This finding supports the hypothesis that a fetal inflammatory response to antenatal infection may be part of the etiology of severe ROP.
Abstract
Purpose.
To test the hypothesis that the presence of bacteria and/or histologic inflammation in the placenta of infants born preterm is associated with an increased risk for severe retinopathy of prematurity (ROP).
Methods.
This was a prospective cohort study. Exploratory and multivariable data analyses were used, including logistic regression models with interaction terms. Main outcomes were four definitions of severe ROP: stage 3 or higher, any ROP in zone I, prethreshold/threshold, and plus disease.
Results.
Individually, placenta bacteria and histologic inflammation were not associated with severe ROP in univariable analyses among 1064 infants with gestational age <28 weeks or among 715 infants with gestational age <27 weeks (we excluded infants with a gestational age of 27 weeks because of the very small number of ROP cases). However, the co-occurrence of bacteria and inflammation was associated with an increased risk for ROP in zone I (odds ratio, 3.1; 95% confidence interval, 1.02–9.5). Among 339 infants with any placental bacteria, the co-occurrence of (1) inflammation and a gestational age of 23 to 24 weeks and (2) inflammation and hyperoxia were associated with prominent increases in risk for all definitions of severe ROP.
Conclusions.
While antenatal exposure to infection or inflammation alone does not appear to convey risk information for severe ROP, their co-occurrence does. This finding supports the hypothesis that a fetal inflammatory response to antenatal infection might be part of the etiology of severe ROP.
Retinopathy of prematurity (ROP) is a disorder of abnormal retinal vasoproliferation in preterm infants.1 The long-term visual outcome among children with ROP includes an increased risk for blindness2 and visual disability.3 Identifying the antecedents is crucial to preventing ROP.
Low birth weight, low gestational age (GA), and hyperoxemia are associated with an increased risk for ROP.4–7 An alternative, but not mutually exclusive, etiologic scenario postulates that exposures to infectious and inflammatory stimuli are associated with an increased risk for ROP.8 Among preterm infants, neonatal infections and sepsis are common complications9,10 that are associated with ROP and vision impairment.11–13 Systemic fungal infection has repeatedly been linked to ROP in ELBW neonates,14–17 as has systemic bacterial infection.18–24 We recently reported that the ROP risk associated with hyperoxemia appears to be especially prominent among infants of 23 to 25 weeks' GA, whereas the ROP risk associated with neonatal sepsis was elevated among infants born at 28 to 29 weeks.25 Late neonatal bacteremia appears to be a more prominent risk factor for severe ROP than early neonatal bacteremia.26 Moreover, proinflammatory cytokines in the neonatal systemic circulation are associated with ROP.27
Although some reports associate neonatal infection/inflammation with ROP occurrence and/or severity,11,17–19,22–24,28 we know of no large cohort study of the relationship between antenatal infection and ROP. In one study of 388 infants <1000 g or <28 weeks' GA, clinical or histologic chorioamnionitis was not associated with ROP >stage 3, but it was associated with a 25% risk for long-term visual impairment (16% among the unexposed; not significant).29 In a smaller study (n = 177) of infants <30 weeks' gestation, histologic chorioamnionitis was associated with a 68% risk for ROP (42% among the unexposed; P = 0.005).28 Another study of 122 infants of a wide range of GAs (26–35 weeks) suggests an association between histologic chorioamnionitis and umbilical cord inflammation and ROP, although results were not adjusted for confounders.30 Although low GA continued to be the most prominent antecedent for ROP in one of our own studies in 73 infants of <32 weeks' gestation, the additional individual or joint exposure to clinical chorioamnionitis or systemic neonatal inflammation added appreciably to the risk of progression to high-grade disease.31
We therefore suggest that ROP's etiology and pathogenesis might begin in utero and hypothesize that both recovery of organisms from the placenta and histologic indicators of inflammation are associated with an increased risk of ROP. In this article, we report results of our exploration of associations between antenatal infection/inflammation and ROP in the Extremely Low Gestational Age Newborn (ELGAN) study.32 Particular emphasis is on interaction analysis and multivariable modeling.
Methods
The ELGAN study was designed to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in ELGANS.32 During the years 2002 to 2004, women who delivered before 28 weeks' gestation at one of 14 participating institutions in 11 cities in five states were asked to enroll in the study. The enrollment and consent processes were approved by the individual institutional review boards. The research complied with the Declaration of Helsinki.
Mothers were approached for consent either on antenatal admission or shortly after delivery, depending on clinical circumstance and institutional preference: 1249 mothers of 1506 infants consented. The sample for this study consists of the 1064 ELGANs for whom we had information about ROP diagnosis, placenta bacteriology, and placenta histology.
