Choroidal blood flow compensates poorly for systemic blood pressure variations in aged pigeons. The resulting choroidal blood flow abnormalities may cause defective waste removal and outer retinal oxidative and ischemic injury, thereby contributing to age-related outer retina decline.
Abstract
Purpose.
Choroidal vessels compensate for changes in systemic blood pressure (BP) so that choroidal blood flow (ChBF) remains stable over a BP range of approximately 40 mm Hg above and below basal. Because of the presumed importance of ChBF regulation for maintenance of retinal health, we investigated if ChBF compensation for BP fluctuation in pigeons fails with age.
Methods.
Transcleral laser Doppler flowmetry was used to measure ChBF during spontaneous BP fluctuation in anesthetized pigeons ranging in age from 0.5 to 17 years (pigeons can live approximately 20 years in captivity).
Results.
ChBF in <8-year-old pigeons remained near 100% of basal ChBF at BPs ranging 40 mm Hg above and below basal BP (95 mm Hg). Baroregulation failed below approximately 50 mm Hg BP. In ≥8-year-old pigeons, ChBF compensation was absent at >90 mm Hg BP, with ChBF linearly following BP. Over the 60 to 90 mm Hg range, ChBF in ≥8-year-old pigeons was maintained at 60–70% of young basal ChBF. Below approximately 55 mm Hg, baroregulation again followed BP linearly.
Conclusions.
Age-related ChBF baroregulatory impairment occurs in pigeons, with ChBF linear with above-basal BP, and ChBF failing to adequately maintain ChBF during below-basal BP. Defective autonomic sympathetic and parasympathetic neurogenic control, or defective myogenic control, may cause these baroregulatory defects. In either case, overperfusion during high BP may cause oxidative injury to the outer retina, whereas underperfusion during low BP may result in deficient nutrient supply and waste removal, with both abnormalities contributing to age-related retinal pathology and vision loss.
Basal choroidal blood flow (ChBF) declines with age in humans, and diverse mammalian and nonmammalian species,1–4 and the possibility that these declines contribute to age-related retinal dysfunction and pathology has been raised.5–7 In our work in pigeons, we have shown that ChBF declines precede and thus may contribute to photoreceptor and acuity loss.1,2 Although the basis of age-related decline in ChBF is uncertain, we have shown that age-related loss in parasympathetic choroidal innervation occurs in both pigeons and humans.2,8 Parasympathetic choroidal innervation, however, not only serves to maintain basal choroidal tone, but is also responsible for adaptive ChBF regulation. Loss of choroidal innervation may thus harm the retina by both reducing basal ChBF and impairing adaptive ChBF control.
Studies in rats, rabbits, humans, and pigeons have shown that a major choroidal regulatory process involves compensation for systemic blood pressure (BP) fluctuation, so as to maintain stable blood flow despite the BP variation.9–15 The ChBF compensation for BP fluctuations occurs over a range of ±40–50% of basal BP, and ensures appropriate choriocapillary exchange. The vascular compensation for the low choroidal perfusion pressure resulting from low systemic BP is mediated by choroidal vasodilation and prevents retinal ischemia, whereas the compensation for the high choroidal perfusion pressure resulting from high systemic BP is mediated by vasoconstriction and prevents retinal hyperoxygenation and edema.16,17 The phenomenon of blood flow stability during systemic BP variation has typically been termed autoregulation because of the view that such compensation is mediated by intrinsic vascular mechanisms. There is, however, considerable evidence that neurogenic mechanisms contribute to choroidal compensation for variation in systemic BP.9,13,14,18,19 For this reason, we use the term “baroregulation” to refer to blood flow stability during BP variation, since it is more descriptive of the phenomenon and avoids the possibly erroneous implications of the term “autoregulation.”
In the present study, we determined whether ChBF baroregulation becomes defective in pigeons as they age. To this end, we examined ChBF responses to spontaneously occurring variation in BP in anesthetized pigeons ranging in age from 0.5 to 17 years. The results indicate that ChBF in young pigeons remains near 100% of basal ChBF over an approximately ±40 mm Hg range around basal BP. Baroregulation remains intact in 2- to 7-year-old pigeons, but is impaired in ≥8-year-old pigeons. This baroregulatory defect manifests as failure to maintain ChBF at young basal levels over a 60 to 90 mm Hg range, and BP-dependent increase in ChBF at above-basal BP.
Methods
Adult White Carneaux pigeons, ranging in age from 0.5 to 17 years (n = 81), that had been housed in 12-hour light/12-hour dark diurnal lighting were used. Birds older than 2 years were provided by William Hodos of the University of Maryland, John E. R. Staddon of Duke University, and Jeffrey R. Smiley of the University of Kentucky. Other birds were obtained from Palmetto Pigeon Plant (Sumter, SC) or Bowman Gray School of Medicine (Winston-Salem, NC). All procedures were approved by the Animal Care and Use Committee of University of Tennessee Health Science Center, followed National Institutes of Health guidelines, and adhered to the ARVO Statement on Animal Research.
For our studies of young adult pigeons (<1 year), the 28 pigeons we previously used to describe avian baroregulation13 were reanalyzed, together with 22 additional young adult pigeons, to more fully assess the pigeon baroregulatory range. Twenty-two pigeons equal to or older than 8 years and 9 pigeons ranging from 2 years to younger than 8 years were newly analyzed. The oldest pigeons ranged from 8 to 17 years, with a mean of 10.9 years (±0.61 SEM), and the 2 to <8-year group had a mean age of 3.9 years (±0.60 SEM). These two age ranges were empirically determined, based on the current data. To monitor ChBF and BP, pigeons were anesthetized with ketamine (66 mg/kg, administered intraperitoneally [IP], Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (33 mg/kg IP; Butler Company, Columbus, OH), with supplemental doses every 30–40 minutes. ChBF was continuously monitored transclerally at the superior aspect of the eye by laser Doppler flowmetry using a blood perfusion monitor (Laserflo, model BPM 403A or BPM2; Vasamedics, St. Paul, MN), and systemic arterial BP (ABP) was continuously monitored via a brachial artery catheter and a blood pressure analyzer (BPA-100; MicroMed Inc., Louisville, KY), as further detailed in our prior studies.20–23 For reasons explained previously,21,23 the relative blood flow values are referred to as blood flow units (BFUs). Artificial respiration was not used, but our pulse oximetry in a subset of pigeons shows that blood oxygenation (measured at the left foot) remained at 90–95% over the typical 2- to 4-hour duration ChBF recording session, even with a 30 mm Hg drop in BP.
In one set of studies, we determined if ChBF baroregulation occurred during BP fluctuation above and below basal ABP (approximately 95 mm Hg in anesthetized pigeons) in the three pigeon age groups.13,14 Simultaneous 5-second samples of ChBF and ABP were taken at approximately 1- to 2-minute intervals over the course of a 2- to 4-hour recording session. Note that ABP and ChBF samples were taken only during periods when neither was changing rapidly (defined as <1 mm Hg/s), since different mechanisms may be involved in dynamic and static phases of baroregulation.24–26 The average ABP range sampled (i.e., difference between means for most extreme bins) for the young pigeons was 29.4 ± 2.8 mm Hg (SEM), the average ABP range sampled for the pigeons ranging in age from >1 year but <8 years was 38.6 ± 9.4 mm Hg, and the average ABP range sampled for the ≥8-year-old pigeons was 33.6 ± 5.4 mm Hg. In one of the ≥8-year-old pigeons, blood was withdrawn and reinfused to modulate BP, whereas in a second pigeon saline was infused, again to modulate BP. In two of the <1-year-old pigeons, blood was withdrawn to reduce BP. Otherwise, in these birds and all others, BP was allowed to fluctuate freely. In the BP-manipulated birds, the range of variation was no different from the average for their groups.
