This study demonstrates for the first time that long-term therapy (∼1 year) is achievable in a mammalian model of GC1 deficiency. These data provide additional justification for the development of an AAV-based gene therapy vector for the clinical treatment of LCA1.
Abstract
Purpose.
The authors previously showed that subretinal delivery of AAV5 vectors containing murine guanylate cyclase-1 (GC1) cDNA driven by either photoreceptor-specific (hGRK1) or ubiquitous (smCBA) promoters was capable of restoring cone-mediated function and visual behavior and preserving cone photoreceptors in the GC1 knockout (GC1KO) mouse for 3 months. Here, the authors compared therapy conferred by the aforementioned vectors to that achieved with the highly efficient capsid tyrosine mutant AAV8(Y733F) and asked whether long-term therapy is achievable in this model.
Methods.
AAV5-hGRK1-mGC1, AAV5-smCBA-mGC1, or AAV8(Y733F)-hGRK1-mGC1 was delivered subretinally to GC1KO mice between postnatal day (P)14 and P25. Retinal function was assayed by electroretinography. Localization of AAV-mediated GC1 expression and cone survival were assayed with immunohistochemistry, and the spread of vector genomes beyond the retina was quantified by PCR of optic nerve and brain tissue.
Results.
Cone function was restored with all vectors tested, with AAV8(Y733F) being the most efficient. Electroretinographic responses were clearly measurable out to 1 year after treatment. AAV-mediated expression of GC1 was found exclusively in photoreceptors out to 15 months after injection. Cones were preserved for at least 11 months after treatment. AAV5- and AAV8(733)–delivered vector genomes were recovered primarily from optic nerve of the treated eye and, in only instance, from brain (1 of 20 samples).
Conclusions.
The authors demonstrate for the first time that long-term therapy (∼1 year) is achievable in a mammalian model of GC1 deficiency. These data provide additional justification for the development of an AAV-based gene therapy vector for the clinical treatment of Leber congenital amaurosis-1.
Retinal guanylate cyclase-1 (GC1) encoded by GUCY2D (Gucy2e in mouse) is expressed in the outer segments of rod and cone photoreceptors of human, monkey, and mouse retinas.1–3 GC1 plays a vital role in light-dark and recovery cycles, anchoring, through cGMP, the feedback loop linking intracellular calcium levels and the polarization state of photoreceptors.4–8 Like other membrane guanylate cyclases, it contains an N′-terminal signal sequence, an extracellular domain, a single transmembrane domain, a kinase-like homology domain, a dimerization domain, and a C′-terminal catalytic domain and is present likely as homomeric dimers.9 Mutations in GUCY2D are associated with recessive Leber congenital amaurosis-1 (LCA1) as well as dominant and recessive forms of cone-rod dystrophy, CORD6 and CORD, respectively.10–16 LCA1 is a severe, early-onset, autosomal recessive blinding disorder characterized by extinguished electroretinogram, which precedes photoreceptor degeneration.17,18 CORD6 is a dominant disorder characterized by progressive degeneration of photoreceptors beginning with cones causing early loss of visual acuity and color vision followed by degeneration of rods leading to progressive night blindness and peripheral visual field loss.12,13 CORD6 mutations are restricted to the dimerization domain and generally cause an increase in guanylate cyclase activating protein (GCAP)–mediated activation of GC1.19–21 A recently found recessive CORD-causing mutation is located in the catalytic domain of GC1 and is thought to reduce overall enzyme function.16 LCA1-causing mutations are distributed throughout GC1.22 These mutations alter enzyme structure and stability, may impact retrograde transport of other peripheral membrane-associated proteins, and are frequently null.22
The GC1 knockout (GC1KO) mouse carries a null mutation in Gucy2e, the murine homolog of GUCY2D. Like LCA1 patients, loss of cone function in this model precedes cone degeneration.23 Rods retain 30% to 50% of their function and do not degenerate because of the presence of guanylate cyclase-2 (GC2), another functional guanylate cyclase in murine photoreceptors.9,22–25 We have previously shown that subretinal injection of serotype 5 adeno-associated viral (AAV5) vectors containing the murine GC1 cDNA driven by either the photoreceptor-specific human rhodopsin kinase (hGRK1) or the ubiquitous (smCBA) promoter were capable of restoring cone-mediated function and visual behavior and preserving cone photoreceptors in the GC1KO mouse for 3 months.26 In the present study, we evaluated whether AAV-mediated gene replacement was capable of providing therapy to the GC1KO mouse over the long term. AAV5-hGRK1-mGC1 and AAV5-smCBA-mGC1 (identical vectors used in our previous study) and the highly efficient capsid tyrosine mutant vector AAV8(Y733F)-hGRK1-mGC1 were delivered subretinally to GC1KO mice between postnatal day (P) 14 and P25. Our findings demonstrate for the first time that long-term therapy (∼1 year) is achievable in a mammalian model of GC1 deficiency. We also studied vector genome biodistribution for AAV5 and AAV8(733) vectors. These findings have direct bearing on the development of an AAV-based gene therapy clinical trial for LCA1 (and possibly for cone-rod dystrophies) and help to develop a standardized vector design for a wide range of recessive retinal degenerations mediated by defects in photoreceptor-associated genes.
Materials and Methods
Experimental Animals
GC1KO and congenic+/+ controls derived from heterozygous matings of GC1+/− mice provided by The Jackson Laboratory (Bar Harbor, ME) were bred and maintained at the University of Florida Health Science Center Animal Care Services Facility under a 12-hour/12-hour light/dark cycle. Food and water were available ad libitum. All experiments were approved by the University of Florida's Institutional Animal Care and Use Committee and were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and with National Institutes of Health regulations. For a detailed summary of total animal numbers, experiments and time points, see Table 1.
Table 1.
Summary of Experiments
| Time after Injection (months) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 15 | |
| AAV5-hGRK1-mGC1 | ERG, n = 6 | ERG, n = 6 | ERG, n = 6 | ERG, n = 6 | ERG, n = 6 | ERG, n = 6 | ERG, n = 6 B, n = 1 | ERG, n = 4 | ERG, n = 3 | ERG, n = 2 B/retinal IHC, n = 1 | ERG, n = 2 | ERG, n = 2 | Retinal IHC, n = 1 |
| AAV5-smCBA-mGC1 | ERG, n = 10 | ERG, n = 10 | ERG, n = 10 | ERG, n = 10 | ERG, n = 10 | ERG, n = 10 | ERG, n = 10 B, n = 1 | ERG, n = 9 | ERG, n = 9 | ERG, n = 2 B/retinal IHC, n = 5 | ERG, n = 2 IHC retinal wholemount, n = 1 | ERG, n = 2 B/mRNA assay, IHC optic nerves, n = 1 | |
| AAV8(Y733F)-hGRK1-mGC1 | ERG, n = 6 | ERG, n = 6 | ERG, n = 6 | ERG, n = 6 B, n = 2 | ERG, n = 4 | ERG, n = 4 | ERG, n = 2 B/retinal IHC, n = 1 WB, n = 1 | ERG, n = 2 | ERG, n = 2 B/mRNA assay, IHC optic nerves, n = 1 | ||||
| GC1KO, no Tx* | ERG, n = 22 | ERG, n = 22 | ERG, n = 22 | ERG, n = 22 B, n = 1 | ERG, n = 20 | ERG, n = 20 | ERG, n = 18 B, n = 1 retinal IHC, n = 1 | ERG, n = 15 | ERG, n = 14 | ERG, n = 4 B, n = 1 | ERG, n = 4 IHC retinal wholemount, n = 1 | ERG, n = 3 B/mRNA assay, IHC optic nerves, n = 1 | |
| GC1+/+, no Tx | ERG, n = 8 | ERG, n = 8 | ERG, n = 8 | ERG, n = 6 | ERG, n = 6 | ERG, n = 5 | ERG, n = 4 retinal IHC, n = 1 WB, n = 1 | ERG, n = 3 | B/mRNA assay, n = 1 | ||||
B, biodistribution; IHC, immunohistochemistry; Tx, treatment, WB, Western blot.
