elevated mood, arousal, or energy levels, with or without one or more depressive episodes. Numerous genes have been linked to bipolar disorder, including ATP1A3 that encodes the Na+,K+-ATPase α3 sodium pump, but no clear causal relationships have been established for any genetic factor (1). To enhance understanding of the neurobiology of the disorder and aid the development of novel therapies, fully validated and appropriate animal models are urgently needed.
The Na+,K+-ATPase (NKA) is a membrane-bound enzyme abundant in brain tissue and comprised of a catalytic α-subunit and regulatory β-subunit. Three α-isoforms are present in the brain: α1 and α2 are expressed in neurons and glia, and α3 is expressed exclusively in neurons. NKA activity maintains and restores electrochemical gradients necessary for neuronal function by active exchange of Na+ and K+, which consumes 40% to 50% of total brain ATP. In addition, the NKA is linked to intracellular signal transduction pathways in the brain (2).
Cardiotonic steroids, commonly referred to as ouabain-like compounds (OLC), selectively bind and inhibit NKA α-isoforms (3). Endogenous OLCs include the structurally similar molecules ouabain, digoxin, marinobufagenin, and proscillaridin A (4). All human α-isoforms and rodent α2 and α3 isoforms, have a high binding affinity for endogenous OLCs, which regulate Na+,K+-ATPase activity and initiate signaling (4–6). Intracerebroventricular (ICV) administration of ouabain to rats stimulates locomotor hyperactivity and NKA signaling and is considered as a model of mania (7–9), but the similar affinities of α2 and α3 for ouabain have precluded conclusive pharmacological studies of isoform specificity. ICV ouabain also excites the sympathetic nervous system and elevates blood pressure and heart rate in mice (10); however, these effects are mediated by the α2 isoform (11, 12). The binding of OLC to neuronal NKA initiates intracellular Ca2+ signaling, and the phospho-activation of extracellular signal regulated kinase (ERK) and Akt (8, 9, 13). Therefore, genetic changes that decrease NKA activity could alter neuronal signaling, both directly and through pleiotropic effects on downstream pathways.
Postmortem gene-expression analysis of bipolar disorder patients has revealed lower expression of NKA α2 in the temporal cortex (14) and α3 in the prefrontal cortex (15). Genetic studies have reported an association between bipolar disorder and variants of the genes encoding α1, α2, and α3 (1, 16), but the functional effects of these genetic changes remain unknown. There is also evidence that abnormal regulation of endogenous OLC may influence NKA activity in bipolar disorder. Relative to healthy controls, bipolar individuals show lower ouabain levels in serum (17, 18) but higher ouabain levels and binding in the parietal cortex (19). Finally, digitalis toxicity can be accompanied by manic and depressive symptoms in healthy humans (20).
By dint of the links between NKA and bipolar disorder, we assessed whether heterozygous Myshkin (Atp1a3Myk/+; Myk/+) mice that carry a missense mutation in the neuron-specific NKA α3 isoform exhibit mood-related behavioral abnormalities. Briefly, the Myk/+ mutation was created through N-nitroso-N-ethylurea mutagenesis and results in a normally expressed but inactive enzyme, leading to a 36% to 42% reduction in total NKA activity in the brain (21). Mutations in the ATP1A3 gene have been identified in rapid-onset dystonia parkinsonism; however, a known rapid-onset dystonia parkinsonism mutation reduces Na+ binding, whereas the Myk/+ mutation is inactivating (22). Because abnormal behaviors are the primary diagnostic indicators of bipolar disorder, we undertook a detailed analysis of the behavioral phenotype of Myk/+ mice in assays that model its fundamental symptoms. Herein, we report that Myk/+ mice display behavioral, pharmacological, and biochemical phenotypes associated with mania observed in bipolar patients.
Results
Absence of Stress-Induced Seizures in Myshkin Mice Backcrossed 20 Generations to C57BL/6NCr Strain.
Previously, we reported that Myk/+ mice backcrossed 12 generations (N12) to the C57BL/6NCr strain display increased susceptibility to stress-induced seizures (21). In the current study, we used Myk/+ mice that were backcrossed to the seizure-resistant C57BL/6NCr strain (23) for 20 generations (N20). Myk/+ mice with this genetic background have increased total brain NKA activity (Fig. S1) and do not exhibit stress-induced seizure activity in electrocorticography (ECoG) recordings.
Myshkin Mice Display Increased Exploratory Locomotion and Sensitivity to Amphetamine.
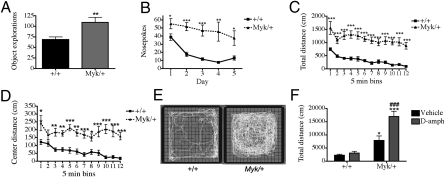
Within a novel environment, manic humans explore novel objects more frequently, travel longer distances (hyperambulation), and show a chaotic path of exploration compared with healthy individuals (24). We observed similar behavior in Myk/+ mice. In a novel-object test and a hole-board test, Myk/+ mice explored objects and nosepoked more frequently than wild-type (+/+) mice (Fig. 1 A and B). In contrast to +/+ mice, Myk/+ mice did not habituate hole-board exploration (Fig. 1B). In a novel open field, Myk/+ mice exhibited hyperambulation, faster walking speed, and decreased freezing than +/+ mice (Fig. 1C and Fig. S2). Hyperambulation in Myk/+ mice was not greater in response to light; instead, they were more hyperactive in the dark (Fig. S2). Although both genotypes had similar total rearing activity, the amount of rearing decreased over time in +/+ mice but increased over time in Myk/+ mice, suggesting a deficiency in habituation (Fig. S2). Finally, the walking path of Myk/+ mice was chaotic and they had greater locomotor activity in the center compared with +/+ mice (Fig. 1 D and E), suggesting decreased anxiety-like behavior.
