Photophobia resulting from light-induced sensitization of the trigeminal system can occur after sectioning of the optic nerve.
Abstract
Purpose.
The authors investigated whether trigeminal sensitization occurs in response to bright light with the retina disconnected from the rest of the central nervous system by optic nerve section.
Methods.
In urethane-anesthetized rats, trigeminal reflex blinks were evoked with air puff stimuli directed at the cornea in darkness and at three different light intensities. After normative data were collected, the optic nerve was lesioned and the rats were retested. In an alert rat, reflex blinks were evoked by stimulation of the supraorbital branch of the trigeminal nerve in the dark and in the light.
Results.
A 9.1 × 103 μW/cm2 and a 15.1 × 103 μW/cm2 light significantly enhanced the magnitude of reflex blinks relative to blinks evoked by the same trigeminal stimulus when the rats were in the dark. In addition, rats exhibited a significant increase in spontaneous blinking in the light relative to the blink rate in darkness. After lesioning of the optic nerve, the 15.1 × 103 μW/cm2 light still significantly increased the magnitude of trigeminal reflex blinks.
Conclusions.
Bright lights increase trigeminal reflex blink amplitude and the rate of spontaneous blinking in rodents. Light can modify trigeminal activity without involving the central visual system.
Light can initiate a continuum of negative sensations in the orbit. People can experience disability glare in which light scattering reduces visual contrast and produces a sensation of asthenopia (eyestrain).1–3 An example of this is the effect of facing oncoming headlights when driving at night. Humans typically compensate for glare by squinting and increasing the rate of spontaneous blinking.2,4 Bright lights can also produce ocular discomfort that varies from mild to intolerable pain.5 Persons with diseases involving the anterior portion of the eye (e.g., uveitis or conjunctivitis) that sensitize trigeminal nociceptors report increased sensitivity to light stimuli.6–9 Central trigeminal sensitization such as occurs with migraine and blepharospasm also reduce tolerance to bright light.10–17 Conversely, bright light can exacerbate the effect of trigeminal stimuli.18 Although these data reveal a clear link between light and the trigeminal system in ocular discomfort, the pathophysiology of ocular discomfort is uncertain and probably involves several processes.
One group of explanations of the neural bases of photophobia focuses on central visual structures. Noseda et al.19 show that afferents from melanopsin-containing retinal ganglion cells innervating the posterior thalamus could be responsible for the ability of light to exacerbate migraine discomfort. Other investigators13,20 propose that photophobia also results from modifications in the visual cortex. Another group of investigator21,22 identify a role for the sympathetic system in photophobia. Okamoto et al.22 provide evidence that the activation of spinal trigeminal nociceptive neurons by visual stimuli results from trigeminal nociceptors responding to choroidal blood vessel dilation. Their study indicated that pretectal olivary neurons that are known to receive input from melanopsin-containing retinal ganglion cells regulate the sympathetic nervous system's control of choroidal blood flow.22 Although these explanations can explain the majority of photophobia characteristics, they have difficulty accounting for photophobia experienced by persons without light perception.
Two studies describe patients without light perception who experience pronounced photophobia.23,24 One possible explanation is that these patients maintained visual input to midbrain regions but were unconscious of this visual sensitivity.19 Some of the patients, however, did not exhibit a pupillary light reflex23 that would be expected to be present if the midbrain inputs were intact. Another possibility, however, is that intraretinal processes independent of central visual centers can produce an enhanced trigeminal response to light. To determine whether an intraretinal mechanism acts in concert with other processes to produce photophobia, we characterized the effects of bright light on reflex and spontaneous blinking before and after lesioning the optic nerve in rats.