Restricted Sample for Multivariable Analyses
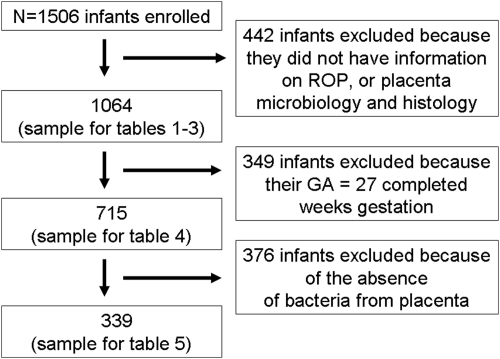
Less than 10 of the 349 infants born at 27 weeks developed ROP in zone 1, prethreshold or worse, or plus disease. To avoid problems that arise from the very small number of ROP cases in infants with a GA of 27 weeks, we limited our main analyses to the 715 infants with a GA between 23 and 26 weeks. A second set of multivariable analyses was restricted to 339 infants with any bacteria in their placentas (Fig. 1).
Figure 1.
Flow diagram of participants in the study. Of the 1506 newborns enrolled in the study, 1064 infants had information on ROP as well as placenta bacteriology/histology available. Of those, 715 infants had <27 weeks' GA. Final restriction was to 339 infants with bacteria present in their placentas.
Demographic and Pregnancy Variables
After birth, a trained research nurse interviewed each mother in her native language using a structured data collection form according to procedures outlined in a manual. Shortly after the mother's discharge, the research nurse reviewed the maternal chart using a second structured data collection form. The medical record was relied on for events after admission. The clinical circumstances that led to each maternal admission and ultimately to each preterm delivery were operationally defined using both data from the maternal interview and data abstracted from the medical record.33
Newborn Variables
The GA estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). When those were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), last menstrual period (LMP) without fetal ultrasound (7%), and GA recorded in the log of the neonatal intensive care unit (1%).
We defined hyperoxemia as an arterial blood gas oxygen measurement in the highest quartile for GA (in groups of 23–24, 25–26, and 27 weeks) on at least 2 of the first 3 postnatal days.6 Since infants who did not have blood gas measurements on postnatal day 3 are less likely than others to have extreme measurements on postnatal day 2, we assigned these newborns to the group with nonextreme measurements on postnatal day 3. This allowed us to include children with no measurement on the third postnatal day, thereby avoiding inflating odds ratios inappropriately. We measured blood gases by arterial blood sampling and then calculated extreme values that defined hyperoxemia as an arterial oxygen concentration in the highest GA-specific quartile.
Placentas
Placentas were placed in a sterile examination basin and transported to a sampling room. Eighty-two percent of the samples were obtained within 1 hour of delivery. The area to be sampled was at the midpoint of the longest distance between the cord insertion and the edge of the placental disc. Once the area was identified, the uppermost layer of the membranes (amnion) was lifted with a set of sterile forceps and cut with sterile scissors. With forceps, the amnion was gently peeled away from the underlying trophoblast tissue by gently pulling on it. A piece of tissue was removed by cutting at the base of the section with the sterile scissors and placed into a sterile 2-mL cryovial. The sample was immediately flash frozen in liquid nitrogen and then stored in −80°C until processing. The sample was then removed from the freezer and allowed to thaw at room temperature. Subsequently, it was placed into a sterile Petri dish. With sterile forceps and a disposable sterile scalpel, a portion of approximately 1 cm2 was removed and weighed. Sterile phosphate-buffered saline (PBS) was added to the vial containing the sample to achieve a 10-fold weight-to-volume dilution of the sample. The sample was then homogenized (PRO Scientific, Inc, Oxford, CT) until the placenta tissue was completely dispersed in the PBS. Serial 10-fold dilutions of the homogenized sample were made in PBS and aliquots of the sample and dilutions were plated onto selective and nonselective media.
Microbiologic Assessment
The culture medium for recovering anaerobes was prereduced Brucella-base agar with 5% sheep blood enriched with hemin and vitamin K1; for facultative anaerobes, tryptic soy agar with 5% sheep blood was used (PML; Microbiologicals, Mississauga, ON, Canada). Chocolate agar was used for the recovery of fastidious microorganisms, and A-7 agar (Northeast Laboratory, Waterville, ME) was used for the detection of Mycoplasma and Ureaplasma. Anaerobic culture plates and A-7 plates were incubated in an anaerobic chamber with an atmosphere of 10% carbon dioxide, 10% hydrogen, and 80% nitrogen for a minimum of 120 hours at 35°C before enumeration. Tryptic soy agar plates were incubated in air and chocolate plates in 5% carbon dioxide for 48 hours. After incubation, the various colony types were enumerated, isolated, and identified by established criteria. We recorded the “number of organisms” as the number of different species of bacteria isolated, thereby referring to polymicrobial infections.
Gram-positive, catalase-negative microaerophilic or anaerobic bacilli that produced a large amount of lactic acid, as determined by gas–liquid chromatography, were classified as lactobacillus sp. Obligate anaerobes were classified by Gram's stain and by gas–liquid chromatographic analysis of glucose fermentation products, and the final identification was determined (Microbial Identification System; MIDI, Inc., Newark, NJ, or the Rapid ANA II system; Remel, Inc., Lenexa, KS). Mycoplasma and Ureaplasma were identified based on their differentiating morphologic characteristics of growth on A-7 agar.