GC1KO ERGs were those measured from the untreated contralateral eyes of treated mice. For all other experimental procedures, naive, age-matched GC1KO mice were used.
Construction of AAV Vectors
Serotype 5 adeno-associated virus (AAV5) vector plasmids containing either the ubiquitous (smCBA) or the photoreceptor-specific (hGRK1) promoter driving murine GC1 (mGC1) cDNA were generated according to previously described methods.26 Site-directed mutagenesis of surface-exposed tyrosine residues on the AAV2 capsid have been reported.27 Similar methods were used to generate the AAV8(Y733F) capsid mutant described here. All vectors were packaged, purified, and titered according to previously described methods.28,29 Resulting titers for AAV5-smCBA-mGC1, AAV5-hGRK1-mGC1, and AAV8(Y733F)-hGRK1-mGC1 were 4.69 × 1012 vector genomes per milliliter (vg/mL), 4.12 × 1013 vg/mL, and 1.08 × 1013 vg/mL, respectively.
Subretinal Injections
One microliter of AAV5-smCBA-mGC1 (4.69 × 109 vector genomes), AAV5-hGRK1-mGC1(4.12 × 1010 vector genomes), or AAV8(Y733F)-hGRK1-mGC1 (1.08 × 1010 vector genomes) was injected subretinally into one eye of GC1KO mice between P14 and P25. The contralateral control eye remained uninjected. Subretinal injections were performed as previously described.30 Further analysis was carried out only on animals that received comparable, successful injections (>60% retinal detachment with minimal complications). By this criterion approximately 75% of mice injected received “successful ” injections. It is well established that the area of vector transduction corresponds to at least the area of retinal detachment.30,31
Electroretinographic Analysis
Scotopic and photopic electroretinograms of treated GC1KO and age-matched, congenic (+/+) controls were recorded using a PC-based control and recording unit (Toennies Multiliner Vision; Jaeger/Toennies, Höchberg, Germany) according to methods previously described, with minor modifications.3,26 Recordings of AAV5-smCBA-mGC1-treated GC1KO mice (n = 10), AAV5-hGRK1-mGC1-treated GC1KO mice (n = 6), AAV8(Y733F)-treated GC1KO mice (n = 6), and congenic (+/+) controls (n = 8) began on different dates; therefore, each subset of mice was monitored for slightly different lengths of time (Table 1). Mice were removed from the study at different time points throughout the experiment for various postmortem studies (biodistribution studies, retinal immunohistochemical analysis, real time RT-PCR of retinal tissue) or unexpected sickness/death. We present quantified electroretinographic (ERG) data for making statistical comparisons between AAV vectors for groups of animals with an n > 3. Remaining treated mice continued to exhibit cone ERG responses; however, sample sizes were sufficiently reduced such that statistical analysis was no longer practical. The average of intraindividual treated versus untreated rod a- and b- wave amplitudes were used to calculate AAV-mGC1–mediated improvements in scotopic, rod-mediated function. As such, any value greater than 1 indicated AAV-mGC1 treatment improved the rod response. Ratios were calculated using amplitudes generated with a 1 cd · s/m2 stimulus. Photopic, cone-mediated, b-wave maximum amplitudes in injected and uninjected eyes of all treated GC1KO mice and congenic (+/+) control mice generated at 12 cd · s/m2 were averaged at each time point and used to generate standard errors. The standard t-test was used to calculate P values between data sets. Significant difference was defined as a P < 0.05.
Biodistribution
The spread of vector DNA in tissues of the treated GC1KO mice was determined in samples collected at kill according to previously described methods, with minor modifications that can be found in the Supplementary Methods, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7867/-/DCSupplemental.29 Biodistribution studies were performed at various time points, which are summarized in Table 1. Criteria for reporting vector genome copies were established according to previously described methods.29
Tissue Preparation, Immunohistochemistry, and Microscopy
Eyes designated for cryosectioning and wholemount immunostaining were processed and immunostained according to previously described methods, with minor modifications, which can be found in the Supplementary Methods, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7867/-/DCSupplemental.3,32
Immunoblot Analysis
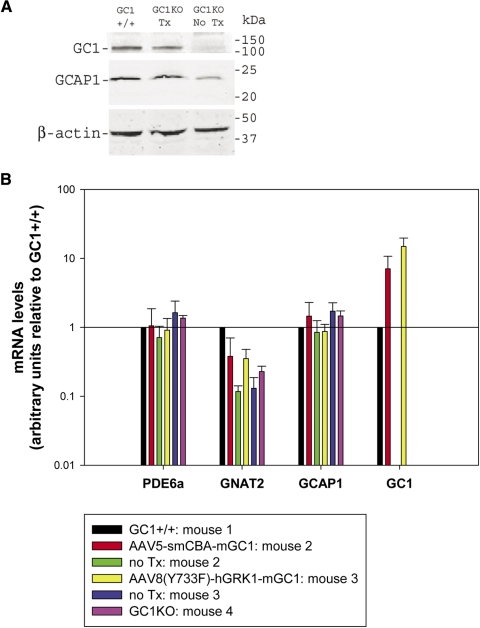
At 7 months after injection, one mouse injected with AAV8(Y733F)-hGRK1-mGC1 and an age-matched, congenic GC1+/+ (control) mouse were killed, and their eyes were enucleated and processed according to previously described methods with minor modifications.33 Briefly, eyes were placed in 1× PBS. Retinas were immediately dissected and processed as follows. Individual retinas were solubilized in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) with 1% Triton X-100 and complete protease inhibitor (Roche) for 1 hour at 4°C, followed by centrifugation at 14,000 rpm. The protein concentration of the supernatant was determined by BCA (Pierce, Rockford, IL), and 15 μg each sample was separated on a 12% polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred onto transfer membranes (Immobilon-FL; Millipore, Billerica, MA) for 1 hour in transfer buffer (25 mM Tris, 192 mM glycine) containing 15% methanol. Blots were treated with blocking buffer (Li-Cor, Lincoln, NE) and labeled for 1 hour with a mouse monoclonal antibody recognizing GC1 (IS4, 1:3000, generously provided by Kris Palczewski, Case Western Reserve University) and rabbit polyclonal antibodies raised against GCAP1 (pAb UW14, 1:25,000, generously provided by Wolfgang Baehr, University of Utah) and β-actin (1:5000; Abcam, Cambridge, MA). Secondary antibodies (goat anti-mouse immunoglobulin conjugated to CW800 and goat anti-rabbit conjugated with IR680) were applied for 1 hour, and blots were imaged with an infrared imaging system (Odyssey; Li-Cor). The goal of this experiment was not to compare GC1 levels across treatment groups but rather to compare levels of vector-mediated GC1 expression to those in a wild-type animal. Similarly, we evaluated the effects of AAV-delivered GC1 on GCAP1 expression.