Methods
The effects of light on spontaneous and reflex blinks were measured in six male Sprague-Dawley rats. All experiments were carried out in adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Visual research and received institutional review board approval. For acute experiments, five rats were anesthetized with 1.5 g/kg urethane and were placed in a stereotactic device. Orbicularis oculi electromyelography (OOemg) recordings were performed with a pair of fluoropolymer (Teflon; DuPont, Wilmington, DE)–coated stainless steel wires (0.003 inch diameter bare, 0.0055 inch coated; A-M Systems, Everett, WA) implanted into the orbicularis oculi muscle near the lateral canthus.25 Under general anesthesia (ketamine 90 mg/kg, xylazine 10 mg/kg), one rat was prepared for chronic recording of the OOemg and stimulation of the supraorbital branch of the trigeminal nerve.26 This rat received analgesics (ketorolac, 7 mg/kg) for 24 hours after surgery. The rat was alert and eating within the first day after surgery, but at least 1 week passed before the experiments began. Data were collected during the rat's subjective night.
Procedures
Blinking was measured under dark and light conditions. To evoke trigeminal reflex blinks in urethane anesthetized rats, a 50- to 150-ms air puff (20 psi at the source) was delivered through a pipette placed 3 to 4 cm in front of the cornea. The air puff stimulus directed at the cornea elicited a single burst of OOemg activity (Fig. 1A).27 To evoke trigeminal reflex blinks in the alert rat, we delivered a 100-μs electrical stimulus to the supraorbital nerve at twice the threshold current required to evoke a reliable blink. Electrical stimulation of the supraorbital nerve evoked two bursts of OOemg activity, R1 and R2 (see Fig. 6A).26 The difference in the pattern of OOemg activity evoked by corneal and supraorbital nerve stimulation reflected the activation of different trigeminal blink circuits.28–30 For both anesthetized and alert rats, reflex-evoking stimuli were presented every 40 seconds during data collection.
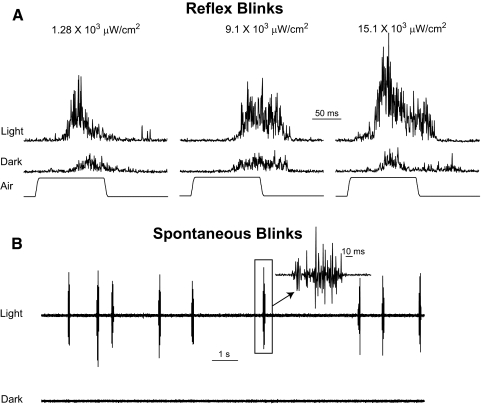
Figure 1.
Effect of light on air puff–evoked reflex blinks. (A) Each trace is the average of five consecutive rectified OOemg responses evoked in the dark or in light levels of 1.28 × 103, 9.1 × 103, or 15.1 × 103 μW/cm2. (B) A 15-second recording of unrectified OOemg activity in 15.1 × 103 μW/cm2 light and in the dark. (inset) Spontaneous blink at a higher temporal resolution.
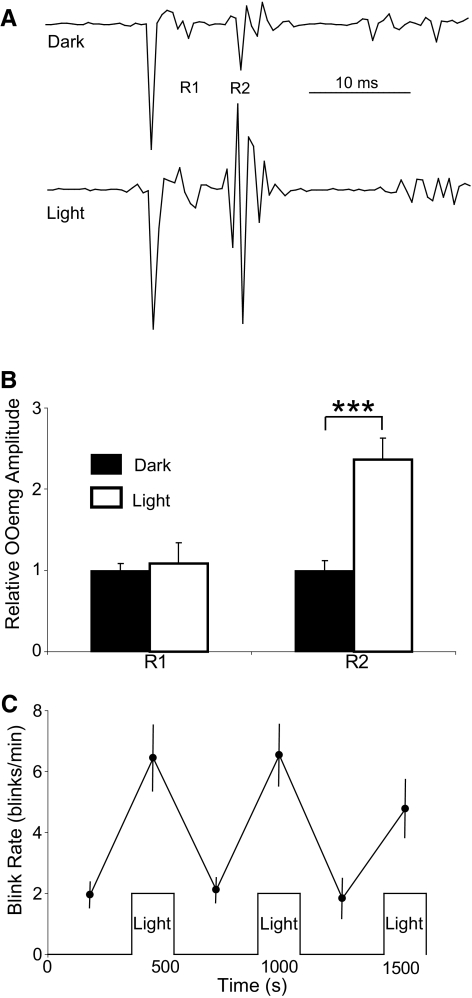
Figure 6.
