The response of uveal melanoma cells to inhibition of B-Raf, MEK, and Akt depends on their genotype. These observations support the design of clinical trials of these inhibitors for the treatment of carefully selected UM patients and provide a rationale for personalized therapy.
Abstract
Purpose.
Inhibitors of B-Raf and MEK kinases hold promise for the management of cutaneous melanomas harboring BRAF mutations. BRAF mutations are rare in uveal melanomas (UMs), but somatic mutations in the G protein α subunits Gαq and Gα11 (encoded by GNAQ and GNA11, respectively) occur in a mutually exclusive pattern in ∼80% of UMs. The impact of B-Raf and MEK inhibitors on Gα-mutant UMs remains unknown.
Methods.
The impact of the B-Raf inhibitor PLX4720, the MEK inhibitor AZD6244, and the Akt inhibitor MK2206 on UM cell lines was assessed with the use of cell viability, proliferation, and apoptosis assays and immunoblot analysis.
Results.
BRAF-mutant UM cells were sensitive to both PLX4720 and AZD6244, undergoing cell cycle arrest but not apoptosis. UM cells with a Gα-protein mutation (GNAQ or GNA11) were mildly sensitive to AZD6244 but completely resistant to PLX4720. In fact, PLX4720 paradoxically increased ERK phosphorylation in Gα-mutant UM cells. The combination of AZD6244 with PLX4720 had synergistic anticancer activity in BRAF-mutant cells but not in Gα-mutant cells. The Akt inhibitor MK2206 sensitized BRAF-mutant cells to both PLX4720 and AZD6244 and sensitized Gα-mutant cells to AZD6244 but did not overcome the resistance of the Gα-mutant cells to PLX4720.
Conclusions.
The response of UM cells to inhibition of B-Raf, MEK, and Akt depends on their genotype. Future use of such targeted therapies in clinical trials of UM patients will require careful design and patient selection based on genotype to provide personalized and effective therapy.
Somatic activating mutations in the RAS/RAF/MEK/ERK signaling pathway are frequent in cutaneous melanomas (CMs), with 50% to 70% of them harboring BRAF mutations (usually the V600E substitution).1 Mutations in RAS genes occur in approximately another 20% to 30% of CMs (most frequently in NRAS) and are usually mutually exclusive with BRAFV600E.2–6 BRAFV600E, the most frequent oncogenic protein kinase mutation known, activates the MEK/ERK cascade and represents a promising therapeutic target for melanomas and for thyroid, colon, and ovarian carcinomas and other malignancies harboring this mutation.1,7–10 Kinase inhibitors targeting B-Raf (in particular the V600E mutant), including PLX4720 (Plexxikon Inc., Berkeley, CA)11 and the related PLX4032 (vemurafenib, RG7204), are in clinical development.12 Preclinical and clinical evidence suggests that these B-Raf inhibitors suppress ERK phosphorylation and induce cell cycle arrest and apoptosis in BRAFV600E-bearing CM cells, whereas in RAS-mutant/BRAF wild-type CM cells they can paradoxically enhance ERK phosphorylation and promote cell proliferation through a cRaf-mediated mechanism.11,13–18 In phase 1 and 2 clinical trials of PLX4032 in patients with metastatic CM, complete or partial tumor regression was observed in the majority of patients with a BRAFV600E tumor.12,19 In a phase 3 trial of patients with advanced-stage CM with BRAFV600E mutations who were randomly assigned to PLX4032 or dacarbazine, the hazard ratios for overall survival and progression-free survival were 0.37 and 0.26, respectively, both favoring PLX4032.20 Therefore, B-Raf inhibitors are very promising targeted therapeutics specifically for BRAFV600E CMs and careful patient selection is crucial.12,20 However, it should be emphasized that, even in BRAFV600E CMs, clinical responses with BRAF inhibitors are usually short-lived because of the emergence of compensatory oncogenic signaling pathways.21–24
Furthermore, preclinical evidence suggests that BRAFV600E CMs are highly sensitive to MEK inhibition, whereas CMs with a wild-type BRAF/mutant NRAS status exhibit variable and usually lower sensitivity and those that are wild-type for both BRAF and NRAS are uniformly resistant to MEK inhibition.25 These data again confirm the “oncogenic addiction” of BRAF-mutant CM cells to this activated pathway and provide another therapeutic method for targeting it in patients with metastatic CMs. Clinical trials of MEK inhibitors such as AZD6244 (AstraZeneca, Wilmington, DE) in BRAFV600E tumors are ongoing.
However, in uveal melanomas (UMs), BRAFV600E mutations are rare.26–28 Instead, somatic mutations in the G protein α subunits Gαq and Gα11 (encoded by GNAQ and GNA11, respectively)29 are present in a mutually exclusive pattern in ∼80% of UMs. Gαq and Gα11 are 90% homologous and transmit signals between G-protein–coupled receptors and downstream effectors. Their mutations occur most commonly in exon 5, affecting codon Q209 (for both proteins)30,31 in their Ras-like domain, abolishing their GTPase activity in a manner similar to that for the NRASQ61R mutation, and resulting in a constitutively active Gα protein that functions as a bona fide oncogene.30,31 A second hot spot for mutations has been discovered in exon 4, affecting codon R183 (for both proteins).31 The presence of these mutations in tumors at all stages of malignant progression suggests that they are early events in UM.32 However, the sensitivity of Gα-mutant UM cells to the B-Raf and MEK inhibitors currently undergoing clinical development for the management of CM remains unknown.
