Abstract
The synthesis of glycosylated Fmoc amino acids by reaction of mono- and disaccharide peracetates with Fmoc amino acids having free carboxyl groups was rapidly promoted by Lewis acids (SnCl4, BF3·Et2O) under microwave irradiation. The products are useful building blocks for the synthesis of glycopeptides.
Keywords: Fmoc amino acids, Glycosylation, Microwave irradiation
Carbohydrates are important components of glycoproteins, playing pivotal roles in many biological processes, such as cell adhesion, inflammation, the immune response, and tumor metastasis.1 It is therefore of great interest to synthesize glycopeptides. Currently, there are two approaches to synthesize glycopeptides including the convergent approach2 and the building block approach.3 The former is based on glycosylation of a peptide in solution or on solid-phase. This direct O-glycosylation of a peptide often results in low yields.4 Alternatively, the versatile and general building block approach has a lot of advantages. A glycosylated amino acid building block is easily introduced into a solid phase peptide synthesis using peptide chemistry. This method has been widely applied to prepare a large variety of complex glycopeptides and even larger glycopeptides libraries.5
Several methods exist for the preparation of glycosylated Fmoc amino acid building blocks.6 However, the previous approaches to glycosylated amino acids require the synthetic glycosyl donors and amino acids to be protected at both the α-carboxyl and α-amino groups. Kihlberg and co-workers reported that Fmoc amino acids with unprotected carboxyl groups could be directly glycosylated with commercial carbohydrate 1, 2-trans peracetates.7 However, these methods suffered from low yields or long reaction times. Microwave irradiation has been demonstrated to dramatically accelerate the conversion in synthetic chemistry.8 Seibel et al. reported on the glycosylation of Fmoc-Ser-OBn in a microwave oven.9 These building blocks cannot be directly used for the solid-phase synthesis of glycopeptides. Therefore, the direct synthesis of glycosylated Fmoc amino acids from Fmoc amino acids with unprotected carboxyl groups and mono- and disaccharide peracetates should be efficiently carried out under microwave irradiation.
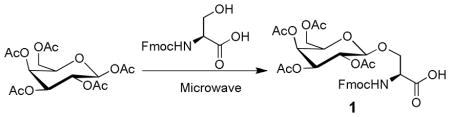
It has been reported that Fmoc amino acids are glycosylated with peracetylated glycosides under the influence of Lewis acids such as FeCl3, SnCl4 or BF3·Et2O as promoters and CH2Cl2, CH3CN and toluene as solvents.7, 9 A microwave reactor (Personal Chemistry, Emrys Optimizer) is compatible with various solvents and reaction temperatures. Initially, glycosylated Fmoc-Ser-OH with β-D-galactose pentaacetate was investigated to determine which Lewis acid and solvent could afford higher yield (Table 1) using microwave reactor. Fmoc-Ser-OH (0.01 mmol) and β-D-galactose pentaacetate (0.01 mmol) were added to a 5-mL microwave vial, followed by addition of 2 mL of solvent and Lewis acid (0.01 mmol). The mixture was irradiated in the microwave oven for 5min. The product 1 was purified by preparative HPLC and confirmed to be of the β-configuration by 1HNMR spectroscopy. The reaction conversion was directly determined by HPLC. FeCl3 provided lower conversion, suggesting heterogeneous system (FeCl3/CH2ClCH2Cl or C6H6) may limit the reaction rates. The use of CH2Cl2 as solvent resulted in a higher conversion than that of reactions performed in CH2ClCH2Cl, C6H6 or CH3CN.
Table 1.