Effects of light on alert rat. (A) Blink evoked by stimulation of the supraorbital nerve (SO) in the dark and in a 15.1 × 103 μW/cm2 light. R1 and R2 identify the two components of the trigeminal-evoked blink. (B) Relative OOemg amplitudes of the R1 and R2 components over all days and trials in the dark (solid bars) and light (open bars). (C) Average spontaneous blink rate in the light and dark trials across all days. ***P < 0.001.
To present light to the anesthetized rats, a light pipe was placed 5 cm directly in front of the eye. Three light stimuli were used (1.28 × 103 μW/cm2, 9.1 × 103 μW/cm2, and 15.1 × 103 μW/cm2). To minimize corneal heating, an infrared cutoff filter (Edmund Optics, Barrington, NJ) was attached at the base of the light pipe to block infrared waves. To determine whether the light heated the cornea significantly, we placed a thermometer at the same place as the cornea in front of the IR-filtered light pipe. A 4-minute presentation of the maximal light stimulus produced less than a 0.5°C increase in temperature. With the light on, the temperature at the cornea was always below 35°C. This temperature was not reported to activate corneal heat receptive nociceptors.31 In humans, it is necessary to increase corneal temperature 1.2°C ± 0.2°C to produce a sensation of heating.32
For the anesthetized rats, a cycle of data was collected continuously over a 30-minute period. After 2 minutes in darkness in which baseline data were collected, the light was turned on for 4 minutes, followed by 6 minutes of darkness. This light-dark pattern was repeated three times. Only one light intensity was presented during each data cycle collection. Each rat went through three cycles of data collection to determine the effects of the three different light intensities. The order of light intensity presentation was 9.1 × 103 μW/cm2, 1.28 × 103 μW/cm2, and 15.1 × 103 μW/cm2. After three cycles of data collection, we lesioned the optic nerve in each rat. The skull was removed around bregma, and all brain tissue overlying the optic chiasm was aspirated to expose the optic nerves. After visualizing the nerves with an operating microscope, we severed the nerves taking care not to disrupt the blood supply to the eyeball. The brain defect was packed with cotton. The pupil was checked to see that it was dilated and unreactive to light. Approximately 1 hour after the lesion, we repeated the three cycles of light stimulation. At the conclusion of the experiment, we killed each rat and confirmed complete sectioning of the optic nerve. Four of the five rats had complete optic nerve sections.
For the alert rat, the light-dark pattern was 6 minutes of darkness followed by 3 minutes of light. This 9-minute pattern was repeated three times. Each day, the rat received two of these 27-minute light-dark cycles. For the alert rat, light was delivered through two light pipes placed at either end of the rat's home cage. During the experiment, the rat moved freely about its cage. Infrared filters were not used on these light pipes because they were not close to the rat's cornea. Although the light intensity for the alert rat was 15.1 × 103 μW/cm2 1 cm from the light pipe, the rat did not experience the full light intensity because it moved freely about its cage. At the center of the cage, light intensity was 75% of the intensity at either end of the cage. Data were collected for 2 weeks.
All OOemg data were sampled at 2 kHz per channel (Data Translation, Marlboro, MA; 12-bit analog-to-digital resolution) and were stored for later analysis using laboratory-developed software. We calculated the following: OOemg amplitude, integration of the OOemg activity for the entire blink; OOemg duration, blink duration in ms; and OOemg amplitude/ms, OOemg amplitude divided by OOemg duration. OOemg amplitude/ms revealed increases in OOemg magnitude independent of blink duration. Results are presented as mean ± SEM. Statistical analyses were performed with a commercial software package (SPSS; IBM, Somers, NY). Post hoc testing of ANOVA was performed with the Tukey HSD.
