Daily brief periods of unrestricted vision during early monocular form deprivation reduced the impact of amblyopia, and this preventive effect was most closely associated with reduced ocular dominance imbalance and binocular suppression in V2.
Abstract
Purpose.
Providing brief daily periods of unrestricted vision during early monocular form deprivation reduces the depth of amblyopia. To gain insights into the neural basis of the beneficial effects of this treatment, the binocular and monocular response properties of neurons were quantitatively analyzed in visual area 2 (V2) of form-deprived macaque monkeys.
Methods.
Beginning at 3 weeks of age, infant monkeys were deprived of clear vision in one eye for 12 hours every day until 21 weeks of age. They received daily periods of unrestricted vision for 0, 1, 2, or 4 hours during the form-deprivation period. After behavioral testing to measure the depth of the resulting amblyopia, microelectrode-recording experiments were conducted in V2.
Results.
The ocular dominance imbalance away from the affected eye was reduced in the experimental monkeys and was generally proportional to the reduction in the depth of amblyopia in individual monkeys. There were no interocular differences in the spatial properties of V2 neurons in any subject group. However, the binocular disparity sensitivity of V2 neurons was significantly higher and binocular suppression was lower in monkeys that had unrestricted vision.
Conclusions.
The decrease in ocular dominance imbalance in V2 was the neuronal change most closely associated with the observed reduction in the depth of amblyopia. The results suggest that the degree to which extrastriate neurons can maintain functional connections with the deprived eye (i.e., reducing undersampling for the affected eye) is the most significant factor associated with the beneficial effects of brief periods of unrestricted vision.
The developing visual system is exquisitely sensitive to degradation of retinal images. Depriving one eye of clear vision soon after birth results in a severe loss of visual sensitivities in the affected eye (form-deprivation amblyopia).1–7 However, recent studies in nonhuman primates showed that the magnitude of form-deprivation amblyopia is dramatically reduced if a brief daily period of unrestricted vision is provided during the deprivation period.8 Similar beneficial effects of brief periods of “normal” vision have been reported in cats.9–11
The neural mechanisms underlying amblyopia or the observed preventive effects of unrestricted vision in amblyopic subjects are not well understood, partly because previous neurophysiological studies of the neural basis of amblyopia in nonhuman primates focused mostly on how the primary visual cortex (V1) is reorganized by early abnormal visual experience. The primate brain has vast cortical areas beyond V1 that process information required for visual perception. Moreover, the functional development of the visual brain is thought to proceed in a hierarchical order and therefore, a higher order visual area (e.g., V2) is likely to be more plastic than a lower area (e.g., V1) at a given developmental age.12–18 For example, our recent study of strabismic amblyopia in macaque monkeys revealed that whereas the ocular dominance distribution of V1 neurons in amblyopic monkeys was balanced between the two eyes, there was a significant ocular dominance shift in V2 neurons away from the deviating eye in these same amblyopic monkeys.18 A previous comprehensive fMRI study on anisometropic and strabismic amblyopia reported that fMRI responses to stimulation of the amblyopic eye were progressively reduced in higher order visual areas.19 Also nearly all neurons in extrastriate visual areas are driven by either eye (i.e., binocular cells) and often exhibit more complex spatial vision processing than V1 neurons.20–24 Moreover binocular signal interactions in V2 are far more robust than in V1, and V2 neurons, unlike V1 neurons, are known to be directly involved in binocular depth perception.25,26
Taken together, studies on higher stages of cortical processing during early development are necessary to better understand the neural basis of amblyopia and therefore, the mechanisms of preventive effects from brief daily periods of unrestricted vision during early monocular form deprivation. In the present study, we asked whether developing V2 neurons are more sensitive than V1 neurons to the protective effects of brief daily periods of unrestricted vision during early monocular form deprivation.
Methods
All experimental and animal care procedures conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Houston.
Subjects
The details of the rearing procedures have been published.8,27 Briefly, between 3 and 18 weeks of age, infant monkeys wore a helmet that secured a diffuser spectacle lenses in front of one eye and plano lenses in front of the other. The rearing regimen included a daily period of unrestricted vision for 0 (n = 3), 1 (n = 2), 2 (n = 2), or 4 (n = 2) hours. The daily lens removal occurred near the midpoint of the normal lighting cycle. The experimental monkeys experienced unrestricted vision between birth and 3 weeks of age and between the end of the rearing period (18 weeks of age) and the microelectrode experiment at approximately 4 years of age. Assessment of the eye alignment with infrared photoretinoscopy, which records the position of the first Purkinje image relative to the center of the pupil,28 indicated that all of our experimental monkeys were orthotropic.
Psychophysical Methods
The details of behavioral testing methods are described elsewhere.8,29,30 Briefly, spatial contrast sensitivity functions (CSFs) were obtained for each eye when the monkeys were at least 18 months of age, using operant procedures. During the daily experimental sessions, the monkeys were seated in a primate chair fitted with a response lever on the waist plate and a drink spout on the neck plate. The animal's optimal spectacle correction, which was determined for each eye independently by retinoscopy and a subjective refraction procedure was held in a facemask at about a 14-mm vertex distance for all training and testing procedures. For monocular viewing, the lens well for one of the eyes was occluded with an opaque disc.
