Abstract
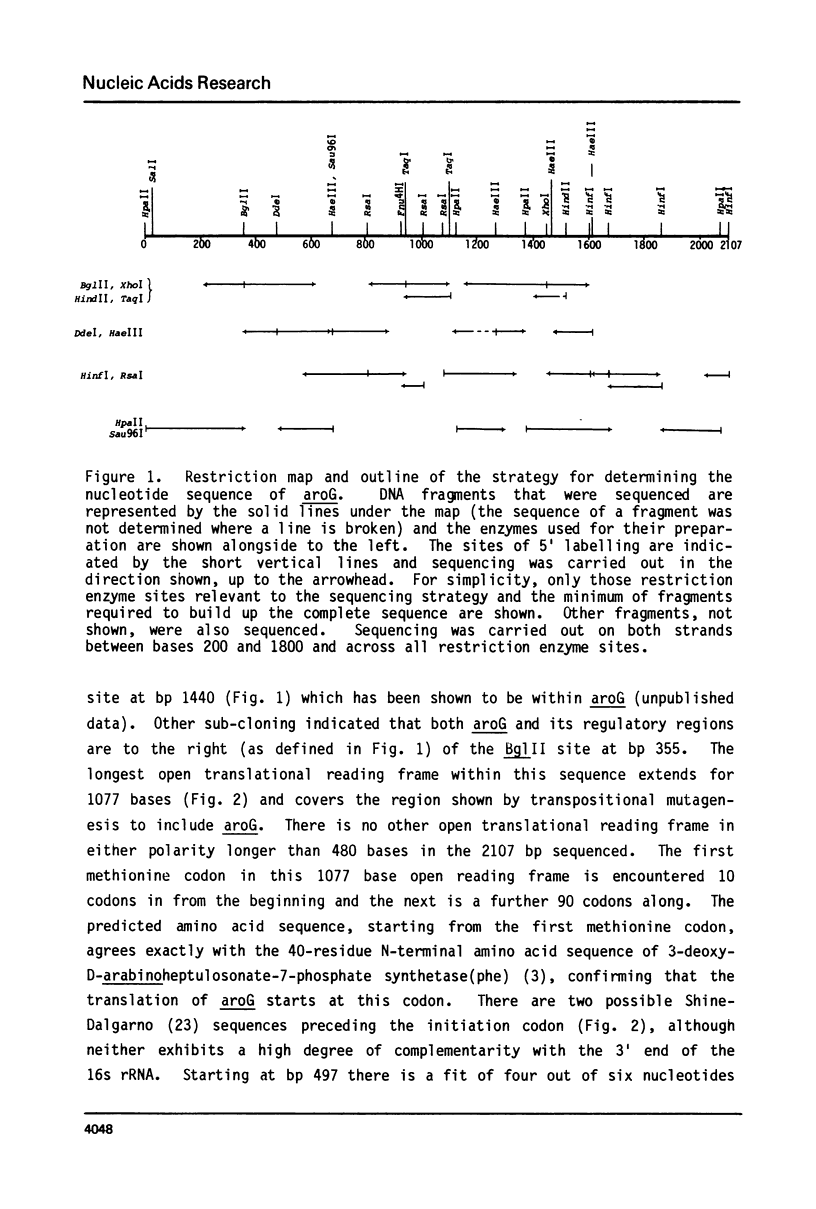
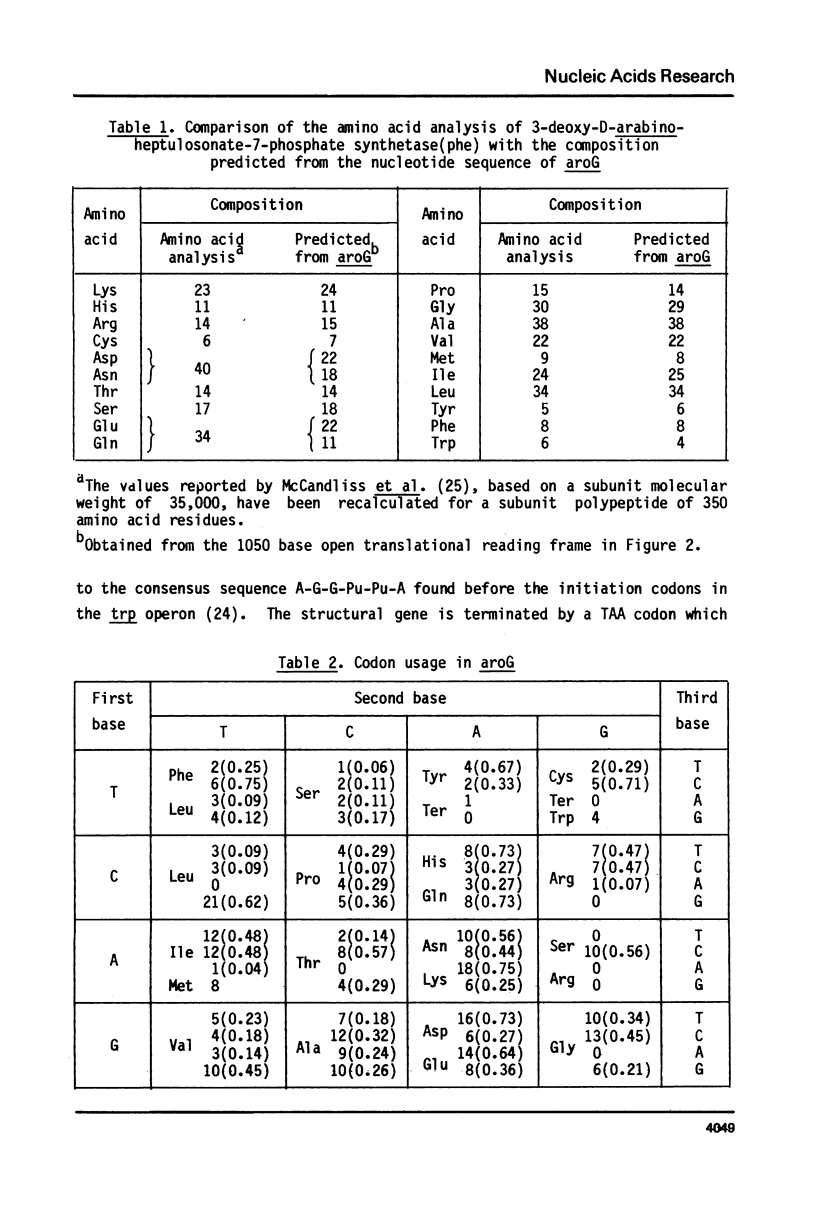
We have determined the nucleotide sequence of aroG, the gene coding for 3-deoxy-D-arabinoheptulosonate-7-phosphate synthetase(phe), one of three isoenzymes that catalyse the first step of the biosynthesis of aromatic amino acids and vitamins in Escherichia coli K12. The DNA sequence agrees with previously published data on the N-terminal sequence, amino acid composition, and subunit molecular weight of 3-deoxy-D-arabinoheptulosonate-7-phosphate synthetase(phe). There is significant identity in the nucleotide sequences of aroG and aroH (the gene for 3-deoxy-D-arabinoheptulosonate-7-phosphate synthetase(trp)), indicating that these two genes have evolved from a common ancestral gene. There is no attenuator structure in the leader region of aroG.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Squires C., Yanofsky C. Transcription termination in vivo in the leader region of the tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):319–337. doi: 10.1016/0022-2836(76)90315-6. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Somerville R. L. Repression of aromatic amino acid biosynthesis in Escherichia coli K-12. J Bacteriol. 1971 Oct;108(1):386–399. doi: 10.1128/jb.108.1.386-399.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camakaris H., Camakaris J., Pittard J. Regulation of aromatic amino acid biosynthesis in Escherichia coli K-12: control of the aroF-tyrA operon in the absence of repression control. J Bacteriol. 1980 Aug;143(2):613–620. doi: 10.1128/jb.143.2.613-620.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo A. B., Dayan J., Sprinson D. B. Purification and kinetics of tyrosine-sensitive 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthetase from Salmonella. J Biol Chem. 1973 Apr 10;248(7):2344–2353. [PubMed] [Google Scholar]
- Gardner J. F. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1706–1710. doi: 10.1073/pnas.76.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Im S. W., Davidson H., Pittard J. Phenylalanine and tyrosine biosynthesis in Escherichia coli K-12: mutants derepressed for 3-deoxy-D-arabinoheptulosonic acid 7-phosphate synthetase (phe), 3-deoxy-D-arabinoheptulosonic acid 7-phosphate synthetase (tyr), chorismate mutase T-prephenate dehydrogenase, and transaminase A. J Bacteriol. 1971 Oct;108(1):400–409. doi: 10.1128/jb.108.1.400-409.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S. W., Pittard J. Phenylalanine biosynthesis in Escherichia coli K-12: mutants derepressed for chorismate mutase P-prephenate dehydratase. J Bacteriol. 