Abstract
GABAA receptors are the major ionotropic inhibitory neurotransmitter receptors. The endocannabinoid system is a lipid signaling network that modulates different brain functions. Here we show a direct molecular interaction between the two systems. The endocannabinoid 2-arachidonoyl glycerol (2-AG) potentiates GABAA receptors at low concentrations of GABA. Two residues of the receptor located in the transmembrane segment M4 of β2 confer 2-AG binding. 2-AG acts in a superadditive fashion with the neurosteroid 3α, 21-dihydroxy-5α-pregnan-20-one (THDOC) and modulates δ-subunit–containing receptors, known to be located extrasynaptically and to respond to neurosteroids. 2-AG inhibits motility in CB1/CB2 cannabinoid receptor double-KO, whereas β2-KO mice show hypermotility. The identification of a functional binding site for 2-AG in the GABAA receptor may have far-reaching consequences for the study of locomotion and sedation.
Keywords: retrograde signaling, electrophysiology, allosteric modulation
GABAA receptors are chloride ion channels composed of five subunits (1), mediating fast synaptic and tonic inhibition in the mammalian brain. A total of 19 different subunit isoforms have been identified, with the major receptor type in mammalian adult brain consisting of α1, β2, and γ2 subunits (1, 2). GABAA receptors are the target of numerous sedating and anxiolytic drugs such as benzodiazepines (3). The currently known endogenous ligands include GABA, neurosteroids (4), and possibly oleamide (5). The pharmacological properties of this chloride ion channel strictly depend on receptor subunit composition (2) and arrangement (6).
Synaptic GABAA receptors mediate phasic inhibition, whereas extrasynaptic receptors mediate tonic inhibition (7, 8). The δ-subunit–containing GABAA receptors occur only extrasynaptically and are particularly sensitive to modulation by neurosteroids (4, 9, 10). GABAA receptors containing the δ-subunit have been implicated in changing seizure susceptibility and states of anxiety during the ovarian cycle (11) and in postpartal depression (12).
The endocannabinoid system (ECS) is part of a complex lipid signaling network involving the G protein-coupled receptors CB1 and CB2 (13, 14). The CB1 receptor is ubiquitously expressed in the central nervous system, whereas the CB2 receptor is primarily expressed in peripheral tissues (13, 15). The two best-characterized endogenous cannabinoid ligands are anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) (15, 16). Endocannabinoids are released postsynaptically and modulate neurotransmission by activating presynaptic CB1 receptors. Thus, they act as retrograde signals. 2-AG is the major CB1 receptor agonist in the brain, where it is found at micromolar concentrations (17). Physiological and pharmacological studies provide evidence that the ECS is involved in the regulation of GABA and glutamate release (18, 19). The major central action of endocannabinoids is analgesia (20, 21), but the ECS has also been implicated in anxiety and movement disorders (22).
Results
Endocannabinoid 2-AG Potentiates GABAA Receptors.
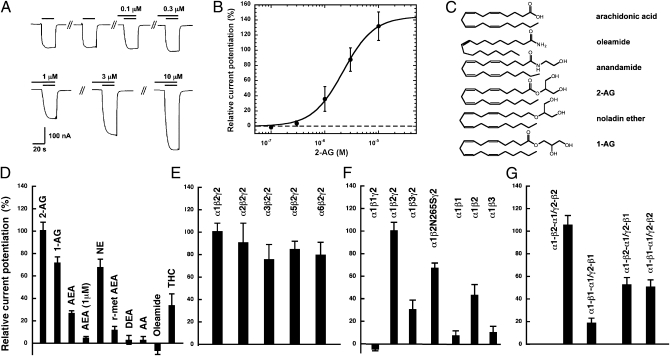
Recombinant α1β2γ2 GABAA receptors were functionally expressed in Xenopus oocytes. We found that currents elicited by 1 μM GABA were potentiated by 2-AG in a concentration-dependent way (Fig. 1 A and B). Fitting of the concentration–response curve indicated a maximal potentiation of 138 ± 21% (SEM) and an EC50 value of 2.1 ± 0.5 μM (n = 5). The resulting Hill coefficient of 2.2 ± 0.2 indicates that more than one molecule of 2-AG interacts with one receptor. Potentiation by 3 μM 2-AG was determined at different GABA concentrations—0.5 μM (EC∼0.9), 1 μM (EC∼2.3), 10 μM (EC∼35), and 100 μM (EC∼92)—and amounted to 86 ± 13% (n = 3), 82 ± 25% (n = 6), 10 ± 6% (n = 3) and 2 ± 6% (n = 3), respectively. This shows that only currents elicited by low GABA concentrations are potentiated by 2-AG.
Fig. 1.