mRNA Quantification by Real time-PCR, Retinal Genome Recovery, and Optic Nerve Immunohistochemistry
Treated eyes with optic nerves attached were harvested from GC1KO mice 1 year after treatment with either AAV8(Y733F)-hGRK1-mGC1 or AAV5-smCBA-mGC1 and an age-matched, untreated GC1+/+ mouse. Retinas were immediately dissected from the eye and snap frozen in liquid nitrogen. Optic nerves were dissociated from the eyes, fixed in 4% paraformaldehyde overnight at 4°C, immersed in 30% sucrose for 2 hours at 4°C, and then quick frozen in cryostat compound (Tissue Tek OCT 4583; Sakura Finetek USA, Inc., Torrance, CA) in a bath of dry ice/ethanol. Optic nerves were sectioned at 10 μm and stained according to previously described methods.26 Retinas were homogenized in 350 mL lysis buffer (Buffer RLT; RNeasy Protect Mini Kit; Qiagen Inc., Valencia, CA) plus BME for 45 seconds. Samples were centrifuged, and the lysate was split in half (one half designated for genome recovery and the other half for RNA extraction).34 Genome recovery was performed as described. RNA extraction was performed with a stabilization and purification kit (RNeasy Protect Mini Kit; Qiagen Inc.). RNA was reverse transcribed (iScript cDNA synthesis kit; Bio-Rad Laboratories) and used in real-time PCR (iQ SYBR Green Supermix and MyiQ real-time PCR detection system interfaced with iCycler thermal cycler; Bio-Rad Laboratories) to measure the following retinal-specific mRNAs: GC1, GCAP1, cone transducin alpha (GNAT2), rod cGMP-specific 3′,5′ cyclic phosphodiesterase subunit alpha (PDE6α), and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primer sequences and a detailed description of target validation are provided in the Supplementary Methods, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7867/-/DCSupplemental, and Supplementary Figure S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7867/-/DCSupplemental. Results are the average of 3 replicate reactions and were calculated using the 2−ΔΔCT method35 with the GAPDH signal used to normalize samples and the GC1+/+ sample serving as the calibrator. Standard deviations were calculated from the three replicate reactions performed for each sample. Data are presented as the fold change in mRNA levels relative to the GC1+/+ sample.
Results
Long-term, Photoreceptor-Specific GC1 Expression Is Achievable in GC1KO Mice Treated with AAV5 or AAV8(Y733F) Vectors
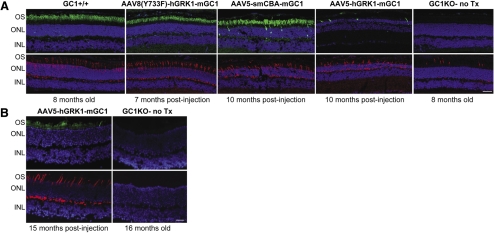
Immunostaining with an antibody directed against GC1 (green) revealed that AAV-vectored therapeutic protein expression persisted exclusively in photoreceptors of treated GC1KO mice for a significant fraction of the animal's lifetime; AAV8(Y733F)-hGRK1-mGC1 for at least 7 months, AAV5-smCBA-mGC1 for at least 10 months, and AAV5-hGRK1-mGC1 for at least 15 months (Figs. 1A, 1B). GC1 expression was limited to the outer segments of rods and cones treated with AAV8(Y733F)-hGRK1-mGC1 vector, whereas it was found in both outer segments and more rarely in photoreceptor cell bodies of eyes treated with AAV5-smCBA-mGC1. GC1 expression was absent from the untreated GC1KO retina (Figs. 1A, 1B). Orientation of eyes before kill allowed for the evaluation of GC1 expression in similar areas of treated or untreated retinas (inferior retina), with care taken to avoid analysis in the far periphery. Two examples of retinal thinning were observed. The first was a GC1KO retina treated with AAV5-smCBA-mGC1 (4.69 × 109 total vector genomes delivered) (Fig. 1A). The outer nuclear layer (ONL) was slightly thinned relative to that seen in naive GC1KO or GC1+/+ control retinas (both 8 months of age). The second was a GC1KO retina treated with the more concentrated AAV5-hGRK1-mGC1, which showed photoreceptor-specific GC1 expression and profound thinning of the ONL (Fig. 1A). Another AAV5-hGRK1-mGC1–treated GC1KO mouse from the same cohort was killed at 15 months after injection. In this case, persistent photoreceptor-specific GC1 expression was observed with no appreciable retinal thinning (Fig. 1B). However, evaluation of additional treated GC1KO mice is warranted before determining categorically that no retinal thinning occurs after injection with this particular vector, at this particular concentration. The number of outer segments expressing GC1 varied across samples. For example, only sparse GC1-positive outer segments were found in the AAV5-hGRK1-mGC1- injected retina (Fig. 1A). Based on the finding that an additional mouse from this cohort exhibited no appreciable retinal thinning at 15 months after injection (Fig. 1B), it is likely that the small number of GC1-positive outer segments found in the former example was due to overall reduction in photoreceptor outer segments after injection-related damage.
Figure 1.
AAV-mediated GC1 expression persists over the long term exclusively in photoreceptors of the GC1KO mouse. (A, B) Immunohistochemistry of frozen retinal cross-sections was used to localize expression of GC1 (green, top row) and cone arrestin (red, bottom row) in GC1KO mice treated with AAV8(Y733F)-hGRK1-mGC1 (7 months after injection), AAV5-smCBA-mGC1 (10 months after injection), or AAV5-hGRK1-mGC1 (10 months after injection) vectors and in retinas from 8-month-old untreated GC1KO and GC1+/+ control mice (A). An additional mouse treated withAAV5-hGRK1-mGC1 was similarly evaluated at 15 months after injection (B). Nuclei were stained with DAPI (blue). All sections were imaged at 20× magnification and exposed at identical settings. OS, outer segments; ONL, outer nuclear layer; INL, inner nuclear layer. Scale bars, 30 μm.