Results
Effect of Light in Normal Rats
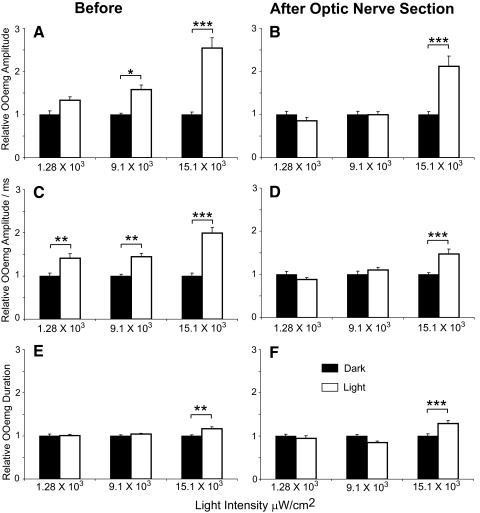
In urethane-anesthetized rats, the same air puff stimulus evoked a larger blink in the light than in the dark (Fig. 1A). OOemg activity increased in a light intensity–dependent manner. ANOVA (F(3,341) = 21.9; P < 0.001) demonstrated that OOemg amplitude increased significantly at the 9.1 × 103 μW/cm2 (P < 0.05) and 15.1 × 103 μW/cm2 (P < 0.001) light intensities relative to OOemg amplitude in the dark before the first light presentation (Fig. 2A). OOemg duration, however, increased significantly only at the highest light intensity (F(3,341) = 6.2; P < 0.005; Fig. 2E). ANOVA (F(3,341) = 15.75, P < 0.001) demonstrated a significant increase in OOemg amplitude per millisecond at all light intensities relative to OOemg amplitude per millisecond in the dark before the first light presentation (Fig. 2C). Thus, bright lights exaggerated the response to trigeminal stimuli in anesthetized rats, as occurs in humans experiencing photophobia.33
Figure 2.
Effect of light on reflex blinks before (A, C, E) and after (B, D, F) optic nerve section. (A) OOemg amplitude relative to the average OOemg amplitude of the first five dark trials at the three different light intensities. (C) OOemg amplitude divided by OOemg duration relative to the average OOemg amplitude per millisecond of the first five dark trials at the three different light intensities. (E) OOemg duration relative to the average OOemg duration of the first five dark trials at the three different light intensities. (B) OOemg amplitude relative to the average OOemg amplitude of the first five dark trials at the three different light intensities. (D) OOemg amplitude divided by OOemg duration relative to the average OOemg amplitude per millisecond of the first five dark trials at the three different light intensities. (F) OOemg duration relative to the average blink duration of the first five dark trials at three different light intensities. (filled bars) Data collected in the dark. (open bars) Data collected in the light. *P < 0.05; **P < 0.005; ***P < 0.001.
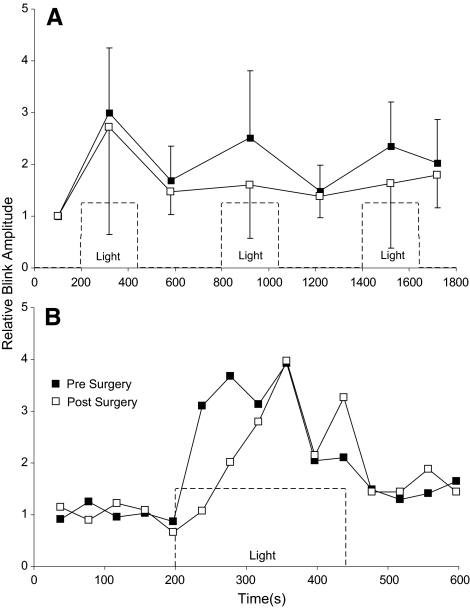
Blink amplitude facilitation was not uniform across all three of the 15.1 × 103 μW/cm2 light presentations in a cycle of data collection (Fig. 3A). Although there was a trend for the first light presentation to produce the largest reflex blink potentiation when averaged across all rats, this effect was not significant (F(69) = 0.67; P > 0.05). In individual rats, however, 2 of the 4 rats with a complete lesion exhibited a significantly larger blink potentiation with the first light presentation than with the second or third presentation (F(15) = 15.04, P < 0.001; F(15) = 7.9, P < 0.01). Because the air puff stimulus occurred at fixed intervals, it was not possible to determine how long the light had to be on for blink potentiation to develop. Nevertheless, potentiation had to develop in <37 seconds when the first air puff stimulus was delivered after light onset (Fig. 3B).