The basis of the spontaneous variation in BP we observed in anesthetized pigeons is uncertain, but we previously saw similar variation in anesthetized rats.14 The ABP tended to fluctuate around a midpoint in most birds, with the ABP fluctuations in some of the young and middle-aged reversed by compensatory changes in heart rate. ABP fluctuations about an apparent set point are consistent with the baroregulatory mechanisms known to exist in birds.27–31 In some individual pigeons, the ABP varied over a range much greater than 30 mm Hg. In these, ABP and heart rate typically declined toward the end of the session, perhaps as a consequence of blood loss and/or the cumulative anesthetic doses. With regard to an impact of cumulative anesthetic dose as a contributor to hypotension in some of our birds, xylazine is known to depress blood pressure in mammals and birds.32,33
In all pigeon groups, the data were organized into 10 mm Hg bins over the 90 to 150 range, and 5 mm Hg bins over the 20 to 90 range. For each animal, all ABP values within a bin were averaged to calculate the mean ABP for that bin, as were all ChBF values occurring within that same ABP range. The mean ABP for the 90 to 100 mm Hg range was taken as the basal ABP for the 50 young pigeons,22 and the mean ChBF for this same bin was defined as basal ChBF. The basal ABP and ChBF values for the 50 young pigeons were used to calculate ABP and ChBF as a percentage of basal for the young (≤1 year old), middle-aged (2–7 years old), and aged (≥8 years old) pigeons. Note that given the limited range of the spontaneous BP fluctuation in any given pigeon (approximately 30 mm Hg on average), no individual pigeon had mean ABP and ChBF values that fell within each ABP bin. For the purposes of Table 1 (young), Table 2 (middle-aged), and Table 3 (aged), the mean ABP and ChBF for each bin were based on those animals whose ABP had included values within that ABP range. The number of animals whose values fell within each bin is shown in the tables (column 2 of each table), as is the mean number of samples per animal for each ABP range (column 3 of each table). ABP and ChBF for each range are shown in the tables in mm Hg and BFUs (±SEM), as well as a percentage of basal. The data are graphed as a percentage of basal, with choroidal blood flow expressed as a percentage of young basal ChBF and ABP expressed as a percentage of young basal ABP, since this most readily depicts baroregulation. For Figures 1, 2, and 3, the 50 pigeons younger than 1 year were used to define basal ChBF (37.8 BFUs) and ABP (94.4 mm Hg), whereas for Figure 4 all 59 pigeons younger than 8 years were used to define basal ChBF (37.9 BFUs) and ABP (94.4 mm Hg). The tables show all data collected, whereas the corresponding graphs plot bins only for which we have more than two animals.
Table 1.
Baroregulation in Normal Young Pigeons (≤1 year; n = 50)
| ABP Range | Pigeons per ABP Range | Mean Samples per Pigeon | Mean ABP ± SEM per ABP Range | Mean ChBF ± SEM per ABP Range | Mean ABP per ABP Range as % of Basal ABP | Mean ChBF per ABP Range as % of Basal ChBF | ChBF Resistance per ABP Range as % of Basal |
|---|---|---|---|---|---|---|---|
| 140–150 | 1 | 1.0 | 143.9 | 41.2 | 152.3% | 79.8% | 190.9% |
| 130–140 | 5 | 22.0 | 134.9 ± 1.42 | 41.2 ± 10.08 | 143.0% | 108.8% | 131.4% |
| 120–130 | 10 | 9.3 | 125.2 ± 0.70 | 35.7 ± 3.89 | 132.7% | 94.5% | 140.4% |
| 110–120 | 13 | 10.3 | 114.1 ± 0.50 | 32.4 ± 3.26 | 120.9% | 85.5% | 141.4% |
| 100–109 | 23 | 9.6 | 103.1 ± 0.48 | 38.9 ± 3.88 | 109.3% | 102.7% | 106.5% |
| 90–100 | 29 | 13.3 | 94.4 ± 0.39 | 37.8 ± 3.32 | 100.0% | 100.0% | 100.0% |
| 85–90 | 24 | 7.9 | 86.8 ± 0.32 | 34.5 ± 2.90 | 92.0% | 91.2% | 100.9% |
| 80–85 | 33 | 9.2 | 82.4 ± 0.23 | 32.7 ± 2.51 | 87.4% | 86.4% | 101.1% |
| 75–80 | 28 | 9.7 | 77.7 ± 0.16 | 32.2 ± 3.19 | 82.3% | 87.8% | 93.8% |
| 70–75 | 28 | 5.8 | 72.5 ± 0.17 | 35.3 ± 3.52 | 76.8% | 93.2% | 82.4% |
| 65–70 | 25 | 6.1 | 67.5 ± 0.19 | 31.5 ± 3.42 | 71.5% | 83.2% | 85.9% |
| 60–65 | 19 | 4.7 | 62.6 ± 0.31 | 33.6 ± 3.11 | 66.4% | 88.7% | 74.8% |
| 55–60 | 13 | 7.8 | 57.4 ± 0.36 | 34.4 ± 4.82 | 60.8% | 90.9% | 66.9% |
| 50–55 | 8 | 9.8 | 53.0 ± 0.28 | 31.3 ± 6.75 | 56.1% | 82.7% | 67.9% |
| 45–50 | 6 | 6.7 | 47.8 ± 0.35 | 28.4 ± 7.49 | 50.7% | 75.0% | 67.6% |
| 40–45 | 4 | 3.0 | 43.2 ± 0.64 | 23.7 ± 9.71 | 45.8% | 62.7% | 73.0% |
Tabulation of choroidal blood flow (ChBF) during fluctuation in arterial blood pressure (ABP) in the 50 normal young pigeons (<12 months old) analyzed. As can be seen, ChBF remained near basal levels over the 135 to 50 mm Hg ABP range, and choroidal resistance declined linearly over this range.
Table 2.
Baroregulation in Middle-Aged Pigeons (2–7 years; n = 9)
| ABP Range | Pigeons per ABP Range | Mean Samples per Pigeon | Mean ABP ± SEM per ABP Range | Mean ChBF ± SEM per ABP Range | Mean ABP per ABP Range as % of Basal ABP | Mean ChBF per ABP Range as % of Basal ChBF | ChBF Resistance per ABP Range as % of Basal |
|---|---|---|---|---|---|---|---|
| 140–150 | 1 | 1.8 | 146.4 | 41.4 | 155.2% | 109.3% | 141.9% |
| 130–140 | |||||||
| 120–130 | |||||||
| 110–120 | 2 | 4.5 | 117.0 ± 1.91 | 32.4 ± 2.59 | 124.0% | 88.2% | 140.7% |
| 100–109 | 3 | 4.3 | 104.1 ± 0.77 | 37.0 ± 15.76 | 110.3% | 97.9% | 112.7% |
| 90–100 | 4 | 15.5 | 94.8 ± 0.65 | 38.6 ± 13.10 | 100.5% | 101.9% | 98.6% |
| 85–90 | 6 | 11.0 | 87.1 ± 0.28 | 38.8 ± 8.73 | 92.3% | 102.4% | 90.1% |
| 80–85 | 6 | 13.5 | 82.3 ± 0.19 | 41.5 ± 6.83 | 87.3% | 109.6% | 79.6% |
| 75–80 | 6 | 16.7 | 77.5 ± 0.42 | 37.2 ± 8.08 | 82.2% | 98.3% | 83.6% |
| 70–75 | 6 | 13.3 | 72.0 ± 0.46 | 35.2 ± 6.69 | 76.3% | 93.0% | 82.0% |
| 65–70 | 8 | 8.4 | 67.6 ± 0.51 | 32.8 ± 6.81 | 71.6% | 86.7% | 82.6% |
| 60–65 | 5 | 15.0 | 62.4 ± 0.53 | 36.9 ± 11.21 | 66.1% | 97.5% | 67.8% |
| 55–60 | 6 | 9.8 | 57.7 ± 0.42 | 36.7 ± 9.53 | 61.1% | 96.9% | 63.1% |
| 50–55 | 4 | 10.0 | 52.7 ± 0.37 | 40.7 ± 14.39 | 55.8% | 107.5% | 51.9% |
| 45–50 | 4 | 4.3 | 48.1 ± 0.25 | 39.7 ± 15.03 | 50.9% | 105.0% | 48.5% |
| 40–45 | 1 | 8.0 | 42.06 | 19.45 | 44.6% | 19.5% | 86.7% |
| 35–40 | 1 | 1.0 | 39.00 | 12.20 | 41.3% | 32.3% | 128.1% |
Tabulation of ChBF during fluctuation in ABP in the 9 normal pigeons analyzed in the 2- to 7-year age range. As can be seen, ChBF showed baroregulation over the 50 to 145 mm Hg ABP range, and the choroidal resistance declined linearly over this range.