Microwave-assisted glycosylation of Fmoc-Ser-OH under different reaction conditions.
 | ||||
|---|---|---|---|---|
| Entry | Promoter (1 equiv) | Solvent (2 mL) | Temperature (°C) | Conversion (%) |
| 1 | FeCl3 | CH2ClCH2Cl | 150 | 19 |
| 2 | SnCl4 | CH2ClCH2Cl | 150 | 41 |
| 3 | FeCl3 | CH3CN | 150 | 7 |
| 4 | SnCl4 | CH3CN | 150 | 24 |
| 5 | FeCl3 | C6H6 | 150 | 0 |
| 6 | SnCl4 | C6H6 | 150 | 40 |
| 7 | FeCl3 | CH2Cl2 | 100 | 35 |
| 8 | SnCl4 | CH2Cl2 | 100 | 44 |
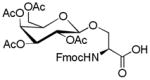
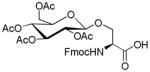
In order to further improve yields, a number of conditions including reaction time and promoter (Table 2) were conducted in CH2Cl2. An increase in the amount of SnCl4 from 1 equivalent to 2 equivalents resulted in higher glycosylation. A further increase in the amount of SnCl4, however, did not increase the yield of glycosylated Fmoc amino acid. Interestingly, variations in the amount of BF3·Et2O did not lead to any change in yield, and prolonged microwave irradiation was discovered to give side products. Furthermore, a wider range of amino acids and mono- and oligosaccharides were explored to synthesize various glycosylated Fmoc amino acid building blocks under microwave irradiation for 5 min using SnCl4 or BF3·Et2O (2 equiv) as promoters and CH2Cl2 as solvent. Their conversions are presented in Table 3. Glycosylation of Fmoc-Ser-OH with different peracetylated glycosylation donors such as galactose, glucose, xylose, lactose and maltose gave higher yields (52 – 72%). In comparison, lower yields (31 – 47%) were obtained in the glycosylation of Fmoc-Tyr-OH under identical conditions. It was noteworthy that disaccharide peracetates of lactose and maltose as glycosyl donors were found to give lower yields than monosaccharide peracetates of galactose, glucose, and xylose.
Table 2.
Microwave-assisted glycosylation of Fmoc-Ser-OH under different reaction conditions.
| Entry | Promoter | Time (min) | Conversion (%) |
|---|---|---|---|
| 1 | SnCl4 (2 equiv) | 5 min | 73 |
| 2 | SnCl4 (3 equiv) | 5 min | 68 |
| 3 | SnCl4 (4 equiv) | 5 min | 64 |
| 4 | SnCl4 (5 equiv) | 5 min | 63 |
| 5 | BF3·Et2O (1 equiv) | 5 min | 72 |
| 6 | BF3·Et2O (2 equiv) | 5 min | 72 |
| 7 | BF3·Et2O (4 equiv) | 5 min | 73 |
| 8 | BF3·Et2O (6 equiv) | 5 min | 71 |
| 9 | SnCl4 (2 equiv) | 15 min | 64 |
| 10 | SnCl4 (2 equiv) | 30 min | 60 |
| 11 | BF3·Et2O (6 equiv) | 15 min | 55 |
| 12 | BF3·Et2O (6 equiv) | 30 min | 36 |
Table 3.