The Gabor detection stimuli consisted of sine-phase vertical sinusoidal gratings windowed by a 2° Gaussian envelope and were presented on an 11° × 14° video monitor with a space-averaged luminance of 60 cd/m2. The stimulus contrast was defined as (Lmax − Lmin)/(Lmax + Lmin), where Lmax and Lmin represent the maximum and minimum luminance of the grating, respectively. The behavioral paradigm was a temporal–interval detection task that required the monkey to press and hold down the response lever to initiate a trial and then to release the lever within a criterion response interval of 900 ms after the presentation of the grating stimulus to score a “hit.” Data were collected using an adaptive decreasing-contrast staircase procedure where each hit was followed by a 0.1-log unit reduction in contrast and two consecutive misses were followed by a 0.6-log unit increase in contrast causing staircase reversals to converge to a contrast where the probability of a hit was 29%, and this contrast was taken as the threshold. Contrast detection thresholds were measured as a function of grating spatial frequency from 0.25 to 16 cyc/deg in 0.15-log unit steps.
Amblyopia index (AI) values8,31 were calculated by the following formula: The AI is the area under the CSF of the nonamblyopic eye minus the area under CSF of the amblyopic eye divided by the area under the CSF of the nonamblyopic eye. This index ranges from 0 (no deficit) to 1.0 (no measurable sensitivity in the treated eye).
Neurophysiology
Preparation.
The surgical preparation and the recording and stimulation methods have been described in detail elsewhere.17,18 Briefly, the monkeys were anesthetized initially with an intramuscular injection of ketamine hydrochloride (15–20 mg/kg) and acepromazine maleate (0.15–0.2 mg/kg). After the completion of all surgical procedures, the animals were paralyzed by an IV infusion of vecuronium bromide (a loading dose of 0.1–0.2 mg/kg followed by a continuous infusion of 0.1–0.2 mg/kg/h) and artificially respired with a mixture of 59% N2O, 39% O2, and 2% CO2. Anesthesia was maintained during the experiments by the continuous infusion of a mixture of sufentanil citrate (0.05 μg/kg/h) and propofol (4 mg/kg/h). The core body temperature was kept at 37.6°C. Cycloplegia was produced by 1% atropine sulfate, and the animals' corneas were protected with rigid, gas-permeable, extended-wear contact lenses. Retinoscopy was used to determine the contact lens parameters needed to focus the eyes on the stimulus screen.
Recording and Visual Stimulation.
Tungsten-in-glass microelectrodes (Frederick Haer, Bowdoin, ME) were used to record single-unit activity or multiunit activity from which responses from single cortical neurons were isolated by using custom spike-sorting software. Drifting gratings (contrast, 50%) were displayed on a monochrome monitor with ultrashort persistence (frame rate = 140 Hz; 800 × 600 pixels, screen size = 20° × 15° at 114 cm, or 40° × 30° at 57 cm, and mean luminance = 50 cd/m2), and neuronal responses were sampled at a rate of 140 Hz (7.14-ms bin widths) and compiled into peristimulus time histograms (PSTHs) that were equal in duration to, and synchronized with, the temporal cycle of the grating.
Data Analysis
Orientation Bias and Spatial Frequency Tuning.
Orientation bias was calculated by using the vector summation methods.18,32,33 To determine each cell's optimal spatial frequency and spatial resolution, the spatial frequency response data were fitted with Gaussian functions34:
where f0 is the spatial frequency, m1 is the response amplitude, f2 is the optimal spatial frequency, and f3 is the standard deviation of the Gaussian function. The optimal spatial frequency was determined from the fitted functions.
Ocular Dominance.
The ocular dominance index (ODI) of a neuron was quantitatively determined from the spatial frequency tuning functions by using the following formula: ODI = (Rl − noise)/[(Rr − noise) + (Rl − noise)], where Rl is the peak response amplitude for left eye stimulation, Rr is the peak response amplitude for right eye stimulation, and noise is the spontaneous maintained activity.35,36 ODI values range from 0.0 (right eye response alone) to 1.0 (left eye response alone), with 0.5 indicating perfect binocular balance. An ocular imbalance index (OII) was quantified for all units with the formula OII = 2 * [ODI − 0.5].37 The OII value ranges from 0.0 (no imbalance) to 1.0 (complete monocular dominance) and shows the difference in relative strength of the two eyes in driving a unit. Since the OII value does not indicate which eye is dominant, each unit was assigned according to its ODI value to be dominated by the amblyopic (right in normal monkeys) eye (ODI < 0.5) or the fellow (left) eye (ODI ≥ 0.5) group. Then all OII values of units dominated by each eye were summed. The relative ratio (log2) of the summed OII value for units dominated by the nonamblyopic eye over that dominated by the amblyopic eye was defined as the relative ocular imbalance index (ROII).
Binocular Interactions.
To determine the strength and the nature of binocular interactions, responses were collected for dichoptic sine wave gratings of the optimal spatial frequency, orientation, and direction of drift as a function of the relative interocular spatial phase disparity of the grating pair. The sensitivity to relative interocular spatial phase disparities was quantified using a binocular interaction index (BII) that was calculated from the sine function fit to the binocular phase tuning data (BII = amplitude of the fitted sine wave divided by the average binocular response amplitude).36,38 To characterize whether binocular signal interactions were facilitatory or suppressive in nature, the peak binocular response amplitude/dominant monocular response amplitude ratios (peak B/M) were calculated for each unit and expressed in terms of relative strength (decibels)—that is, 10 log peak B/M. Negative peak B/M values signify binocular suppression, and positive values indicate binocular facilitation.
Results
We recorded from 385 V2 neurons in nine experimental monkeys and 176 units in three normal monkeys. For each neuron, we quantitatively determined the unit's ocular dominance, orientation, and spatial frequency-tuning functions for both eyes and their binocular interaction properties.