2-AG affects the function of GABAA receptors. Receptors were expressed in Xenopus oocytes. Currents were measured using electrophysiological tools. (A) Concentration–response curve of 2-AG at α1β2γ2 GABAA receptors. Increasing concentrations of 2-AG (upper bars) were preapplied for 30 s and subsequently coapplied with 1 μM GABA (lower bars) after two control applications of GABA. Concentrations of 2-AG are indicated above the upper bars. Original current traces are shown. (B) Concentration–response curve of 2-AG. Mean values ± SEM are shown for experiments with five oocytes from three batches of oocytes. (C) Molecular structures of tested compounds. (D) Relative current potentiation by different agents sharing structural or functional similarity with 2-AG. Current potentiation by 3 μM of 2-AG, 1-AG, AEA, NE, r-met AEA, AA, DEA, oleamide, THC, and 1 μM of AEA. (E) Lack of a role of the type of α-subunit in recombinant αxβ2γ2 (x = 1, 2, 3, 5, 6) GABAA receptors for 2-AG effect. Relative current potentiation was determined at 3 μM of 2-AG. (F) Role of the type of β-subunit in recombinant α1βy and α1βyγ2 (y = 1, 2, 3) GABAA receptors. (G) Concatenated receptors, containing two β1, two β2, or one β1 and one β2 subunit in different positions, were modulated by 2-AG as earlier. Data are shown as mean values ± SEM (n = 4). For all experiments shown in this figure, GABA was used at a concentration eliciting 1.0% to 3.2% of the maximal current amplitude [EC(2.1 ± 1.1)] in each receptor form.
Identification of Other Endocannabinoids That Target GABAA Receptors.
To determine if this effect was only observed with 2-AG, we also evaluated physiologically relevant compounds sharing close structural similarity with 2-AG (Fig. 1C). The spontaneous isomerization product 1(3)-AG (stereoisomeric mixture produced by acyl migration) showed a similar potentiation as 2-AG. Whereas AEA and 2-methyl-2′-F-AEA (r-met AEA) only weakly affected the response to 2-AG, 2-arachidonoyl glyceryl ether (noladin ether; NE), which is a minor putative endogenous CB1 receptor agonist (23), was similarly active at GABAA receptors (Fig. 1D). Docosatetraenylethanolamide (DEA) and arachidonic acid (AA) showed only a weak if any potentiation (Fig. 1D). Not unexpectedly, oleamide, which was previously shown to only weakly potentiate GABAA receptors (5), was ineffective at 3 μM. The phytocannabinoid Δ9-tetrahydrocannabinol (THC) at 3 μM only weakly potentiated the response to GABA, at 34 ± 10% (Fig. 1D). These data uncover the significant effect of 2-AG and NE at GABAA receptors and indicate the importance of the glycerol moiety for the GABAA receptor interaction.
Potentiation by 2-AG Is Selective for GABAA Receptors Containing the β2 Subunit.
Next, we investigated the GABAA receptor subunit selectivity of 2-AG. The α1 subunit in α1β2γ2 was replaced by α2, α3, α5, or α6. This had little effect on the current potentiation by 3 μM 2-AG (Fig. 1E). However, drastic effects were observed upon replacement of the β2 subunit by β1 or β3 (Fig. 1F). Whereas, in receptors containing β1, potentiation was abolished, it was reduced to approximately one third in receptors containing β3. To test whether 2-AG shares the binding site with loreclezole (24), another allosteric activator of GABAA receptors, we evaluated the point mutation β2N265S in α1β2γ2 receptors that abolishes the potentiation by loreclezole. As shown in Fig. 1F, this mutation only partially reduced the effect of 2-AG, thus indicating that endocannabinoids act through a different site. Potentiation was also strongly reduced upon omission of γ2 in α1β2γ2 to give the dual combination α1β2. Possibly, in receptors with two adjacent β2 subunits the binding site for 2-AG is compromised in these subunits. Replacement of the β2 subunit in dual subunit combinations by β1 or β3 in both cases led to an additional strong reduction of the potentiation (Fig. 1F). For all experiments, GABA was used at a concentration eliciting 1.0% to 3.2% of the maximal current amplitude in each receptor form.
We then investigated the concatenated receptors (25, 26) α1-β1-α1/γ2-β1, α1-β2-α1/γ2-β2, α1-β1-α1/γ2-β2, and α1-β2-α1/γ2-β1, in which the subunit arrangement is predefined by covalent linkage. These receptors have been described in detail previously (27). As shown in Fig. 1G, in receptors containing two β1 subunits stimulation by 2-AG was strongly reduced compared with receptors containing two β2 subunits. In the receptors containing one β2 subunit, irrespective of the position, the potentiation was reduced by approximately 50%, indicating that two sites for 2-AG may be located on one α1β2γ2 receptor.
Identification of Amino Acid Residues Conferring Subunit Selectivity of 2-AG.