Long-term Cone Photoreceptor Survival Is Achieved by AAV-Vectored GC1
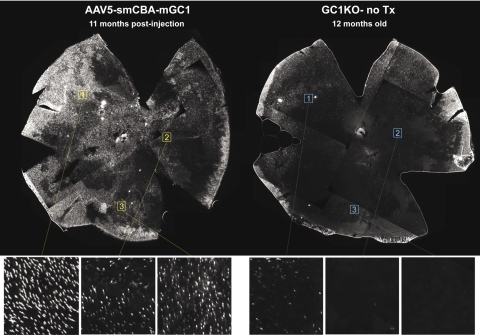
Cone photoreceptors in treated and untreated GC1KO mice as well as GC1+/+ controls were identified by staining for mouse cone arrestin (red). Here we show that cone photoreceptor densities were markedly reduced in untreated GC1KO retinas by 8 months of age (Fig. 1) and confirm previous reports that cones are lost in a topographically specific manner in this mouse model36 (Fig. 2). Representative retinal cross-sections from the inferior retinas of treated mice revealed increased cone densities relative to those of an untreated control (Fig. 1). At 16 months of age, no cone arrestin expression was seen in a cross-section taken from the inferior retina of an untreated GC1KO eye. This does not exclude the possibility that some cones remained in the superior retina. Wholemount analysis revealed the 12-month-old untreated retina exhibited sparse cone density, with residual cones found exclusively in superior retinal regions whereas the partner P14-treated retina retained much higher cone density throughout, with the exception of a small patch of temporal retina that likely was not exposed to vector during subretinal injection and therefore did not contain transgene product (Fig. 2). Representative 100-μm2 boxes were placed on identical areas of inferior, central, and superior retinas of both eyes and blown up for a high-resolution depiction of individual cones in those areas (Fig. 2). Quantification of cone photoreceptors revealed significant differences in cone cell densities in the inferior (P < 0.001), central (P < 0.001), and superior (P < 0.001) retinal regions of treated and untreated GC1KO retains. Average cone cell densities per square millimeter in the treated GC1KO retina were 1597 (inferior), 1432 (central), and 1790 (superior). Average cone cell densities per square millimeter in the untreated GC1KO retina were 2 (inferior), 7 (central), and 637 (superior). These results show that AAV-mGC1 treatment is capable of preserving cone photoreceptors for at least 11 months after treatment and suggest this that preservation of cones is possible over the lifetime of the animal.
Figure 2.
Long-term preservation of cone photoreceptors is achievable in AAV-mGC1–treated GC1KO mice. Immunostaining of retinal wholemounts from one GC1KO mouse 11 months after treatment with AAV5-smCBA-mGC1 (one eye only) with an antibody against cone arrestin revealed marked preservation of cone photoreceptors in the treated eye compared with the untreated contralateral control eye. Retinal wholemounts were oriented similarly, with their temporal portions in the 12 o'clock position. Portions of wholemounts were imaged at 10× magnification and merged together for final presentation. Representative 100-μm2 boxes were placed on identical areas of superior, central, and inferior retinas of each eye and blown up for a high-resolution depiction of individual cone photoreceptors.
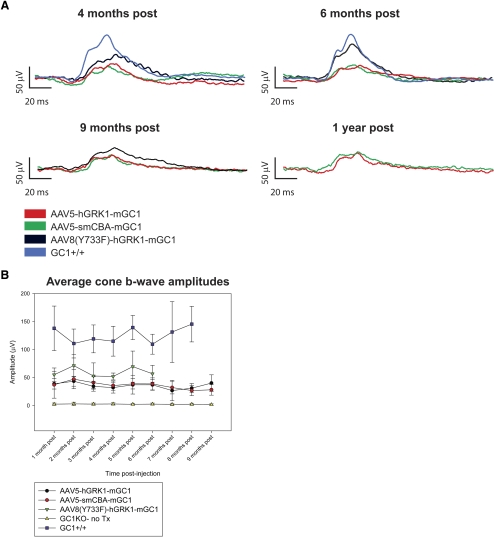
Long-term Restoration of Photoreceptor Function (ERG) Is Achieved in AAV-Treated GC1KO Mice
We previously showed that average photopic b-wave amplitudes in AAV-mGC1–treated mice were partially restored at 4 weeks after injection and remained stable for 3 months.26 Here we statistically compare cone-mediated responses out to 9 months after treatment in cohorts of mice treated with AAV5 vector containing hGRK1 or smCBA promoters, the identical vectors used in our previous 3-month study.26 We also compare, out to 6 months, cone responses in mice treated with AAV8(Y733F)-hGRK1-mGC1 to these cohorts. Cone responses were stable over time and were significantly higher than responses generated from untreated contralateral controls (P < 0.001), suggesting that restoration of cone function is possible over the lifetime of the animal (Fig. 3). Consistent with our previous report, the level of restoration achieved after delivery of the photoreceptor-specific promoter (hGRK1)-containing AAV5 vector was not significantly different from that achieved with the ubiquitous promoter (smCBA)-containing AAV5 vector at any post-treatment time point.26 Beyond the period of statistical comparison (9 months after treatment), AAV5-mGC1–treated mice continued to exhibit cone-mediated function (Fig. 3A). Additionally, we show that cone photoreceptor function was stably restored for at least 6 months after injection with AAV8(Y733F)-hGRK1-mGC1. Cone b-wave amplitudes in GC1KO mice injected with this strong, fast-acting AAV8 tyrosine capsid mutant were higher than those seen in GC1KO mice injected with either AAV5 vector at every time point evaluated. At 6 months after treatment, the latest time point at which all vectors could be compared in parallel, there was a significant difference between cone b-wave amplitudes in AAV8(Y733)-hGRK1-mGC1 versus AAV5-hGRK1-mGC1–treated mice (P = 0.033) and AAV(Y733F)-hGRK1-mGC1 versus AAV5-smCBA-mGC1–treated mice (P = 0.025). A representative trace recorded 9 months after injection with AAV8(Y733F)-hGRK1-mGC1 (n = 1) was noticeably smaller than that recorded at 6 months after injection. Whether AAV8(Y733F)-mediated therapy remains stable beyond 6 months after treatment awaits further study.
Figure 3.
Cone-mediated electroretinograms are restored in AAV-mGC1–treated GC1KO mice over the long term. (A) Representative cone-mediated traces elicited by a 12 cd · s/m2 light stimulus from GC1KO eyes treated with AAV5-hGRK1-mGC1 (red line), AAV5-smCBA-mGC1 (green line), or AAV8(Y733F)-hGRK1-mGC1 (black line) or age-matched, untreated GC1+/+ control eyes reveal long-term AAV-mGC1–mediated restoration of cone function. Representative traces generated between 4 months and 1 year after treatment are shown. (B) Maximum cone b-wave amplitudes (those generated at 12 cd · s/m2) were calculated from each mouse and averaged monthly in each treatment group and in age-matched, untreated GC1KO and GC1+/+ controls. Comparisons were made between groups of animals with an n > 3. All AAV treatment groups were statistically compared for 6 months after treatment. AAV5 vector–treated eyes were statistically compared for 9 months after treatment. Error bars represent ±1 SD.