1971 Jun;106(3):784–790. doi: 10.1128/jb.106.3.784-790.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The calculation of partial specific volumes of proteins in guanidine hydrochloride. Arch Biochem Biophys. 1974 Nov;165(1):268–273. doi: 10.1016/0003-9861(74)90164-7. [DOI] [PubMed] [Google Scholar]
- Lynn S. P., Cohen L. K., Kaplan S., Gardner J. F. RsaI: a new sequence-specific endonuclease activity from Rhodopseudomonas sphaeroides. J Bacteriol. 1980 May;142(2):380–383. doi: 10.1128/jb.142.2.380-383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCandliss R. J., Poling M. D., Herrmann K. M. 3-Deoxy-D-arabino-heptulosonate 7-phosphate synthase. Purification and molecular characterization of the phenylalanine-sensitive isoenzyme from Escherichia coli. J Biol Chem. 1978 Jun 25;253(12):4259–4265. [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Camakaris J., Wallace B. J. Inhibition of 3-deoxy-d-arabinoheptulosonic acid-7-phosphate synthetase (trp) in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1242–1247. doi: 10.1128/jb.97.3.1242-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Davidson B. E. Studies on 3-deoxy-D-arabinoheptulosonate-7-phosphate synthetase(phe) from Escherichia coli K12. 1. Purification and subunit structure. Eur J Biochem. 1976 Nov 15;70(2):493–500. doi: 10.1111/j.1432-1033.1976.tb11040.x. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Davidson B. E. Studies on 3-deoxy-D-arabinoheptulosonate-7-phosphate synthetase(phe)from Escherichia coli K12. 2. Kinetic properties. Eur J Biochem. 1976 Nov 15;70(2):501–507. doi: 10.1111/j.1432-1033.1976.tb11041.x. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S. R., Calvo J. M. Control of leu operon expression in Escherichia coli by a transcription attenuation mechanism. J Mol Biol. 1981 Jul 15;149(4):579–597. doi: 10.1016/0022-2836(81)90348-x. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Platt T., Crawford I. P., Nichols B. P., Christie G. E., Horowitz H., VanCleemput M., Wu A. M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981 Dec 21;9(24):6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Gunsalus R. P., Brown K. D., Yanofsky C. Structure and regulation of aroH, the structural gene for the tryptophan-repressible 3-deoxy-D-arabino-heptulosonic acid-7-phosphate synthetase of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):47–73. doi: 10.1016/0022-2836(81)90334-x. [DOI] [PubMed] [Google Scholar]