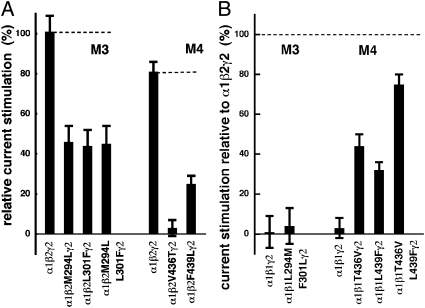
Next, we identified the amino acid residues (AARs) mediating the selectivity of 2-AG for the β2 subunits. We aligned the protein sequences of the three β-subunits and identified homologous AAR in predicted transmembrane regions that were identical in β1 and β3, but different from those in β2. These were the AAR corresponding to β2M294 and β2L301 in transmembrane region M3, and β2V436 and β2F439 in M4. Mutation of these residues in β2 to the respective residues present in β1 and β3 were prepared and coexpressed with α1 and γ2 subunits, and the resulting receptors screened for their potentiation by 3 μM of 2-AG (Fig. 2A and SI Appendix, Fig. S1). All mutations significantly reduced potentiation by 2-AG. In the mutant receptor α1β2V436Tγ2, potentiation was even completely abolished, but the EC50 of GABA was not significantly altered. In a second step, the corresponding residues in β1 were mutated to the homologous residue present in β2 (Fig. 2B). A critical residue located in the binding site would be expected to lead to a loss of potentiation in α1β2Mγ2 receptors and to a gain in potentiation in α1β1Mγ2 receptors. Our results clearly show that AAR V436 and, to a smaller extent, also F439 in M4 of β2 mediate the functional effect of 2-AG, thus pinpointing the receptor binding site. Along the same line, both mutations combined resulted in almost complete recovery. Although allosteric effects by the mutations cannot be fully excluded, we consider this possibility unlikely. Both AARs are predicted to be located in cytoplasmic leaflet of the M4 α-helix, in an angle of approximately 60° and a distance in the direction of the α-helix of 4.5 Å. Intriguingly, in a homology model described by Ernst et al. (28), all four mentioned residues face the same cavity in the receptor.
Fig. 2.
Point mutations affecting the stimulation by 2-AG. (A) The point mutation α1β2V436Tγ2 abolishes potentiation by 2-AG. Impact of selected point mutations in M3 and M4 of the β2 subunit of α1β2Mγ2 receptors on the potentiation by 2-AG. The values are given relative to the current elicited by GABA (mean of 101% and 81%, respectively, in two different experiments). (B) The β1 subunit, which is naturally not able to confer potentiation to α1β1γ2 receptors, is partially converted to a competent subunit by the mutations β1T436V and β1L439F alone and to a larger extent by the combined mutation. The mutations did not affect the EC50 for GABA. GABA was used at a concentration eliciting 1.0% to 3.2% of the maximal current amplitude [EC(2.1 ± 1.1)]. Data are shown as mean values ± SEM. Measurements of the mutations in M3 were performed in at least four oocytes, and those of the mutations in M4 in five to eight oocytes from two independent batches of oocytes.
δ-Subunit–Containing Receptors Are Responsive to 2-AG.
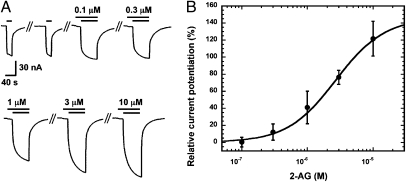
In agreement with the finding that 2-AG mediates its effect via β2, α1β2δ receptors in which γ2 in α1β2γ2 was replaced by the δ-subunit also responded in a concentration-dependent way to 2-AG (Fig. 3A). Fitting of the concentration–response curve carried out with 1 μM GABA (EC∼8) indicated a maximal potentiation of 150 ± 51% (n = 3), an EC50 value of 2.9 ± 1.8 μM, and a Hill coefficient of 1.25 ± 0.15 (Fig. 3B). In additional experiments, α1β2δ receptors responded with a potentiation of 114 ± 19% (n = 4; SI Appendix, Fig. S2) to 3 μM 2-AG. In α1β2δ receptors, the subunits may be arranged differently to form a pentamer, and the different receptor isoforms are characterized by distinct properties, some of them being strictly dependent on the presence of neurosteroids and low GABA concentrations (29). Together, these results establish 2-AG as an endogenous allosteric activator of GABAA receptors and identify M4 of the β2 subunit as the primary molecular target for 2-AG.
Fig. 3.
Effect of 2-AG on α1β2δ GABAA receptors. (A) Concentration response curve of 2-AG at α1β2δ GABAA receptors. Increasing concentrations of 2-AG (upper bars) were preapplied for 30 s and subsequently coapplied with 1 μM GABA (EC∼8; lower bars) after two control applications of GABA. Concentrations of 2-AG are indicated above the upper bars. Original current traces are shown. Please note that preapplication of 2-AG resulted in small currents by itself. Currents did not saturate completely within the time of application and saturation of potentiation was not reached experimentally. Time of application and concentrations used were both limited by the available 2-AG. (B) Three concentration–response curves of 2-AG were averaged. Mean values ± SD are shown for each point.