Glycosylation of Fmoc amino acids
| Entry | Fmoc amino acid | Donor | Product | Conversion (%) | |
|---|---|---|---|---|---|
| SnCl4 | BF3·Et2O | ||||
| 1 | Fmoc-Ser-OH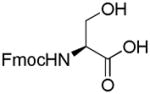
|
β-D-Galactose pentaacetate |
|
73 | 72 |
| 2 | β-D-Glucose-pentaacetate |
|
64 | 61 | |
| 3 | D-Xylose tetraacetate |
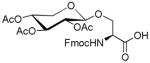
|
62 | 64 | |
| 4 | β-D-Lactose octaacetate |
|
49 | 57 | |
| 5 | β-D-Maltose octaacetate |
|
52 | 52 | |
| 6 | Fmoc-Thr-OH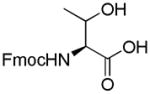
|
β-D-Galactose pentaacetate |
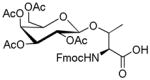
|
54 | 58 |
| 7 | β-D-Glucose- pentaacetate |
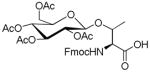
|
54 | 49 | |
| 8 | D-Xylose tetraacetate |
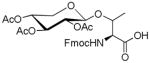
|
45 | 62 | |
| 9 | β-D-Lactose octaacetate |
|
45 | 43 | |
| 10 | β-D-Maltose octaacetate |
|
45 | 43 | |
| 11 | Fmoc-Tyr-OH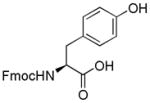
|
β-D-Galactose pentaacetate |
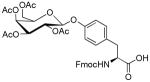
|
34 | 38 |
| 12 | β-D-Glucose- pentaacetate |
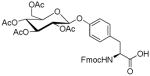
|
33 | 47 | |
| 13 | D-Xylose tetraacetate |
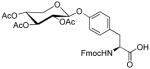
|
35 | 40 | |
| 14 | β-D-Lactose octaacetate |
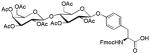
|
24 | 35 | |
| 15 | β-D-Maltose octaacetate |
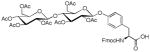
|
28 | 31 | |
In conclusion, we have developed a microwave-assisted glycosylation of Fmoc amino acids with unprotected carboxyl groups, which can be used to efficiently synthesize glycosylated Fmoc amino acid building blocks. To our knowledge, this is the first report of glycosylation of Fmoc amino acids without prior protection of the amino acid carboxyl groups under microwave conditions. The reactions were readily carried out under microwave irradiation for only 5 min, resulting in β-glycosides in moderate yields. The products could be easily purified via flash chromatography. Furthermore Fmoc amino acids and carbohydrate peracetates are commercially available. This method provides easy assess to numerous valuable building blocks, which can be directly used for constructing parallel and combinatorial glycopeptide libraries as well as the synthesis of various glycopeptides.
1. Experimental
1.1. General Methods
All solvents and chemical reagents were purchased from Sigma – Aldrich Chemical Co. (Milwaukee, WI) and were of analytical grade. NMR spectra were recorded on a Bruker DRX spectrometer in DMSO-d6 at 25 °C (500 M Hz for 1H NMR). HRMS was performed with a Finnigan LTQ FT™ instrument. Analytical HPLC was performed on a Waters 2996 HPLC system equipped with a 4.6 × 150 mm Waters Xterra® MS C18 5.0-μm column, and employed a 20-min gradient from 100% aqueous H2O (0.1% TFA) to 100% CH3CN (0.1% TFA) at a flow rate of 1.0 mL/min. Preparative HPLC was performed on a System Gold 126NMP Solvent Module (Beckman) with a C18 column (Vydac, 5μM, 2.5 cm i.d. × 25 cm). A gradient elution of 0 – 60% B over 25 min, followed by 60 – 100% B over 25 min, followed by 100% B for 5 min, was used at a flow rate of 7 mL/min (solvent A: H2O – 0.1% TFA; B: Acetonitrile – 0.1% TFA).
1.2. General procedure for glycosylation of Fmoc amino acids
Fmoc amino acids (0.2 mmol) and mono- and disaccharide peracetates (0.26 mmol) were added to a 5-mL round-bottom microwave vial, followed by addition of 3 mL of CH2Cl2 and SnCl4, or BF3·Et2O (0.4 mmol). The vessel was sealed and heated to 100 °C for 5 min in a microwave reactor (Personal Chemistry, Emrys Optimizer). The reaction temperature increased from 25 to 100 °C in 90 s and was maintained at 100 °C for the duration of the reaction. The reaction conversion was determined by analytical HPLC. The mixture was concentrated and purified by preparative HPLC. All of the products were characterized by ESIMS and 1H NMR spectroscopy.