Effects of Brief Daily Periods of Unrestricted Vision on the Development of Amblyopia
Figure 1 shows the spatial contrast sensitivity functions of our experimental monkeys and the relationship between the duration of the daily brief periods of unrestricted normal vision during the deprivation period and the severity of amblyopia. To quantify the depth of amblyopia in each monkey, we calculated an AI by comparing the contrast sensitivity function for the affected and fellow eyes.18,31 Not surprisingly, monkeys that experienced continuous monocular form deprivation (0 hours of unrestricted vision) exhibited severe amblyopia in the deprived eye (e.g., MK-274, AI = 0.99). One hour of unrestricted vision during the daily 12 hours of monocular deprivation had no effect in one monkey (MK-306, AI = 0.99), whereas it reduced the severity of amblyopia in another monkey substantially (MK-307, AI = 0.80). Two hours of unrestricted vision was far more effective in reducing the depth of amblyopia (MK-300, AI = 0.7 and MK-311, AI = 0.45), and 4 hours of unrestricted vision virtually prevented the development of amblyopia in two monkeys (MK-308, AI = 0.0 and MK-309, AI = 0.0).
Figure 1.
Effects of the duration of brief daily periods of unrestricted vision during early monocular form deprivation on the depth of amblyopia. AIs of individual monkeys were plotted as a function of the duration of unrestricted vision (middle panel). Spatial contrast sensitivity functions for the deprived eye (filled circles) and the fellow eye (open circles) are also illustrated for the representative monkeys for each treatment group (top and bottom panels).
Effects of Brief Daily Periods of Unrestricted Vision on V2 Neuronal Responses
Ocular Dominance Imbalance.
To determine the effectiveness of each eye in driving a V2 unit, we quantitatively measured its ODI. The ODIs were placed into one of the seven evenly spaced bins that match the traditional seven categories of ocular dominance.39 The OD category 0 in Figure 2 represents neurons exclusively driven by the amblyopic eye (A), whereas category 1 signifies units driven only by the fellow eye (F). The ROII values then were calculated for each treatment group to quantify the overall ocular dominance imbalance (Fig. 2).
Figure 2.
Effects of the duration of brief daily periods of unrestricted vision on the ocular dominance of V2 neurons. (A) Ocular dominance distribution for each treatment group. ROII values calculated for each group are shown. A, amblyopic eye. F, fellow eye. (B) The average AI as a function of rearing histories for each group (r = 0.96, P = 0.03).
For the monocularly deprived monkeys that wore the diffuser constantly (0 hours of unrestricted vision), there was an obvious shift in ocular dominance in V2 away from the amblyopic eye (Fig. 2A). One hour of unrestricted vision each day during the period of monocular form deprivation did not significantly alter the ocular dominance shift in V2 neurons, although one of these monkeys behaviorally showed a moderate reduction in the depth of amblyopia (Fig. 1, middle panel, MK-307). The ocular dominance imbalance for these experimental monkeys with 1 hour of unrestricted vision was slightly larger (ROII = 3.69) than that for monkeys reared without any unrestricted vision (ROII = 2.84). However, the difference was not statistically significant (χ2 test, P > 0.1).
With 2 hours of unrestricted vision, there was a substantial improvement in the ocular dominance distribution; a larger percentage of V2 neurons showed robust responses to stimulation of the deprived eye and as a result, the ocular dominance imbalance between the two eyes was substantially smaller (ROII = 1.95; Fig. 2A). With 4 hours of unrestricted vision, the effect of monocular deprivation was virtually absent; the OD distribution was very similar to that for normal monkeys except that the prevalence of units dominated by the deprived eye was slightly higher than in normal monkeys. However, again, this difference was not statistically significant (χ2 test, P > 0.05) and probably reflects a sampling bias in recording.
Figure 2B plots the average depth of amblyopia (AI) for each subject group as a function of their relative ocular dominance imbalance values (ROII). There was a relatively strong correlation between the depth of amblyopia and the ocular dominance imbalance in V2 (r = 0.92, P = 0.03).
Monocular Receptive-Field Properties.
The poor acuity of amblyopic animals reared with strabismus or anisometropia is thought to result at least in part from the abnormal spatial frequency tuning of V1 neurons dominated by the amblyopic eye3,31,40–42 and also from the abnormal spatial frequency and orientation bias of V2 neurons.18 In the present study, we examined whether brief daily periods of normal vision during the diffuser-rearing period prevents or reduces the impact of form deprivation on the monocular spatial properties of V2 neurons.
Orientation Tuning.
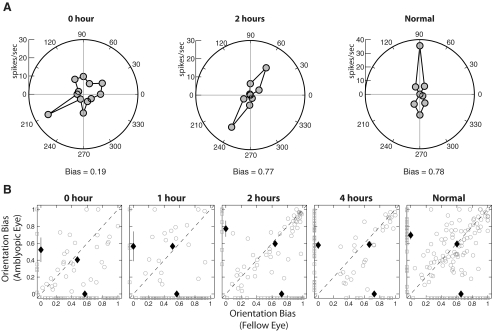
Figure 3 illustrates the orientation-tuning functions of representative V2 units. The orientation-tuning function for the unit from one of the monkeys that experienced continuous form deprivation with 0 hours of unrestricted vision exhibited a reduced orientation bias, resulting from the cell's irregular responses to stimulus orientations (Fig. 3A). On the other hand, the units from a deprived monkey that had 2 hours of unrestricted vision each day and the neuron from a normal monkey showed a sharp orientation-tuning function.
Figure 3.