2-AG Acts in a Superadditive Manner with Neurosteroids or Diazepam.
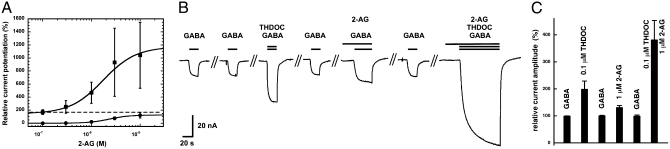
Next, we investigated a possible functional interaction between 2-AG and neurosteroids at α1β2γ2 GABAA receptors. Concentration–response curves for the potentiation by 2-AG were generated in the presence of 0.1 μM 3α, 21-dihydroxy-5α-pregnan-20-one (THDOC; Fig. 4A). GABA (0.5 μM) was applied alone and then in combination with 0.1 μM THDOC, followed by applications containing GABA, THDOC, and increasing concentrations of 2-AG. As shown in Fig. 4A potentiation by 0.1 μM THDOC alone amounted to 164 ± 44% (n = 4). As mentioned earlier, the maximal potentiation by 2-AG amounted to 138 ± 21 (n = 5). Combined application of 2-AG and THDOC resulted in a curve characterized by an EC50 of 1.7 ± 0.5 μM and a maximal potentiation of 1,041 ± 505% (n = 4). This indicates that THDOC acts by increasing the maximal potentiation without significantly affecting the EC50 for 2-AG. Combined application of 0.1 μM THDOC and 1 μM 2-AG resulted in a superadditive potentiation, thus suggesting a synergism between these two agents. Further experiments were performed to support a superadditivity between THDOC and 2-AG. Fig. 4B shows an experiment in which currents were potentiated by low concentrations of THDOC, 2-AG, or a combination of both. We observed a strong superadditive effect between the two endogenous modulators (Fig. 4C). Superadditivity was also observed in three additional experiments carried out at 0.05 μM THDOC (SI Appendix, Fig. S3). To assess whether the agonist site or the modulatory site for neurosteroids (30) was involved in the superadditivity, we applied 0.1 μM THDOC together with 1 μM 2-AG in the absence of GABA. Currents amplitudes amounting to less than 4 nA were elicited in oocytes expressing more than 10,000 nA maximal GABA current. It is therefore unlikely that the agonist site for neurosteroids is involved in the superadditivity of 2-AG and THDOC effects.
Fig. 4.
Superadditivity between 2-AG and the neurosteroid THDOC. Recombinant α1β2γ2 GABAA receptors were functionally expressed in Xenopus oocytes. (A) Cumulative concentration response curve of 2-AG without (circles) and with potentiation with 0.1 μM THDOC (squares). The dashed line indicates the stimulation by 0.1 μM THDOC alone. (B) GABA 0.5 μM (EC0.2–0.5) was applied twice alone, followed by the same concentration of GABA in combination with 0.1 μM THDOC, GABA alone, GABA in combination with 1 μM 2-AG, GABA alone, and GABA in combination with 0.1 μM THDOC and 1 μM 2-AG. Responses to 2-AG, alone or in combination, did not reach equilibrium, presumably because 2-AG is hydrophobic and accumulates in the membrane. (C) Four such experiments were averaged. Data are shown as mean values ± SD (n = 4).
Next, we investigated a possible interaction of 2-AG with the benzodiazepine diazepam. Concentration–response curves for the potentiation by 2-AG were generated in the presence of 0.3 μM diazepam (SI Appendix, Fig. S4). GABA (0.5 μM) was applied alone and then in combination with 0.3 μM diazepam, followed by several applications containing GABA, diazepam, and increasing concentrations of 2-AG. In these experiments, potentiation by 0.3 μM diazepam alone amounted to 128 ± 7% (n = 3). The EC50 for 2-AG in the presence of diazepam was 0.8 ± 0.3 μM, indicating a small increase in apparent affinity for 2-AG, and the maximal additional potentiation relative to this level achieved with diazepam alone was 210 ± 35% (n = 3). In additional experiments, currents were potentiated by 0.3 μM diazepam or 2-AG, respectively, or by a combination of both. Again, we observed a significant superadditivity between the two modulators (SI Appendix, Fig. S5). These results clearly suggest a superadditivity between the modulation by diazepam and 2-AG.
Locomotor Activity Is Suppressed by 2-AG and NE in CB1/CB2 Double-KO Mice, and β2 KO Mice Show Hypermotility.