Table 3.
Baroregulation in Aged Pigeons (≥8 years; n = 22)
| ABP Range | Pigeons per ABP Range | Mean Samples per Pigeon | Mean ABP ± SEM per ABP Range | Mean ChBF ± SEM per ABP Range | Mean ABP per ABP Range as % of Basal ABP | Mean ChBF per ABP Range as % of Basal ChBF | ChBF Resistance per ABP Range as % of Basal |
|---|---|---|---|---|---|---|---|
| 140–150 | 2 | 4.0 | 141.9 ± 0.42 | 47.3 ± 41.68 | 150.4% | 125.1% | 120.3% |
| 130–140 | 2 | 6.0 | 132.8 ± 0.22 | 52.8 ± 24.62 | 140.8% | 139.7% | 100.8% |
| 120–130 | 6 | 7.0 | 124.7 ± 1.25 | 45.3 ± 5.67 | 132.2% | 119.7% | 110.4% |
| 110–120 | 6 | 5.8 | 114.3 ± 1.25 | 41.2 ± 4.78 | 121.2% | 108.9% | 111.2% |
| 100–109 | 8 | 8.4 | 105.0 ± 0.87 | 29.4 ± 5.00 | 111.2% | 77.8% | 143.0% |
| 90–100 | 12 | 12.8 | 94.0 ± 0.64 | 24.0 ± 3.58 | 99.7% | 63.4% | 157.3% |
| 85–90 | 12 | 10.9 | 87.3 ± 0.33 | 27.0 ± 2.74 | 92.5% | 71.3% | 129.6% |
| 80–85 | 12 | 11.6 | 82.8 ± 0.23 | 26.2 ± 2.11 | 87.7% | 69.1% | 126.9% |
| 75–80 | 15 | 16.9 | 77.2 ± 0.21 | 25.3 ± 2.18 | 81.8% | 66.7% | 122.6% |
| 70–75 | 14 | 10.8 | 72.5 ± 0.29 | 26.3 ± 2.31 | 76.9% | 69.4% | 110.8% |
| 65–70 | 13 | 7.1 | 67.5 ± 0.36 | 30.5 ± 2.37 | 71.6% | 80.6% | 88.7% |
| 60–65 | 9 | 14.7 | 62.3 ± 0.24 | 26.7 ± 1.94 | 66.1% | 70.7% | 93.5% |
| 55–60 | 10 | 13.7 | 56.6 ± 0.58 | 26.4 ± 2.07 | 59.9% | 69.8% | 85.8% |
| 50–55 | 8 | 11.6 | 52.4 ± 0.94 | 26.1 ± 2.77 | 55.5% | 68.9% | 80.6% |
| 45–50 | 3 | 5.0 | 48.0 ± 1.30 | 23.9 ± 0.95 | 50.9% | 63.1% | 80.7% |
| 40–45 | 3 | 6.8 | 43.6 ± 0.83 | 21.0 ± 6.05 | 46.2% | 55.5% | 83.2% |
| 35–40 | 2 | 2.0 | 36.9 ± 0.34 | 20.5 ± 11.03 | 39.1% | 54.3% | 72.1% |
| 30–35 | 1 | 1.0 | 31.5 | 12.2 | 33.4% | 32.2% | 103.9% |
| 25–30 | 1 | 1.0 | 27.1 | 9.0 | 28.7% | 24.3% | 120.8% |
| 20–25 | 1 | 1.0 | 21.9 | 12.2 | 23.2% | 32.8% | 72.0% |
Tabulation of ChBF during fluctuation in ABP in the 22 normal aged pigeons (≥8 years old) analyzed. As can be seen, ChBF values linearly followed ABP increases at ABP from 90 to 140 mm Hg, and were maintained at approximately 65% of basal over the 50 to 90 mm Hg range. Choroidal resistance changes with ABP variation reflected these ChBF trends, with resistance not increasing linearly with ABP > 90, and resistance being high, but showing a linear decline with ABP from 50 to 90 mm Hg.
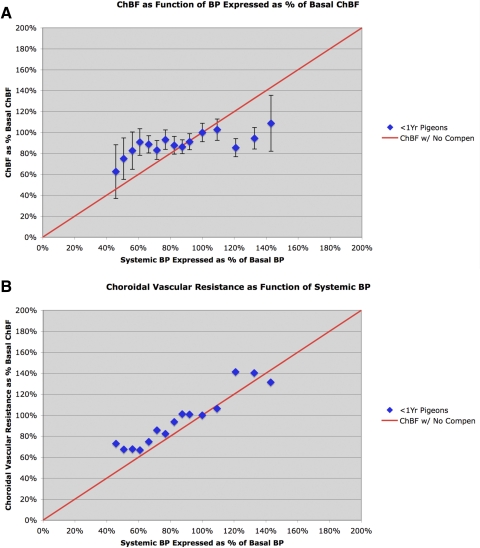
Figure 1.
Graphs showing mean ChBF (A) and choroidal resistance (B) (±SEM) graphed as a function of the corresponding ABP, over an ABP range of 40 to 135 mm Hg (45% to 145% of basal ABP) in 50 normal young pigeons less than 1 year old. The mean ChBF and ABP are graphed as a percentage of basal ChBF and ABP for the 50 <1-year-old pigeons. The red line in A shows ChBF as it would be if it linearly followed ABP (i.e., with no compensation). Note that from approximately 30% above and 40% below basal ABP, ChBF remains between 80% and 100% of basal. The red line in B shows choroidal resistance as it would be if it linearly followed ABP (i.e., with baroregulatory compensation). Note that choroidal resistance in the young pigeons decreased linearly as ABP declined from 40% above to 50% below basal ABP.
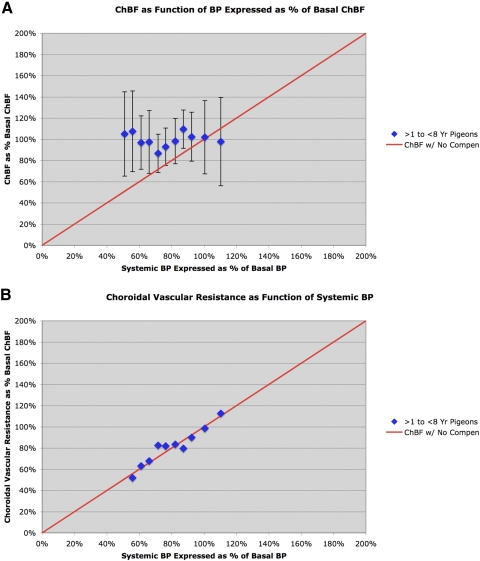
Figure 2.
Graph showing ChBF (A) and choroidal resistance (B) (±SEM) graphed as a function of the corresponding ABP, over an ABP range of 50 to 120 mm Hg (48% to 117% of basal ABP) in 9 normal young pigeons ranging in age from 2 to 7 years (mean, 3.9 years). The mean ChBF and ABP are graphed as a percentage of basal ChBF and ABP for the 50 <1 year old pigeons. The red line in A shows ChBF as it would be if it linearly followed ABP (i.e., with no compensation), whereas the red line in B shows choroidal resistance as it would be if it linearly followed ABP (i.e., with compensation). As can be seen, ChBF remained near basal levels over the 50 to 120 mm Hg ABP range, and vascular resistance (calculated from the means for each bin) linearly followed ABP over this range.