Histologic Assessment
Histologic examination of the placenta was performed according to College of American Pathologists Guidelines.34 Representative sections were taken from all abnormal areas; we also sampled routine sections of the umbilical cord and a membrane roll and full-thickness sections from the center and a paracentral zone of the placental disc.
Inflammation of the membranes was described in detail by using a scoring system similar to that proposed by Redline et al.35 At the chorionic plate of the disc, acute inflammation was assigned a stage from 0 to 3 (0, none; 1, neutrophils collecting in the chorionic plate; and 3, neutrophils up to the amnionic epithelium) and a grade (1, 1–9 neutrophils/×20 fields, 2, 10–19 neutrophils/×20 fields; and 3, >20 neutrophils/×20 fields). In the external membranes, inflammation of the chorion/decidua was graded from 0 to 4 (0, none; 1, a single focus of 5–10 neutrophils; 2, several small foci or a single focus of >10 neutrophils; 3, numerous large or confluent foci, and 4, necrosis).
Chorionic plate vasculitis was defined as neutrophilic infiltration of the fetal stem vessels in the chorionic plate. Umbilical cord vasculitis was defined by the presence of neutrophils in a cord vessel graded from 0 (none) to 5. The quality and interrelatedness of microbiologic and histologic findings in the ELGAN cohort have been documented.36,37
Eye Examinations
Participating ophthalmologists helped prepare a manual and data collection form, and then participated in certification conference calls, in which representative ROP images were reviewed in an effort to minimize observer variability. Definitions of terms were those accepted by the International Committee for Classification of ROP.19,38,39 In keeping with guidelines,14,40 the first ophthalmic examination was between the 31st and 33rd postmenstrual week. We did not include information about treatment because guidelines for timing of laser treatment changed during study recruitment, in response to results from the Early Treatment for ROP (ETROP) study.41
As in previous analyses,6 we used four categories to describe ROP severity: stage 3 or higher, zone I disease, any prethreshold or worse, and plus disease. In risk analyses modeling for ROP stage 3 or higher, the comparison group has children with ROP stages 1 or 2 or no ROP. In analyses modeling risk for ROP in zone 1, children in the comparison group have no ROP in zone 1, but can have ROP in zones 2 or three. In essence, all case groups are defined by the presence of the characteristic defining severe ROP, whereas the control groups are characterized by its absence. All comparison groups were allowed to include infants with ROP stages 1 and 2.
Data Analysis
We calculated crude percentages of infants with severe ROP in groups defined by the presence or absence of antenatal and postnatal characteristics (Tables 1–3).
Table 1.
The Distribution of ROP in Categories of the Newborns' Characteristics
| Antecedent | ROP |
Category Total n | ||||
|---|---|---|---|---|---|---|
| None | Stages 3–5 | Zone I | Prethreshold/Threshold | Plus Disease | ||
| Sex | ||||||
| Male | 26 | 29 | 10 | 16 | 11 | 570 |
| Female | 28 | 28 | 5 | 14 | 11 | 494 |
| GA, wk | ||||||
| 23–24 | 7 | 50 | 15 | 30 | 24 | 229 |
| 25–26 | 23 | 31 | 9 | 17 | 12 | 468 |
| 27 | 45 | 10 | 1 | 3 | 3 | 349 |
| Birth weight | ||||||
| ≤750 | 12 | 46 | 13 | 26 | 20 | 406 |
| 751–1000 | 28 | 23 | 7 | 12 | 8 | 445 |
| >1000 | 53 | 6 | 0.5 | 2 | 2 | 213 |
| Any organism | ||||||
| Yes | 25 | 29 | 7 | 16 | 14 | 522 |
| No | 28 | 27 | 9 | 14 | 9 | 542 |
| Any inflammation | ||||||
| Yes | 28 | 29 | 7 | 15 | 12 | 421 |
| No | 26 | 28 | 8 | 15 | 11 | 641 |
| Hyperoxemia | ||||||
| Yes | 18 | 36 | 11 | 20 | 14 | 438 |
| No | 29 | 26 | 6 | 14 | 12 | 451 |
| Maximum n | 286 | 301 | 82 | 162 | 121 | 1064 |
These are the row percents. (n = 1064).
Table 2.