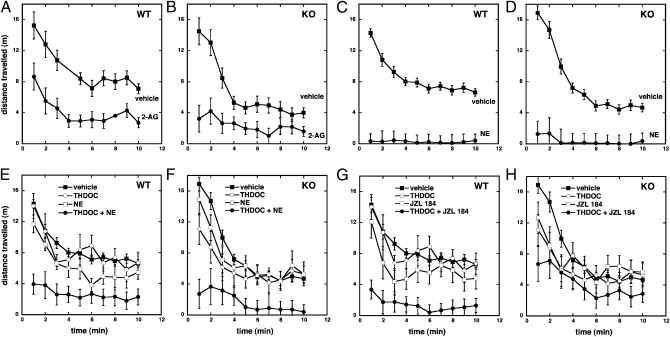
The sedating effects of GABAA receptor activation can be readily determined in vivo by evaluating exploratory locomotor behaviors (31). To eliminate the cannabinoid receptor-mediated effects by 2-AG, the experiments were carried out in cannabinoid receptor (Cnr1−/−/Cnr2−/−) double-KO mice. Additionally, WT mice were investigated. Vehicle-injected control animals of both genotypes showed a characteristic open-field exploratory behavior. Locomotor activity was initially high and gradually decreased over time, as the animals habituated to the new environment (Fig. 5 A and B). As anticipated, 2-AG–treated animals (10 mg⋅kg−1 i.v.) showed a strong hypomotility in WT and in KO animals (P < 0.0001; Fig. 5 A and B). Similarly, NE (10 mg⋅kg−1 i.v.), a metabolically stable ether-linked analogue of 2-AG (23), led to potent hypomotility (Fig. 5 C and D). After 2-AG treatment in several cases, a brief anesthetic effect (i.e., loss of righting reflex) was detected (WT, three of eight; KO, five of eight). NE treatment in all cases led to a brief loss of righting reflex. Thus, these data provide evidence for the cannabinoid receptor-independent sedative effect of 2-AG and NE.
Fig. 5.
Behavioral effects of 2-AG and NE in WT and Cnr1−/−/Cnr2−/− double-KO mice. The spontaneous locomotor activity was determined in WT (A) and Cnr1−/−/Cnr2−/− double-KO mice (B) after treatment with 2-AG (10 mg⋅kg−1 i.v.; circles) or vehicle (squares) and in WT (C) and Cnr1−/−/Cnr2−/− double-KO mice (D) after treatment with NE (10 mg⋅kg−1 i.v.; circles) or vehicle (squares). Data are shown as the mean distance traveled in 1-min time bins ± SEM. Repeated-measures ANOVA was used to assess the effect of 2-AG and NE treatment on locomotor activity (n ≥ 8; P < 0.0001). Superadditivity between THDOC and NE in WT and Cnr1−/−/Cnr2−/− double-KO mice. The spontaneous locomotor activity was determined in WT (E) and Cnr1−/−/Cnr2−/− double-KO mice (F) after treatment with vehicle (closed squares), THDOC (2 mg⋅kg−1 i.v.; open circles), NE (5 mg⋅kg−1 i.v.; open squares), or combined treatment with THDOC and NE (closed circles). Superadditivity between THDOC and JZL184 in WT and Cnr1−/−/Cnr2−/− double-KO mice. The spontaneous locomotor activity was determined in WT (G) and Cnr1−/−/Cnr2−/− double-KO mice (H) after treatment with vehicle (closed squares), THDOC (2 mg⋅kg−1 i.v.; open circles), JZL184 (16 mg⋅kg−1 i.p.; open squares), or combined treatment with THDOC and JZL184 (closed circles). Data are shown as the mean distance traveled in 1-min time bins ± SEM. Repeated-measures ANOVA was used to assess significance of the superadditive effect after habituation (P < 0.0001).
Next, we addressed the potential pharmacological superadditivity between 2-AG and THDOC. Given that 2-AG injection leads to rapid degradation of 2-AG in the brain (32) we used an indirect approach through pharmacological inhibition of 2-AG metabolism. Treatment of WT and cannabinoid receptor (Cnr1−/−/Cnr2−/−) double-KO mice with the selective monoacylglycerol lipase inhibitor JZL184 (16 mg⋅kg−1 i.p.), a compound that inhibits the enzymatic hydrolysis of 2-AG (32, 33), or THDOC (2 mg⋅kg−1 i.v.) alone, only partially inhibited locomotor activity after habituation to the test cage (P = 0.08 and P = 0.156, respectively; Fig. 5 E and F). However, when the animals received THDOC (2 mg⋅kg−1 i.v.) 2 h after the JZL184 treatment, they presented strong hypolocomotion after habituation (P < 0.0001). These data show that elevated endogenous 2-AG in brain mediates a superadditive effect with externally administered THDOC, independent of CB receptors (Fig. 5 E and F). In WT mice, the superadditive effect was more pronounced than in KO mice, and may also involve CB receptors. JZL184 16 mg⋅kg−1 i.p. did not trigger the full effect on 2-AG elevation, which is approximately 10-fold (33), and showed significant hypomotility only during the habituation phase (Fig. 5 G and H). Upon completion of the experiments, the JZL184-treated (Cnr1−/−/Cnr2−/−) double-KO mice showed a fourfold increase of 2-AG in brain, and the free AA was significantly reduced (SI Appendix, Fig. S6). As expected, a more potent superadditive effect was observed in the late phase in animals treated with combinations of low doses of NE (5 mg/kg−1 i.v.) and THDOC (2 mg⋅kg−1 i.v.; P < 0.0001; Fig. 5 G and H), as a result of the better metabolic stability of NE vs. 2-AG (34). It should be noted that nontreated KO mice (vehicle controls in Fig. 5 B, D, F, and H) display a lower motility than WT mice (vehicle controls in Fig. 5 A, C, E, and G; P < 0.0001).