We investigated the impact of B-Raf and MEK inhibition on UM cell lines using the small molecule inhibitors PLX4720 and AZD6244, respectively, either as monotherapy or in combination with each other and the Akt inhibitor MK2206 (Merck, North Wales, PA).33–35 We found that the BRAF-mutant UM cells behave similarly to their cutaneous counterparts, with high sensitivity to inhibition of either B-Raf or MEK that can be further enhanced by concurrent Akt inhibition. However, Gα-mutant UM cells are less sensitive to MEK inhibition (but can be further sensitized by concurrent Akt inhibition) and completely resistant to B-Raf inhibition (even in the presence of the Akt inhibitor). In fact, the B-Raf inhibitor PLX4720 paradoxically increased ERK phosphorylation in Gα-mutant UM cells. Our data demonstrate that the response of UM cells to the inhibition of B-Raf and MEK is genotype dependent. Future use of targeted therapies in clinical trials of UM patients will require careful design and patient selection based on genotype to provide personalized and effective therapy.
Materials and Methods
Cell Lines and Tissue Culture
The genotype of OMM1.3 and Mel202 UM cells (both GNAQ-mutant at Q209 and BRAF-wt) has been previously reported.30 The OMM1 UM cells carry a GNA11 Q209 mutation and are wild-type for BRAF and GNAQ. The OCM3 (BRAFV600E/GNAQ-wt/GNA11-wt) UM cell line and the A375 and M14 (both BRAFV600E/GNAQ-wt/GNA11-wt) CM cell lines were also used. All cell lines examined in this study were carefully genotyped by Sanger sequencing using a cycle sequencing kit (BigDye Terminator v1.1; Applied Biosystems, Foster City, CA) and a genetic analyzer (3130; Applied Biosystems) for GNAQ exons 4 and 5, GNA11 exons 4 and 5, and BRAF exon 15, and their mutation status was confirmed (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7398/-/DCSupplemental). Overall, BRAF, GNAQ, and GNA11 mutations are mutually exclusive. All cells were grown in DMEM/F12 (Invitrogen, Carlsbad, CA) with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% FBS (Invitrogen).
MTT, Proliferation, and Apoptosis Assays
The B-Raf inhibitor PLX4720, the MEK inhibitor AZD6244, and the Akt inhibitor MK2206 were reconstituted in dimethyl sulfoxide (DMSO) and were stored at −20°C until use. Cells were plated in 24-well plates in medium containing 10% FBS. Drugs were added 24 hours later, and the cells were incubated for 96 more hours. Cell numbers were quantified by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO) as described previously,8 and were expressed as a percentage of the value of control wells. In parallel experiments, the proliferation rate in cells treated with AZD6244 or PLX4720 was quantified by measurement of the amount of BrdU incorporated into DNA (BrdU Cell Proliferation Assay; Calbiochem, San Diego, CA) according to the instructions of the manufacturer and as previously performed.36
Apoptosis was detected in UM cells treated with AZD6244 or PLX4720 for 72 hours using the terminal dUTP nick-end labeling (TUNEL) method with a cell death detection kit (In Situ Cell Death Kit, Fluorescein; Roche Applied Science, Indianapolis, IN), in accordance with the instructions of the manufacturer, and were run on a flow cytometer (BD FACSCanto II; BD Biosciences, San Jose, CA). Flow cytometry software (BD FACSDiva; BD Biosciences) was used to analyze the data.
As a positive control for the induction of apoptosis, treatment with bortezomib (100 nM for 72 hours) was used. The pan-caspase inhibitor ZVAD-FMK was purchased from Calbiochem and used at 20 μM.
Immunoblot Analysis
Immunoblot analysis was performed as previously described.8 The following antibodies were purchased from Cell Signaling (Danvers, MA): phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) rabbit monoclonal antibody; p27 Kip1 (SX53G8.5) mouse monoclonal antibody; cyclin D1 (92G2) rabbit monoclonal antibody; phospho-Rb (Ser780) rabbit monoclonal antibody; phospho-Akt (Ser473) (D9E) rabbit monoclonal antibody; phospho-Akt (Thr308) (C31E5E) rabbit monoclonal antibody; and Akt (pan) (C67E7) rabbit monoclonal antibody. The anti–β-actin clone AC-15 mouse monoclonal antibody was from Sigma-Aldrich.
Statistical Analysis
To evaluate the differences across various experimental conditions in the viability experiments, one-way analysis of variance was performed, and post hoc tests (Duncan and Dunnett's T3 tests) served to evaluate differences between pairs of experimental conditions (e.g., vehicle-treated cells vs. cells treated with each concentration of each agent). The additive or synergistic nature of the interaction between agents used in combination was evaluated by isobologram analysis using dose-effect analyzer software (Calcusyn; Biosoft, Ferguson, MO). In all analyses, P < 0.05 was considered statistically significant.