Percentage of Infants Classified by the Organisms in Their Placentas Who Also Had ROP as Defined at the Top of Each Column
| Placenta Bacteriology | ROP |
Category Total n | ||||
|---|---|---|---|---|---|---|
| None | Stages 3–5 | Zone I | Prethreshold/Threshold | Plus Disease | ||
| Organisms isolated, n* | ||||||
| 0 | 28 | 27 | 9 | 14 | 9 | 542 |
| 1 | 30 | 28 | 6 | 15 | 12 | 251 |
| 2+ | 21 | 30 | 7 | 17 | 16 | 271 |
| Any anaerobe | ||||||
| Yes | 24 | 30 | 7 | 18 | 15 | 304 |
| No | 28 | 28 | 8 | 14 | 10 | 760 |
| Any aerobe | ||||||
| Yes | 23 | 29 | 7 | 16 | 14 | 338 |
| No | 29 | 28 | 8 | 15 | 10 | 726 |
| Any Mycoplasma | ||||||
| Yes | 26 | 33 | 8 | 18 | 16 | 112 |
| No | 27 | 28 | 8 | 15 | 11 | 952 |
| Skin organisms† | ||||||
| Yes | 25 | 29 | 7 | 17 | 14 | 217 |
| No | 27 | 28 | 8 | 15 | 11 | 847 |
| Vaginal organisms‡ | ||||||
| Yes | 23 | 31 | 6 | 19 | 16 | 172 |
| No | 28 | 28 | 8 | 14 | 11 | 892 |
| Overall | 27 | 28 | 8 | 15 | 11 | |
| Maximum number of infants | 286 | 301 | 82 | 162 | 121 | 1064 |
These are row percents.
Number of different species of bacteria.
Corynebacterium sp, Propionebacterium sp, Staphylococcus sp.
Prevotella bivia, Lactobacillus sp, Peptostrep magnus, Gardnerella vaginalis.
Table 3.
The Percentage of Infants Whose Placentas Had the Histologic Characteristics Listed on the Left Who Also Had ROP as Defined at the Top of Each Column
| Placenta Histology | ROP |
Category Total n | ||||
|---|---|---|---|---|---|---|
| None | Stages 3–5 | Zone I | Prethreshold/threshold | Plus Disease | ||
| Inflammation chorionic plate* | ||||||
| Yes | 33 | 24 | 5 | 12 | 11 | 201 |
| No | 26 | 29 | 8 | 16 | 12 | 845 |
| Inflammation external membranes† | ||||||
| Yes | 28 | 29 | 7 | 15 | 13 | 382 |
| No | 26 | 28 | 8 | 15 | 11 | 663 |
| Neutrophl infiltrate in fetal stem vessels | ||||||
| Yes | 29 | 28 | 6 | 14 | 11 | 259 |
| No | 26 | 28 | 8 | 16 | 12 | 779 |
| Umbilical cord vasculitis‡ | ||||||
| Yes | 38 | 23 | 6 | 12 | 10 | 172 |
| No | 25 | 29 | 8 | 16 | 12 | 848 |
| Thrombosis of fetal stem vessels | ||||||
| Yes | 39 | 22 | 13 | 20 | 11 | 54 |
| No | 26 | 28 | 7 | 15 | 11 | 984 |
| Infarct | ||||||
| Yes | 24 | 24 | 7 | 13 | 10 | 184 |
| No | 28 | 29 | 8 | 16 | 12 | 871 |
| Increased syncytial knots | ||||||
| Yes | 27 | 28 | 5 | 12 | 9 | 211 |
| No | 27 | 28 | 8 | 16 | 12 | 848 |
| Decidual hemorrhage/fibrin deposition | ||||||
| Yes | 18 | 3 | 6 | 17 | 14 | 171 |
| No | 28 | 27 | 8 | 15 | 11 | 872 |
| Overall | 27 | 28 | 8 | 15 | 11 | |
| Maximum number of infants | 286 | 301 | 82 | 162 | 121 | 1064 |
These are row percents.
Stage 3 and severity 3.
Grades 3 and 4.
Grades 3, 4, and 5.
In the first of two sets of multivariable logistic regression analyses (Table 4), we calculated odds ratios and 95% confidence intervals in three ways. First, we calculated crude odds ratios. Second, we looked at models that included three terms: one for low GA (23–24 weeks compared to 25–26 weeks), one for any placental bacterial colonization, and one for any antenatal inflammation, defined as any of the following: inflammation of the chorionic plate (stage 3 and severity 3), inflammation of the external (chorion) membranes (grades 3 and 4), chorionic plate vasculitis (neutrophilic infiltration of the fetal vessels of the plate), and umbilical cord vasculitis (grades 3, 4, and 5). Third, we looked at the interactions between any two of these three variables, while adjusting for the three main effects.
Table 4.