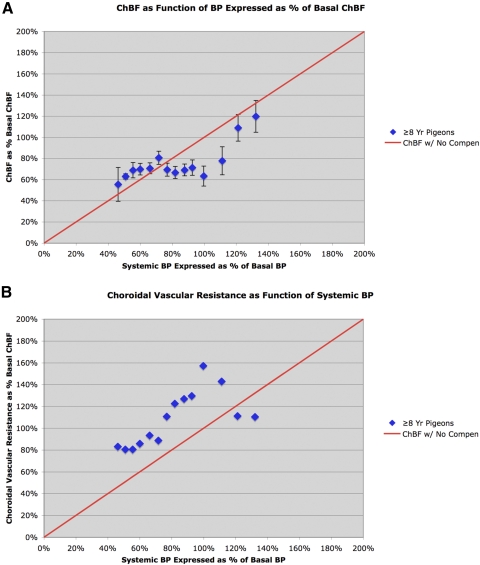
Figure 3.
Graphs showing ChBF (A) and choroidal resistance (B) (±SEM) graphed as a function of the corresponding ABP, over an ABP range of 35 to 140 mm Hg in 22 aged pigeons whose mean age was 10.9 years. The mean ChBF and ABP are graphed as a percentage of basal ChBF and ABP for the 50 <1-year-old pigeons. The red line in A shows ChBF as it would be if it linearly followed ABP, that is, with no compensation. As can be seen, ChBF in aged pigeons linearly followed ABP at >90 mm Hg, and was maintained at approximately 65% of basal ABP over the 50 to 90 mm Hg range. The red line in B shows choroidal resistance as it would be if it linearly followed ABP (i.e., with baroregulatory compensation). Graph B shows that choroidal resistance over the 50 to 90 mm Hg range was elevated, but nonetheless declined linearly with ABP. Note that choroidal vascular resistance in the aged birds was unusually high at 90 to 110 mm Hg, but insufficient for choroidal baroregulation above 110 mm Hg.
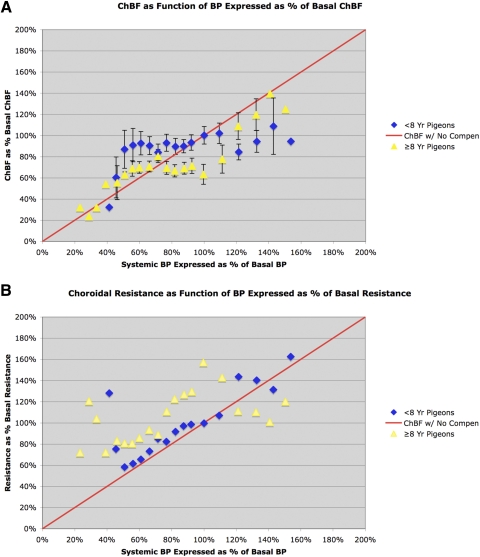
Figure 4.
Graphs comparing ChBF (A) and choroidal resistance (B) (±SEM) as a function of corresponding ABP in young pigeons (<8 years) and old pigeons (≥8 years) over an ABP range of 20 to 150 mm Hg (20% to 150% of basal ABP). The blue diamonds show the ChBF for young pigeons, whereas the yellow triangles show the ChBF for the old pigeons. The mean ChBF and ABP are graphed as a percentage of basal ChBF and ABP for all 59 <8-year-old pigeons. The green line in A shows ChBF as it would be if it linearly followed ABP (i.e., with no compensation), whereas the green line in B shows choroidal resistance as it would be if it linearly followed ABP (i.e., with baroregulatory compensation). As detailed in the text, ChBF and choroidal resistance with ABP differed significantly in the old pigeons from that in the young pigeons, and baroregulation was clearly impaired. All bins for which data were collected for ≥8-year-old birds and all bins for which we have >one bird in the <8-year-old birds are plotted in these graphs, but SEMs are shown only for bins with >two birds.
Additionally, choroidal resistance was calculated for each ABP range, by dividing mean ChBF into mean ABP for each range. For ease of interpretation, resistance is shown in the tables and figures as a percentage of basal resistance, where 100% represents basal resistance for the 90 to 100 mm Hg range. The calculated choroidal resistance is shown in the last column of each table. Note that in using ABP to estimate choroidal perfusion pressure, we did not take into account the effect of intraocular pressure (IOP) on choroidal perfusion pressure for two reasons. First, the impact of IOP changes on choroidal perfusion pressure during a ChBF recording session or across animals of different ages is likely to be small because: (1) based on published data in young adult Columba livia34 and our own measurements of 57 eyes from 0.5- to 15-year-old Columba livia, their IOP was only 13 to 15 mm Hg, and (2) IOP did not increase with age in our pigeons, nor was IOP significantly correlated with age (r = 0.100). Second, we do not know the extent to which changes in extraocular vessels feeding the pigeon choroid (i.e., the ophthalmotemporal and choroidal arteries) versus the intrachoroidal branches of these vessels contribute to changes in ChBF during ABP variation,35 and perfusion pressure in extraocular vessels would not be directly affected by IOP.
The relationship between ABP and ChBF is assessed by regression analysis, and the difference between ChBF in young and old per ABP bin by repeated-measures ANOVA (SAS Institute Inc., Cary, NC), with one-tailed Fisher's protected least significant difference test used to assess ChBF differences between ABP bins within age group or within ABP bin between age groups.
Results
Young Adult Pigeons (0.5–1 Year of Age)
In young adult pigeons, mean ChBF remained near 100% of basal ChBF over an ABP range from approximately 135 to approximately 55 mm Hg (i.e., from approximately 50% above to approximately 45% below basal ABP) (Table 1, Fig. 1A). Beginning at 55 mm Hg, ChBF linearly followed ABP and rapidly dropped below 80% of basal ChBF. These findings extend on our prior observations in two ways.13 First, they show that baroregulation in pigeons, as in mammals, extends well into the range above basal BP, as well as to a comparable extent below basal ABP. Second, they show that baroregulation in young adult pigeons (approximately 0.5 to 1 year old) maintains ChBF near (i.e., within 80–90% of) basal ChBF levels at ABPs as low as approximately 40 mm Hg below basal ABP. Statistical analysis confirms these observations, since ChBF was not significantly correlated with ABPs over the 125 to 55 mm Hg range (r = 0.3637, n = 11), and the slope of the linear regression line was flat (0.0955). Above 125 mm Hg, our sample of young birds is insufficient to statistically assess if ChBF remains flat, but the limited sample suggests it remains flat out to approximately 145 mm Hg (or 50% above basal ABP). The calculated choroidal resistance showed similar evidence of baroregulation over the 55 to 125 mm Hg range (Table 1, Fig. 1B). As confirmed by regression analysis, from 125 to 55 mm Hg, the choroidal vascular resistance linearly followed ABP (r = 0.9612, n = 11), and the slope of the linear regression line showed a nearly unitary relationship of choroidal vascular resistance with ABP (0.9999), resulting in effective ChBF compensation over this range. Below 55 mm Hg, the resistance did not decrease further (vasodilation was apparently maximal), and ChBF was thus unable to compensate for ABP increasingly below 55 mm Hg.
Middle-Aged Pigeons (2 to 7 Years of Age)
We evaluated the effects of age on choroidal baroregulation by studying pigeons equal to or older than 2 years. The data showed that baroregulation was normal in pigeons ranging in age from 2 to 7 years, but impaired in pigeons equal to or older than 8 years. We thus present data for these two groups of birds separately, beginning with the former. In birds ranging in age from 2 to 7 years, ChBF remained at the basal level typical of young pigeons from ABPs ranging from approximately 105 to approximately 50 mm Hg (Table 2, Fig. 2A). Over this range, ChBF was uncorrelated with systemic BP (r = 0.0264, n = 11), and the slope of the linear regression line was flat (0.0095). Additionally, vascular resistance over this range was highly correlated with ABP (r = 0.9656, n = 11), and the slope of the ABP correlation with vascular resistance was nearly unitary (0.9888) (Table 2, Fig. 2B). Limited data suggest baroregulation in these middle-aged pigeons was effective up to an ABP of approximately 145 mm Hg. Thus, baroregulation appears normal in pigeons through the age of 7 years, which is approximately one third into the maximum pigeon lifespan in captivity.