Results
Impact of MEK Inhibition on UM Cell Lines Depends on Their Genotype
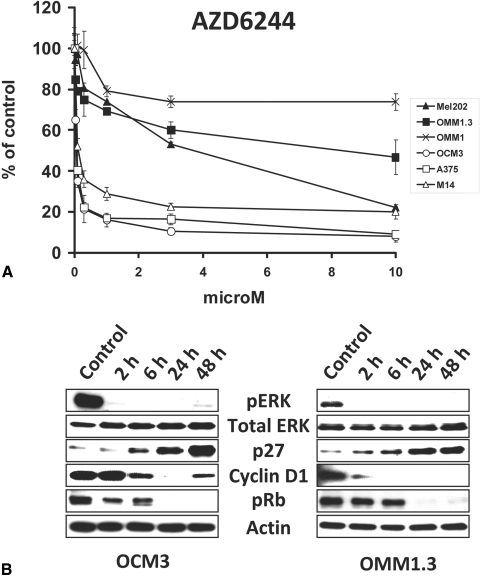
We tested the impact of the MEK inhibitor AZD6244 on UM cell lines using MTT assay and immunoblotting with the BRAF-mutant CM cell lines A375 and M14 serving as controls. We found that the BRAF-mutant OCM3 cells were very sensitive to AZD6244, similar to their CM counterparts (Fig. 1A). The Gα-mutant UM cell lines were less sensitive (higher IC50) than the BRAF-mutant cells but still responded with growth arrest (Fig. 1A).
Figure 1.
The MEK inhibitor AZD6244 suppresses the growth of BRAF-mutant and Gα-mutant UM cell lines. (A) The BRAF-mutant OCM3 cells, the GNAQ-mutant OMM1.3 and Mel202 cells, and the GNA11-mutant OMM1 cells were treated with AZD6244 at the indicated concentrations for 96 hours in medium containing 10% FBS. Cell number was quantified with the MTT assay and expressed as percentage of control (DMSO) wells (average ± SD). The BRAF-mutant CM cell lines A375 and M14 served as controls. The BRAF-mutant OCM3 cells were very sensitive to AZD6244, similar to their CM counterparts. The Gα-mutant UM cell lines were less sensitive than the BRAF-mutant cells but still responded with growth arrest. (B) Immunoblot analysis revealed that AZD6244 (1 μM) decreased levels of phosphorylated ERK, decreased levels of cyclin D1, increased levels of p27, and decreased levels of phosphorylated Rb in both BRAF-mutant (OCM3) and Gα-mutant (OMM1.3) UM cell lines.
Immunoblot analysis revealed that AZD6244 decreased levels of phosphorylated ERK, decreased levels of cyclin D1, increased levels of the cyclin-dependent kinase inhibitor p27, and decreased levels of phosphorylated Rb in both BRAF-mutant (OCM3) and Gα-mutant (OMM1.3) UM cell lines (Fig. 1B). All these changes are consistent with and probably mediate the growth-suppressive effect of AZD6244, as has been previously reported for BRAF-mutant CM cell lines.25
Impact of B-Raf Inhibition on UM Cell Lines Depends on Their Genotype
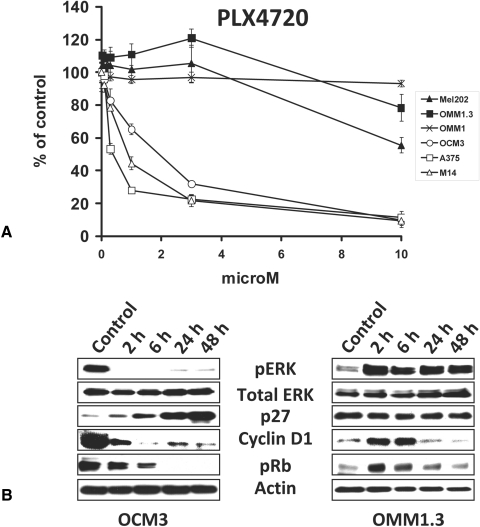
We tested the impact of the B-Raf inhibitor PLX4720 on UM cell lines using cell survival assays and immunoblot analysis with the BRAF-mutant CM cell lines A375 and M14 serving as controls. We found that the BRAF-mutant OCM3 cells were sensitive to PLX4720, similar to their CM counterparts (Fig. 2A). However, the Gα-mutant UM cell lines were resistant to the anticancer effect of PLX4720, and, in fact, OMM1.3 cells even exhibited a paradoxical mild increase in proliferation (Fig. 2A). This stimulatory effect of PLX4720 occurred both in the presence of 10% FBS and in serum-free conditions.
Figure 2.