Effects of the duration of brief daily periods of unrestricted vision on the orientation bias of V2 neurons. (A) Representative orientation-tuning functions for the affected eye from a monkey reared with continuous form deprivation (0 hours of unrestricted vision) (left), a deprived monkey with 2 hours of unrestricted vision (middle), and a normal monkey (right). (B) Interocular comparisons of the orientation biases of binocular units (ODI = 0.2–0.8; open circles). Filled symbols: mean values (±SE). Open squares on the x- and y-axes indicate comparable data for monocular units (ODI = 0.0–0.2 or 0.8–1.0). Filled symbols on the x- and y-axes show the mean values (±SE) for monocular units and filled symbols near the unity line indicate the mean values (±SE) for binocular units. If error bars are not visible, the standard errors are smaller than the size (length and width) of the symbols.
For analysis purposes, we divided all units into binocular units (ODI = 0.2–0.8; Fig. 3B, open circles) or monocular units (ODI = 0.0–0.2 or 0.8–1.0, Fig. 3B, open squares along the x- and y-axes). The scatter plots in Fig. 3B illustrate the orientation bias for individual units, specifically the interocular differences in orientation bias in binocular units, and the orientation bias for the dominant eye of monocular units. There were no significant interocular differences in the overall orientation biases for any animal group (two-way ANOVA, P > 0.1). However, V2 neurons in the deprived monkeys with 0 hours of normal vision had significantly lower orientation bias for the deprived eye than did the V2 neurons of normal monkeys (two-way ANOVA, P < 0.01). Interestingly, the biases for the nondeprived eyes of the monkeys reared with continuous form deprivation were also significantly lower than those for normal monkeys. Finally, 2 and 4 hours, but not 1 hour, of unrestricted vision during deprivation periods significantly improved the orientation biases of V2 neurons in the experimental monkeys (two-way ANOVA, P < 0.01).
Preferred Spatial Frequency.
We did not find significant interocular differences in the preferred spatial frequency for any subject group (Fig. 4). However, the cell population analysis showed that the preferred spatial frequencies of units for the deprived eye in the monkeys reared with continuous form deprivation were significantly lower than those for normal monkeys (two-way ANOVA, P < 0.01).
Figure 4.
Effects of the duration of brief daily periods of unrestricted vision on the spatial frequency tuning of V2 neurons. (A) Representative spatial frequency tuning functions for the affected eye from a monkey reared with continuous form deprivation (0 hours of unrestricted vision) (left), a deprived monkey with 2 hours of unrestricted vision (middle), and a normal monkey (right). (B) Interocular comparisons of the optimal spatial frequency of binocular units (ODI = 0.2–0.8) (open circles). Filled symbols: mean values (±SE). Open squares: on x- and y-axes indicate comparable data for monocular units (ODI = 0.0–0.2 or 0.8–1.0). Filled symbols along x- and y-axes show the mean values (±SE) for monocular units and filled symbols near the unity line indicate the mean values (±SE) for binocular units. If error bars are not visible, the standard errors are smaller than the size (length and width) of the symbols.
Binocular Signal Interactions.
Early binocular imbalance is known to result in abnormal binocular functions including a loss of binocular summation and disparity sensitivity and an increased prevalence of interocular suppression.1,2 Monkeys reared with early form deprivation, anisometropia, or strabismus exhibit anomalous ocular dominance shifts, reduced disparity sensitivity, and an abnormally high prevalence of binocular suppression in V1 and V2.15,18,31,36,43,44 The question we asked in the present study was whether brief periods of normal vision during monocular form deprivation influence the degree of these anomalous binocular signal interactions in V2.
Disparity Sensitivity.
Figure 5A illustrates typical interocular spatial phase disparity functions of a V2 neuron in a monkey that experienced continuous form deprivation (left), a form deprived monkey reared with 2 hours of normal vision each day (middle), and a normal monkey (right). Unlike in normal units, neurons from a monkey that experienced continuous form deprivation were not sensitive to phase disparity (BII = 0.15), and all binocular responses were nearly one half that of the dominant monocular (DM) response (peak B/M = −4.8 dB), thus exhibiting a severe loss of disparity sensitivity and strong binocular suppression. However, the unit from the deprived monkey that had the benefit of 2 hours of unrestricted vision each day retained binocular disparity sensitivity (BII = 0.40), although the binocular responses were lower than the dominant monocular response.
Figure 5.
Effects of the duration of brief daily periods of unrestricted vision on binocular signal interactions in V2 neurons. (A) Representative binocular disparity sensitivity functions of a V2 neuron from a monkey reared with continuous form deprivation (left), a monocularly form deprived monkey with 2 hours of unrestricted vision (middle), and a normal monkey (right). BII, binocular interaction index. Dotted line with an open triangle indicates the response level of the dominant eye. Open triangle at the bottom right indicates the response level of the nondominant eye. Open square: the noise level (spontaneous firing). (B) Average (±SE) BII values for each subject group. (C) Average (±SE) peak B/M values for each group. (D) Proportion of binocularly suppressive units (peak B/M < 0.0 dB).