Fig. 1.
Exploration and ambulation in Myk/+ mice. Myk/+ show an increased number of explorations of (A) novel objects (+/+ mice n = 10, Myk/+ n = 11) and (B) nosepokes compared with +/+ mice and do not habituate (+/+ mice n = 8, Myk/+ n = 7). (C) In an open field Myk/+ travel a further distance and (D) spend more time in the center (+/+ mice n = 27, Myk/+ n = 22) in 5-min bins compared with +/+ mice. (E) Myk/+ exhibit unusual walking patterns compared with +/+ mice. (F) In 60 min in the open field, Myk/+ increase locomotor activity in response to d-amph, but +/+ mice do not (+/+ vehicle n = 9, +/+ d-amph n = 9; Myk/+ vehicle n = 8, Myk/+ d-amph n = 9). All data are presented as mean SEM, *P < 0.05, **P < 0.01, ***P < 0.001 compared with +/+ mice, ###P < 0.001 compared with Myk/+ vehicle mice.
Bipolar patients exhibit a greater response to amphetamine (25). Amphetamine exacerbates hyperactivity in bipolar disorder, but decreases locomotor activity in attention-deficit hyperactivity disorder (26). Mice were treated with an acute dose of d-amphetamine (0.5 mg/kg) and locomotor activity was assessed in an open field. As expected (27), the behavior of +/+ mice was unchanged by a low dose of d-amphetamine, but Myk/+ mice showed increased ambulation (Fig. 1F), rearing, stereotypy, and circling behavior (Fig. S2), suggestive of an increase in dopamine signaling. This enhanced sensitivity of Myk/+ mice to d-amphetamine is consistent with mania, rather than attention-deficit hyperactivity disorder.
Myshkin Mice Display Sleep and Circadian Rhythm Abnormalities.
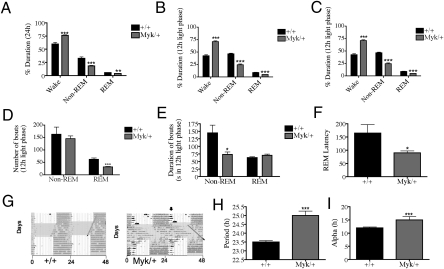
A decreased need for sleep while maintaining energy is the most common symptom of mania (28). Incidentally, we found that Myk/+ mice have more wake time than +/+ mice across 24 h, at the expense of non-rapid eye movement (non-REM) and REM sleep (Fig. 2A). Myk/+ mice showed a deficit in the amount of sleep only during the light phase (Fig. 2 B and C). In the light phase, Myk/+ mice exhibited a reduced number of REM sleep bouts and shorter non-REM sleep bout length (Fig. 2 D and E). Furthermore, similar to humans, REM sleep latency, as measured by the average duration of non-REM sleep that precedes entrance into REM sleep, was significantly reduced in Myk/+ mice (Fig. 2F).
Fig. 2.
Sleep and endogenous circadian period in Myk/+ mice. (A) Myk/+ (n = 6) experience more wake time than +/+ mice (n = 6) across 24 h with a reduction of both non-REM and REM sleep, as assessed by EEG and EMG. (B and C) Myk/+ show deficits in sleep duration only during the light phase; (D) Myk/+ have fewer REM sleep bouts but no change in non-REM bouts. (E) Non-REM bout length is reduced and REM bout length was unchanged in Myk/+. (F) REM sleep latency is reduced in Myk/+. (G) Wheel running actograms from +/+ and Myk/+ mice. Animals were held on a light-dark (LD) cycle for 14+ d, released into constant dark for 7 d to assess free running period, and reentrained to a LD12:12 cycle. Shaded area represents dark portion of LD cycle. Vertical arrows indicate continuation of nocturnal activity into light. (H) Endogenous period is extended in Myk/+ because of longer periods of (I) activity (α). All data are presented as means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 compared with +/+ mice.
The majority of bipolar individuals have altered circadian functions (29). Myk/+ mice successfully entrain to light and show normal circadian periods in a 12-h light:12-h dark environment. However, when external zeitgebers are removed, +/+ mice show an expected endogenous circadian period of 23.5 h (30) but Myk/+ mice show an extended endogenous circadian period of 25 h because of an increase in activity (Fig. 2 G–I).
Myshkin Mice Display Lowered Anxiety and a Greater Preference for Reward.