Old Pigeons (≥8 Years of Age)
ChBF was abnormal and its baroregulation was impaired in the oldest (≥8 years) pigeons (Table 3, Fig. 3). For example, baroregulation was seemingly absent at ABP > 90 mm Hg, since at ABP above basal (i.e., approximately 94 mm Hg) ChBF followed ABP linearly. Moreover, at ABP between 90 and 60 mm Hg, ChBF remained between 60% and 70% of the basal ChBF observed in young pigeons; thus, baroregulation over this range (while present) was inadequate for maintaining ChBF at the levels observed in young pigeons. Below an ABP of approximately 60 mm Hg, ChBF followed ABP in the ≥8-year-old pigeons, as was the case in young birds as well. Statistical analysis confirms the partial failure of choroidal baroregulation in aged pigeons, since the ChBF was significantly correlated with ABP over the 125 to 85 mm Hg range (r = 0.9316, n = 5), and the slope of the linear regression line relating ChBF and ABP was somewhat >1 (1.438), indicating that ChBF was significantly affected by ABP over the high end of the ABP range. By contrast, from approximately 85 to 55 mm Hg, ChBF was uncorrelated with ABP (r = 0.0625, n = 8), and the slope of the relationship between ChBF and ABP was flat (0.020). Thus, ChBF baroregulated over this range, although ChBF was maintained at only approximately 65% of young basal levels. Below 55 mm Hg, ChBF was again highly correlated with ABP (r = 0.934, n = 7), and the slope of the ChBF relationship to ABP was again near unitary (1.376).
The defective choroidal baroregulation was also evident from the choroidal resistance in the aged pigeons. For example, as ABP dropped from 145 to 110 mm Hg, resistance remained flat, showing no decline with declining ABP. Over the 110 to 90 mm Hg range, however, resistance increased as ABP declined. As a result, over the 125 to 95 mm Hg range, choroidal resistance was inversely correlated with ABP (r = −0.9600, n = 4), with resistance increasing as ABP decreased (slope = −1.460). Note that because resistance was >100%, ChBF over this range was lower than predicted from a one-to-one association of ChBF and ABP. By contrast, over the 90 to 55 mm Hg range, choroidal resistance steadily declined in association with ABP (r = 0.693, n = 8), with a slope approaching unitary (0.6477) (Fig. 3, Table 3). Below 55 mm Hg, choroidal resistance declined no further and was largely flat. Note that choroidal resistance below basal ABP (i.e., approximately 94 mm Hg) remained higher than that in young pigeons at the same ABPs. Thus, over this range, vasodilation was less effective in old than that in young pigeons, and accounts for the low ChBF seen in older pigeons over this range.
We used ANOVA to further assess the ChBF defect in pigeons equal to or older than 8 years over the ABP range examined. For this statistical analysis, we combined the data for all pigeons younger than 8 years (n = 59) and used two-way ANOVA with post hoc comparisons for each ABP range to assess the effects of ABP on ChBF in young and middle-aged (<8 years) and aged (≥8 years) pigeons (Fig. 4). The comparisons were carried out over the range for which we had obtained more than three samples for a given ABP bin (i.e., 50 to 130 mm Hg). ANOVA revealed an overall significant effect of age on ChBF (P = 0.0481, degrees of freedom = 78, F = 4.03). Post hoc planned comparison revealed that ChBF over the 50 to 130 ABP range in <8-year-old pigeons was not significantly different from basal ChBF (i.e., ChBF over the 90 to 100 ABP range). Thus, ChBF baroregulated over the 50 to 130 ABP range in the young pigeons. By contrast, in ≥8-year-old pigeons, ChBF over the 110 to 130 mm Hg range was significantly greater than basal ChBF at an ABP of 90 to 100 mm Hg. ChBF over the 50 to 110 mm Hg range, however, was not significantly different from the ChBF at an ABP of 90 to 100 mm Hg in ≥8-year-old pigeons. Over part of this range (70 to 100 mm Hg), ChBF in the aged birds was significantly less than that in the younger birds. Thus, comparison of the young and aged birds by ANOVA confirms that baroregulation was absent above basal ABP (approximately 95 mm Hg) in the aged birds (i.e., ChBF was above basal values), and whereas baroregulation was present at and below basal ABP in the aged birds, ChBF during systemic hypotension was lower than that in the younger birds.
Discussion
Extending on our prior findings,13 we found that ChBF in young and middle-aged pigeons (<8 years old) is maintained near 100% of basal ChBF over a blood pressure range of approximately ±45 mm Hg above and below basal BP. The baroregulation at high BP is mediated by a vasoconstriction that serves to counteract the effects of the high BP on blood flow, whereas the baroregulation at low BP is mediated by vasodilation that serves to counteract the consequences of diminished perfusion pressure. In contrast, choroidal baroregulation was defective in both the high and the low BP ranges at or beyond about halfway into the maximum pigeon lifespan. Note that pigeons in captivity live approximately 20 years, but in the wild their life expectancy is only approximately 7 years.1 Above basal ABP of approximately 95 mm Hg, baroregulation in anesthetized ≥8-year-old pigeons was absent, with ChBF increasing as ABP increased, whereas at ABP ranging from approximately 55 to 90 mm Hg ChBF was stable, but the vasodilation during systemic hypotension was able to maintain ChBF only at approximately 65% of that in young pigeons.
Both myogenic and neurogenic mechanisms have been proposed to play a role in choroidal baroregulation.10,13,14,17,19 Myogenic mechanisms involve a response of arteriolar smooth muscle to variation in stretch caused by variation in perfusion pressure, with stretch thought to open calcium channels that cause constriction of arteriole vessel walls and thereby maintain flow within a preferred range.36–38 The neurogenic contribution involves sympathetic adrenergic constriction with high systemic BP, and parasympathetic vasodilation during low systemic BP.13,14,18,39 Blood pressure dependent adaptive autonomic control of the cardiovascular system in both birds and mammals is mediated by specialized aortic and carotid receptors that detect vessel stretch and relay this information to the nucleus of the solitary tract (NTS) via the vagal and glossopharyngeal nerves, although birds appear to rely more on aortic baroreceptors than do mammals.27–31
Given that the sympathetic nervous system heavily innervates avian choroid,40 a failure of this input to vasoconstrict the choroid as ABP rose seems likely to be a contributor to the high-ABP baroregulatory defect observed in aged pigeons. This interpretation is consistent with the observation that the sympathetic nervous system prevents choroidal overperfusion during high BP in cats,39 and with the finding that the sympathetic nervous system plays a major role in cerebral blood flow compensation for high systemic blood pressure.41,42 The circuitry by which BP signals might influence the sympathetic input from the superior cervical ganglion to choroidal vessels is unknown, but presumably involves a multisynaptic circuit between the baroreceptive part of the NTS and those sympathetic preganglionic neurons of the upper thoracic spinal cord that innervate superior cervical neurons supplying choroidal blood vessels and possibly extraocular vessels that give rise to the choroidal vessels.39,40,43,44 Note that myogenic mechanisms too may play a role in ChBF compensation to high systemic BP in younger pigeons. If so, the linearity of the ABP ChBF curve at >90 mm Hg BP in ≥8-year-old pigeons suggests that both neurogenic and myogenic mechanisms contributing to choroidal baroregulation during high BP were impaired in our older pigeons.
The circuitry of the parasympathetic choroidal innervation is consistent with a neurogenic contribution to choroidal baroregulation during below-basal BP in birds and mammals. The autonomic subdivision of the facial nucleus in birds and mammals, termed the superior salivatory nucleus (SSN), provides preganglionic input to the pterygopalatine ganglion (PPG),17,35,45 which dilates choroidal vessels using nitric oxide and vasoactive intestinal polypeptide.39,46,47 The choroidal preganglionic neurons of the SSN receive input from the baroreceptive part of the NTS in both birds and mammals.18,30,43,48–53 Our observation that baroregulation in pigeons failed below approximately 60 mm Hg and the evidence that baroreceptors unload at this ABP in rats54 are consistent with a role of the NTS SSN PPG circuit in choroidal baroregulation during low BP.