The cell population analysis shows that monocular form deprivation with 0 hours of normal vision virtually eliminated disparity sensitivity in V2 neurons (Fig. 5B). The average disparity tuning in the 0-hour monkeys was significantly lower than that in normal monkeys (one-way ANOVA, P < 0.01). More important, having brief daily periods of unrestricted vision during the period of form deprivation nearly tripled the average disparity sensitivity (BII) of the V2 neurons (Fig. 5B). This improvement was statistically significant (one-way ANOVA, P < 0.01). However, the average disparity sensitivities for all the deprived monkeys, regardless of the duration of unrestricted vision, were significantly lower than that for normal monkeys (one-way ANOVA, P < 0.01). Interestingly, these results parallel previous clinical observations for human infants with congenital unilateral cataracts; increased daily periods of binocular visual experience (from reduced patching therapy) improved disparity sensitivity, whereas their overall disparity sensitivity was significantly lower than that for normal infants.45–47
Binocular Suppression.
The peak B/M responses of V2 neurons from all experimental monkeys were significantly lower than that in normal monkeys, which reflected the increased prevalence of suppressive binocular interactions (Fig. 5C; one-way ANOVA, P < 0.01). Although 2 and 4 hours of normal vision improved the average peak B/M values, the difference did not reach statistical significance (P > 0.05).
The prevalence of binocularly suppressive V2 units (peak B/M < 0.0 dB) in monocularly form-deprived monkeys with 0 hours of unrestricted vision each day was much higher (35.7%) than that in normal monkeys (10.5%; χ2 test, P < 0.001; Fig. 5D). Daily periods of unrestricted vision during monocular deprivation reduced the proportion of suppressive units, but there were no statistically significant differences between the results for the three deprived subject groups that experienced unrestricted vision. Together, daily brief periods of normal vision during monocular deprivation reduced the prevalence of binocularly suppressive units in V2, but the duration of unrestricted vision had inconsistent effects.
Discussion
The important finding of this study was that the ocular dominance imbalance of V2 neurons favoring the nondeprived eye was the neuronal change most closely associated with the reductions in the depth of amblyopia and, therefore, with the degree of the preventive effects from daily brief periods of unrestricted vision during monocular form deprivation.
V2 versus V1
Although substantial evidence for the association between V2 neurophysiology and amblyopia was revealed, our study did not allow us to directly establish a causal link between the severity of V2 deficits and the protective effects of differing durations of daily unrestricted vision on a degree of amblyopia. However, it is possible to infer from our data which alterations in V2 neurophysiology (ocular dominance imbalance, binocular suppression, lower optimal spatial frequency, and/or reduced orientation biases) had the strongest impact in limiting the visual performance of our amblyopic monkeys and also reflecting the protective effects of unrestricted vision. To analyze this association between behavior and physiology, the ocular imbalance (ROII), the proportion of the binocularly suppressive unit, the average optimal spatial frequency, and the average orientation bias of V2 neurons in individual monkeys were first normalized to the respective maximum value, and then the degree of amblyopia was examined by linear regression (Fig. 6). Comparisons of the normalized regression lines relating the four major cortical deficits in V2 with the depth of amblyopia show that the function relating ocular dominance imbalance and the depth of amblyopia (AI) had a slope of 0.76 and was closest to the unity line relating normalized physiological deficits with the AI, which suggests that this association is the strongest among all the response properties examined in this study. The prevalence of suppression in V2 was also relatively close to the unity line, but the slope was shallower (0.42) and above the unit line—that is, the physiological deficits were greater than perceptual deficits. The slopes of the functions relating the monocular response properties of V2 neurons (orientation bias and optimal spatial frequency) with amblyopia were much shallower, and these functions are located far below the diagonal, indicating that each of these neural deficits alone had far less consistent impact on limiting visual performance.
Figure 6.
Comparisons of the relative magnitude of V2 (left) and V1 (right) deficits with the depth of amblyopia (AI). The ocular imbalance (ROII), the proportion of the binocularly suppressive unit, the average orientation bias, and the average optimal spatial frequency of neurons in individual monkeys were first normalized to the respective maximum value and then were fit to obtain a regression line for each cortical deficit. The slope (±SE) is given for each function.
We previously reported that intermittent unrestricted vision during monocular deprivation had similar preventive effects against the development of amblyopia,8 and that these perceptual effects were generally associated with changes in the responses of V1 neurons.48 As previously mentioned, the neuronal deficits of strabismic monkeys exhibiting varying degrees of amblyopia are more severe in V2 than in V1.18 Therefore, we asked whether the neuronal deficits in V2 are more closely associated with the protective effects of daily periods of unrestricted vision during monocular deprivation (i.e., the depth of amblyopia) than V1 deficits. We performed the same analysis as described above to analyze the strength of the association between behavior and physiology using the data from our previous study in V148 (Fig. 6). The ocular dominance imbalance (ROII) in V1 had the slope closest to the unity line (0.55) although it was shallower than the comparable function in V2 (0.76), primarily because the ocular dominance imbalance in this study was amplified in V2. The normalized change in ROII in V1 was only approximately 30% compared with more than 40% in V2—hence, the shallower slope in V1. Other response properties in V1 had slopes that were shallower or similar to those for V2. These comparisons suggest that although ocular dominance imbalance was the most critical neural factor for both cortical areas, the protective effects of brief periods of unrestricted vision had a greater impact on V2 than on V1 neurons.
The present study adds support for the idea that amblyopia can be best explained as a cascade of cortical deficits over several processing stages that begin at V1.2,3,18,19,31,49 This study suggests that V2 is more sensitive to brief daily periods of unrestricted vision during form deprivation than is V1.