Main Effects and Interaction of Three Risk Factors for ROP
| Main-Effects Models |
Interaction Models |
|||||
|---|---|---|---|---|---|---|
| 23–24 wk | Any Organism | Any Inflammation | 23–24 wk × Any Organism | 23–24 wk × Any Inflammation | Any Organism × Any Inflammation | |
| ROP stages 3–5 | ||||||
| Crude | 2.0 (2.0–4.1) | 1.1 (0.8–1.5) | 0.8 (0.6–1.1) | — | — | — |
| Main | 2.3 (1.7–3.3) | 0.9 (0.7–1.3) | 0.8 (0.6–1.2) | — | — | — |
| With interaction | 2.0 (1.1–3.5) | 0.8 (0.5–1.4) | 0.6 (0.4–1.1) | 1.0 (0.5–1.9) | 1.5 (0.8–3.0) | 1.3 (0.7–2.5) |
| ROP zone I disease | ||||||
| Crude | 1.8 (1.1–2.8) | 0.6 (0.4, 0.96) | 0.9 (0.5–1.2) | — | — | — |
| Main | 2.1 (1.3–3.3) | 0.5 (0.3–0.9) | 0.8 (0.5–1.4) | — | — | — |
| With interaction | 1.9 (0.9–3.9) | 0.4 (0.2–0.98) | 0.3 (0.1–0.8) | 0.6 (0.2–1.7) | 2.3 (0.8–7.1) | 3.1 (1.02–9.5) |
| ROP prethreshold/threshold | ||||||
| Crude | 2.0 (1.4–2.9) | 1.0 (0.7–1.5) | 0.8 (0.6–1.2) | — | — | — |
| Main | 2.1 (1.4–3.0) | 1.0 (0.7–1.4) | 0.8 (0.5–1.1) | — | — | — |
| With interaction | 1.8 (0.96–3.4) | 1.0 (0.6–1.7) | 0.5 (0.2–0.9) | 0.7 (0.3–1.6) | 2.2 (0.99–4.9) | 1.3 (0.6–3.0) |
| ROP plus disease | ||||||
| Crude | 2.4 (1.8–3.6) | 1.5 (0.99–2.2) | 1.0 (0.7–1.5) | — | — | — |
| Main | 2.3 (1.5–3.5) | 1.3 (0.9–2.1) | 0.9 (0.6–1.3) | — | — | — |
| With interaction | 2.7 (1.3–5.5) | 1.6 (0.8–3.0) | 0.5 (0.2–1.2) | 0.5 (0.2–1.2) | 1.8 (0.7–4.3) | 1.4 (0.6–3.7) |
Data are the OR (95% CI). The three risk factors are gestational age 23–24 wk, any placenta organism, and any placenta inflammation. Bold data indicate appreciably increased/decreased odds ratios with lower/upper 95% confidence limits that come very close to or exclude the null (1.0). n = 715.
In the second set of multivariable analyses (Table 5), we restricted our sample to infants with any bacteria in their placentas (n = 339), and evaluated the contribution of low GA, any antenatal inflammation, and hyperoxemia (defined as a PaO2 in the highest quartile for GA on at least two of the first 3 postnatal days), as well as combinations of two of these three variables.
Table 5.
Main Effects and Interaction of Three Risk Factors on ROP among ELGANs with Any Placenta Organism
| Main-Effects Models |
Interaction Models |
|||||
|---|---|---|---|---|---|---|
| 23–24 wk | Any Inflammation | Hyperoxemia | 23–24 wk × Any Inflammation | 23–24 wk × Hyperoxemia | Any Inflammation × Hyperoxemia | |
| ROP stages 3–5 | ||||||
| Crude | 2.1 (1.3–3.3) | 1.1 (0.7–1.7) | 1.4 (0.9–2.2) | — | — | — |
| Main | 2.1 (1.3–3.2) | 1.0 (0.7–1.6) | 1.4 (0.9–2.2) | — | — | — |
| With interaction | 1.2 (0.5–2.7) | 0.4 (0.2–0.9) | 0.9 (0.4–1.8) | 2.7 (1.1–6.9) | 1.0 (0.4–2.4) | 2.4 (0.97–6.0) |
| ROP zone I disease | ||||||
| Crude | 2.0 (0.9–4.2) | 1.3 (0.6–2.9) | 1.3 (0.6–2.7) | |||
| Main | 1.9 (0.9–4.1) | 1.3 (0.6–2.8) | 1.3 (0.6–2.6) | |||
| With interaction | 0.9 (0.2–4.1) | 0.2 (0–1.3) | 1.1 (0.3–4.2) | 10 (1.5–68) | 0.3 (0–1.9) | 3.4 (0.5–23) |
| ROP prethreshold/threshold | ||||||
| Crude | 1.9 (1.1–3.1) | 1.0 (0.6–1.6) | 1.3 (0.9–2.2) | — | — | — |
| Main | 1.9 (1.1–3.1) | 0.9 (0.5–1.6) | 1.3 (0.8–2.2) | — | — | — |
| With interaction | 0.8 (0.3–2.2) | 0.3 (0.1–0.8) | 0.7 (0.3–1.7) | 3.5 (1.2–10) | 1.1 (0.4–3.4) | 2.7 (0.9–8.0) |
| ROP plus disease | ||||||
| Crude | 1.8 (1.05–3.1) | 1.1 (0.8–1.9) | 1.2 (0.7–2.1) | — | — | — |
| Main | 1.8 (1.04–3.1) | 1.0 (0.6–1.8) | 1.2 (0.7–2.1) | — | — | — |
| With interaction | 0.8 (0.3–2.2) | 0.3 (0.1–0.9) | 0.5 (0.2–1.3) | 2.8 (0.9–9.0) | 1.5 (0.5–4.7) | 3.2 (1.00–10) |
Data are the OR (95% CI). The three risk factors are gestational age 23–24 wk, any placenta inflammation, and pO2. Bold data indicate appreciably increased/decreased odds ratios with lower/upper 95% confidence limits that come very close to or exclude the null (1.0). n = 339.