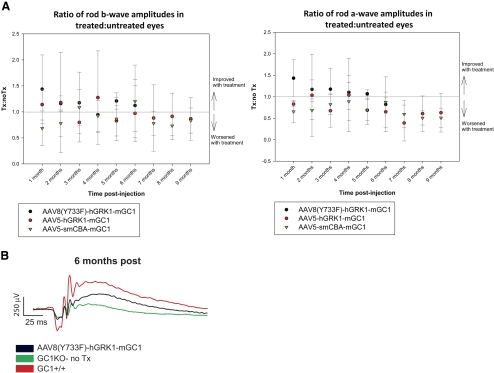
Calculating the average ratios of rod b-wave amplitudes in AAV8(Y733F)-hGRK1-mGC1–treated versus untreated eyes resulted in values >1.0, with one exception at 4 months after treatment (Fig. 4). Rod b-wave ratios of AAV5-treated versus untreated eyes were only occasionally >1.0. Similarly, rod a-wave ratios were consistently >1.0 in AAV8(Y733F)-hGRK1-mGC1–treated mice whereas they were, for the most part, <1.0 after treatment with either AAV5 vector (Fig. 4). Representative rod-mediated scotopic ERG traces elicited by a 1 cd · s/m2 stimulus show AAV8(Y733F)-mediated improvements in rod ERG amplitudes and indicate that aside from the reduced amplitude, treated-eye response kinetics resemble that seen in the GC1+/+ control (Fig. 4).
Figure 4.
Rod-mediated electroretinograms are generally unchanged in AAV-treated GC1KO mice. Rod a- and b-wave amplitudes (A) elicited by a 1 cd · s/m2 stimulus under scotopic conditions were determined in the treated and untreated eyes of GC1KO mice treated with AAV8(Y733F)-hGRK1-mGC1 (black circles), AAV5-hGRK1-mGC1 (red circles), or AAV5-smCBA-mGC1 (green triangles). Intra-mouse ratios of treated and untreated eyes were generated by dividing the maximum a- or b-wave amplitude in the treated eye by the maximum amplitude in the untreated eye. These ratios were averaged monthly in all treatment groups. Comparisons were made between groups of animals with an n > 3. All AAV treatment groups were statistically compared for 6 months. AAV5 vectors were also statistically compared for 9 months. Vector-mediated improvement was defined by an average ratio >1.0. Representative rod-mediated ERG traces from one GC1KO mouse (B) reveal that rod responses from the AAV8(Y733F)-hGRK1-mGC1–treated eye (black line) were higher than those recorded from the untreated contralateral control eye (green line). This treated rod response was restored to ∼50% that of the normal GC1+/+ rod response (red line). Error bars represent ±1 SD.
Vector Biodistribution
Optic nerve from injected and uninjected eyes and portions of left and right brain that contained visual pathways were analyzed for the presence of vector genomes. AAV5 vectors were injected in the right eyes of GC1KO mice. Accordingly, vector genomes were detected in the right optic nerve of AAV5-treated mice at both 7 and 10 months after injection. At 7 months after injection, vector genomes were also detected in the left brain of one mouse injected with AAV5-hGRK1-mGC1. No vector genomes were detected from the right brain of that animal. The observation that right (injected) optic nerve and left brain were positive is anatomically consistent because the left hemisphere is predominantly “wired” to the right eye. By 10 months after injection, AAV5-delivered vector genomes were still detected in right (injected) optic nerve but were absent from both brain hemispheres. AAV8(Y733) vector was injected into the left eyes of GC1KO mice. Accordingly, AAV8(Y733F)-delivered vector genomes were detected in the left optic nerves at both 4 and 7 months after injection. At no time point were vector genomes in the AAV8(733)-treated mouse detected in either brain hemisphere. We detected a higher average number of vector genomes in the optic nerves of eyes injected with AAV5-GRK1-mGC1 compared with AAV5-smCBA-mGC1. Only AAV5-hGRK1-mGC1–delivered genomes were detected in brain tissue over the course of our study (1 of 16 samples). As expected, no AAV vector genomes were recovered from any tissue of naive GC1KO control mice (Table 2).
Table 2.
Detection of rAAV Vector Sequences by PCR in GC1KO Tissue Samples
| M1* |
M2† |
M3† |
M4‡ |
M5‡ |
M6‡ |
M7‡ |
M8‡ |
M9† |
M10‡ |
M11† |
M12‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AAV8(733)-hGRK1 | AAV8(733)-hGRK1 | AAV5-smCBA | AAV5-smCBA | AAV5-smCBA | AAV5-smCBA | AAV5-smCBA | AAV5-smCBA | AAV5-hGRK1 | AAV5-hGRK1 | No Tx | No Tx |
| N/A | N/A | +160 | +1000 | +970 | +1890 | N/A | +395 | +3140 | +1695 | — | — |
| +632 | +25,885 | — | N/A | N/A | N/A | N/A | N/A | — | N/A | N/A | N/A |
| — | — | — | — | — | — | — | — | — | — | — | — |
| — | — | — | — | — | — | — | — | +252 | — | — | — |
AAV8(733)-hGRK1-mGC1 injections were performed in left eyes; AAV5-mGC1 injections were performed in right eyes. M, animal; Tx, treatment; —, no PCR amplification; +, PCR amplification of vector sequence (replicated value shown is average, copy number per microgram of DN/A); N/A, not available (not determinable because of an unacceptable spike-in).
Four months after injection.
Seven months after injection.
Ten months after injection.
AAV-mGC1 Treatment Restores Wild-Type Levels of GC1 and GCAP1 to Treated GC1KO Retina
GC1 protein was absent from the untreated eye of the GC1KO mouse. In contrast, at 7 months after injection with AAV8(Y733F)-hGRK1-mGC1, the level of GC1 in the treated eye was comparable to that seen in the GC1+/+ control (Fig. 5A). Consistent with previous reports that GCAP1 is post-translationally downregulated in the GC1KO mouse, we show that GCAP1 was downregulated in the untreated GC1KO retina relative to the GC1+/+ control37 (Fig. 5A). However, AAV-mediated delivery of GC1 leads to an upregulation in GCAP1 expression in the treated GC1KO mouse retina. Levels of GCAP1 expression were also comparable to those seen in GC1+/+ controls (Fig. 5A).
Figure 5.
AAV-mGC1 treatment restores wild-type levels of GC1 and GCAP1 protein, increases GC1 mRNA relative to normal, and increases cone-specific cone transducin alpha (GNAT2) mRNA levels in GC1KO mice. (A) Immunoblot of retinal lysates from one GC1KO mouse eye 10 months after treatment with AAV8(Y733F)-hGRK1-mGC1 probed with anti–GC1 and anti–GCAP1 antibodies reveal that AAV-mGC1 treatment restores wild-type levels of GC1 and GCAP1 protein to the treated GC1KO mouse retina. Anti–β-actin antibody was used as an internal loading control. (B) Semiquantitative real time RT-PCR was used to assay several transcripts (GC1, GCAP1, GNAT2, and PDE6α) in one GC1KO retina treated with AAV5-smCBA-mGC1, one GC1KO retina treated with AAV8(Y733F)-hGRK1-mGC1 vector, and individual untreated GC1KO or GC1+/+ control retinas. Samples were run in triplicate using Gapdh-specific primers as a standard. Data are presented as the fold change in mRNA levels relative to the GC1+/+ control. Levels of AAV-delivered GC1 mRNA were between 7- to 14-fold higher than levels of endogenous GC1 mRNA in GC1+/+ retina. Levels of cone-specific GNAT2 were increased in AAV-mGC1–treated GC1KO mice relative to that seen in untreated eyes. Levels of PDE6α and GCAP1 were relatively unchanged across samples.