We previously observed that nitric oxide synthase inhibition in young pigeons impairs the ability of ChBF to compensate for low BP (i.e., <95 mm Hg) caused by blood withdrawal.14 The present results show that residual baroregulatory mechanisms in ≥8-year-old pigeons are present over the 60 to 90 mm Hg range, but insufficient to keep ChBF at >70% of basal levels. It may be that a defect in the functioning of the parasympathetic nitrergic input to choroid underlies the deficient baroregulation in the hypotensive BP range. It is also possible, however, that deficient myogenic control contributes to the deficient choroidal baroregulation in the hypotensive BP range. In either event, the fact that ChBF is maintained at approximately 70% of basal over the 60 to 90 mm Hg range suggests that neurogenic or myogenic mechanisms remain somewhat functional in the ≥8-year-old birds.
The basis of the exaggerated vascular resistance over the 90 to 110 mm Hg ABP range in ≥8-year-old pigeons is uncertain. It is possible that there may normally be interplay between vasoconstrictive and vasodilatory mechanisms over this range, with increasing vasodilatory action normally serving to increase flow as BP drops, by counteracting residual sympathetic vasoconstrictive action. In the aged pigeons, the increased resistance at and just above basal ABP thus may stem from a combination of increased choroidal vascular resistance over the 90 to 150 ABP range in the older birds and a failure of normal vasodilation over the 90 to 110 mm Hg ABP range.
In principle, the defects in choroidal vasoconstriction and vasodilation in aged pigeons could stem from a defect at the level of the vessel smooth muscle, a defect in autonomic nerve function, or a defect in the baroreceptive signaling pathways from the aortic baroreceptors to the NTS to the preganglionic neurons controlling sympathetic vasoconstriction and parasympathetic vasodilation. Baroreceptor and baroreflex functions decline with age in humans and animals, especially in the range around basal BP,55–58 as does the ability of smooth muscle to relax and produce vascular dilation in response to vasodilators and in response to myogenic signals.58 Moreover, diminished PPG parasympathetic and/or superior cervical sympathetic input to the choroidal and cerebral vasculature have been demonstrated in several species,8,59 as has impaired hypertensive choroidal baroregulation in patients with age-related macular degeneration (AMD).60
We have previously shown that regulation of ChBF by a second parasympathetic circuit is impaired as pigeons age. That circuit involves the nucleus of Edinger Westphal input to ciliary ganglion neurons that innervate choroid, and the circuit appears to mediate ChBF increases as a function of retinal activity.20,21 The age-related dysfunction in this circuit precedes the major age-related photoreceptor and acuity losses that occur in pigeons, and thus may be causal to them.1,2 Our present data show that an age-related defect also develops in pigeons in the mechanisms that hold ChBF steady as systemic blood pressure rises or falls. ChBF baroregulation prevents overperfusion-related edema and oxidative injury during above-basal ABP, and underperfusion-related ischemia in the retina during low ABP.10,16,17 Blood pressure recorded telemetrically has been reported to vary by as much as 100 mm Hg in quiescent turkeys, and vary even more with activity.61 Physical activity is known to greatly increase ABP in pigeons (as in other animals),62 and based on the above-cited data in turkeys and on telemetric data in rodents,61,63–65 ABP is likely to fluctuate during the course of the day even in quiescent caged pigeons over a relatively broad range, with BP lower during the inactive night period than that during the active day period. Given then such daily BP variation, the defect in baroregulation in aged pigeons over the course of a day would yield many episodes of overperfusion in the high ABP range and underperfusion in the low ABP range. Such episodes are likely to harm the retina, based on published findings66 and our own data (FitzGerald PG, et al. IOVS 2010;51:ARVO E-Abstract 6323) showing that surgical disruption of sympathetic or parasympathetic control of ChBF in rats both harm the retina.
Age-related declines in PPG parasympathetic innervation of choroid occur in humans,8 which might impair choroidal vasodilation as BP drops below basal levels. Since modest reductions in choroidal blood flow (as achieved by raising IOP) or in its oxygen content adversely affect retinal metabolism and function,67–70 impaired baroregulatory choroidal vasodilation is likely to acutely affect retinal function during low BP. Moreover, a chronic defect of this nature could cause ischemic oxidative injury to retinal pigmented epithelium (RPE) cells and impair transport between retina and choroid, possibly leading to the waste accumulation in and along Bruch's membrane seen in normal aging retina.71–73 In more severe cases, the sub-RPE debris may take the form of the basal linear deposits and drusen in Bruch's membrane seen in AMD. The accumulation of such waste is thought to trigger the inflammatory response that is the proximate cause of the severe RPE and photoreceptor death in AMD, given pro-AMD genetic predispositions in the alternate complement cascade or lipid metabolism.5–7 Defective baroregulation in the high BP range would further exacerbate outer retinal injury by causing hyperoxygenation, promoting accumulation of oxidized lipids waste products such as the carboxyethylpyrrole adducts formed by oxidative damage to docosahexanoic acid.74 Defective aortic baroreceptor function that would cause impaired neurogenic ChBF baroregulation develops with age, smoking, hypertension, and diabetes.57,75–77 Consistent with this, ChBF baroregulation is impaired in human smokers,78 and profound declines in ChBF, as well as in its baroregulation, occur in AMD, with the ChBF declines increasing in severity with AMD severity.60,79–82 Thus, impaired ChBF baroregulation may be an underlying commonality that makes age, smoking, hypertension, and diabetes nongenetic risk factors for AMD.6 Better understanding of the occurrence of deficient ChBF baroregulation in humans and its basis might thus be important for addressing the adverse impact of age, smoking, hypertension, and diabetes on retinal health.
Acknowledgments
The authors thank Seth V. Jones, Toya Kimble, Sherry L. Cuthbertson, Julia Jones, Rebeca-Ann Weinstock, Amanda Taylor, Marion Joni, Ting Wong, and Aminah Henderson for their invaluable technical assistance; William Hodos for providing some of the older pigeons used here and for helpful discussion on pigeon aging; Yunping Deng for assistance with statistical analysis; and Chris Murphy and David Hamilton for their discussion and input on avian physiology.
Footnotes
Supported in part by National Institutes of Health Grants NIH-EY-05298 (AR), NIH/NEI-5P30EY013080, and NIH-AG-10538 (MECF); the University of Tennessee Neuroscience Institute (CL); and Department of Ophthalmology, University of Tennessee Health Science Center unrestricted grant from Research to Prevent Blindness (MECF).