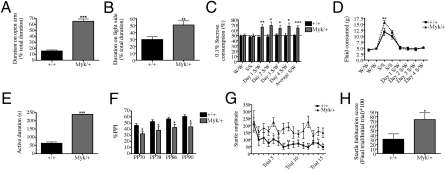
Low levels of anxiety, greater risk-taking, and greater impulsivity are core symptoms of mania (31). To assess levels of anxiety-like behavior, we used the elevated plus maze (EPM) and light-dark box (LDB). In the EPM, general locomotor activity did not differ between Myk/+ and +/+ mice (Fig. S3); however, Myk/+ mice made more open-arm entries and exploratory head dips (Fig. S3) and demonstrated a preference for the open arms (Fig. 3A). In the LDB, Myk/+ mice spent a higher percentage of time in the light (Fig. 3B), and did not show a preference for the dark compartment. However, +/+ mice appeared to be driven by anxiety, but Myk/+ mice were focused on exploration in unprotected spaces. Because NKA α3 is expressed in all neuronal-type cells of the retina (32) and anxiety-related behaviors are affected by visual impairment (33), we assessed the head-tracking response of Myk/+ mice in an optokinetic drum (34), but found no difference between genotypes in this test of visual acuity (Fig. S4).
Fig. 3.
Mania-like behavior in Myk/+ mice. (A) Myk/+ prefer to explore the open arm of the EPM (+/+ mice n = 29, Myk/+ n = 19) and (B) the light side of the LDB (+/+ mice n = 27, Myk/+ n = 18) for longer durations than +/+ mice. (C) Myk/+ (n = 8) show a higher preference for 0.1% sucrose than +/+ mice (n = 16) over 4 d and (D) consume more sucrose on the first presentation of sucrose. W, water; S, sucrose. (E) Myk/+ (n = 14) are active for a longer duration than +/+ mice (n = 15) in the Porsolt forced swim test. (F) PPI scores are impaired in Myk/+ (n = 20) compared with +/+ mice (n = 33) at all prepulse intensities tested. (G and H) Myk/+ (n = 16) had a startle habituation deficit compared with +/+ when presented with a repeated auditory stimulus (n = 12). All data are presented as means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 compared with +/+ mice.
Excessive motivation, such as increased reward-seeking behavior or drive to perform or to achieve goals, is common during mania (35). To assess preference for a natural reward, we tested sucrose preference. We found that Myk/+ mice consumed more sucrose solution relative to water than +/+ mice (Fig. 3C). In addition, Myk/+ mice initially consumed more sucrose solution before the choice test (Fig. 3D). The increased preference for sucrose is indicative of a hyperhedonic state common to mania. To assess drive and motivation, we used the Porsolt forced swim test, which involves measurement of escape-directed behavior. In this test, Myk/+ mice spent a longer time active than +/+ mice (Fig. 3E). Antidepressant drugs have been shown to increase the duration of mobility in the forced swim test (36) and the increased escape-directed behavior of Myk/+ mice suggests a lower level of depressive-like behavior, which correlates with their increase in preference for rewarding stimuli.
Manic individuals and their unaffected siblings show abnormal deficits in prepulse inhibition (PPI) and habituation of startle (37, 38). We found that Myk/+ mice demonstrate deficits in both PPI and startle habituation (Fig. 3 F–H), suggesting that they share the abnormal sensorimotor gating observed in bipolar patients. The behavioral profile of Myk/+ mice is remarkably similar to that of bipolar patients in the manic state (Table S1).
Manic-Like Behavior of Myshkin Mice Can Be Attenuated with Mood Stabilizers and Transgenic Restoration of NKA α3.
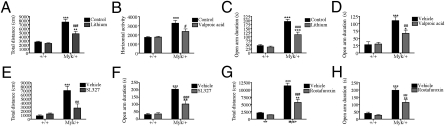
Lithium and valproic acid (VPA) are mood stabilizers that are effective in treating mania (39). We found the behavioral abnormalities of Myk/+ mice were reduced by chronic lithium carbonate and VPA treatment, but the behavior of +/+ mice was unaffected. In the open field, lithium and VPA reduced the total distance traveled by Myk/+ mice (Fig. 4 A and B). Lithium and VPA also reduced duration on the open arms (Fig. 4 C and D), entries to the open arms, and exploratory head dips (Fig. S3) by Myk/+ mice in the EPM. In the LDB, lithium reduced the time spent in the light by Myk/+ mice (Fig. S3).
Fig. 4.
Attenuation of mania-like behavior by lithium and VPA in Myk/+ mice. (A) Chronic treatment with lithium reduces total distance traveled by Myk/+ (n = 20) compared to untreated Myk/+ (n = 22) over 30 min in the open field and had no effect in +/+ mice (n = 28 control, n = 27 lithium). (B) VPA reduced total distance traveled by Myk/+ (n = 20) compared to vehicle-treated Myk/+ (n = 22), and had no effect on +/+ mice (n = 28 vehicle, n = 27 VPA) over 30 min. (C) Lithium reduces open-arm duration in Myk/+ (n = 18) compared with control Myk/+ (n = 19), but had no effect on +/+ mice (n = 29 control, n = 29 lithium) in the EPM. (D) VPA reduces open-arm duration in Myk/+ (n = 12) compared with vehicle-treated Myk/+ (n = 10) but had no effect on +/+ mice (n = 12 control, n = 12 VPA) in the EPM. (E) ERK inhibitor SL327 decreased distance traveled in Myk/+ (n = 12) compared with vehicle-treated Myk/+ (n = 9), and had no effect in +/+ mice (n = 12 vehicle, n = 12 SL327) in the open field. (F) SL327 reduced open-arm duration in Myk/+ (n = 6) compared with Myk/+ vehicle (n = 6) but had no effect on +/+ mice (n = 6 control, n = 6 SL327) in the EPM. (G) Rostafuroxin decreased distance traveled in Myk/+ (n = 12) compared with vehicle treatment (n = 12) and had no effect in +/+ mice (n = 13 vehicle, n = 15 rostafuroxin) in the open field. (H) In the EPM rostafuroxin reduced open arm duration in Myk/+ (n = 12) compared with vehicle treated Myk/+ (n = 12) and had no effect in +/+ mice (n = 12 vehicle, n = 15 rostafuroxin). All data are presented as means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 compared with +/+ mice, #P < 0.05, ##P < 0.01, ###P < 0.001 compared with Myk/+ vehicle mice.