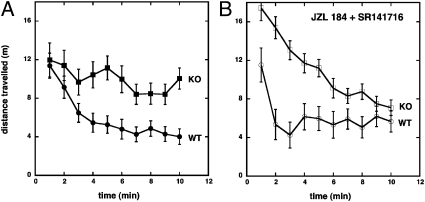
As 2-AG acts via the β2 subunit, we studied β2 KO mice. These mice displayed a very pronounced hypermotility (P < 0.0001; Fig. 6A), which is in agreement with a previous report (35). This may indicate the involvement of a tonic activation of β2 subunit-containing GABAA receptors by 2-AG in WT C57BL6/J/129SvEv mice. When 2-AG levels were increased by JZL184 and CB1 receptors simultaneously blocked by low concentrations of the CB1 antagonist SR141716 at which GABAA receptors were not affected (36), WT mice showed hypomotility, whereas the β2 KO mice showed hypermotility during the habituation phase (Fig. 6B). This clearly indicates that physiological concentrations of 2-AG (as modulated by JZL184) can exert hypolocomotion in these mice via both β2 and CB1 receptors. However, treatment of β2 KO mice with NE (10 mg⋅kg−1 i.v.) and SR141716 (3 mg⋅kg−1 i.v.) likewise induced strong hypomotility (SI Appendix, Fig. S7), thus indicating additional receptors for NE.
Fig. 6.
Hypermotility of β2-KO mice and effects of JZL184 and SR141716. The spontaneous locomotor activity was determined before (A) and after (B) treatment with JZL184 (16 mg⋅kg−1 i.v.) plus SR141716 (3 mg⋅kg−1 i.v.) in WT (open circles) and β2-KO mice (open squares). Data are shown as the mean distance traveled in 1-min time bins ± SEM (n = 6 each).
Discussion
In addition to the well known retrograde GABAergic signaling of endocannabinoids mediated via the CB1 receptor at central synapses (19), we show an unexpected direct molecular interaction between 2-AG and GABAA receptors. 2-AG is an endogenous ester formed from the omega-6 fatty acid AA and glycerol by different biosynthetic pathways (16). 2-AG, unlike AEA, is present at high levels in the central nervous system; it is the most abundant molecular species of monoacylglycerol found in mouse and rat brain (∼5–10 μmol/kg tissue) (16). Our finding that elevated 2-AG levels upon JZL184 treatment inversely correlate with free AA levels is consistent with a previous study (32) and clearly indicates that 2-AG is a major AA metabolite in mouse brain. AEA levels in brain are generally low (up to 0.1 μmol/kg wet weight) (17), and at 1 μM of AEA, only minor and irrelevant effects on GABAA receptors were detected in this study. This is in line with our previous observation that AEA fails to modulate motility in Cnr1−/−/Cnr2−/− double-KO mice (37) even though it inhibits locomotion in CB1 KO mice (38). In this study, NE produced stronger pharmacological effects in Cnr1−/−/Cnr2−/− double-KO mice than 2-AG, which may be explained by its better metabolic stability (34) or additional receptor targets. The latter is also indicated by the fact that, in β2 KO mice, NE showed similar effects as in WT mice in the presence of CB1 receptor blockage by SR141716 (SI Appendix, Fig. S7). In agreement with a previous study (39), we were not able to detect NE in mouse brain. Other groups have reported only low levels (in nmol/kg) of NE in rat brain (40). This suggests that NE is not a major AA brain metabolite, and physiological interactions with GABAA receptors are therefore rather unlikely.
The superadditivity between the effects of THDOC and 2-AG at GABAA receptors is intriguing and may suggest that 2-AG can modulate the action of neurosteroids at GABAA receptors. A study showing that 2-AG distribution in rat brain does not match the CB1 receptor sites describes high amounts of 2-AG in brainstem and hippocampus (41). We also explored the superadditivity of 2-AG and THDOC in animal experiments to obtain additional evidence for the physiological relevance of our finding. Low concentrations of THDOC in combination with partially elevated 2-AG levels or low concentrations of NE administered i.v. resulted in significant superadditive inhibition of locomotion both in WT and KO mice. Intriguingly, β2 KO mice showed an overall pronounced hyperlocomotion compared with WT mice, indicating a role for β2 in 2-AG–mediated locomotion behavior. Blocking of 2-AG degradation in the presence of SR141716 in β2 KO mice resulted in hyperlocomotion during the habituation phase, which was not observed in WT mice. On the contrary, WT mice showed inhibition of locomotion during this phase, which is in agreement with the hypothesis that 2-AG can mediate behavioral effects also via β2. Nevertheless, the expected lack of locomotion inhibition by 2-AG in the later phase in β2 KO mice was not observed. Although our data with β2 KO mice demonstrate the involvement of β2-containing GABAA receptors during locomotion habituation, the overall strong hypolocomotion induced by NE in the β2 KO mice suggests the involvement of additional receptors, possibly from the cys-loop family.