Effects on Binocular Vision
Can daily brief periods of unrestricted vision during early monocular form deprivation prevent a loss of binocular depth perception? A recent study in cats was revealing. A loss of binocular depth perception could not be prevented by brief daily periods of normal vision during monocular deprivation.50 In this study, we found a threefold improvement in the overall disparity sensitivity of V2 neurons in our experimental monkeys reared with brief daily periods of unrestricted vision over those exposed to continuous form deprivation. Whereas V2 neurons are known to be more directly involved in binocular depth perception than V1 neurons,25 it is unclear whether the observed retention of disparity sensitivity in V2 associated with periods of unrestricted vision is sufficient to support stereoscopic vision in these monkeys until they are behaviorally tested for binocular functions. Interestingly, a new study from our laboratory demonstrated that 2 hours of daily normal binocular vision during 12 hours of optical strabismus largely preserved local and global stereopsis in infant monkeys.51 This finding suggests that the cortical mechanisms supporting stereopsis are very sensitive to the protective effects of periods of normal binocular vision. Although the nature of early abnormal visual experience is vastly different between the two studies, it may be worthwhile to behaviorally test the binocular capacities of monkeys reared with early intermittent form deprivation.
Normal Vision before and after Treatment
Our experimental monkeys experienced normal vision until 3 weeks of age when our rearing regimen began. This rearing strategy was necessary to obtain interocular alignment and, therefore, to ensure the restorative power of intermittent unrestricted binocular vision. Abnormal visual experience imposed from birth that prevents binocularly matched visual stimulation (e.g., prism-rearing52 binocular deprivation,53 or alternating monocular deprivation54) is known to produce permanent strabismus in monkeys53,55 that would have reduced or complicated functional recovery.56
The prolonged periods of normal vision between the end of the rearing regimen and behavioral testing in our study could have contributed to the prevention of amblyopia and restoration of balanced ocular dominance; hence, it is difficult to assess the true benefit of intermittent unrestricted vision.11 This possibility is highly unlikely for several reasons. First, the dramatic effects of monocular deprivation on spatial vision in monkeys require only a few weeks of treatment.57,58 Moreover, it is important to keep in mind that all experimental monkeys in this and previous studies received the same recovery period after the treatment. Also monkeys reared with optical strabismus showed that prolonged recovery periods, similar to those in this study, had little beneficial effects in restoring the binocular response properties of, at least, V1 neurons—that is, spontaneous recovery was minimal.15,36,43,44 Moreover, Mitchell and Sengpiel11 conducted similar experiments in cats and found comparable degrees of the protective effects from intermittent periods of unrestricted vision in cats without prolonged normal binocular visual experience. Finally, even if there were a substantial spontaneous recovery due to prolonged normal vision after the end of our rearing regimen, such recovery would promote the benefits of earlier interventions to eliminate abnormal visual experience in human infants.
Clinical Implications
Abnormal visual experience in newborn infants that deprives clear vision includes congenital cataract, ptosis, and excessive hyperopic anisometropia. There is no debate on the opinion that removal of these conditions at the earliest possible time is the most desirable course of treatment.59–64 The results of this and our previous studies suggest that if permanent solutions are not possible, temporary, or intermittent removal of these abnormal visual conditions, if possible, may promote the reduction of amblyopic image degradation and myopic refractive errors.8,27 More specifically, when compliance with corrective lens treatment after infantile cataract surgery or with spectral wear for hyperopic anisometropia is difficult in infants and young children, our data suggest that even brief periods of daily wear of corrective lenses may have beneficial effects for the developing visual system.
Footnotes
Supported by National Institutes of Health Research Grants R01-EY008128 (YMC), R01-EY003611 (ELS), R01-EY001139 (RSH), and Core Grant P30 EY007751.
Disclosure: B. Zhang, None; X. Tao, None; J.M. Wensveen, None; R.S. Harwerth, None; E.L. Smith III, None; Y.M. Chino, None
References
- 1. Chino YM. Developmental visual deprivation. In: Kaufman PL, Alm A. eds. Adler's Physiology of the Eye. Edinburgh: Elsevier; 2011:732–749 [Google Scholar]
- 2. Chino YM, Bi H, Zhang B. The postnatal development of the neuronal response properties in primate visual cortex. In: Kaas J, Collins C. eds. Primate Vision. Boca Raton, FL; 2004:81–108 [Google Scholar]
- 3. Kiorpes L, Movshon JA. Neural limitations on visual development in primates. In: Chalupa LM, Werner JS. eds. The Visual Neurosciences. Cambridge, MA: MIT Press; 2003:159–173 [Google Scholar]
- 4. Anderson SJ, Swettenham JB. Neuroimaging in human amblyopia. Strabismus. 2006;14:21–35 [DOI] [PubMed] [Google Scholar]
- 5. Daw NW. Visual Development. 3rd ed New York: Springer; 2006 [Google Scholar]
- 6. Kiorpes L. Visual processing in amblyopia: animal studies. Strabismus. 2006;14:3–10 [DOI] [PubMed] [Google Scholar]