To verify a causal link between the Atp1a3Myk mutation—with its reduction in NKA activity—and the observed phenotype, we attempted to rescue the mania-like behavioral phenotype of Myk/+ mice by transgenic restoration of functional NKA α3. To achieve this verification, we crossed Myk/+ mice with Tg-Atp1a31Stcl transgenic (Tg) mice to yield +/+, Tg/+, Myk/+, and Myk/+/Tg littermates. Inheritance of the Tg-Atp1a31Stcl transgene by Myk/+/Tg mice was previously shown to increase brain NKA activity to 74% of wild-type levels (21). We found that transgenic Myk/+/Tg mice perform at wild-type levels in the open field, EPM, and LDB (Fig. S5). This normalization of the behavioral phenotype shows that NKA α3 function is important in the regulation of mania-like behavior. We deduce the Tg-Atp1a31Stcl transgene is located on the X chromosome (Table S2).
Signal-Transduction Pathways Downstream of NKA α3 Are Up-Regulated in Myshkin Mice.
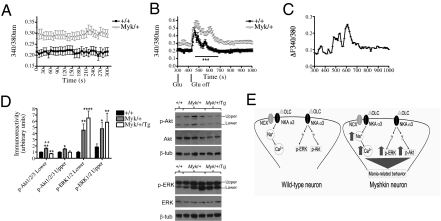
The binding of ouabain to NKA induces calcium (Ca2+) release from intracellular stores via the activation of the inositol 1,4,5-trisphosphate receptor (40, 41). We used fura-2 microfluorometry to compare intracellular free Ca2+ ([Ca2+]i) in cortical neurons cultured from Myk/+ and +/+ mice. We found that Myk/+ neurons exhibit higher resting [Ca2+]i, as measured by the fura-2 fluorescence emission ratio F340/F380 (Fig. 5A). Application of 10 μM glutamate evoked transient [Ca2+]i increases that were qualitatively similar in neurons from both genotypes. However, neurons from Myk/+ mice demonstrated markedly prolonged glutamate-evoked [Ca2+]i transients, as revealed by comparing the normalized fura-2 ratio over time (Fig. 5 B and C).
Fig. 5.
Free intracellular Ca2+ and Ca2+-dependent signaling in Myk/+ mice. (A) Mean resting intracellular ([Ca2+]i) is stably elevated in cortical cells cultured from Myk/+ (n = 47) than +/+ mice (n = 19), as measured by the ratio of fura-2 fluorescence emission upon 340-nm and 380-nm excitation (P < 0.01). (B) Myk/+ cortical neurons show a prolonged peak in [Ca2+]i compared with +/+ in response to bath superfusion of 10 μM glutamate (Glu). (C) When normalized to baseline [Ca2+]i, glutamate-evoked [Ca2+]i transients were prolonged in neurons from Myk/+ compared with neurons from +/+ (D) Immunoreactivity of p-Akt1/2/3 and p-ERK1/2 was elevated in Myk/+ compared with +/+ hippocampus. Transgenic overexpression of NKA α3 in Myk/+/Tg mice did not alter hippocampal levels of p-ERK1/2 but reduced p-Akt1/2/3. +/+, n = 8 (Akt, ERK); Myk/+, n = 11 (Akt), n = 10 (ERK); Myk/+/Tg, n = 5 (Akt, ERK). (E) Model of NKA α3 signaling at the synapse in +/+ and Myk/+ mice. Myk/+ mice have reduced NKA activity that augments [Ca2+]i and activation of p-ERK and p-Akt. These intracellular signals may independently, additively or synergistically contribute to behavioral phenotypes of mania. All data are presented as means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with +/+ mice, ++P < 0.01 compared with Myk/+/Tg mice.
ICV administration of 1 mM ouabain to rats induces locomotor hyperactivity and phosphorylation of ERK and Akt in the hippocampus (7–9). We expected ouabain-treated rats and Myk/+ mice to show similarities. To determine the phosphorylation level of ERK and Akt, hippocampal extracts were subjected to Western blot analysis. We found that the immunoreactivity of p-ERK1/2 and p-Akt1/2/3 normalized to the corresponding total protein was elevated in Myk/+ mouse hippocampus (Fig. 5D), although the degree of phospho-activation of ERK in Myk/+ samples was variable (Fig. S6). Transgenic overexpression of NKA α3 in Myk/+/Tg mice, with 26% lower brain NKA activity than +/+ mice, showed normalized hippocampal levels of p-Akt but not p-ERK (Fig. 5D). The persistent reduction in NKA activity may explain why the increase in ERK activation is maintained in the Myk/+/Tg mice.