In Treated GC1KO Mice, GC1 mRNA Is Present and GNAT2 mRNA Levels Are Increased Compared with Untreated GC1KO Mice
At 1 year after treatment, levels of GC1 mRNA in treated retinas were approximately 7-fold (AAV5-treated) and 14-fold (AAV8[YY733F]-treated) higher than those seen in the age-matched GC1+/+ control mouse (Fig. 5B). We found that high levels of GC1 mRNA in treated retinas corresponded to recovery of many vector genomes: 1.57 × 107 vg/μg DNA forAAV8(Y733F) and 4.7 × 106 vg/μg for AAV5. Despite the high levels of GC1 mRNA in treated retinas, no GC1 expression was detected in optic nerves of treated eyes (data not shown). This result further supports the notion that vectors evaluated in this study did not result in off-target transgene expression. Consistent with previous reports that the reduction of GCAP1 in GC1KO mice is post-translational (i.e., mRNA levels are unchanged), we found no substantial changes in the levels of GCAP1 mRNA across samples (Fig. 5B).37
GNAT2 RNA in untreated GC1KO samples was reduced relative to GC1+/+ controls, a result likely due to the loss of cone photoreceptors in these retinas (Fig. 5B). In contrast, there were appreciable increases in GNAT2 mRNA levels in both AAV5- and AAV8(Y733F)-treated eyes, a result that further supports that cone photoreceptors are preserved in AAV-mGC1–treated GC1KO mice (Fig. 5B). Levels of rod PDE6α were relatively unchanged across samples, likely because rod photoreceptors do not degenerate in the GC1KO mouse (Fig. 5B).
Discussion
Here we show that persistent AAV-mediated GC1 expression is capable of restoring retinal function and preserving cone photoreceptors in the GC1KO mouse over the long term (∼1 year). This therapeutic longevity was characterized by a number of different criteria: the existence of GC1 protein in treated eyes at 15 months after treatment, the restoration of cone function as measured by electroretinography at 1 year after treatment, the increased cone survival in treated eyes at 11 months after treatment, and the recovery of vector genomes and GC1 mRNA in retinas at 1 year after treatment. When viewed as individual, discrete analyses, the sample sizes used in these assays were often small. However, when all are considered as correlates of therapeutic efficacy in mice exhibiting clear functional rescue (ERG), the sample size was effectively much larger. Within this context, therefore, we would suggest therapy persists beyond the period during which different vector serotypes were statistically compared for ERG rescue. This is the first demonstration of long-term therapy in a model of GC1 deficiency (∼1 year) and builds on our previous report that AAV-mediated delivery of murine GC1 restored cone-mediated function and behavior and preserved cone photoreceptors in this model for 3 months.26
Cone photoreceptor function was significantly improved over the long term after AAV-mGC1 treatment, with AAV8(Y733F) vector being the most efficient. This result complements our previous report that an AAV8(Y733F) vector stably restored retinal structure and function to the rd10 mouse, a model refractory to treatment with standard AAV vectors.36 Quantifying differences in rod amplitudes between treated and untreated eyes in the GC1KO mouse is complicated by the fact that rod function in this model is partially subserved by GC2 and by the large inter-animal variation in rod responses.38 Some improvements in rod-mediated responses in AAV8(Y733F)-treated GC1KO mice were observed more consistently than those recorded from AAV5-treated mice; however, these improvements did not reach statistical significance. This suggests that more aggressive expression of GC1 in the GC1KO eye can supplement the partial effect of GC2 on murine rod function.
Long-term preservation of cone photoreceptors was demonstrated 11 months after gene replacement. This result suggests that cone preservation is achievable over the lifetime of the animal. Although the preserved cones in treated GC1KO retina were not examined on an ultrastructural level (e.g., electron microscopy), our observation that cones remained functional over time by ERG analysis suggests that they were structurally intact. Long-term preservation of cone photoreceptors mediated by therapeutic AAV-GC1 has obvious clinical relevance because it suggests the potential to preserve macular cones and to restore usable daytime/color vision to patients with GC1 deficiency.
AAV-mediated GC1 expression persisted for at least 15 months after treatment (the latest time point evaluated by immunohistochemistry). Regardless of the serotype used or whether a photoreceptor-specific (hGRK1) or ubiquitous (smCBA) promoter drove its expression, GC1 was located exclusively in photoreceptors. Although transgene expression was limited to the target cell type, it should be noted that the hGRK1 promoter resulted in GC1 localization mainly within the proper compartment of the target cell (photoreceptor outer segments). This result, along with other successful proof-of-concept studies using this promoter, suggests that the hGRK1 promoter should be considered in the design of a clinical AAV vector targeting photoreceptors.39,40 However, because of the existence of multiple GRK isoforms across species that could limit the activity of hGRK1 in photoreceptor subtypes41 and instances in which promoter activity in the mouse retina does not translate perfectly to the nonhuman primate (NHP) retina,42 the activity of this promoter first must be thoroughly evaluated in the NHP retina.
Some evidence of retinal thinning was observed in two GC1KO retinal sections at 10 months after treatment with AAV5-smCBA-mGC1 or AAV5-hGRK1-mGC1. However, no appreciable thinning was observed in an older mouse that was evaluated 15 months after treatment with AAV5-hGRK1-mGC1. The small number of retinas analyzed precludes determination of the cause of thinning (injection-related damage or vector/transgene-related toxicity). Clearly, dose de-escalation experiments in larger cohorts of mice will be required to determine whether retinal thickness can be preserved at lower therapeutic doses. This will be an essential experiment for firmly establishing any dose-limiting toxicity, a critical parameter in designing a safe dosing schedule for a clinical trial.