Disclosure: A. Reiner, None; N. Del Mar, None; Y. Zagvazdin, None; C. Li, None; M.E.C. Fitzgerald, None
References
- 1. Fitzgerald ME, Tolley E, Frase S, et al. Functional and morphological assessment of age-related changes in the choroid and outer retina in pigeons. Vis Neurosci. 2001;18:299–317 [DOI] [PubMed] [Google Scholar]
- 2. Fitzgerald ME, Tolley E, Jackson B, et al. Anatomical and functional evidence for progressive age-related decline in parasympathetic control of choroidal blood flow in pigeons. Exp Eye Res. 2005;81:478–491 [DOI] [PubMed] [Google Scholar]
- 3. Grunwald JE, Hariprasad SM, DuPont J. Effect of aging on foveolar choroidal circulation. Arch Ophthalmol. 1998;116:150–154 [DOI] [PubMed] [Google Scholar]
- 4. Ito YN, Mori K, Young-Duvall J, Yoneya S. Aging changes of the choroidal dye filling pattern in indocyanine green angiography of normal subjects. Retina. 2001;21:237–242 [DOI] [PubMed] [Google Scholar]
- 5. Feigl B. Age-related maculopathy: linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog Retin Eye Res. 2009;28:63–86 [DOI] [PubMed] [Google Scholar]
- 6. Hageman GS, Gehrs K, Johnson LV, Anderson D. Age-related macular degeneration (AMD). May be accessed at http://webvision.med.utah.edu/Hagerman.html; 2009 [PubMed]
- 7. Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:Art. 32 [PMC free article] [PubMed] [Google Scholar]
- 8. Jablonski MM, Iannaccone A, Reynolds DH, et al. Age-related decline in VIP-positive parasympathetic nerve fibers in the human submacular choroid. Invest Ophthalmol Vis Sci. 2007;48:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiel JW. Modulation of choroidal autoregulation in the rabbit. Exp Eye Res. 1999;69:413–429 [DOI] [PubMed] [Google Scholar]
- 10. Kiel JW, Shepherd AP. Autoregulation of choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1992;33:2399–2410 [PubMed] [Google Scholar]
- 11. Kiel JW, van Heuven WA. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1995;36:579–585 [PubMed] [Google Scholar]
- 12. Lovasik JV, Kergoat H, Riva CE, Petrig BL, Geiser M. Choroidal blood flow during exercise-induced changes in the ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2003;44:2126–2132 [DOI] [PubMed] [Google Scholar]
- 13. Reiner A, Zagvazdin Y, Fitzgerald ME. Choroidal blood flow in pigeons compensates for decreases in arterial blood pressure. Exp Eye Res. 2003;76:273–282 [DOI] [PubMed] [Google Scholar]
- 14. Reiner A, Li C, Del Mar N, Fitzgerald MEC. Choroidal blood flow compensation in rats for arterial blood pressure decreases is neuronal nitric oxide-dependent but compensation for arterial blood pressure increases is not. Exp Eye Res. 2010;90:734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riva CE, Titze P, Hero M, Movaffaghy A, Petrig BL. Choroidal blood flow during isometric exercises. Invest Ophthalmol Vis Sci. 1997;38:2338–2343 [PubMed] [Google Scholar]
- 16. Johnson PC. The myogenic response. In: Bohr DF, Somlyo AP, Sparks HV., Jr eds. Handbook of Physiology, Section 2: The Cardiovascular System. Vol. II. Vascular Smooth Muscle. Bethesda, MD: American Physiological Society; 1980:409–442 [Google Scholar]
- 17. Kiel JW. Choroidal myogenic autoregulation and intraocular pressure. Exp Eye Res. 1994;58:529–543 [DOI] [PubMed] [Google Scholar]
- 18. Cuthbertson S, LeDoux MS, Jones S, et al. Localization of preganglionic neurons that innervate choroidal neurons of pterygopalatine ganglion. Invest Ophthalmol Vis Sci. 2003;44:3713–3724 [DOI] [PubMed] [Google Scholar]
- 19. Hardy P, Lamireau D, Hou X, et al. Major role for neuronal nitric oxide synthase in curtailing choroidal blood flow autoregulation in newborn pigs. J Appl Physiol. 2001;91:1655–1662 [DOI] [PubMed] [Google Scholar]
- 20. Fitzgerald MEC, Vana BA, Reiner A. Control of choroidal blood flow by the nucleus of Edinger-Westphal in pigeons: a laser Doppler study. Invest Ophthalmol Vis Sci. 1990;31:2483–2492 [PubMed] [Google Scholar]
- 21. Fitzgerald MEC, Gamlin PD, Zagvazdin Y, Reiner A. Central neural circuits for the light-mediated reflexive control of choroidal blood flow in the pigeon eye: a laser Doppler study. Vis Neurosci. 1996;13:655–669 [DOI] [PubMed] [Google Scholar]
- 22. Zagvazdin YS, Fitzgerald MEC, Sancesario G, Reiner A. Neural nitric oxide mediates Edinger-Westphal nucleus evoked increase in choroidal blood flow in the pigeon. Invest Ophthalmol Vis Sci. 1996;37:666–672 [PubMed] [Google Scholar]
- 23. Zagvazdin Y, Fitzgerald MEC, Reiner A. Role of muscarinic cholinergic transmission in Edinger-Westphal nucleus-induced choroidal vasodilation in pigeon. Exp Eye Res. 2000;70:315–327 [DOI] [PubMed] [Google Scholar]
- 24. Blaber AP, Bondar RL, Stein F, et al. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke. 1997;28:1686–1692 [DOI] [PubMed] [Google Scholar]
- 25. Panerai RB, White RP, Markus HS, Evans DH. Grading of cerebral dynamic autoregulation from spontaneous fluctuations in arterial blood pressure. Stroke. 1998;29:2341–2346 [DOI] [PubMed] [Google Scholar]
- 26. Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol. 1998;274:H233H241. [DOI] [PubMed] [Google Scholar]
- 27. Abdel-Magied EM, Taha AAM, King AS. An ultrastructural investigation of a baroreceptor zone in the common carotid artery of the domestic fowl. J Anat. 1982;135:463–475 [PMC free article] [PubMed] [Google Scholar]
- 28. Taha AAM, Abdel-Magied EM, King AS. Ultrastructure of aortic and pulmonary baroreceptors in the domestic fowl. J Anat. 1983;137:197–207 [PMC free article] [PubMed] [Google Scholar]
- 29. Bagshaw RJ, Cox RH. Baroreceptor control of heart rate in chickens (Gallus domesticus). Am J Vet Res. 1986;47:293–295 [PubMed] [Google Scholar]
- 30. Smith FM. Blood pressure regulation by aortic baroreceptors in birds. Physiol Zool. 1994;67:1402–1425 [Google Scholar]
- 31. Lucitti JL, Hedrick MS. Characterization of baroreflex gain in the domestic pigeon (Columba livia). Comp Biochem Physiol A Mol Integr Physiol. 2006;143:103–111 [DOI] [PubMed] [Google Scholar]
- 32. Ludders JW, Rode J, Mitchell GS, Nordheim EV. Effects of ketamine, xylazine, and a combination of ketamine and xylazine in Pekin ducks. J Vet Res. 1989;50:245–249 [PubMed] [Google Scholar]
- 33. Mostachio GQ, de-Oliveira LD, Carciofi AC, Vicente WR. The effects of anesthesia with a combination of intramuscular xylazine-diazepam-ketamine on heart rate, respiratory rate and cloacal temperature in roosters. Vet Anaesth Analg. 2008;35:232–236 [DOI] [PubMed] [Google Scholar]
- 34. Korbel R. Tonometrie am Vogelauge. DVG-Tagung Vogelkrankheiten, München. 1992;VIII:281–291 [Google Scholar]
- 35. Cuthbertson S, Jackson B, Toledo C, et al. Innervation of orbital and choroidal blood vessels by the pterygopalatine ganglion in pigeons. J Comp Neurol. 1997;386:422–442 [PubMed] [Google Scholar]
- 36. Faraci FM, Baumbach GL, Heistad DD. Myogenic mechanisms in the cerebral circulation. J Hypertens Suppl. 1989;7:S61–S65 [PubMed] [Google Scholar]
- 37. Kolb B, Rotella DL, Stauss HM. Frequency response characteristics of cerebral blood flow autoregulation in rats. Am J Physiol Heart Circ Physiol. 2007;292:H432–H438 [DOI] [PubMed] [Google Scholar]
- 38. Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol Heart Circ Physiol. 1992;263:H647–H659 [DOI] [PubMed] [Google Scholar]
- 39. Bill A. Some aspects of the ocular circulation. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1985;26:410–424 [PubMed] [Google Scholar]
- 40. Guglielmone R, Cantino D. Autonomic innervation of the ocular choroid membrane in the chicken: a fluorescence-histochemical and electron-microscopic study. Cell Tissue Res. 1982;222:417–431 [DOI] [PubMed] [Google Scholar]
- 41. Gotoh F, Tanaka K. Regulation of cerebral blood flow. In: Bruyn GW, Vinken PJ. eds. Handbook of Clinical Neurology. Amsterdam: Elsevier; 1988:47–77 [Google Scholar]