The B-Raf inhibitor PLX4720 suppressed the growth of BRAF-mutant but not Gα-mutant UM cell lines. (A) The BRAF-mutant OCM3 cells, the GNAQ-mutant OMM1.3 and Mel202 cells, and the GNA11-mutant OMM1 cells were treated with PLX4720 at the indicated concentrations for 96 hours in medium containing 10% FBS. Cell number was quantified with the MTT assay and expressed as percentage of control (DMSO) wells (average ± SD). The BRAF-mutant CM cell lines A375 and M14 served as controls. The BRAFV600E OCM3 cells were sensitive to PLX4720, similar to their CM counterparts. The Gα-mutant UM cell lines were resistant to the anticancer effect of PLX4720 and, in fact, exhibited a paradoxical mild increase in proliferation. (B) Immunoblot analysis revealed that PLX4720 (3 μM) decreased levels of phosphorylated ERK, decreased levels of cyclin D1, increased levels of p27, and decreased levels of phosphorylated Rb in BRAF-mutant (OCM3) cells. On the contrary, in Gα-mutant (OMM1.3) UM cells, PLX4720 induced a paradoxical increase in phosphorylated ERK and Rb levels and an early increase in cyclin D1 levels but did not stimulate p27 levels.
Immunoblot analysis revealed that PLX4720 decreased levels of phosphorylated ERK, decreased levels of cyclin D1, increased levels of p27, and decreased levels of phosphorylated Rb only in BRAF-mutant (OCM3) cells. On the contrary, in Gα-mutant (OMM1.3) UM cells, PLX4720 induced a paradoxical increase in phosphorylated ERK and an early increase in cyclin D1 and pRb levels and did not stimulate p27 levels (Fig. 2B).
Collectively, our findings suggest that the B-Raf inhibitor PLX4720 does not exert anticancer activity against Gα-mutant melanoma cell lines (in fact, it can stimulate ERK signaling).
BrdU Incorporation
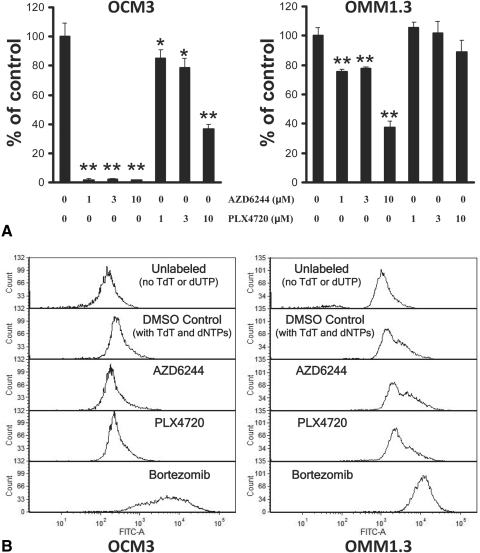
Using the BrdU cell proliferation assay, we studied the impact of AZD6244 and PLX4720 on UM DNA synthesis (S phase). Overall, we obtained parallel results to our MTT data. We found that the BRAF-mutant OCM3 cells were very sensitive to AZD6244, whereas OMM1.3 cells still responded with decreased DNA synthesis (but to a lesser degree; Fig. 3A). We also found that OCM3 cells were sensitive to PLX4720. However, the Gα-mutant OMM1.3 cells were resistant to the anticancer effect of PLX4720 (Fig. 3A).
Figure 3.
Growth arrest without apoptosis in UM cell lines treated with AZD6244 or PLX4720 monotherapy. (A) Using the BrdU cell proliferation assay, we studied the impact of AZD6244 and PLX4720 on UM DNA synthesis (S phase). The BRAF-mutant OCM3 cells and the Gα-mutant OMM1.3 cells were treated with AZD6244 or PLX4720 (or DMSO) at the indicated concentrations in 24-well plates for 48 hours. Subsequently, BrdU was added to the wells for an additional 24 hours, and the amount of incorporation into DNA was quantified according to the instructions of the manufacturer and was expressed as percentage of control (DMSO) wells (average ± SD). We found that the BRAF-mutant OCM3 cells were sensitive to AZD6244 and PLX4720, whereas Gα-mutant OMM1.3 cells still responded with decreased DNA synthesis to AZD6244 (but to a lesser degree than OCM3 cells) though they were resistant to the anticancer effect of PLX4720 (*P < 0.05, **P < 0.001 vs. respective DMSO controls). (B) Using the TUNEL assay, we studied the impact of AZD6244 and PLX4720 on UM cell apoptosis. UM cells were treated with AZD6244 or PLX4720 at 3 μM (or DMSO control) for 72 hours and then labeled for apoptosis using the enzyme terminal deoxynucleotidyl transferase (TdT) and the fluorescein-dUTP. We did not detect any shift in fluorescence (TUNEL labeling) in OCM3 or OMM1.3 cells treated with AZD6244 or PLX4720 monotherapy, suggesting a lack of apoptosis. The proteasome inhibitor bortezomib (100 nM for 72 hours) served as a positive control for the induction of apoptosis. Cells incubated without TdT or fluorescein-dUTP served as a negative control.