1.3. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)-L-serine (1)
White powder, Purity, 97%; 1H NMR (500 M Hz, DMSO-d6):δ 7.90(d, J 5 Hz, 2H), 7.72(d, J 10 Hz, 2H), 7.43 (m, 2H), 7.33 (m, 2H), 5.25 (d, J 2.5 Hz, 1H), 5.15 (dd, J 9 Hz, 3.0 Hz, 1H), 4.91 (dd, J 9 Hz, 6.5 Hz, 1H), 4.76 (d, J 6.5 Hz, 1H), 4.28–4.34 (m, 2H), 4.19–4.24 (m, 3H), 4.04 (m, 1H), 3.90 (m, 1H), 3.79 (m, 1H), 2.11 (s, 3H), 2.00 (s, 3H), 1.93 (s, 3H), 1.91 (s, 3H). HRESIFTMS [M+K]+: calcd for C32H35KNO14 696.1695; found: 696.1697
1.4. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)-L-serine (2)
White powder, purity, 99%; 1H NMR (500 M Hz, DMSO-d6): δ 7.90(d, J 5 Hz, 2H), 7.72 (d, J 5 Hz, 2H), 7.43 (m, 2H), 7.33 (m, 2H), 5.25 (t, J 10 Hz, 1H), 4.90 (dd, 1H), 4.87 (d, J 10 Hz, 1H), 4.73 (t, J 5 Hz, 1H), 4.16–4.33 (overlap, 5H), 3.99 (m, 1H), 3.90 (m, 1H), 3.77 (dd, 1H), 2.01 (s, 3H), 1.98(s, 3H), 1.93(s, 3H), 1.90(s, 3H). HRESIFTMS [M+K]+: calcd for C32H35KNO14 696.1695; found: 696.1696.
1.5. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)-L-serine (3)
White powder, purity, 96%; 1H NMR (500 M Hz, DMSO-d6): δ 7.90( d, J 10 Hz, 2H), 7.73(d, J 10 Hz, 2H), 7.43(m, 2H), 7.33(m, 2H), 5.12(t, J 5 Hz, 1H), 4.80(d, J 5 Hz, 1H), 4.73(m, 2H), 4.25–4.33(m, 2H), 4.20–4.23(m, 2H), 3.88(m, 1H), 3.74(m, 1H), 2.00(s, 3H), 1.96(s, 3H), 1.93(s, 3H). HRESIFTMS [M+K]+: calcd for C29H31KNO12, 624.1483, found: 624.1486
1.6. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-[(2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)-β-D-glucopyranosyl]-L-serine (4)
White powder, purity, 98%; 1H NMR (500 M Hz, DMSO-d6): δ 7.90(d, J 10 Hz, 2H), 7.71(d, J 10 Hz, 2H), 7.43(m, 2H), 7.33(m, 2H), 5.22(brs, 1H), 5.15(m, 2H), 4.84(dd, 1H), 4.80(d, J 5.0 Hz, 1H), 4.73(d, J 10 Hz, 1H), 4.65(dd, 1H), 4.29(m, 3H), 4.21(m, 3H), 4.05(m, 1H), 4.00(m, 2H), 3.81–3.88(m, 2H), 3.75–3.79(m, 2H), 2.11(s, 3H), 2.07(s, 3H), 2.00(s, 6H), 1.93(s, 3H), 1.89(s, 6H). HRESIFTMS [M+K]+: calcd for C44H51KNO22, 984.2540, found: 984.2542.
1.7. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-[(2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D- glucopyranosyl)-β-D-glucopyranosyl]-L-serine (5)
White powder, purity, 97%; 1H NMR (500 M Hz, DMSO-d6): δ 7.90( d, J 10 Hz, 2H), 7.71(d, J 10 Hz, 2H), 7.43(m, 2H), 7.33(m, 2H), 5.29(m, 1H), 5.27(brs, 1H), 5.22(t, J 10 Hz, 1H), 4.98(dd, 1H), 4.87(dd, 1H), 4.84(d, J 10 Hz, 1H), 4.63(t, J 5 Hz, 1H), 4.14–4.36(m, 4H), 3.86–4.01(m, 4H), 3.76(dd, 1H), 3.40–3.70(overlap), 2.06(s, 3H), 2.01(s, 3H), 1.98(s, 6H), 1.95(s, 3H), 1.91(s, 3H), 1.88(s, 3H). HRESIFTMS [M+K]+: calcd for C44H51KNO22, 984.2540, found: 984.2542.