- 42. Morita Y, Hardebo JE, Bouskela E. Influence of cerebrovascular sympathetic, parasympathetic, and sensory nerves on autoregulation and spontaneous vasomotion. Acta Physiol Scand. 1995;154:121–130 [DOI] [PubMed] [Google Scholar]
- 43. Katz DM, Karten HJ. Visceral representation within the nucleus of the tractus solitarius in the pigeon, Columba livia. J Comp Neurol. 1983;218:42–73 [DOI] [PubMed] [Google Scholar]
- 44. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346 [DOI] [PubMed] [Google Scholar]
- 45. Schrödl F, Brehmer A, Neuhuber WL, Nickla D. The autonomic facial nerve pathway in birds: a tracing study in chickens. Invest Ophthalmol Vis Sci. 2006;47:3225–3233 [DOI] [PubMed] [Google Scholar]
- 46. Nilsson SF. Nitric oxide as a mediator of parasympathetic vasodilation in ocular and extraocular tissues in the rabbit. Invest Ophthalmol Vis Sci. 1996;37:2110–2119 [PubMed] [Google Scholar]
- 47. Nilsson SF. The significance of nitric oxide for parasympathetic vasodilation in the eye and other orbital tissues in the cat. Exp Eye Res. 2000;70:61–72 [DOI] [PubMed] [Google Scholar]
- 48. Agassandian K, Fazan VP, Margaryan N, Dragon DN, Riley J, Talman WT. A novel central pathway links arterial baroreceptors and pontine parasympathetic neurons in cerebrovascular control. Cell Mol Neurobiol. 2003;23:463–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arends JJ, Wild JM, Zeigler HP. Projections of the nucleus of the tractus solitarius in the pigeon (Columba livia). J Comp Neurol. 1988;278:405–429 [DOI] [PubMed] [Google Scholar]
- 50. Ciriello J. Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neurosci Lett. 1983;36:37–42 [DOI] [PubMed] [Google Scholar]
- 51. Rogers RF, Paton JF, Schwaber JS. NTS neuronal responses to arterial pressure and pressure changes in the rat. Am J Physiol Regul Integr Comp Physiol. 1993;265:R1355–R1368 [DOI] [PubMed] [Google Scholar]
- 52. Jaccoby S, Koike TI, Cornett LE. c-fos expression in the forebrain and brainstem of White Leghorn hens following osmotic and cardiovascular challenges. Cell Tissue Res. 1999;297:229–239 [DOI] [PubMed] [Google Scholar]
- 53. Steinle JJ, Krizsan-Agbas D, Smith PG. Regional regulation of choroidal blood flow by autonomic innervation in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R202–R209 [DOI] [PubMed] [Google Scholar]
- 54. Brown AM. Receptors under pressure. An update on baroreceptors. Circ Res. 1980;46:1–10 [DOI] [PubMed] [Google Scholar]
- 55. Brown CM, Hecht MJ, Weih A, Neundorfer B, Hilz MJ. Effects of age on the cardiac and vascular limbs of the arterial baroreflex. Eur J Clin Invest. 2003;33:10–16 [DOI] [PubMed] [Google Scholar]
- 56. Andresen MC. Short- and long-term determinants of baroreceptor function in aged normotensive and spontaneously hypertensive rats. Circ Res. 1984;54:750–759 [DOI] [PubMed] [Google Scholar]
- 57. Hajduczok G, Chapleau MW, Johnson SL, Abboud FM. Increase in sympathetic activity with age. I. Role of impairment of arterial baroreflexes. Am J Physiol Heart Circ Physiol. 1991;260:H1113–H1120 [DOI] [PubMed] [Google Scholar]
- 58. Marin J. Age-related changes in vascular responses: a review. Mech Ageing Dev. 1995;79:71–114 [DOI] [PubMed] [Google Scholar]
- 59. Omar NM, Marshall JM. Age-related changes in the sympathetic innervation of cerebral vessels and in carotid vascular responses to norepinephrine in the rat: in vitro and in vivo studies. J Appl Physiol. 2010;109:314–322 [DOI] [PubMed] [Google Scholar]
- 60. Pournaras CJ, Logean E, Riva CE, et al. Regulation of subfoveal choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:1581–1586 [DOI] [PubMed] [Google Scholar]
- 61. Krista LM, Beckett SD, Branch CE, McDaniel GR, Patterson RM. Cardiovascular responses in turkeys as affected by diurnal variation and stressors. Poult Sci. 1981;60:462–468 [DOI] [PubMed] [Google Scholar]
- 62. Butler PJ, West NH, Jones DR. Respiratory and cardiovascular responses of the pigeon to sustained, level flight in a wind-tunnel. J Exp Biol. 1977;71:7–26 [Google Scholar]
- 63. van den Brandt J, Kovács P, Klöting I. Blood pressure, heart rate and motor activity in 6 inbred rat strains and wild rats (Rattus norvegicus): a comparative study. Exp Anim. 1999;48:235–240 [DOI] [PubMed] [Google Scholar]
- 64. Curtis KS, Krause EG, Contreras RJ. Cardiovascular function and circadian patterns in rats after area postrema lesions or prolonged food restriction. Neurosci Lett. 2003;350:46–50 [DOI] [PubMed] [Google Scholar]
- 65. Basset A, Laude D, Laurent S, Elghozi JL. Contrasting circadian rhythms of blood pressure among inbred rat strains: recognition of dipper and non-dipper patterns. J Hypertens. 2004;22:727–737 [DOI] [PubMed] [Google Scholar]
- 66. Steinle JJ, Lindsay NL, Lashbrook BL. Cervical sympathectomy causes photoreceptor-specific cell death in the rat retina. Auton Neurosci. 2005;120:46–51 [DOI] [PubMed] [Google Scholar]
- 67. Steinberg RH. Monitoring communications between photoreceptors and pigment epithelial cells: effects of “mild” systemic hypoxia. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1987;28:1888–1904 [PubMed] [Google Scholar]
- 68. Yancey CM, Linsenmeier RA. The electroretinogram and choroidal PO2 in the cat during elevated intraocular pressure. Invest Ophthalmol Vis Sci. 1988;29:700–707 [PubMed] [Google Scholar]
- 69. Yancey CM, Linsenmeier RA. Oxygen distribution and consumption in the cat retina at increased intraocular pressure. Invest Ophthalmol Vis Sci. 1989;30:600–611 [PubMed] [Google Scholar]
- 70. Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208 [DOI] [PubMed] [Google Scholar]
- 71. Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339 [DOI] [PubMed] [Google Scholar]
- 72. Curcio CA, Johnson M, Huang JD, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009;28:393–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tso MOM. Photic injury to the retina and pathogenesis of age-related macular degeneration. In: Tso MOM. ed. Retinal Diseases: Biomedical Foundations and Clinical Management. Philadelphia: Lippincott; 1988:187–214 [Google Scholar]
- 74. Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation, and outer retinal disease. Invest Ophthalmol Vis Sci. 2010;51:1276–1281 [DOI] [PubMed] [Google Scholar]
- 75. Brown AM, Saum WR, Tuley FH. A comparison of aortic baroreceptor discharge in normotensive and spontaneously hypertensive rats. Circ Res. 1976;39:488–496 [DOI] [PubMed] [Google Scholar]
- 76. Do Carmo JM, Huber DA, Castania JA, Fazan VP, Fazan R, Jr, Salgado HC. Aortic depressor nerve function examined in diabetic rats by means of two different approaches. J Neurosci Methods. 2007;161:17–22 [DOI] [PubMed] [Google Scholar]
- 77. Fazan VPS, Salgado HC, Bareira AA. Aortic depressor nerve myelinated fibers in acute and chronic experimental diabetes. Am J Hypertens. 2006;19:153–160 [DOI] [PubMed] [Google Scholar]
- 78. Wimpissinger B, Resch H, Berisha F, Weigert G, Polak K, Schmetterer L. Effects of isometric exercise on subfoveal choroidal blood flow in smokers and nonsmokers. Invest Ophthalmol Vis Sci. 2003;44:4859–4863 [DOI] [PubMed] [Google Scholar]
- 79. Ciulla TA, Harris A, Martin BJ. Ocular perfusion and age-related macular degeneration. Acta Ophthalmol Scand. 2001;79:108–115 [DOI] [PubMed] [Google Scholar]
- 80. Friedman E, Krupsky S, Lane AM, et al. Ocular blood flow velocity in age-related macular degeneration. Ophthalmology. 1995;102:640–646 [DOI] [PubMed] [Google Scholar]
- 81. Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46:1033–1038 [DOI] [PubMed] [Google Scholar]
- 82. Metelitsina TI, Grunwald JE, DuPont JC, Ying GS, Brucker AJ, Dunaief JL. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]