Despite the photoreceptor-exclusive nature of AAV-mediated GC1 expression, vector genomes were detected in the optic nerves and, in one instance, the brains of treated GC1KO mice. Our biodistribution data were collected from “diseased” animals. This is important because of evidence that patterns of vector transduction are different in diseased versus healthy retina43and that this difference was due in part to vector distribution being enhanced in diseased retina. This is the first evaluation of biodistribution for an AAV vector containing a capsid surface–exposed tyrosine mutation. Despite the fact that the titer of AAV8(Y733F)-hGRK1-mGC1 vector used (1.08 × 1013 vg/mL) was lower than that of the AAV5-hGRK1-mGC1 vector, we detected a higher average number of vector genomes in optic nerves of AAV8(Y733F)-treated eyes. Although AAV5 is known to be ineffective for transducing ganglion cells of the mouse retina,44 we have shown that AAV8 does transduce this cell type.45 Some exposure of vector to retinal ganglion cells is expected as the syringe transverses the inner retina during subretinal injection and because the ratio of injection volume to total eye size is high in mouse. The higher number of vector genomes detected in optic nerves of AAV8(Y733F)-treated eyes, therefore, could be due to the increased affinity of AAV8(Y733F) for retinal ganglion cells. At only one time point did we detect AAV5-delivered vector genomes in the brain of a treated GC1KO mouse with recovery only from the hemisphere opposite the injected eye. Our results (only 1 of 16 samples positive) are consistent with a previous study that reported a lack of AAV5-delivered sequence in brains of subretinally injected rats and dogs.46 By 10 months after injection, no vector genomes were recovered from brains of AAV5-treated GC1KO mice or from brains of mice treated with AAV8(Y733F) at any time point. Vectors used in this study were delivered at full strength. Future experiments involving dose de-escalation may show that a lower vector concentration is sufficient to affect therapy without spread to the brain. In general, other studies evaluating AAV biodistribution suggest that there is a progressive loss of vector sequences with time distal from the site of administration.29,47 Studies in rat, dog, and primate show that ocularly delivered AAV (serotypes 2, 4, and 5) do not spread widely outside the injected eye, with optic nerve and brain the most common exceptions.29,46,47 The lack of genome recovery from brains of treated GC1KO mice suggests that AAV5 and AAV8(Y733F) vectors used in this study have biodistribution patterns consistent with those reported for AAV2 by Jacobson et al.47
Despite recovering vector genomes from optic nerves of treated eyes, immunostaining revealed a lack of GC1 expression in optic nerves of eyes treated with either AAV5-smCBA-mGC1 or AAV8(Y733F)-hGRK1-mGC1vectors. A previous study by Stieger et al.48 detected transgene expression in optic nerves and brains of rats and dogs at 2 months and 4 weeks after subretinal injection with AAV8 containing green fluorescent protein (GFP). Taking into account that our AAV8(Y733F) vector contained the photoreceptor-specific hGRK1 promoter and our previous finding that GC1 expression was limited to photoreceptors even when under the control of a ubiquitous promoter such as smCBA, a lack of GC1expression in optic nerves is not unexpected. Stieger et al.48 incorporated the strong, ubiquitous cytomegalovirus (CMV) promoter into their vector to drive GFP, a protein capable of being stably expressed in a wide variety of tissues when delivered by viral vectors.
Although both AAV5 and AAV8(Y733F) vectors were capable of providing long-term therapy to the GC1KO mouse, there are apparent advantages associated with using AAV8(Y733F). First and foremost, AAV8(Y733F) with a photoreceptor-specific promoter conferred significantly higher cone ERG responses to treated mice than either AAV5 vector. The reason for this may be the ability of AAV8 vectors to transduce areas outside the injection bleb in the rodent retina, whereas the area of retina transduced by AAV5 remains largely confined to the bleb.48 Thus AAV8(733F) may simply transduce, on average, a larger area of retina relative to AAV5. Although we did not evaluate the kinetics of vector-mediated GC1 expression in cones transduced with AAV8(733) or AAV5 vectors in this study, it is known that AAV8(Y733F) supports a much faster onset of transgene expression than AAV5 vectors and that this temporal difference is important in other retinal degenerative models in terms of ability to preserve function.36,45 In the GC1KO mouse, the earlier availability of GC1 in AAV8(Y733F)–treated mice might have led to a higher overall number of preserved cone photoreceptors than that achieved in the AAV5-treated mice and, therefore, to more robust cone function. Again, experiments in NHP comparing the two serotypes are needed before firm conclusions can be drawn regarding which vector should be considered in a clinical setting.
This study provides insights into the effects of GC1 delivery to a mammalian model of GC1 deficiency. However, evaluating a gene replacement strategy for LCA1 in the GC1KO mouse has limitations. Rod photoreceptor structure and function are relatively preserved in this mouse, whereas LCA1 is characterized by extinguished cone and rod electroretinograms and by the degeneration of both cones and rods.23 With the goal to treat photoreceptors in an affected LCA1 retina, therapeutic vector transduction profiles should first be evaluated in a model that is more degenerative (i.e., exhibits more than just a loss of cone photoreceptors). Studies are under way to characterize these vectors in the recently described GC1/GC2 double knockout (GCdko) mouse, which exhibits loss of rod/cone function and both rod and cone degeneration.38 These studies should provide critical information regarding the recovery of rod function and whether, when treated together, both subclasses of photoreceptors are capable of being preserved.
The results presented here support further development of an AAV-based gene replacement therapy for LCA1 and potentially for GC1-related autosomal recessive CORD patients. Interestingly, our results may suggest that LCA12 patients could benefit from gene replacement as well. A recent report49 has shown that rd3, a gene associated with autosomal recessive photoreceptor degeneration in the rd3 mouse, rcd2 dog, and LCA12 patients, codes for RD3, a protein required for the export of GC1 from the endoplasmic reticulum to endosomal vesicles.49 Specifically, photoreceptor degeneration in the rd3 mouse is caused by impaired RD3-mediated guanylate cyclase expression and trafficking and is similar to the pattern exhibited by the GCdko mouse.49 Given the parallels in biochemical dysfunction, our results showing AAV-GC1 arrests cone degeneration and restores visual function would suggest that rd3 gene replacement may also be successful in the rd3 mouse and the rcd2 dog. Future gene therapy experiments using AAV-GC1 to treat GCdko mice will perhaps lend further support to this concept.
Supplementary Material
Acknowledgments
The authors thank Vince Chiodo, Thomas Doyle, and Ron Mandel at the University of Florida for their technical support, and Wolfgang Baehr at the University of Utah and Cheryl Craft at the University of Southern California for providing materials. WWH and the University have a financial interest in the use of AAV therapies and own equity in a company (AGTC, Inc.) that might, in the future, commercialize some aspects of this work.
Footnotes
Supported in part by National Institutes of Health Grants EY13729, EY11123, and EY08571; Foundation Fighting Blindness, Macula Vision Research Foundation, and Research to Prevent Blindness, Inc.