Given the increased p-ERK in Myk/+ mice, we investigated the behavioral effects of acute SL327, an inhibitor of ERK, at a dose shown to reduce ERK activity in +/+ mice and have no effect on locomotion (42). SL327 reduced total distance traveled in the open field, duration on the open arm, and the number of exploratory head dips in the EPM in Myk/+ mice (Fig. 4 E and F and Fig. S7). We also investigated the behavioral effects of rostafuroxin (PST-2238; Sigma-Tau/Rostaquo), a compound that selectively displaces ouabain from the NKA in a rat model of hypertension (43). We expected that a reduction in endogenous ouabain binding would increase NKA activity or reduce NKA signaling in Myk/+ mice and restore behavior. Chronic rostafuroxin reduced total distance traveled in the open field, duration on the open arms, and exploratory head dips in the EPM, and minimized light side duration in the LDB in Myk/+ mice (Fig. 4 G and H and Fig. S7). These findings suggest a possible relationship between the mania-like behavioral phenotype and NKA signaling pathways in the Myk/+ brain (Fig. 5E).
Discussion
The behavioral profile of Myk/+ mice carrying an inactivating mutation in the neuron-specific α3 isoform of the NKA is remarkably similar to bipolar patients during the manic state, including their treatment by lithium and VPA. In light of emerging evidence implicating abnormal NKA function in mania, Myk/+ mice represent a convincing model of human mania, with construct validity and significant face and predictive validity. Myk/+ mice are behaviorally similar to other genetic models of mania, including reduction of Clock, ERK, and GluR2, and overexpression of glycogen synthase kinase-3β (GSK3β) (44–47). These genes may be interconnected in a pathway regulating mania-like behaviors.
Increasing the contribution of the seizure-resistant C57BL/6NCr strain (23) to the genetic background of Myk/+ mice had a significant phenotypic impact. In contrast to N12 C57BL/6NCr Myk/+ mice, Myk/+ mice at N20 C57BL/6NCr did not show stress-induced seizure activity in ECoG recordings and had increased total brain NKA activity. These results support our previous finding that an increase in NKA activity contributes to seizure resistance (21). Nonetheless, the possibility remains that unobserved subcortical epileptiform discharges contribute to mania-like behavior in Myk/+ mice. Interestingly, epilepsy and bipolar disorder can be comorbid in humans (48) and they share a common pathophysiology (49). Given that Myk/+ mice and ICV ouabain-treated rats exhibit mania-like behavior and increased susceptibility to seizures (50, 51), these models may help to explain why these debilitating conditions can be comorbid and suggest that increasing NKA activity may serve as therapy for mania and epilepsy.
Thus far, there have been no indications that Myk/+ mice cycle between mania and depression, and future studies may determine whether depression-like symptoms occur after stress, sleep deprivation, or administration of antidepressants. However, we have shown that mice heterozygous for a point mutation in Atp1a3 intron 4 (Atp1a3tm1Ling) exhibit increased susceptibility to depression-related behavior and a 33% reduction of brain NKA activity following chronic variable stress (52). Taking these data together, our work suggests that mood is significantly correlated with reductions in brain NKA activity. Theoretically, as previously hypothesized (53, 54), modulation of NKA activity could account for changes in mood in bipolar disorder.
Myk/+ mice may be a valuable tool for the development of novel mood stabilizers. We expected that the reduction in neuronal NKA activity in Myk/+ brain would increase NKA signal transduction given that NKA-dependent signal transduction pathways are Ca2+-dependent (13). Cortical neurons cultured from Myk/+ mice demonstrated higher resting and glutamate-evoked [Ca2+]i signals. Similarly, NKA activity is reduced and [Ca2+]i is elevated in erythrocytes during manic and depressed states (55, 56). Elevated [Ca2+]I may be studied as a drug target in Myk/+ mice. Calcium channel blockers, such as nimodipine, are prescribed as treatments for bipolar disorder (57), and variation in calcium channel genes, such as CACNA1C and TRPM2, has shown strong association with bipolar disorder (58, 59), underscoring a possible role for dysregulation of the influx and efflux of calcium in mood disorders.
Similar to an ouabain model of mania (8), phospho-activation of the ERK signaling cascade was enhanced in Myk/+ hippocampus, possibly by increasing transmitter-evoked Ca2+ signaling. However, p-ERK levels were not restored in Myk/+/Tg mice, suggesting that multiple signals contribute to mania-like behavior. In parallel with previous findings (42), the dose of SL327 that we used had no effect on locomotor activity in +/+ mice. In contrast, a higher dose of SL327 (60) or deletion of the Mapk3 (ERK1) gene (44, 61) have been shown to increase locomotor activity in rats and mice, respectively. These results suggest that the degree of ERK activation is correlated with mania-like behavior in animal models, and support the ERK pathway as a promising target for mood stabilizers (62). Also in accordance with an ouabain model of mania (9), the Myk/+ hippocampus showed elevated phospho-activation of Akt. p-Akt was reduced in Myk/+/Tg mice, suggesting it may contribute to the regulation of mania-like behavior. Activated Akt phosphorylates and inhibits the activity of GSK-3β, a well-known molecular target of lithium and VPA (63), mood stabilizing drugs that diminished many of the behavioral abnormalities of Myk/+ mice. Because Akt/GSK-3β signaling is regulated by dopamine (64), our results suggest that Akt may also be a promising target for mood stabilizers. Myk/+ mice could be used as a tool to investigate the potential of ERK and Akt modulators as antimanic therapies and to assess the prophylactic effect of novel mood stabilizers. Finally, because Myk/+ mice and ouabain-treated rats show similar p-ERK and p-Akt increases in the hippocampus, the genetic Myk/+ model of mania may replace the pharmacological ouabain model of mania, thus providing a less laborious model for exploring potential therapeutic approaches.