1.8. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)-L-threonine (6)
White powder, purity, 99%; 1H NMR(500 M Hz, DMSO-d6): δ 7.90( d, J 10 Hz, 2H), 7.73(d, J 10 Hz, 2H), 7.42(t, J 10 Hz, 2H), 7.33(t, J 10 Hz, 2H), 6.60(d, J 5 Hz, 1H), 5.25(d, J 5.0 Hz, 1H), 5.17(dd, J 10 Hz, 5.0 Hz, 1H), 4.87(dd, 1H), 4.79(d, J 10 Hz, 1H), 4.30(m, 1H), 4.29(m, 1H), 4.22(m, 2H), 4.17(t, J 10 Hz, 1H), 4.10(dd, J 10 Hz, 5.0 Hz, 1H), 4.02(t, J 5 Hz, 2H), 2.09(s, 3H), 1.99(s, 3H), 1.92(s, 3H), 1.13(d, J 5 Hz, 3H). HRESIFTMS [M+K]+: calcd for C33H37KNO14, 710.1851, found: 710.1854.
1.9. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)-L-threonine (7)
White powder, purity, 97%; 1H NMR(500 M Hz, DMSO-d6): δ 7.90( d, J 10 Hz, 2H), 7.74(d, J 5 Hz, 2H), 7.42(t, J 5 Hz, 2H), 7.32(t, J 5 Hz, 2H), 6.75(d, J 5 Hz, 1H), 5.26(t, J 10 Hz, 1H), 4.90(dd, 1H), 4.88(d, J 10 Hz, 1H), 4.70(t, J 5 Hz, 1H), 4.27–4.31(m, 2H), 4.17–4.24(m, 3H), 4.11(dd, 1H), 3.97(m, 1H), 1.98(s, 6H), 1.97(s, 3H), 1.94(s, 3H), 1.13(d, J 5 Hz, 3H). HRESIFTMS [M+K]+: calcd for C33H37KNO14, 710.1851, found: 710.1854.
1.10. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)-L-threonine (8)
White powder, purity, 98%; 1H NMR (500 M Hz, DMSO-d6): δ 7.90(d, J 10 Hz, 2H), 7.73(d, J 5 Hz, 2H), 7.42(t, J 5 Hz, 2H), 7.32(t, J 5 Hz, 2H), 6.75(d, J 5 Hz, 1H), 5.10(t, J 5 Hz, 1H), 4.76(d, J 5 Hz, 1H) 4.75(m, 1H), 4.69(t, J 5 Hz, 1H), 4.23–4.31(m, 4H), 4.12(dd, J 5 Hz, 5 Hz, 1H), 4.93(dd, J 5 Hz, 5 Hz, 1H), 2.01(s, 3H), 2.00(s, 6H), 1.12(d, J 5 Hz, 3H). HRESIFTMS [M + K]+:calcd for C30H33KNO12, 638.1640, found: 638.1642.
1.11. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-[(2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)- β-D-glucopyranosyl]-L-threonine (9)
White powder, purity, 96%; 1H NMR(500 M Hz, DMSO-d6): δ 7.90(d, J 10 Hz, 2H), 7.72(d, J 10 Hz, 2H), 7.42(t, J 10 Hz, 5 Hz, 2H), 7.33(t, J 5 Hz, 2H), 6.69(d, J 10 Hz, 1H), 5.22(brs, 1H), 5.12–5.17(m, 2H), 4.82–4.85(m, 2H), 4.73(d, J 5 Hz, 1H), 4.63(t, J 10 Hz, 1H), 4.22–4.29(m, 6H), 4.11(dd, 1H), 4.00–4.05(m, 3H), 3.75–3.79(m, 2H), 2.10(s, 3H), 2.05(s, 3H), 2.00(s, 6H), 1.97(s, 3H), 1.95(s, 3H), 1.89(s, 3H), 1.12(d, J 5 Hz, 3H). HRESIFTMS [M+H]+: calcd for C45H54NO22, 960.3137, found: 960.3140.