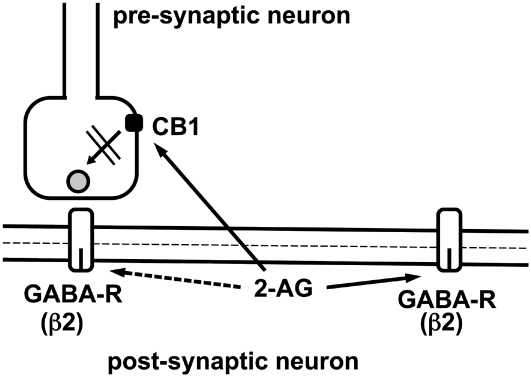
2-AG is biosynthetically generated in postsynaptic neurons directly from constituents of the cell membrane where GABAA receptors are located (42). Given that 2-AG is synthesized in the membrane and cannot easily cross the aqueous barrier, it is tempting to speculate that 2-AG accumulates and interacts locally with GABAA receptors within the postsynaptic neuron (Fig. 7). Based on our finding that 2-AG acts exclusively at low concentrations of GABA and that it synergizes with neurosteroids, it is likely that extrasynaptic GABAA receptors may be preferentially targeted by 2-AG. As it has been shown in hippocampus, not all synapses are silenced by 2-AG to the same degree, and residual synaptic activity, especially in dendritic synapses, may additionally underlie modulation by 2-AG (43), at least during the late phase of the inhibitory postsynaptic current. Fig. 7 summarizes these possible actions of 2-AG. The fact that modulation of GABAA receptors by 2-AG is exclusively observed at low concentrations of GABA may be the reason why we were not able to establish conditions to measure robust effects of 2-AG in brain slice experiments.
Fig. 7.
Hypothetical scheme showing how newly synthesized 2-AG may diffuse laterally and act in the postsynaptic membrane at extrasynaptic and, to some degree, also at synaptic β2 subunit-containing GABAA receptors. Only after diffusion or transport to the presynaptic membrane, 2-AG inhibits the release of GABA upon activation of CB1 receptors.
In this study, we have identified the AAR V436 and F439 in the inner membrane leaflet of the M4 helix of the β2 subunit as the molecular site of action of 2-AG. The discovery of a modulatory site for 2-AG on a specific set of GABAA receptor subtypes adds another level of complexity to endocannabinoid and GABA action and provides important insight into their molecular mechanisms.
Materials and Methods
Materials.
2-AG was obtained from Cayman. According to GC-MS analyses, 2-AG was 92% pure. The remaining 8% was determined as the spontaneous breakdown product 1(3)-AG. 2-AG was prepared as a 10-mM stock solution in DMSO and was dissolved in the experimental solutions, resulting in a maximal final DMSO concentration of 0.1%. 1-AG/3-AG, AEA, r-met AEA, oleamide, and DEA were obtained from Cayman and were at least 95% pure. THC was obtained from THC Pharm (>95% purity). THDOC was purchased from Fluka.
Electrophysiological Experiments.
Preparation of RNA, isolation of Xenopus oocytes, culturing of the oocytes, injection of cRNA, defolliculation, and two-electrode voltage-clamp measurements were performed as described earlier (44). 2-AG was preapplied for 30 s. Relative current potentiation by 2-AG was determined as (I2-AG + GABA / IGABA − 1) * 100%. Unless indicated otherwise, potentiation by 2-AG was determined at all receptors at a similar relative GABA concentration relative to the maximal current amplitude at EC(2.1 ± 1.1) in the each receptor form. Concentration response curves were fitted with the following equation:
where P is the current potentiation, c, the concentration of 2-AG, max is the maximal current potentiation, the EC50 is the concentration of 2-AG at which half-maximal potentiation was observed, and n is the Hill coefficient. The perfusion system was cleaned between two experiments by washing with 100% DMSO after application of 2-AG or THDOC to avoid contamination.
Behavioral Experiments and Quantification of 2-AG and AA.
Methods of behavioral experiments and quantification of 2-AG and AA are provided in the SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. V. Niggli for carefully reading the manuscript and B. P. Lüscher for frog surgery. This work was supported by Swiss National Science Foundation Grants 31003A_132806/1 (to E.S.) and 31003A_120672 (to J.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113444108/-/DCSupplemental.