Figure 3.
(A) Relative OOemg amplitude for the four rats with complete optic nerve sections before (■) and after (□) optic nerve section as a function of time. Each point is the average relative OOemg amplitude for all the reflex blinks evoked during that light or dark time interval. (B) The relative blink amplitude of blinks made during the first light presentation averaged across all four rats. Dashed lines: onset and offset of 15.1 × 103 μW/cm2 light.
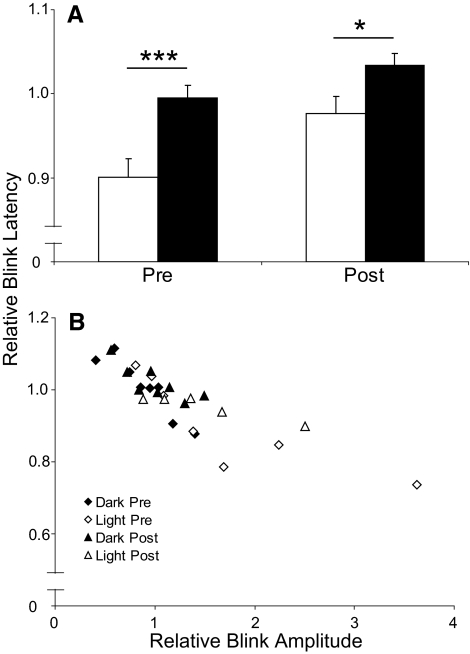
The presence of the brightest light, 15.1 × 103 μW/cm2, significantly decreased the latency of air puff–evoked blinks relative to blinks evoked in the dark (Figs. 1A, 4). Among individual animals, the average blink latency in the dark ranged from 55.1 ms to 85.3 ms. To enable comparisons between animals, blink latency was normalized to the average blink latency in the first period of darkness for each animal. In all animals, the brightest light significantly decreased blink latency by 10% relative to blinks evoked in the dark (Fig. 4A; t(178) = −3.71; P < 0.001). The amount of latency shortening, however, depended on blink amplitude (Fig. 4B). Plotting the blink latency relative to the average blink latency during the first period of darkness for each animal as a function of the blink amplitude relative to average blink amplitude during the first period of darkness demonstrated that blink latency decreased with increasing blink amplitude. Consistent with the enhancement of blink amplitude by the light, most of the light-evoked blinks had relative latency values <1, indicating shortened blink latencies (Fig. 4B), whereas most blinks elicited in the dark had values >1 (Fig. 4B). Thus, the reduced latency associated with the brightest light appeared to result from light's potentiation of blink amplitude.
Figure 4.
(A) Latency of air puff–evoked blinks relative to blink latency during the first period of darkness in 15.1 × 103 μW/cm2 light (open bars) and in the dark (solid bars) before (Pre) and after (Post) optic nerve lesion. (B) Latency of air puff–evoked blinks relative to blink latency during the first period of darkness in 15.1 × 103 μW/cm2 light (◊, ▵) and in the dark (⧫, ▴) as a function of blink potentiation before (Pre) and after (Post) optic nerve lesion. *P < 0.05; ***P < 0.001.