Results
Univariable Analyses
Male infants were twice as likely as females to have zone I ROP (Tables 1–3). The percentage of infants with severe ROP (all categories) increased with decreasing GA and birth weight. The incidence of severe ROP, however defined, was not appreciably higher in infants whose placentas harbored any organism or showed signs of inflammation. Hyperoxemia was associated with an appreciably increased risk for ROP stage 3 or worse, zone I, and prethreshold or worse, but with only a slight increase in plus disease.
Only the incidence of plus disease increased as the number of organisms recovered from the placenta increased and then only slightly (Table 2). The presence of an anaerobe, aerobe, Mycoplasma, skin, or vaginal organism was associated with very small increased risks for stages 3 to 5, prethreshold/threshold, and plus disease, but no ROP in zone I.
The incidence of stage 3 to 5 ROP was 3% in the presence of decidual hemorrhage/fibrin deposition and 27% in its absence (Table 3). Otherwise, the incidence of ROP did not vary with the presence or absence of histologic abnormalities in the placenta. Tables 1 to 3 display univariable descriptive data; we did not perform statistical tests.
Multivariable Analyses
In crude analyses, a GA of 23 to 24 weeks was associated with a significantly increased risk for all four definitions of severe ROP (Table 4). Neither an organism in the placenta nor placenta inflammation appeared to be associated with a significant risk increase, both in crude analyses and in regression models that included all three antecedent variables of interest. However, the interaction between any bacteria and any inflammation in the placenta was associated with a significant threefold risk increase for ROP zone I disease (odds ratio [OR], 3.1; 95% confidence interval [CI], 1.02–9.5).
This interaction between infection and inflammation in the placenta prompted us to perform an additional set of analyses within a dataset restricted to infants whose placentas harbored a microorganism (Table 5). Among these infants, the interaction between low GA and placenta inflammation was associated with prominent and significant increases in the risk for virtually all definitions of severe ROP (the 2.8-fold increase in the risk for plus disease did not achieve significance). Moreover, a similarly prominent (but often less significant) risk increase was present for the interaction between inflammation and hyperoxemia.
Discussion
In previous studies, the association between placenta histologic characteristics of inflammation and ROP was modest, not significant,28,30,42 or not present at all.43 Our findings suggest that these previous negative results might be due to an underappreciation of etiologic complexity.
One of our major findings is that placenta infection and inflammation appear to be synergistic in predicting the presence of ROP in zone I. In other words, although the presence of either one alone was not associated with an increased risk, the presence of both was associated with a significant threefold risk increase (Table 4).
ROP is essentially a disorder of retinal blood vessel development, and normal vessel growth progresses from the optic nerve to the retinal periphery.44 Thus, it seems reasonable to assume that the earlier the insult during the process of vessel sprouting, the higher the probability of zone I disease. This idea is supported by the findings that zone I ROP occurs predominantly in infants <28 weeks' gestation in one study45 and only in infants <751 g in another one.46
Our second major finding is that in a sample restricted to infants whose placentas harbored a microorganism, the co-occurrence of either low GA or hyperoxemia on the one hand, and placenta inflammation on the other hand was associated with a significant two- to threefold risk increase for virtually all definitions of ROP (Table 5).
Our third major finding is that in a sample restricted to infants whose placentas harbored a microorganism, the co-occurrence of low GA and placenta inflammation, as well as the co-occurrence of hyperoxemia and placenta inflammation were associated with significant two- to threefold risk increases for virtually all definitions of ROP (Table 5). These observations suggest that antenatal infection and maternal/fetal inflammatory responses are associated with an increased risk for severe ROP and that ROP pathogenesis is a multihit phenomenon.25,31 We found that 57% of infants with any placenta organisms had any sign of inflammation, and 30% of those with placenta inflammation had no organisms recovered from their placentas. At least part of the multihit, inflammation-related pathogenesis of ROP is likely to be mediated by cytokines and growth factors present in the newborn's systemic circulation.27
Our findings are supported by those of others who found associations between clinical/histologic chorioamnionitis and ROP.28,30 Placenta infection and inflammation are likely to trigger the synthesis and release of cytokines into the circulation, which gain access to the retina and might contribute to ROP pathogenesis. Elevated blood concentrations of multiple proinflammatory cytokines over the first few weeks of postnatal life in newborns who develop ROP compared with controls suggests that postnatal systemic inflammation contributes to ROP pathogenesis.27 Moreover, many inflammation-associated genes were upregulated in an oxygen-induced retinopathy mouse model.47 Oxygen levels regulate retinal levels of vascular endothelial growth factor (VEGF), which in turn regulates retinal neovascularization in ROP.48 The isoform VEGF164 that is involved in pathologic neovascularization also triggers local inflammation in the retina.49 This makes room for the speculation that systemic inflammation might exaggerate local inflammation and neovascularization in the developing retina.