References
- 1.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 2.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 3.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 4.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 5.Yost CS, et al. Oleamide potentiates benzodiazepine-sensitive gamma-aminobutyric acid receptor activity but does not alter minimum alveolar anesthetic concentration. Anesth Analg. 1998;86:1294–1300. doi: 10.1097/00000539-199806000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Minier F, Sigel E. Positioning of the α-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:7769–7774. doi: 10.1073/pnas.0400220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 8.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Zheleznova N, Sedelnikova A, Weiss DS. α1β2δ, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Br J Pharmacol. 2008;53:1062–1071. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigel E, Kaur KH, Lüscher BP, Baur R. Use of concatamers to study GABAA receptor architecture and function: application to δ-subunit-containing receptors and possible pitfalls. Biochem Soc Trans. 2009;37:1338–1342. doi: 10.1042/BST0371338. [DOI] [PubMed] [Google Scholar]
- 11.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 12.Maguire JL, Mody I. GABA(A)R plasticity during pregnancy: Relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackie K. Cannabinoid receptors: Where they are and what they do. J Neuroendocrinol. 2008;20(suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 14.De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: From the early to the latest concepts. Best Pract Res Clin Endocrinol Metab. 2009;23:1–15. doi: 10.1016/j.beem.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Di Marzo V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Hanus LO. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med Res Rev. 2009;29:213–271. doi: 10.1002/med.20135. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura T, et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 18.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rea K, Roche M, Finn DP. Supraspinal modulation of pain by cannabinoids: The role of GABA and glutamate. Br J Pharmacol. 2007;152:633–648. doi: 10.1038/sj.bjp.0707440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohmann AG, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 21.Nyilas R, et al. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci. 2009;29:1964–1978. doi: 10.1111/j.1460-9568.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 23.Hanus L, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wafford KA, et al. A novel allosteric modulatory site on the GABAA receptor β subunit. Neuron. 1994;12:775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 25.Minier F, Sigel E. Techniques: Use of concatenated subunits for the study of ligand-gated ion channels. Trends Pharmacol Sci. 2004;25:499–503. doi: 10.1016/j.tips.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Baur R, Minier F, Sigel E. A GABA(A) receptor of defined subunit composition and positioning: Concatenation of five subunits. FEBS Lett. 2006;580:1616–1620. doi: 10.1016/j.febslet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Boulineau N, Baur R, Minier F, Sigel E. Consequence of the presence of two different β subunit isoforms in a GABA(A) receptor. J Neurochem. 2005;95:1724–1731. doi: 10.1111/j.1471-4159.2005.03495.x. [DOI] [PubMed] [Google Scholar]
- 28.Ernst M, Bruckner S, Boresch S, Sieghart W. Comparative models of GABAA receptor extracellular and transmembrane domains: Important insights in pharmacology and function. Mol Pharmacol. 2005;68:1291–1300. doi: 10.1124/mol.105.015982. [DOI] [PubMed] [Google Scholar]
- 29.Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of delta-subunit-containing GABAA receptors. J Biol Chem. 2009;284:7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 31.Bertrand S, Cazalets JR. Presynaptic GABAergic control of the locomotor drive in the isolated spinal cord of neonatal rats. Eur J Neurosci. 1999;11:583–592. doi: 10.1046/j.1460-9568.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- 32.Long JZ, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laine K, Järvinen K, Mechoulam R, Breuer A, Järvinen T. Comparison of the enzymatic stability and intraocular pressure effects of 2-arachidonylglycerol and noladin ether, a novel putative endocannabinoid. Invest Ophthalmol Vis Sci. 2002;43:3216–3222. [PubMed] [Google Scholar]
- 35.Sur C, et al. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baur R, Gertsch J, Sigel E. The cannabinoid receptor CB1 antagonists rimonabant (SR141716) and AM251 directly potentiate GABA(A) receptors. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01405.x. 10.1111/j.1476-5381.2011.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rácz I, et al. Anandamide effects on 5-HT(3) receptors in vivo. Eur J Pharmacol. 2008;596:98–101. doi: 10.1016/j.ejphar.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Di Marzo V, et al. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: Evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- 39.Oka S, et al. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J Neurochem. 2003;85:1374–1381. doi: 10.1046/j.1471-4159.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 40.Fezza F, et al. Noladin ether, a putative novel endocannabinoid: Inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513:294–298. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- 41.Bisogno T, et al. Brain regional distribution of endocannabinoids: Implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- 42.Di Marzo V. Targeting the endocannabinoid system: To enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 43.Lee S-H, Földy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sigel E. Properties of single sodium channels translated by Xenopus oocytes after injection with messenger ribonucleic acid. J Physiol. 1987;386:73–90. doi: 10.1113/jphysiol.1987.sp016523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.