Rostafuroxin is a digitoxigenin derivative that antagonizes the signaling action of endogenous ouabain on the NKA that acts upstream of ERK to reduce ouabain-mediated NKA signaling (43). Its effective reduction of mania-like behavior in Myk/+ mice supports the notion that the phenotype of Myk/+ mice is caused by increased NKA downstream signaling.
Our results highlight the potential involvement of genes regulating NKA activity or downstream signaling pathways that are engaged by this transporter in bipolar etiology, and suggest that at least some manic individuals possess hypofunctional NKA sodium pumps and hyperfunctional NKA signal transduction.
Materials and Methods
All procedures were approved by the Animal Care Committee of the Toronto Centre for Phenogenomics and followed the Province of Ontario Animals for Research Act 1971 and requirements of the Canadian Council on Animal Care. The Myshkin and Tg-Atp1a31Stcl mouse lines have been described previously (21). Lithium carbonate was administered in chow (Harlan Teklad) at 0.4% for 28 d. VPA (Sigma-Aldrich) was administered at 150 mg/kg intraperitoneally for 28 d. The ERK inhibitor SL327 (Enzo Life Sciences) was acutely administered intraperitoneally at 30 mg/kg. Rostafuroxin (Sigma-Tau/Rostaquo) was administered for 21 d by oral gavage at 100 μg/kg. See SI Materials and Methods for more detailed discussion.
Supplementary Material
Acknowledgments
We are indebted to Sigma-Tau/Rostaquo for providing Rostafuroxin; Fatima Kadi for technical assistance; Tatiana Lipina for advice; and the Centre for Modeling Human Disease for producing the founder Myshkin mutant. This work was supported in part by Grant MOP 94856 from the Canadian Institutes of Health Research and a grant from the Amalgamated Transit Union (to J.C.R.); Grant G0900625 from the United Kingdom Medical Research Council and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to S.J.C.); a grant from the Ontario Mental Health Foundation (to G.S.K.); and grants from the Lundbeck Foundation, the Novo Nordisk Foundation (Fabrikant Vilhelm Pedersen og Hustrus Legat), and the Danish Medical Research Council (to B.V.). J.C.R. holds a Canada Research Chair.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.P.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108416108/-/DCSupplemental.
References
- 1.Goldstein I, et al. Association between sodium- and potassium-activated adenosine triphosphatase alpha isoforms and bipolar disorders. Biol Psychiatry. 2009;65:985–991. doi: 10.1016/j.biopsych.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Dobretsov M, Stimers JR. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci. 2005;10:2373–2396. doi: 10.2741/1704. [DOI] [PubMed] [Google Scholar]
- 3.Croyle ML, Woo AL, Lingrel JB. Extensive random mutagenesis analysis of the Na+/K+-ATPase alpha subunit identifies known and previously unidentified amino acid residues that alter ouabain sensitivity—Implications for ouabain binding. Eur J Biochem. 1997;248:488–495. doi: 10.1111/j.1432-1033.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- 4.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Urayama O, Sweadner KJ. Ouabain sensitivity of the alpha 3 isozyme of rat Na,K-ATPase. Biochem Biophys Res Commun. 1988;156:796–800. doi: 10.1016/s0006-291x(88)80914-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Velotta JB, McDonough AA, Farley RA. All human Na(+)-K(+)-ATPase alpha-subunit isoforms have a similar affinity for cardiac glycosides. Am J Physiol Cell Physiol. 2001;281:C1336–C1343. doi: 10.1152/ajpcell.2001.281.4.C1336. [DOI] [PubMed] [Google Scholar]
- 7.Ruktanonchai DJ, El-Mallakh RS, Li R, Levy RS. Persistent hyperactivity following a single intracerebroventricular dose of ouabain. Physiol Behav. 1998;63:403–406. doi: 10.1016/s0031-9384(97)00457-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, et al. Dose-dependent effect of intracerebroventricular injection of ouabain on the phosphorylation of the MEK1/2-ERK1/2-p90RSK pathway in the rat brain related to locomotor activity. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1637–1642. doi: 10.1016/j.pnpbp.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Yu HS, Kim SH, Park HG, Kim YS, Ahn YM. Activation of Akt signaling in rat brain by intracerebroventricular injection of ouabain: A rat model for mania. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:888–894. doi: 10.1016/j.pnpbp.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Huang BS, Huang X, Harmsen E, Leenen FH. Chronic central versus peripheral ouabain, blood pressure, and sympathetic activity in rats. Hypertension. 1994;23:1087–1090. doi: 10.1161/01.hyp.23.6.1087. [DOI] [PubMed] [Google Scholar]