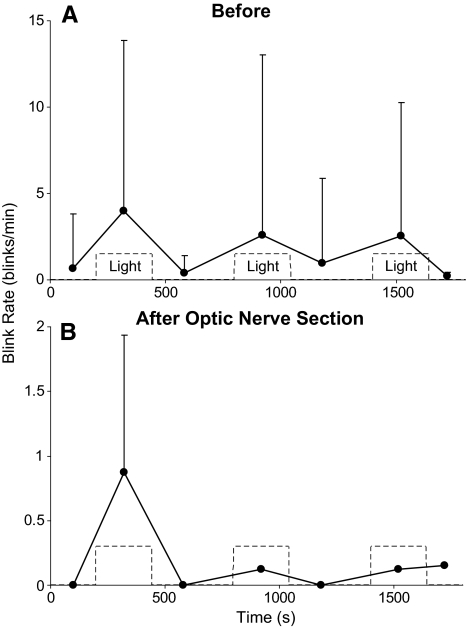
In the light, but rarely in the dark, 4 of the 5 anesthetized rats exhibited spontaneous blinks (Figs. 1B, 5A). The shortest latency from light onset for these blinks, 7.2 seconds, was at least 70-fold longer than the shortest latency of blinks evoked by the onset of bright light (40–100 ms).34–37 In addition, the blinks occurred periodically rather than being linked to light onset. These data indicate that these were spontaneous blinks rather than light-evoked reflex blinks. As with the potentiation of blink amplitude by the 15.1 × 103 μW/cm2 light, there was a trend for spontaneous blinks to be more frequent in the first light presentation (F(9) = 3.84; P = 0.06). Thus, light potentiated trigeminal reflex blinks (Figs. 1–3) and initiated spontaneous blinks (Figs. 1B, 5A) in anesthetized rats.
Figure 5.
(A) Spontaneous blink rate during 240 seconds of 15.1 × 103 μW/cm2 light presentation (Light) and periods of darkness averaged across four anesthetized rats before optic nerve lesion. (B) Spontaneous blink rate during 240-second 15.1 × 103 μW/cm2 light presentations (Light) and periods of darkness averaged across the two rats exhibiting spontaneous blinks after the optic nerve lesion.
Intense light produced similar changes in the blinking of the alert rat, as occurred with anesthetized rats (Fig. 6). In the presence of the 15.1 × 103 μW/cm2 light, there was an insignificant increase in the R1 component of the SO-evoked blink reflex (t(54) = −0.83, P > 0.05; Figs. 6A, 6B) but a significant increase in the amplitude of the R2 component (t(54) = −4.45, P < 0.001; Figs. 6A, 6B) relative to the blinks evoked in the dark before the first light presentation. The light also increased the rate of spontaneous blinking (Fig. 6C). In the dark, the average blink rate was 2.2 ± 0.23, whereas in the light, the average blink rate significantly increased to 6.6 ± 0.46 (t(98) = −8.04; P < 0.001). Thus, as with humans experiencing photophobia, a bright light enhanced trigeminal reflex blink amplitude and increased the rate of spontaneous blinking in urethane-anesthetized and alert rats. Given that the trigeminal reflex circuits involved in producing corneal and supraorbital reflex blinks are different,28–30 light produced a general rather than a localized increase in trigeminal excitability.
Effect of Light in Rats with Optic Nerve Lesions
The 15.1 × 103 μW/cm2 light intensity, but not the intermediate intensity, continued to modify trigeminal reflex blinks after the optic nerve was cut (Figs. 2, 3). Five rats were tested before and after lesioning. Complete sectioning of the optic nerve was verified in 4 of the 5 rats. These four rats were the source of the data reported after lesioning for before and after comparisons. The surgery to lesion the optic nerve reduced blink amplitude an average of 37% relative to blink amplitude before surgery. After the optic nerve lesion, however, the brightest light still significantly increased OOemg amplitude (P < 0.001; Figs. 2B, 3A), OOemg amplitude/ms (P < 0.001; Fig. 2D), and OOemg duration (P < 0.001; Fig. 2F). The relative increase in OOemg amplitude and duration produced by this light intensity was not significantly different before and after optic nerve lesioning (OOemg amplitude, t(160) = 1.24, P > 0.05; OOemg duration, t(160) = −1.6, P > 0.05). The relative increase in OOemg amplitude per millisecond produced by the light, however, was significantly lower after the optic nerve lesion than before it (t(160) = 2.85; P < 0.005). Averaging relative OOemg amplitude for each blink across all 15.1 × 103 μW/cm2 light presentations showed a trend toward a slower development of blink potentiation after the lesion, but the differences did not reach statistical significance because of the small number of animals (Fig. 3B).