In crude analysis, any organism or any inflammation did not appear to be associated with an increased risk for ROP. However, when co-occurring, they were associated with a significantly increased ROP zone I risk (positive interaction). In essence, the risk estimate provided by both factors together is greater than the risk estimate provided by either risk factor alone.
Although our sample consisted of 1064 infants born before the 28th week of gestation, the incidence of each of three definitions of severe ROP (zone I, prethreshold/threshold disease, and plus disease) occurred in 3% or less of the 349 infants born after the 26th week. Thus, to a large extent, our useful sample consisted of the 697 infants born during weeks 23 to 26 of gestation, a considerably smaller sample, and one that might have limited our power to detect a doubling of many risks associated with interaction terms. On the other hand, any statistical significance in a large study such as ours may still be due to chance and deserves confirmation.
In conclusion, very preterm infants who were exposed to multiple proinflammatory stimuli appear to be at increased risk for severe ROP. Future studies of ROP should consider such interaction patterns.
Footnotes
Supported by Cooperative Agreement 5U01NS040069-05 with the National Institute of Neurologic Disorders and Stroke, Grant 1R21EY019253-01 from the National Eye Institute, Program Project Grant NIH-P30-HD-18655 from the National Institute of Child Health and Human Development, and the Richard Saltonstall Charitable Foundation. The sponsor or funding organization had no role in the design or conduct of this study.
Disclosure: M.L. Chen, None; E.N. Allred, None; J.L. Hecht, None; A. Onderdonk, None; D. VanderVeen, None; D.K. Wallace, None; A. Leviton, None; O. Dammann, None
References
- 1. Heidary G, Vanderveen D, Smith LE. Retinopathy of prematurity: current concepts in molecular pathogenesis. Semin Ophthalmol. 2009;24(2):77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steinkuller PG, Du L, Gilbert C, et al. Childhood blindness. J AAPOS. 1999;3(1):26–32 [DOI] [PubMed] [Google Scholar]
- 3. Phelps D. Retinopathy of prematurity. In: Fanaroff AA, Martin RJ. eds. Neonatal-Perinatal Medicine: Diseases of the Fetus. 7th ed. St. Louis: Mosby Year Book; 2002;1595–1602 [Google Scholar]
- 4. Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111(2):339–345 [DOI] [PubMed] [Google Scholar]
- 5. Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326(16):1050–1054 [DOI] [PubMed] [Google Scholar]
- 6. Hauspurg A, Allred EN, Vanderveen DK, et al. Blood gases and retinopathy of prematurity. The ELGAN Study. Neonatology. 2011;99:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wheatley CM, Dickinson JL, Mackey DA, et al. Retinopathy of prematurity: recent advances in our understanding. Arch Dis Child Fetal Neonatal Ed. 2002;87(2):F78–F82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dammann O. Inflammation and retinopathy of prematurity. Acta Paediatr. 2010;99(7):975–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240–247 [DOI] [PubMed] [Google Scholar]
- 10. Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291 [DOI] [PubMed] [Google Scholar]
- 11. Kim TI, Sohn J, Pi SY, Yoon YH. Postnatal risk factors of retinopathy of prematurity. Paediatr Perinat Epidemiol. 2004;18(2):130–134 [DOI] [PubMed] [Google Scholar]
- 12. Klinger G, Levy I, Sirota L, et al. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. 2010;125(4):e736–e740 [DOI] [PubMed] [Google Scholar]
- 13. Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365 [DOI] [PubMed] [Google Scholar]
- 14. Manzoni P, Maestri A, Leonessa M, et al. Fungal and bacterial sepsis and threshold ROP in preterm very low birth weight neonates. J Perinatol. 2006;26(1):23–30 [DOI] [PubMed] [Google Scholar]
- 15. Noyola DE, Bohra L, Paysse EA, et al. Association of candidemia and retinopathy of prematurity in very low birthweight infants. Ophthalmology. 2002;109(1):80–84 [DOI] [PubMed] [Google Scholar]
- 16. Bharwani SK, Dhanireddy R. Systemic fungal infection is associated with the development of retinopathy of prematurity in very low birth weight infants: a meta-review. J Perinatol. 2008;28(1):61–66 [DOI] [PubMed] [Google Scholar]
- 17. Mittal M, Dhanireddy R, Higgins RD. Candida sepsis and association with retinopathy of prematurity. Pediatrics. 1998;101(4):654–657 [DOI] [PubMed] [Google Scholar]
- 18. Chye JK, Lim CT, Leong HL, Wong PK. Retinopathy of prematurity in very low birth weight infants. Ann Acad Med Singapore. 1999;28(2):193–198 [PubMed] [Google Scholar]