TUNEL Assay
UM cells treated with AZD6244 or PLX4720 for 72 hours were tested for apoptosis using the terminal dUTP nick-end labeling (TUNEL) assay. We did not detect any increase in TUNEL labeling in OCM3 or OMM1.3 cells treated with AZD6244 and PLX4720, suggesting lack of apoptosis (Fig. 3B). The proteasome inhibitor bortezomib (100 nM for 72 hours) served as a positive control for the induction of apoptosis.
Collectively, these data suggest that the mechanism of the observed activity is by growth arrest, not apoptosis. In support, we did not detect any cleavage of caspase-3 or PARP in our immunoblotting analysis of UM cells treated with AZD6244 or PLX4720 for 72 hours (not shown), and the anticancer activity of AZD6244 and PLX4720 was not attenuated by pretreatment with the pan-caspase inhibitor ZVAD-FMK, indicating that apoptosis is not substantially involved in their effects (Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7398/-/DCSupplemental).
Impact of Combined B-Raf and MEK Inhibition on BRAF-Mutant and Gα-Mutant UM Cells
We next assessed whether combined B-Raf and MEK inhibition would achieve an enhanced anticancer activity. We found that, in BRAF-mutant OCM3 cells, the combination of sublethal concentrations of PLX4720 and AZD6244 resulted in synergistic anticancer activity (Fig. 4A). However, in Gα-mutant OMM1.3 cells, the addition of AZD6244 could not overcome their resistance to PLX4720 (Fig. 4B). Therefore, the combination of B-Raf and MEK inhibition appears to be a promising approach only for BRAF-mutant cells.
Figure 4.
Impact of combined B-Raf and MEK inhibition on BRAF-mutant and Gα-mutant UM cells. The BRAF-mutant OCM3 cells (A) and the Gα-mutant OMM1.3 (B) and Mel202 cells were treated with the combination of PLX4720 and AZD6244 at the indicated concentrations for 96 hours in medium containing 10% FBS. Cell number was quantified with the MTT assay and expressed as a percentage of control wells (average ± SD). In BRAF-mutant OCM3 cells, the combination of sublethal concentrations of PLX4720 and AZD6244 resulted in synergistic anticancer activity (A) , whereas in Gα-mutant OMM1.3 cells (B) and Mel202 cells (not shown), the addition of AZD6244 could not overcome their resistance to PLX4720.
Impact of B-Raf and MEK Inhibition on the Akt Pathway in BRAF-Mutant and Gα-Mutant UM Cells
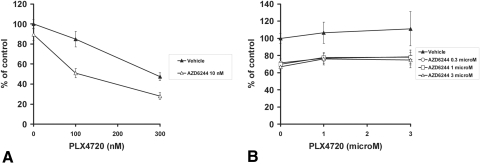
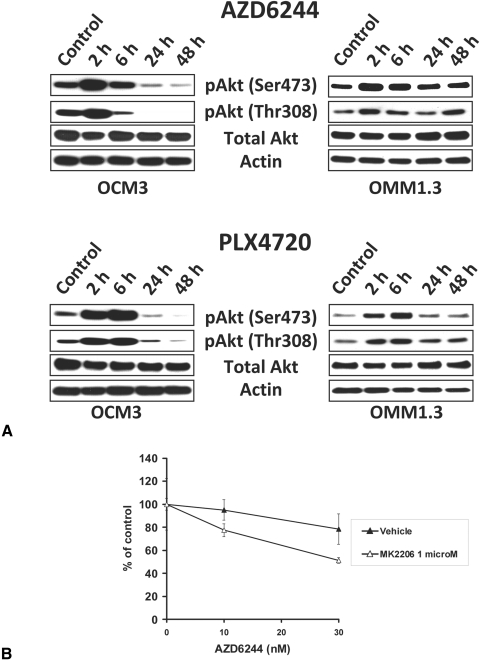
We next investigated the impact of B-Raf and MEK inhibition on the Akt pathway in BRAFV600E and Gα-mutant UM cells. We found that both AZD6244 and PLX4720 induced an early (within 2–6 hours of exposure) increase in the levels of phosphorylated Akt (both Ser473 and Thr308) in both BRAF-mutant (OCM3) and Gα-mutant (OMM1.3) cells (Fig. 5A). Eventually, and with prolonged drug exposure, the phosphorylation of Akt returned to baseline in Gα-mutant OMM1.3 cells and decreased even below baseline levels in BRAF-mutant OCM3 cells (Fig. 5A).
Figure 5.
Impact of B-Raf and MEK inhibition on the Akt pathway in BRAF-mutant and Gα-mutant UM cells. (A) Immunoblot analysis revealed that both AZD6244 and PLX4720 induced an early (within 2–6 hours of exposure) increase in the levels of phosphorylated Akt (at residues Ser473 and Thr308) in both BRAF-mutant OCM3 and Gα-mutant OMM1.3 cells. Eventually, and with prolonged drug exposure, the phosphorylation of Akt returned to baseline in Gα-mutant OMM1.3 cells and decreased even below baseline levels in BRAF-mutant OCM3 cells. (B) The Akt inhibitor MK2206 potently enhanced the anticancer activity of the MEK inhibitor AZD6244 against the BRAF-mutant OCM3 cells. The cells were treated with drugs at the indicated concentrations for 96 hours in medium containing 10% FBS. Cell number was quantified with the MTT assay and expressed as a percentage of control wells (average ± SD).