- 11.Hou X, et al. Enhanced pressor response to increased CSF sodium concentration and to central ANG I in heterozygous alpha2 Na+ -K+ -ATPase knockout mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1427–R1438. doi: 10.1152/ajpregu.00809.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Huysse JW, Yang B, Leenen F. Increased expression of the epithelial sodium channel (ENaC) in the brain and enhanced BP response to central sodium mediated by brain ENaC in mice with a knockout of the neural precursor cell and developmentally downregulated 4-2 gene (Nedd4-2 –/–) FASEB J. 2011;25:823–827. [Google Scholar]
- 13.Dietz RM, Kiedrowski L, Shuttleworth CW. Contribution of Na(+)/Ca(2+) exchange to excessive Ca(2+) loading in dendrites and somata of CA1 neurons in acute slice. Hippocampus. 2007;17:1049–1059. doi: 10.1002/hipo.20336. [DOI] [PubMed] [Google Scholar]
- 14.Rose AM, et al. Alpha 2 isoform of the Na,K-adenosine triphosphatase is reduced in temporal cortex of bipolar individuals. Biol Psychiatry. 1998;44:892–897. doi: 10.1016/s0006-3223(97)00440-x. [DOI] [PubMed] [Google Scholar]
- 15.Tochigi M, et al. Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neurosci Res. 2008;60:184–191. doi: 10.1016/j.neures.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Mynett-Johnson L, et al. Evidence for an allelic association between bipolar disorder and a Na+, K+ adenosine triphosphatase alpha subunit gene (ATP1A3) Biol Psychiatry. 1998;44:47–51. doi: 10.1016/s0006-3223(97)00343-0. [DOI] [PubMed] [Google Scholar]
- 17.El-Mallakh RS, et al. Aberrant regulation of endogenous ouabain-like factor in bipolar subjects. Psychiatry Res. 2010;178:116–120. doi: 10.1016/j.psychres.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Grider G, et al. Endogenous digoxin-like immunoreactive factor (DLIF) serum concentrations are decreased in manic bipolar patients compared to normal controls. J Affect Disord. 1999;54:261–267. doi: 10.1016/s0165-0327(98)00208-0. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein I, et al. Involvement of Na(+), K(+)-ATPase and endogenous digitalis-like compounds in depressive disorders. Biol Psychiatry. 2006;60:491–499. doi: 10.1016/j.biopsych.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Keller S, Frishman WH. Neuropsychiatric effects of cardiovascular drug therapy. Cardiol Rev. 2003;11:73–93. doi: 10.1097/01.CRD.0000053453.89776.2D. [DOI] [PubMed] [Google Scholar]
- 21.Clapcote SJ, et al. Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc Natl Acad Sci USA. 2009;106:14085–14090. doi: 10.1073/pnas.0904817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Einholm AP, Toustrup-Jensen MS, Holm R, Andersen JP, Vilsen B. The rapid-onset dystonia parkinsonism mutation D923N of the Na+, K+-ATPase alpha3 isoform disrupts Na+ interaction at the third Na+ site. J Biol Chem. 2010;285:26245–26254. doi: 10.1074/jbc.M110.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosobud AE, Crabbe JC. Genetic correlations among inbred strain sensitivities to convulsions induced by 9 convulsant drugs. Brain Res. 1990;526:8–16. doi: 10.1016/0006-8993(90)90243-5. [DOI] [PubMed] [Google Scholar]
- 24.Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: Rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev. 2007;31:882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand A, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- 26.Dopheide JA, Pliszka SR. Attention-deficit-hyperactivity disorder: An update. Pharmacotherapy. 2009;29:656–679. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- 27.Helmeste DM, Seeman P. Amphetamine-induced hypolocomotion in mice with more brain D2 dopamine receptors. Psychiatry Res. 1982;7:351–359. doi: 10.1016/0165-1781(82)90072-5. [DOI] [PubMed] [Google Scholar]
- 28.Harvey AG. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- 29.Jones SH. Circadian rhythms, multilevel models of emotion and bipolar disorder—An initial step towards integration? Clin Psychol Rev. 2001;21:1193–1209. doi: 10.1016/s0272-7358(01)00111-8. [DOI] [PubMed] [Google Scholar]
- 30.Possidente B, McEldowney S, Pabon A. Aging lengthens circadian period for wheel-running activity in C57BL mice. Physiol Behav. 1995;57:575–579. doi: 10.1016/0031-9384(94)00298-j. [DOI] [PubMed] [Google Scholar]
- 31.Steiner J. A questionnaire study of risk-taking in psychiatric patients. Br J Med Psychol. 1972;45:365–374. doi: 10.1111/j.2044-8341.1972.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 32.Wetzel RK, Arystarkhova E, Sweadner KJ. Cellular and subcellular specification of Na,K-ATPase alpha and beta isoforms in the postnatal development of mouse retina. J Neurosci. 1999;19:9878–9889. doi: 10.1523/JNEUROSCI.19-22-09878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook MN, Williams RW, Flaherty L. Anxiety-related behaviors in the elevated zero-maze are affected by genetic factors and retinal degeneration. Behav Neurosci. 2001;115:468–476. [PubMed] [Google Scholar]
- 34.Thaung C, Arnold K, Jackson IJ, Coffey PJ. Presence of visual head tracking differentiates normal sighted from retinal degenerate mice. Neurosci Lett. 2002;325:21–24. doi: 10.1016/s0304-3940(02)00223-9. [DOI] [PubMed] [Google Scholar]