1.12. Nα-(9-Fluorenylmethoxycarbonyl)-3-O-[(2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D- glucopyranosyl)- β-D-glucopyranosyl]-L-threonine (10)
White powder, purity, 98%; 1H NMR(500 M Hz, DMSO-d6): δ 7.89(d, J 10 Hz, 2H), 7.73(d, J 10 Hz, 5 Hz, 2H), 7.42(t, J 10 Hz, 2H), 7.32(t, J 5 Hz, 2H), 6.78(d, J 5 Hz, 1H), 5.29(dd, 1H), 5.27(brs, 1H), 4.98(t, J 10 Hz, 1H), 4.88(dd, 1H), 4.87(d, J 10 Hz, 1H), 4.61(t, J 10 Hz, 1H), 4.10–4.31(m, 7H), 3.98–4.09(m, 5H), 2.05(s, 3H), 2.00(s, 3H), 1.99(s, 3H), 1.98(s, 3H), 1.95(s, 3H), 1.94(s, 6H), 1.13(d, J 10 Hz, 3H). HRESIFTMS [M+H]+: calcd for C45H54NO22, 960.3137, found: 960.3140.
1.13. Nα-(9-Fluorenylmethoxycarbonyl)-O-(2, 3, 4, 6-tetra-O-acetyl-β-D-galactopyranosyl)-L-tyrosine (11)
White powder, purity, 99%; 1H NMR(500 M Hz, DMSO-d6): δ 7.88(d, J 10 Hz, 2H), 7.63(dd, J 10 Hz, 5 Hz, 2H), 7.41(m, 2H), 7.31(m, 2H), 7.23(d, J 10 Hz, 2H), 7.00(d, J 10 Hz, 2H), 5.73(d, J 5 Hz, 1H), 5.40(dd, 1H), 5.39(brs, 1H), 5.32(dd, 1H), 5.15(d, J 5 Hz, 1H), 5.13(t, J 5 Hz, 1H), 4.28(dd, 1H), 4.12–4.20(m, 3H), 4.01(dd, J 5 Hz, 5 Hz, 1H), 3.90(dd, J 5 Hz, 5 Hz, 1H), 3.05(dd, J 10 Hz, 5 Hz, 1H), 3.81(dd, J 10 Hz, 5 Hz, 1H), 2.14(s, 3H), 2.04(s, 3H), 1.97(s, 3H), 1.83(s, 3H). HRESIFTMS [M + H]+: calcd for C38H40NO14, 734.2449, found: 734.2451.
1.14. Nα-(9-Fluorenylmethoxycarbonyl)-O-(2,3,4,6-tetra-O-acetyl-β-D-gulcopyranosyl)-L-tyrosine (12)
White powder, purity, 97%; 1H NMR(500 M Hz, DMSO-d6): δ 7.88(d, J 5 Hz, 2H), 7.64(d, J 5 Hz, 2H), 7.40(m, 2H), 7.30(m, 2H), 7.22(d, J 5 Hz, 2H), 6.90(d, J 5 Hz, 2H), 5.73(d, J 5 Hz, 1H), 5.45(dd, 1H), 5.37(t, J 5 Hz, 1H), 5.05(dd, 1H), 5.03(d, J 10 Hz, 1H), 4.97(t, J 5 Hz, 1H), 4.10–4.19(m, 5H), 4.01(dd, 1H), 3.03(dd, 1H), 2.83(dd, 1H), 2.00(s, 3H), 1.99(s, 3H), 1.97(s, 3H), 1.96(s, 3H). HRESIFTMS [M + H]+: calcd for C38H40NO14, 734.2449, found: 734.2451.