- 7. Levi DM. Visual processing in amblyopia: human studies. Strabismus. 2006;14:11–19 [DOI] [PubMed] [Google Scholar]
- 8. Wensveen JM, Harwerth RS, Hung LF, Ramamirtham R, Kee CS, Smith EL., 3rd Brief daily periods of unrestricted vision can prevent form-deprivation amblyopia. Invest Ophthalmol Vis Sci. 2006;47:2468–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell DE, Kind PC, Sengpiel F, Murphy K. Brief daily periods of binocular vision prevent deprivation-induced acuity loss. Curr Biol. 2003;13:1704–1708 [DOI] [PubMed] [Google Scholar]
- 10. Mitchell DE, Kind PC, Sengpiel F, Murphy K. Short periods of concordant binocular vision prevent the development of deprivation amblyopia. Eur J Neurosci. 2006;23:2458–2466 [DOI] [PubMed] [Google Scholar]
- 11. Mitchell DE, Sengpiel F. Neural mechanisms of recovery following early visual deprivation. Philos Trans R Soc Lond B Biol Sci. 2009;364:383–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barone P, Dehay C, Berland M, Bullier J, Kennedy H. Developmental remodeling of primate visual cortical pathways. Cereb Cortex. 1995;5:22–38 [DOI] [PubMed] [Google Scholar]
- 13. Distler C, Bachevalier J, Kennedy C, Mishkin M, Ungerleider LG. Functional development of the corticocortical pathway for motion analysis in the macaque monkey: a 14C-2-deoxyglucose study. Cereb Cortex. 1996;6:184–195 [DOI] [PubMed] [Google Scholar]
- 14. Batardiere A, Barone P, Knoblauch K, et al. Early specification of the hierarchical organization of visual cortical areas in the macaque monkey. Cereb Cortex. 2002;12:453–465 [DOI] [PubMed] [Google Scholar]
- 15. Zhang B, Zheng J, Watanabe I, et al. Delayed maturation of receptive field center/surround mechanisms in V2. Proc Natl Acad Sci U S A. 2005;102:5862–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng J, Zhang B, Bi H, et al. Development of temporal response properties and contrast sensitivity of V1 and V2 neurons in macaque monkeys. J Neurophysiol. 2007;97:3905–3916 [DOI] [PubMed] [Google Scholar]
- 17. Maruko I, Zhang B, Tao X, Tong J, Smith EL, 3rd, Chino YM. Postnatal development of disparity sensitivity in visual area 2 (v2) of macaque monkeys. J Neurophysiol. 2008;100:2486–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bi H, Zhang B, Tao X, Harwerth RS, Smith EL, 3rd, Chino YM. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cereb Cortex. 2011;21:2033–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muckli L, Kiess S, Tonhausen N, Singer W, Goebel R, Sireteanu R. Cerebral correlates of impaired grating perception in individual, psychophysically assessed human amblyopes. Vision Res. 2006;46:506–526 [DOI] [PubMed] [Google Scholar]
- 20. Hegde J, Van Essen DC. Selectivity for complex shapes in primate visual area V2. J Neurosci. 2000;20:RC61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito M, Komatsu H. Representation of angles embedded within contour stimuli in area V2 of macaque monkeys Neurosci. 2004;24:3313–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anzai A, Peng X, Van Essen DC. Neurons in monkey visual area V2 encode combinations of orientations. Nat Neurosci. 2007;10:1313–1321 [DOI] [PubMed] [Google Scholar]
- 23. Dillenburger B, Roe AW. Influence of parallel and orthogonal real lines on illusory contour perception. J Neurophysiol. 2010;103:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willmore BD, Prenger RJ, Gallant JL. Neural representation of natural images in visual area V2. J Neurosci. 2010;30:2102–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cumming BG, Parker AJ. Local disparity not perceived depth is signaled by binocular neurons in cortical area V1 of the Macaque. J Neurosci. 2000;20:4758–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roe AW, Parker AJ, Born RT, DeAngelis GC. Disparity channels in early vision. J Neurosci. 2007;27:11820–11831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith EL, 3rd, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43:291–299 [PubMed] [Google Scholar]
- 28. Quick MW, Boothe RG. Measurement of binocular alignment in normal monkeys and in monkeys with strabismus. Invest Ophthalmol Vis Sci. 1989;30:1159–1168 [PubMed] [Google Scholar]
- 29. Harwerth RS, Boltz RL, Smith EL., 3rd Psychophysical evidence for sustained and transient channels in the monkey visual system. Vision Res. 1980;20:15–22 [DOI] [PubMed] [Google Scholar]
- 30. Smith EL, 3rd, Harwerth RS, Crawford ML. Spatial contrast sensitivity deficits in monkeys produced by optically induced anisometropia. Invest Ophthalmol Vis Sci. 1985;26:330–342 [PubMed] [Google Scholar]
- 31. Kiorpes L, Kiper DC, O'Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998;18:6411–6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levick WR, Thibos LN. Analysis of orientation bias in cat retina. J Physiol. 1982;329:243–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith EL, 3rd, Chino YM, Ridder WH, 3rd, Kitagawa K, Langston A. Orientation bias of neurons in the lateral geniculate nucleus of macaque monkeys. Vis Neurosci. 1990;5:525–545 [DOI] [PubMed] [Google Scholar]
- 34. DeAngelis GC, Ohzawa I, Freeman RD. Spatiotemporal organization of simple-cell receptive fields in the cat's striate cortex. I. General characteristics and postnatal development. J Neurophysiol. 1993;69:1091–1117 [DOI] [PubMed] [Google Scholar]