- 19. Liu PM, Fang PC, Huang CB, et al. Risk factors of retinopathy of prematurity in premature infants weighing less than 1600 g. Am J Perinatol. 2005;22(2):115–120 [DOI] [PubMed] [Google Scholar]
- 20. Cats BP, Tan KE. Retinopathy of prematurity: review of a four-year period. Br J Ophthalmol. 1985;69(7):500–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussain N, Clive J, Bhandari V. Current incidence of retinopathy of prematurity 1989–1997. Pediatrics. 1999;104(3):e26. [DOI] [PubMed] [Google Scholar]
- 22. Gunn TR, Easdown J, Outerbridge EW, Aranda JV. Risk factors in retrolental fibroplasia. Pediatrics. 1980;65(6):1096–1100 [PubMed] [Google Scholar]
- 23. Maheshwari R, Kumar H, Paul VK, et al. Incidence and risk factors of retinopathy of prematurity in a tertiary care newborn unit in New Delhi. Natl Med J India. 1996;9(5):211–214 [PubMed] [Google Scholar]
- 24. Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann Acad Med Singapore. 2005;34(2):169–178 [PubMed] [Google Scholar]
- 25. Chen M, Çitil A, McCabe F, et al. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology. 2010;99(2):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Washburn Tolsma K, Allred EN, Chen M, et al. Neonatal bacteremia and retinopathy of prematurity: the ELGAN study. Arch Ophthalmol. In press [DOI] [PubMed] [Google Scholar]
- 27. Sood BG, Madan A, Saha S, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67(4):394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polam S, Koons A, Anwar M, et al. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Arch Pediatr Adolesc Med. 2005;159(11):1032–1035 [DOI] [PubMed] [Google Scholar]
- 29. Fung G, Bawden K, Chow P, Yu V. Chorioamnionitis and outcome in extremely preterm infants. Ann Acad Med Singapore. 2003;32(3):305–310 [PubMed] [Google Scholar]
- 30. Moscuzza F, Belcari F, Nardini V, et al. Correlation between placental histopathology and fetal/neonatal outcome: chorioamnionitis and funisitis are associated to intraventricular haemorrage and retinopathy of prematurity in preterm newborns. Gynecol Endocrinol. 2011;27(5):319–323 [DOI] [PubMed] [Google Scholar]
- 31. Dammann O, Brinkhaus MJ, Bartels DB, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev. 2009;85(5):325–329 [DOI] [PubMed] [Google Scholar]
- 32. O'Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Driscoll SG, Langston C. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on Methods for Placental Examination. Arch Pathol Lab Med. 1991;115(7):704–708 [PubMed] [Google Scholar]
- 35. Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–448 [DOI] [PubMed] [Google Scholar]
- 36. Onderdonk AB, Hecht JL, McElrath TF, et al. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol. 2008;199(1):52 e1–e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hecht JL, Onderdonk A, Delaney M, et al. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr Dev Pathol. 2008;11(1):15–22 [DOI] [PubMed] [Google Scholar]
- 38. An international classification of retinopathy of prematurity. Pediatrics 1984;74(1):127–133 [PubMed] [Google Scholar]
- 39. Yudkin PL, Aboualfa M, Eyre JA, et al. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52 [DOI] [PubMed] [Google Scholar]
- 40. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119(8):1110–1118 [DOI] [PubMed] [Google Scholar]
- 41. Early Treatment For Retinopathy Of Prematurity Cooperative G Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694 [DOI] [PubMed] [Google Scholar]
- 42. Rocha G, Proenca E, Quintas C, et al. Chorioamnionitis and neonatal morbidity (in Portuguese). Acta Med Port. 2006;19(3):207–212 [PubMed] [Google Scholar]
- 43. Mehta R, Nanjundaswamy S, Shen-Schwarz S, Petrova A. Neonatal morbidity and placental pathology. Indian J Pediatr. 2006;73(1):25–28 [DOI] [PubMed] [Google Scholar]
- 44. Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002;43(2):522–527 [PubMed] [Google Scholar]
- 45. Good WV, Hardy RJ, Dobson V, et al. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116(1):15–23 [DOI] [PubMed] [Google Scholar]
- 46. Choo MM, Martin FJ, Theam LC, U-Teng C. Retinopathy of prematurity in extremely low birth weight infants in Malaysia. J AAPOS. 2009;13(5):446–449 [DOI] [PubMed] [Google Scholar]
- 47. Sato T, Kusaka S, Hashida N, et al. Comprehensive gene-expression profile in murine oxygen-induced retinopathy. Br J Ophthalmol. 2009;93(1):96–103 [DOI] [PubMed] [Google Scholar]
- 48. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10(2):133–140 [DOI] [PubMed] [Google Scholar]
- 49. Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198(3):483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]