1.15. Nα-(9-Fluorenylmethoxycarbonyl)-O-(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)-L-tyrosine (13)
White powder, purity, 98%; 1H NMR(500 M Hz, DMSO-d6): δ 7.88(d, J 5 Hz, 2H), 7.64(d, J 5 Hz, 2H), 7.40(m, 2H), 7.30(m, 2H), 7.22(d, J 10 Hz, 2H), 7.01(d, J 10 Hz, 2H), 5.74(brs, 1H), 5.46(t, J 10 Hz, 1H), 5.03(d, J 10 Hz, 1H) 5.02(m, 1H), 5.01(m, 1H), 4.92(m, 1H), 4.11–4.21(m, 5H), 3.02(dd, 1H), 2.81(dd, 1H), 2.02(s, 3H), 2.00(s, 6H). HRESIFTMS [M+K]+: calcd for C35H35KNO12, 700.1796, found: 700.1798.
1.16. Nα-(9-Fluorenylmethoxycarbonyl)-O-[(2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)- β-D-glucopyranosyl]-L-tyrosine (14)
White powder, purity, 99%; 1H NMR(500 M Hz, DMSO-d6): δ 7.88(d, J 5 Hz, 2H), 7.64(d, J 5 Hz, 2H), 7.40(m, 2H), 7.29(m, 2H), 7.20(d, J 10 Hz, 2H), 7.02(d, J 5 Hz, 1H), 6.87(d, J 10 Hz, 1H), 5.26(dd, 1H), 5.23(brs, 1H), 5.15–5.19(m, 2H), 4.92–4.96(m, 2H), 4.84–4.87(m, 2H), 4.76(d, J 10 Hz, 1H), 3.87–4.26(m, 9H), 3.03(dd, 1H), 2.82(dd, 1H), 2.10(s, 3H), 2.03(s, 3H), 2.02(s, 6H), 1.99(s, 3H), 1.96(s, 3H), 1.89(s, 3H). HRESIFTMS [M+K]+: calcd for C50H55KNO22, 1060.2853, found: 1060.2855.
1.17. Nα-(9-Fluorenylmethoxycarbonyl)-O-[(2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D- gulcopyranosyl)- β-D-gulcopyranosyl]-L-tyrosine (15)
White powder, purity, 98%; 1H NMR(500 M Hz, DMSO-d6): δ 7.88(d, J 5 Hz, 2H), 7.63(d, J 5 Hz, 2H), 7.41(m, 2H), 7.31(m, 2H), 7.23(d, J 10 Hz, 2H), 7.04(d, J 5 Hz, 1H), 6.87(d, J 10 Hz, 1H), 5.40(dd, 1H), 5.39(brs, 1H), 5.30(d, J 5 Hz, 1H), 5.22–5.26(m, 2H), 4.97(t, J 10 Hz, 2H), 4.88–4.93(m, 2H), 4.12–4.22(m, 6H), 3.98–4.03(m, 3H), 3.03(m, 1H), 2.82(m, 1H), 2.02(s, 3H), 2.00(s, 3H), 1.99(s, 6H), 1.96(s, 6H), 1.94(s, 3H). HRESIFTMS [M+K]+: calcd for C50H55KNO22, 1060.2853, found: 1060.2855.