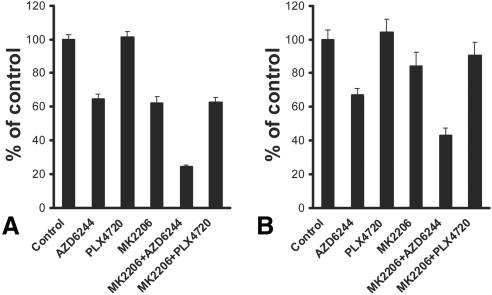
We hypothesized that the early upregulation of pAkt caused by B-Raf or MEK inhibition might attenuate the anticancer activity of AZD6244, PLX4720, or both, and, therefore, concurrent inhibition of the Akt pathway might enhance the anticancer activity of B-Raf and MEK inhibition. To address this hypothesis, we evaluated the effect of the Akt inhibitor MK2206 in combination with AZD6244 or PLX4720. We found that the Akt inhibitor MK2206 potently enhanced the anticancer activity of the MEK inhibitor (Fig. 5B) and the B-Raf inhibitor (not shown) against the BRAF-mutant OCM3 cells. MK2206 also enhanced the anticancer activity of the MEK inhibitor against the Gα-mutant UM cells but did not overcome the resistance of the Gα-mutant UM cells to the B-Raf inhibitor (Figs. 6A, 6B).
Figure 6.
Akt inhibition enhances the anticancer activity of MEK, but not B-Raf, inhibition in Gα-mutant UM cells. A sublethal concentration of the Akt inhibitor MK2206 (1 μM) potently enhanced the anticancer activity of the MEK inhibitor AZD6244 (1 μM) against the Gα-mutant Mel202 cells (A) and OMM1.3 cells (B). However, MK2206 (1 μM) did not sensitize them to PLX4720 (1 μM). The cells were treated with drugs for 96 hours in medium containing 10% FBS. Cell number was quantified with the MTT assay and expressed as a percentage of control wells (average ± SD).
Discussion
Recent advances in our understanding of the molecular pathophysiology of cancer have allowed for rational development of targeted therapies designed to interrupt molecular pathways critical for cell growth and survival.37 Kinases represent such “druggable” targets and an area of very active clinical research in oncology. Various malignancies that exhibit “oncogenic addiction” to select kinase pathways respond clinically to treatment with respective kinase inhibitors (e.g., imatinib in chronic myelogenous leukemia and gastrointestinal stromal tumors, erlotinib in EGFR-mutant non–small cell lung carcinoma, and others).38–41 Because of the biological heterogeneity and interindividual variation in human cancers, molecular profiling of each patient's tumor is necessary to guide selection of the appropriate targeted therapy for the patient most likely to benefit from it.37 The goal of this personalized approach is to avoid exposing patients to drugs from which they are unlikely to benefit, thus sparing them unnecessary toxicity and cost. For example, responses of non–small cell lung carcinomas to EGFR small molecule inhibitors (such as erlotinib) are generally limited to tumors harboring somatic mutations in the EGFR tyrosine kinase domain.39–41 Moreover, colorectal carcinomas harboring certain activating somatic mutations in KRAS are resistant to EGFR-targeting monoclonal antibodies, and use of such agents should be avoided in these particular carcinomas.42 In the present study, we investigated the activity of small molecule inhibitors of MEK, B-Raf and Akt against UM cells in vitro. Our results demonstrate that the sensitivity of UM cells to these inhibitors is genotype dependent and make a strong case for a personalized approach in the management of UMs with targeted therapies.
CMs frequently harbor mutations in BRAF (usually BRAFV600E)1 or NRAS2–6 that cause constitutive activation of the MEK/ERK pathway and cell proliferation. CMs harboring BRAFV600E mutations exhibit increased sensitivity to inhibitors of MEK25 and B-Raf.13–20 We found similar sensitivity to the MEK and B-Raf inhibitors in BRAFV600E UM cells, suggesting that such targeted therapies may represent a promising option for this subset of UM patients as well. In agreement with their CM counterparts,13,25 BRAFV600E UM cells treated with AZD6244 or PLX4720 exhibited decreased levels of phosphorylated ERK, decreased levels of cyclin D1, increased levels of the cyclin-dependent kinase inhibitor p27, and decreased levels of phosphorylated Rb. All these changes are consistent with and probably mediate the growth-suppressive effect (inhibition of cell proliferation) of MEK or B-Raf inhibition.