Disclosure: S.L. Boye, AGTC, Inc. (C), P; T. Conlon, None; K. Erger, None; R. Ryals, None; A. Neeley, None; T. Cossette, None. J. Pang, None; F.M. Dyka, None; W.W. Hauswirth, AGTC, Inc. (C, I), P; S.E. Boye, P
References
- 1. Dizhoor AM, Lowe DG, Olshevskaya EV, et al. The human photoreceptor membrane guanylylcyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352 [DOI] [PubMed] [Google Scholar]
- 2. Liu X, Seno K, Nishizawa Y, et al. Ultrastructural localization of retinal guanylatecyclase in human and monkey retinas. Exp Eye Res. 1994;59:761–768 [DOI] [PubMed] [Google Scholar]
- 3. Haire SE, Pang J, Boye SL, et al. Light-driven cone arrestin translocation in cones of postnatal guanylate cyclase-1 knockout mouse retina treated with AAV-GC1. Invest Ophthalmol Vis Sci. 2006;47:3745–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pugh EN, Jr, Duda T, Sharma RK, et al. Photoreceptor guanylatecyclases: a review. Biosci Rep. 1997;17:429–473 [DOI] [PubMed] [Google Scholar]
- 5. Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca2+ binding proteins in the retina. Trends Neurosci. 1996;19:547–554 [DOI] [PubMed] [Google Scholar]
- 6. Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vis Res. 2008;48:2052–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lamb TD, Pugh EN., Jr Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci. 2006;47:5138–5152 [DOI] [PubMed] [Google Scholar]
- 8. Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187 [DOI] [PubMed] [Google Scholar]
- 9. Yang RB, Garbers DL. Two eye guanylylcyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem. 1997;272:13738–13742 [DOI] [PubMed] [Google Scholar]
- 10. Perrault I, Rozet JM, Calvas P, et al. Retinal-specific guanylatecyclase gene mutations in Leber's congenital amaurosis. Nat Genet. 1996;14:461–464 [DOI] [PubMed] [Google Scholar]
- 11. Perrault I, Rozet JM, Gerber S, et al. Spectrum of retGC1 mutations in Leber's congential amaurosis. Eur J Hum Genet. 2000;8:578–582 [DOI] [PubMed] [Google Scholar]
- 12. Kelsell RE, Gregory-Evans K, Payne AM, et al. Mutation in the retinal guanylatecyclase (RETGC-1) gene in dominant cone-rod dystrophy. Hum Mol Genet. 1998;7:1179–1184 [DOI] [PubMed] [Google Scholar]
- 13. Perrault I, Rozet JM, Gerber S, et al. A retGC1–1 mutation in autosomal dominant cone-rod dystrophy. Am J Hum Genet. 1998;63:651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gregory-Evans K, Kelsell RE, Gregory-Evans CY, et al. Autosomal dominant cone-rod retinal dystrophy (CORD6) from heterozygous mutation of GUCY2D, which encodes retinal guanylatecyalse. Ophthalmology. 2000;107:55–61 [DOI] [PubMed] [Google Scholar]
- 15. Weigell-Weber M, Fokusten S, Torok B, et al. Codons 837 and 838 in the retinal guanylatecyalse gene on chromosome 17p: hot spots for mutations in autosomal dominant cone-rod dystrophy. Arch Ophthalmol. 2000;188:300. [DOI] [PubMed] [Google Scholar]
- 16. Ugur SA, Durlu YK, Tolun A. A novel recessive GUCY2D mutation causing cone-rod dystrophy and not Leber's congenital amaurosis. Eur J Hum Genet. 2010;18:1121–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perrault I, Rozet JM, Gerber S, et al. Leber congenital amaurosis. Mol Genet Metab. 68:200–8, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Chung DC, Traboulsi EI. Leber congenital amaurosis: clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS. 13:587–92, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Payne AM, Morris AG, Downes SM, et al. Clustering and frequency of mutations in the retinal guanylatecyclase (GUCY2D) gene patients with dominant cone-rod dystrophies. J Med Genet. 2001;38:611–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Downes SM, Payne AM, Kelsell RE, et al. Autosomal dominant cone-rod dystrophy with mutations in the guanylatecyclase 2D gene encoding retinal guanylate cyclase-1. Arch Ophthalmol. 2001;119:1667–1673 [DOI] [PubMed] [Google Scholar]
- 21. Wilkie SE, Newbold FJ, Deery E, et al. Functional characterization of missense mutations at codon 838 in retinal guanylatecyclase correlates with disease severity in patients with autosomal dominant cone-rod dystrophy. Hum Mol Genet. 2000;9:3065–3073 [DOI] [PubMed] [Google Scholar]
- 22. Karan S, Frederick JM, Baehr W. Novel functions of photoreceptor guanylatecyclases revealed by targeted deletion. Mol Cell Biochem. 2010;334:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang RB, Robinson SW, Xiong WH, et al. Disruption of a retinal guanylylcyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J Neurosci. 1999;19:5889–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lowe DG, Dizhoor AM, Liu K, et al. Cloning and expression of a second photoreceptor-specific membrane retina guanylylcyclase (RetGC), RetGC-2. Proc Natl Acad Sci U S A. 1995;92:5535–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang RB, Foster DC, Garbers DL, et al. Two membrane forms of guanylylcyclase found in the eye. Proc Natl Acad Sci U S A. 1995;92:602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boye SE, Boye SL, Pang J, et al. Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS One. 2010;5:e11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105:7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zolotukhin S, Potter M, Zolotukhin I, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167 [DOI] [PubMed] [Google Scholar]
- 29. Jacobson SG, Acland GM, Aguirre GD, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006;13:1074–1084 [DOI] [PubMed] [Google Scholar]
- 30. Timmers AM, Zhang H, Squitieri A, et al. Subretinal injections in rodent eyes: effects on electrophysiology and histology of rat retina. Mol Vis. 2001;7:131–137 [PubMed] [Google Scholar]
- 31. Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pang J, Boye SE, Lei B, et al. Self-complimentary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Ther. 2010;17:815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molday LL, Wu WW, Molday RS. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J Biol Chem. 2007;282:32792–32801 [DOI] [PubMed] [Google Scholar]
- 34. Triant DA, Whitehead A. Simultaneous extraction of high-quality RNA and DNA from small tissue samples. J Hered. 2009;100:246–250 [DOI] [PubMed] [Google Scholar]
- 35. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 36. Pang JJ, Dai X, Boye SE, et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19:234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coleman JE, Zhang Y, Brown GA, et al. Cone cell survival and downregulation of GCAP1 protein in the retinas of GC1 knockout mice. Invest Ophthalmol Vis Sci. 2004;45:3397–3403 [DOI] [PubMed] [Google Scholar]
- 38. Baehr W, Karan S, Maeda T, et al. The function of guanylatecyclase 1 and guanylatecyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun X, Pawlyk B, Xu X, et al. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 2010;17:117–131 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Tan MH, Smith AJ, Pawlyk B, et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum Mol Genet. 2009;18:2099–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beltran W, Boye SL, Boye SE, et al. rAAV2/5 gene-targeting to rods: dose-dependent efficiency and complications associated with different promoters. Gene Ther. 2010;17:1162–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin L, Greenberg K, Hunter JJ, et al. Intravitreal injection of AAV2 transduces macaque inner retina. Invest Ophthalmol Vis Sci. 2011;52:2775–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kolstad KD, Dalkara D, Guerin K, et al. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther. 2010;21:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2007;48:353–359 [DOI] [PubMed] [Google Scholar]
- 45. Petrs-Silva H, Dinculescu A, Li Q, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Provost N, Le Meur G, Weber M, et al. Biodistribution of rAAV vectors following intraocular administration: evidence for the presence and persistence of vector DNA in the optic nerve and in the brain. Mol Ther. 2005;11:275–283 [DOI] [PubMed] [Google Scholar]
- 47. Jacobson SG, Boye SL, Aleman TS, et al. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther. 2006;17:845–858 [DOI] [PubMed] [Google Scholar]
- 48. Stieger K, Colle MA, Dubreil L, et al. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther. 2008;16:916–923 [DOI] [PubMed] [Google Scholar]
- 49. Azadi S, Molday LL, Molday RS. RD3, the protein associated with Leber congenital amaurosis type 12, is required for guanylatecyclase trafficking in photoreceptor cells. Proc Natl Acad Sci U S A. 2010;107:21158–21163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.