Acknowledgments
This work was supported by U.S. Department of Defense Breast Cancer Research Program’s Multidisciplinary Postdoctoral Training Award contract no. W81XWH-06-1-0447 (N. Yao), NIH R01CA115483, and U19CA113298. We thank Mr. David Olivos for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Bertozzi CR, Kiessling LL. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]; (b) Zachara NE, Hart GW. Chem Rev. 2002;102:431–438. doi: 10.1021/cr000406u. [DOI] [PubMed] [Google Scholar]; (c) Roth J. Chem Rev. 2002;102:285–304. doi: 10.1021/cr000423j. [DOI] [PubMed] [Google Scholar]; (d) Collins BE, Paulson JC. Curr Opin Chem Biol. 2004;8:617–625. doi: 10.1016/j.cbpa.2004.10.004. [DOI] [PubMed] [Google Scholar]; (e) Gabius HJ, Siebert HC, Andre S, Jimenez-Barbero J, Rudieger H. ChemBioChem. 2004;56:691–702. doi: 10.1002/cbic.200300753. [DOI] [PubMed] [Google Scholar]
- 2.(a) Wang Zh-G, Zhang X, Live D, Danishefsky SJ. Angew Chem Int Ed. 2000;39:3652–3656. doi: 10.1002/1521-3773(20001016)39:20<3652::aid-anie3652>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; (b) Halkes KM, Gotfredsen ChH, GrΦtli M, Miranda LP, Duus J, Meldal M. Chem Eur J. 2001;7:3584–3590. doi: 10.1002/1521-3765(20010817)7:16<3584::aid-chem3584>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (c) Schleyer A, Meldal M, Manat R, Paulsen H, Bock K. Angew Chem Int Ed. 1997;36:1976–1978. [Google Scholar]
- 3.(a) St Hilaire PM, Lowary TL, Meldal M, Bock K. J Am Chem Soc. 1998;120:13312–13320. [Google Scholar]; (b) Mitchell SA, Pratt MR, Hruby VJ, Polt R. J Org Chem. 2001;66:2327–2342. doi: 10.1021/jo005712m. [DOI] [PubMed] [Google Scholar]; (c) Ameijde JV, Albada HB, Liskamp RMJ. J Chem Soc, Perkin Trans. 2002;1:1042–1049. [Google Scholar]
- 4.(a) Hollosi M, Kollat E, Laczko I, Medzihradsky K, Thurin J, Otvos LJ. Tetrahedron Lett. 1991;32:1531–1534. [Google Scholar]; (b) Andrews D, seale P. Int J Pept Prot Res. 1993;42:165–170. doi: 10.1111/j.1399-3011.1993.tb00493.x. [DOI] [PubMed] [Google Scholar]; (c) Paulsen H, Schleyer A, Mathieux N, Meldal M, Bock K. J Chem Soc Perkin Trans. 1997;1:281–293. [Google Scholar]
- 5.(a) Kunz H. Angew Chem Int Ed. 1987;26:294–306. [Google Scholar]; (b) Kihlberg J, Elofsson M. Curr Med Chem. 1997;4:79–110. [Google Scholar]; (c) Herzner H, Reipen T, Schultz M, Kunz H. Chem Rev. 2000;100:4495–4537. doi: 10.1021/cr990308c. [DOI] [PubMed] [Google Scholar]
- 6.(a) Schmidt R. Angew Chem Int Ed. 1986;25:212–235. [Google Scholar]; (b) Bielfeldt T, Peter S, Meldal M, Bock K, Paulsen H. Angew Chem Int Ed. 1992;31:857–859. [Google Scholar]; (c) Mathieux N, Paulsen H, Meldal M, Klaus B. J Chem Soc, Perkin Trans. 1997;1:2359–2368. [Google Scholar]
- 7.(a) Elofsson M, Walse B, Kihlberg J. Tetrahedron Lett. 1991;51:7613–7616. [Google Scholar]; (b) Salvador LA, Elofsson M, Kihlberg J. Tetrahedron. 1995;51:5643–5656. [Google Scholar]
- 8.(a) Miller N, Williams GM, Brimble MA. Org Lett. 2009;11:2409–2412. doi: 10.1021/ol9005536. [DOI] [PubMed] [Google Scholar]; (b) Bagley MC, Davis T, Dix MC, Fusillo V, Pigeaux M, Rokicki MJ, Kipling D. J Org Chem. 2009;74:8336–8342. doi: 10.1021/jo9017155. [DOI] [PubMed] [Google Scholar]; (c) Wang X, Dixon S, Yao N, Kurth MJ, Lam KS. Tetrahedron Lett. 1995;46:5747–5750. [Google Scholar]
- 9.Seibel J, Hillringhaus L, Moraru R. Carbohydr Res. 2005;340:507–511. doi: 10.1016/j.carres.2004.12.014. [DOI] [PubMed] [Google Scholar]