However, BRAFV600E mutations are rare in UMs.26–28 Instead, an equivalent oncogenic event is frequently present in the form of mutually exclusive somatic mutations in the heterotrimeric G protein α-subunit GNAQ (∼40%-50% of primary UMs)30,32,43,44 or its related GNA11 (∼30% of primary UMs).31 These mutations apparently occur early in UM carcinogenesis,32 contrary to other genetic events associated with increased metastatic potential such as the recently described BRCA1-associated protein 1 (BAP1) mutations.45 The GNAQQ209 and GNA11Q209 mutations affect the Ras-like domain of these G proteins, specifically corresponding to NRASQ61R, abolishing GTPase activity, and resulting in constitutive activation. In this study, we examined the sensitivity of GNAQ-mutant and GNA11-mutant UM cell lines to MEK and B-Raf inhibition. We found that Gα-mutant UM cells are sensitive to MEK inhibition (exhibiting decreased levels of pERK, cyclin D1, and pRb, increased levels of p27, and decreased proliferation) but to a lower degree (higher IC50) than BRAF-mutant UM and CM cells. In direct contrast to BRAF-mutant UM and CM cells, Gα-mutant UM cells were resistant to the anticancer effect of the B-Raf inhibitor PLX4720 and, in fact, exhibited a paradoxical increase in pERK signaling.
These findings emphasize the need for a personalized approach to the use of targeted therapies in patients with UM because the Gα-mutant activates the ERK pathway in a manner that cannot be targeted, thus far, by PLX4720, at least when used as a monotherapy. Therefore, new therapeutic approaches are needed for Gα-mutant tumors. The paradoxical activation of the ERK pathway in Gα-mutant UM cells by PLX4720 parallels the similar behavior of NRAS-mutant and other BRAF wild-type CM cell lines.13–16,18 Collectively, these data highlight the importance of choosing the appropriate, personalized targeted therapy for each patient, not only to avoid unnecessary toxicity to normal tissues but also because an inappropriate targeted therapy can enhance the proliferation rate and growth of the cancer cells harboring the wrong genotype, with detrimental effects. Tumor genotyping should be a prerequisite for enrollment of UM patients (and probably all patients) in clinical trials of targeted therapies.18
In view of the resistance of Gα-mutant UM cells to PLX4720, we investigated whether combination of the B-Raf inhibitor with MEK or Akt inhibition would restore sensitivity. Unfortunately, in Gα-mutant UM cells, the addition of the MEK inhibitor AZD6244 or the Akt inhibitor MK2206 did not restore sensitivity to PLX4720. On the contrary, in BRAF-mutant cells, combined B-Raf and MEK inhibition resulted in synergistic anticancer activity. Furthermore, the Akt inhibitor MK2206 enhanced the anticancer activity of both the B-Raf inhibitor and the MEK inhibitor against the BRAF-mutant UM cells and the anticancer activity of the MEK inhibitor against the Gα-mutant UM cells. This finding is in agreement with the early upregulation of phosphorylated Akt that we observed on MEK or B-Raf inhibition in our models and with reports of the synergistic activity of AZD6244 in combination with the inhibition of Akt in BRAFV600E cutaneous melanoma46 and lung carcinoma47 cell lines. Moreover, in UM cells, cotreatment with the PI3K inhibitor LY294003, in combination with the MEK inhibitor U0126 or with the B-Raf inhibitor BAY43–9006, has been reported to result in more potent inhibition of cell proliferation.48 These data highlight the combined inhibition of MEK and Akt pathways as a rationally designed targeted therapeutic approach for Gα-mutant UMs, that deserves investigation in a clinical trial.
In conclusion, we report that the Gα mutations, present in the majority of UMs, are functionally similar to the NRAS mutations seen in CMs in that they are associated with lower sensitivity to MEK inhibition and complete resistance to the anticancer activity of the B-Raf inhibitor PLX4720. Combined inhibition of MEK and Akt results in synergistic anticancer activity and represents a promising targeted therapeutic approach for Gα-mutant UMs. Our findings emphasize the importance of a personalized, genotype-based approach to the use of targeted therapies in clinical trials of patients with UM.
Supplementary Material
Acknowledgments
The authors thank Martine Jager (Department of Ophthalmology, Leiden University Medical Center, Leiden, The Netherlands) for generously providing UM cell lines.
Footnotes
Supported by a fellowship from Jackstaedt Stiftung, Essen (AIR) and by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant 5K01DK081446 (BH).