- 35.Johnson SL. Mania and dysregulation in goal pursuit: A review. Clin Psychol Rev. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.David DJ, Renard CE, Jolliet P, Hascoët M, Bourin M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology (Berl) 2003;166:373–382. doi: 10.1007/s00213-002-1335-4. [DOI] [PubMed] [Google Scholar]
- 37.Giakoumaki SG, et al. Evidence of disrupted prepulse inhibition in unaffected siblings of bipolar disorder patients. Biol Psychiatry. 2007;62:1418–1422. doi: 10.1016/j.biopsych.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50:418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- 39.Shastry BS. On the functions of lithium: The mood stabilizer. Bioessays. 1997;19:199–200. doi: 10.1002/bies.950190304. [DOI] [PubMed] [Google Scholar]
- 40.Miyakawa-Naito A, et al. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J Biol Chem. 2003;278:50355–50361. doi: 10.1074/jbc.M305378200. [DOI] [PubMed] [Google Scholar]
- 41.Yuan Z, et al. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16:4034–4045. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valjent E, Corvol JC, Trzaskos JM, Girault JA, Hervé D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrari P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: An ouabain antagonist that corrects renal and vascular Na+-K+- ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R529–R535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 44.Engel SR, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2009;14:448–461. doi: 10.1038/sj.mp.4002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ackermann TF, Kempe DS, Lang F, Lang UE. Hyperactivity and enhanced curiosity of mice expressing PKB/SGK-resistant glycogen synthase kinase-3 (GSK-3) Cell Physiol Biochem. 2010;25:775–786. doi: 10.1159/000315097. [DOI] [PubMed] [Google Scholar]
- 46.Roybal K, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaltiel G, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mula M, Marotta AE, Monaco F. Epilepsy and bipolar disorders. Expert Rev Neurother. 2010;10:13–23. doi: 10.1586/ern.09.139. [DOI] [PubMed] [Google Scholar]
- 49.Mazza M, et al. Bipolar disorder and epilepsy: A bidirectional relation? Neurobiological underpinnings, current hypotheses, and future research directions. Neuroscientist. 2007;13:392–404. doi: 10.1177/10738584070130041101. [DOI] [PubMed] [Google Scholar]
- 50.Davidson DL, Tsukada Y, Barbeau A. Ouabain induced seizures: Site of production and response to anticonvulsants. Can J Neurol Sci. 1978;5:405–411. doi: 10.1017/s0317167100024185. [DOI] [PubMed] [Google Scholar]
- 51.El-Mallakh RS, et al. Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord. 2003;5:362–365. doi: 10.1034/j.1399-5618.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 52.Kirshenbaum GS, et al. Decreased neuronal Na+, K+ -ATPase activity in Atp1a3 heterozygous mice increases susceptibility to depression-like endophenotypes by chronic variable stress. Genes Brain Behav. 2011;10:542–550. doi: 10.1111/j.1601-183X.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 53.el-Mallakh RS, Wyatt RJ. The Na,K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37:235–244. doi: 10.1016/0006-3223(94)00201-D. [DOI] [PubMed] [Google Scholar]
- 54.Newton JR. Manic-depression neural conduction speeds and action potential event dyscorrelation. Med Hypotheses. 1999;52:77–83. doi: 10.1054/mehy.1997.0610. [DOI] [PubMed] [Google Scholar]
- 55.Warsh JJ, Andreopoulos S, Li PP. Role of intracellular calcium signaling in the pathophysiology and pharmacotherapy of bipolar disorder: Current status. Clin Neurosci Res. 2004;4:201–213. [Google Scholar]
- 56.Looney SW, el-Mallakh RS. Meta-analysis of erythrocyte Na,K-ATPase activity in bipolar illness. Depress Anxiety. 1997;5:53–65. doi: 10.1002/(sici)1520-6394(1997)5:2<53::aid-da1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 57.Hollister LE, Trevino ES. Calcium channel blockers in psychiatric disorders: A review of the literature. Can J Psychiatry. 1999;44:658–664. doi: 10.1177/070674379904400702. [DOI] [PubMed] [Google Scholar]
- 58.Ferreira MA, et al. Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu C, et al. TRPM2 variants and bipolar disorder risk: Confirmation in a family-based association study. Bipolar Disord. 2009;11:1–10. doi: 10.1111/j.1399-5618.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 60.Einat H, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Creson TK, et al. The anterior cingulate ERK pathway contributes to regulation of behavioral excitement and hedonic activity. Bipolar Disord. 2009;11:339–350. doi: 10.1111/j.1399-5618.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- 62.Chen G, Manji HK. The extracellular signal-regulated kinase pathway: An emerging promising target for mood stabilizers. Curr Opin Psychiatry. 2006;19:313–323. doi: 10.1097/01.yco.0000218604.63463.cd. [DOI] [PubMed] [Google Scholar]
- 63.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 64.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.