- 35. Chino YM, Smith EL, 3rd, Hatta S, Cheng H. Postnatal development of binocular disparity sensitivity in neurons of the primate visual cortex. J Neurosci. 1997;17:296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith EL, 3rd, Chino YM, Ni J, Ridder WH, 3rd, Crawford ML. Binocular spatial phase tuning characteristics of neurons in the macaque striate cortex. J Neurophysiol. 1997;78:351–365 [DOI] [PubMed] [Google Scholar]
- 37. DeAngelis GC, Newsome WT. Organization of disparity-selective neurons in macaque area MT. J Neurosci. 1999;19:1398–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohzawa I, Freeman RD. The binocular organization of simple cells in the cat's visual cortex. J Neurophysiol. 1986;56:221–242 [DOI] [PubMed] [Google Scholar]
- 39. Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chino YM, Shansky MS, Jankowski WL, Banser FA. Effects of rearing kittens with convergent strabismus on development of receptive-field properties in striate cortex neurons. J Neurophysiol. 1983;50:265–286 [DOI] [PubMed] [Google Scholar]
- 41. Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque's visual system. III. Physiological observations. J Neurosci. 1987;7:1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Curr Opin Neurobiol. 1999;9:480–486 [DOI] [PubMed] [Google Scholar]
- 43. Kumagami T, Zhang B, Smith EL, 3rd, Chino YM. Effect of onset age of strabismus on the binocular responses of neurons in the monkey visual cortex. Invest Ophthalmol Vis Sci. 2000;41:948–954 [PubMed] [Google Scholar]
- 44. Mori T, Matsuura K, Zhang B, Smith EL, 3rd, Chino YM. Effects of the duration of early strabismus on the binocular responses of neurons in the monkey visual cortex (V1). Invest Ophthalmol Vis Sci. 2002;43:1262–1269 [PubMed] [Google Scholar]
- 45. Gregg FM, Parks MM. Stereopsis after congenital monocular cataract extraction. Am J Ophthalmol. 1992;114:314–317 [DOI] [PubMed] [Google Scholar]
- 46. Jeffrey BG, Birch EE, Stager DR, Jr, Stager DR, Sr, Weakley DR., Jr Early binocular visual experience may improve binocular sensory outcomes in children after surgery for congenital unilateral cataract. J AAPOS. 2001;5:209–216 [DOI] [PubMed] [Google Scholar]
- 47. Brown SM, Archer S, Del Monte MA. Stereopsis and binocular vision after surgery for unilateral infantile cataract. J AAPOS. 1999;3:109–113 [DOI] [PubMed] [Google Scholar]
- 48. Sakai E, Bi H, Maruko I, et al. Cortical effects of brief daily periods of unrestricted vision during early monocular form deprivation. J Neurophysiol. 2006;95:2856–2865 [DOI] [PubMed] [Google Scholar]
- 49. El-Shamayleh Y, Kiorpes L, Kohn A, Movshon JA. Visual motion processing by neurons in area MT of macaque monkeys with experimental amblyopia. J Neurosci. 2010;30:12198–12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mitchell DE, Kennie J, Schwarzkopf DS, Sengpiel F. Daily mixed visual experience that prevents amblyopia in cats does not always allow the development of good binocular depth perception. J Vis. 2009;9:22 21–27 [DOI] [PubMed] [Google Scholar]
- 51. Wensveen JM, Smith EL, 3rd, Hung LF, Harwerth RS. Brief daily periods of unrestricted vision preserve stereopsis in strabismus. Invest Ophthalmol Vis Sci. 2011;52:4872–4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tychsen L, Richards M, Wong AM, et al. Decorrelation of cerebral visual inputs as the sufficient cause of infantile esotropia. Am Orthopt J. 2008;58:60–69 [DOI] [PubMed] [Google Scholar]
- 53. Mustari MJ, Tusa RJ, Burrows AF, Fuchs AF, Livingston CA. Gaze-stabilizing deficits and latent nystagmus in monkeys with early-onset visual deprivation: role of the pretectal not. J Neurophysiol. 2001;86:662–675 [DOI] [PubMed] [Google Scholar]
- 54. Das VE, Mustari MJ. Correlation of cross-axis eye movements and motoneuron activity in non-human primates with “A” pattern strabismus. Invest Ophthalmol Vis Sci. 2007;48:665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Das VE, Fu LN, Mustari MJ, Tusa RJ. Incomitance in monkeys with strabismus. Strabismus. 2005;13:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kind PC, Mitchell DE, Ahmed B, Blakemore C, Bonhoeffer T, Sengpiel F. Correlated binocular activity guides recovery from monocular deprivation. Nature. 2002;416:430–433 [DOI] [PubMed] [Google Scholar]
- 57. Harwerth RS, Smith EL, 3rd, Duncan GC, Crawford ML, von Noorden GK. Multiple sensitive periods in the development of the primate visual system. Science. 1986;232:235–238 [DOI] [PubMed] [Google Scholar]
- 58. Harwerth RS, Smith EL, 3rd, Crawford ML, von Noorden GK. Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behav Brain Res. 1990;41:179–198 [DOI] [PubMed] [Google Scholar]
- 59. Birch EE, Stager D, Leffler J, Weakley D. Early treatment of congenital unilateral cataract minimizes unequal competition. Invest Ophthalmol Vis Sci. 1998;39:1560–1566 [PubMed] [Google Scholar]
- 60. Birch EE. Stereopsis in infants and its developmental relation to visual acuity. In: Simons K. ed. Early Development: Normal and Abnormal. Oxford, UK: Oxford University Press; 1993 [Google Scholar]
- 61. Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996;37:1532–1538 [PubMed] [Google Scholar]
- 62. Birch EE, Stager DR, Wright WW. Grating acuity development after early surgery for congenital unilateral cataract. Arch Ophthalmol. 1986;104:1783–1787 [DOI] [PubMed] [Google Scholar]
- 63. Birch EE, Stager DR. Prevalence of good visual acuity following surgery for congenital unilateral cataract. Arch Ophthalmol. 1988;106:40–43 [DOI] [PubMed] [Google Scholar]
- 64. Horton JC, Hocking DR. Timing of the critical period for plasticity of ocular dominance columns in macaque striate cortex. J Neurosci. 1997;17:3684–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]