Disclosure: N. Mitsiades, None; S.A. Chew, None; B. He, None; A.I. Riechardt, None; T. Karadedou, None; V. Kotoula, None; V. Poulaki, None
References
- 1. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954 [DOI] [PubMed] [Google Scholar]
- 2. Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–6488 [PubMed] [Google Scholar]
- 3. Reifenberger J, Knobbe CB, Sterzinger AA, et al. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. Int J Cancer. 2004;109:377–384 [DOI] [PubMed] [Google Scholar]
- 4. Akslen LA, Angelini S, Straume O, et al. BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J Invest Dermatol. 2005;125:312–317 [DOI] [PubMed] [Google Scholar]
- 5. Edlundh-Rose E, Egyhazi S, Omholt K, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006;16:471–478 [DOI] [PubMed] [Google Scholar]
- 6. Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol. 2006;126:154–160 [DOI] [PubMed] [Google Scholar]
- 7. Karasarides M, Chiloeches A, Hayward R, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–6298 [DOI] [PubMed] [Google Scholar]
- 8. Mitsiades CS, Negri J, McMullan C, et al. Targeting BRAFV600E in thyroid carcinoma: therapeutic implications. Mol Cancer Ther. 2007;6:1070–1078 [DOI] [PubMed] [Google Scholar]
- 9. Halilovic E, Solit DB. Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr Opin Pharmacol. 2008;8:419–426 [DOI] [PubMed] [Google Scholar]
- 10. Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000 [PubMed] [Google Scholar]
- 11. Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joseph EW, Pratilas CA, Poulikakos PI, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–14908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435 [DOI] [PubMed] [Google Scholar]
- 16. Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene. 2011;30(3):366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ribas A, Kim KB, Schuchter LM, et al. BRIM-2: An open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. ASCO Meeting Abstracts. 2011;29:8509 [Google Scholar]
- 20. Chapman PB, Hauschild A, Robert C, et al. Phase III randomized, open-label, multicenter trial (BRIM3) comparing BRAF inhibitor vemurafenib with dacarbazine (DTIC) in patients with V600EBRAF-mutated melanoma. ASCO Meeting Abstracts. 2011;29:LBA4 [Google Scholar]
- 21. Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71:2750–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang CC, Lai F, Thorne RF, et al. MEK-independent survival of B-RAFV600E melanoma cells selected for resistance to apoptosis induced by the RAF inhibitor PLX4720. Clin Cancer Res. 2011;17:721–730 [DOI] [PubMed] [Google Scholar]
- 25. Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rimoldi D, Salvi S, Lienard D, et al. Lack of BRAF mutations in uveal melanoma. Cancer Res. 2003;63:5712–5715 [PubMed] [Google Scholar]
- 27. Kilic E, Bruggenwirth HT, Verbiest MM, et al. The RAS-BRAF kinase pathway is not involved in uveal melanoma. Melanoma Res. 2004;14:203–205 [DOI] [PubMed] [Google Scholar]
- 28. Maat W, Kilic E, Luyten GP, et al. Pyrophosphorolysis detects B-RAF mutations in primary uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:23–27 [DOI] [PubMed] [Google Scholar]
- 29. Hepler JR, Kozasa T, Smrcka AV, et al. Purification from Sf9 cells and characterization of recombinant Gq alpha and G11 alpha: activation of purified phospholipase C isozymes by G alpha subunits. J Biol Chem. 1993;268:14367–14375 [PubMed] [Google Scholar]
- 30. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Onken MD, Worley LA, Long MD, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:5230–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tolcher AW, Yap TA, Fearen I, et al. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST). ASCO Meeting Abstracts. 2009;27:3503 [Google Scholar]
- 34. Yap TA, Patnaik A, Fearen I, et al. First-in-class phase I trial of a selective Akt inhibitor, MK2206 (MK), evaluating alternate day (QOD) and once weekly (QW) doses in advanced cancer patients (pts) with evidence of target modulation and antitumor activity. ASCO Meeting Abstracts. 2010;28:3009 [Google Scholar]
- 35. Chandarlapaty S, Sawai A, Scaltriti M, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poulaki V, Mitsiades CS, Kotoula V, et al. Molecular sequelae of histone deacetylase inhibition in human retinoblastoma cell lines: clinical implications. Invest Ophthalmol Vis Sci. 2009;50:4072–4079 [DOI] [PubMed] [Google Scholar]
- 37. Schilsky RL. Personalized medicine in oncology: the future is now. Nat Rev Drug Discov. 2010;9:363–366 [DOI] [PubMed] [Google Scholar]
- 38. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480 [DOI] [PubMed] [Google Scholar]
- 39. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139 [DOI] [PubMed] [Google Scholar]
- 40. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500 [DOI] [PubMed] [Google Scholar]
- 41. Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096 [DOI] [PubMed] [Google Scholar]
- 43. Lamba S, Felicioni L, Buttitta F, et al. Mutational profile of GNAQQ209 in human tumors. PLoS One. 2009;4:e6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dratviman-Storobinsky O, Cohen Y, Frenkel S, Pe'er J, Goldenberg-Cohen N. Lack of oncogenic GNAQ mutations in melanocytic lesions of the conjunctiva as compared to uveal melanoma. Invest Ophthalmol Vis Sci. 2010;51:6180–6182 [DOI] [PubMed] [Google Scholar]
- 45. Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gopal YN, Deng W, Woodman SE, et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res. 2010;70:8736–8747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meng J, Peng H, Dai B, et al. High level of AKT activity is associated with resistance to MEK inhibitor AZD6244 (ARRY-142886). Cancer Biol Ther. 2009;8:2073–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Babchia N, Calipel A, Mouriaux F, Faussat AM, Mascarelli F. The PI3K/Akt and mTOR/P70S6K signaling pathways in human uveal melanoma cells: interaction with B-Raf/ERK. Invest Ophthalmol Vis Sci. 2010;51:421–429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.