Two of the four rats that exhibited spontaneous blinks in the light continued to generate spontaneous blinks after the optic nerve lesion. The rate of spontaneous blinking in these two rats, however, was only approximately 10% of their rate of blinking before lesioning (Fig. 5B).
Discussion
In the presence of bright light, anesthetized and alert rats exhibit blink modifications like those of people experiencing ocular discomfort and photophobia. Bright light significantly increases the sensitivity of people to trigeminal stimuli,14,15,18 and rats respond more strongly to trigeminal stimuli presented in bright light than in darkness (Figs. 1–4, 6). Bright light significantly increases squinting and the spontaneous blink rate in people,2,33,38,39 and rats generate more spontaneous blinks in the presence of bright lights than in darkness (Figs. 1, 5, 6). Although these parallel behaviors indicate neural mechanisms for photophobia shared by humans and rodents, an alternative explanation for the effect of light is acute stress or fear created by the aversion of rats to bright light. Although stress or fear can increase the spontaneous blink rate of alert humans,40 these emotions cannot explain the increased blink rate of anesthetized rats in bright light. Fear can increase the amplitude of trigeminal reflex blinks in alert animals,41 whereas stress can have no effect42 or can depress reflex blink magnitude.43 Fear, however, does not account for the increased reflex blink amplitude in anesthetized rats (Figs. 1–3, 6). Moreover, the decreased blink amplitude produced by stress42,43 is in contrast to the increased reflex blink amplitude produced by light. The simplest explanation for the effects of bright light on reflex and spontaneous blinking shared by humans and rodents is that bright light activates similar neural circuits in the two species.
There is abundant evidence that photophobia involves interactions between the trigeminal system and portions of the central visual system that receive inputs from melanopsin-containing retinal ganglion cells such as the pretectal olivary nucleus.19,22 One circuit involved in photophobia is a projection of melanopsin-containing retinal ganglion cells to thalamic nuclei that receive nociceptive inputs from the spinal trigeminal system.19 This mechanism can account for the ability of light to exacerbate the pain experienced by migraineurs.17,18,44,45 Through a mechanism involving the pretectal olivary nucleus, bright light increases choroidal blood flow.46,47 Another circuit supporting photophobia is the activation of spinal trigeminal nociceptive neurons by choroidal vessel dilation. Thus, a bright light indirectly activates spinal trigeminal nociceptors.22,48 This mechanism can explain why trigeminal nociceptor sensitization increases the painful quality of bright light.14,18,49,50 In addition to these processes involving central nervous system visual centers, our data reveal an intraretinal process modulating the trigeminal system to produce the ocular discomfort associated with bright light.
Although we have not identified this intraretinal mechanism, associational ganglion cells are a candidate for affecting trigeminal activity independent of central visual pathways. The axons of associational ganglion cells do not enter the optic nerve. Instead they extend into the retinal periphery near the pars plana of the ciliary body.51 This region is richly innervated with trigeminal nociceptors.52–57 Associational ganglion cells may directly activate trigeminal nociceptors to sensitize spinal trigeminal nucleus neurons. An indirect activation of trigeminal nociceptors could occur if associational ganglion cell activity modulates uveal blood flow, which in turn activates trigeminal nociceptors associated with these blood vessels.53,55,56 In primates and cats, associational ganglion cells terminate on pericytes surrounding retinal blood vessels.51 Regardless of the mechanism, the data demonstrate that trigeminal blink enhancement by light can occur after optic nerve lesion and is an additional pathway through which light and the trigeminal system interact to create photophobia. Thus, blind persons may experience photophobia independently of central visual pathways.
Acknowledgments
The authors thank Donna Schmidt for technical assistance and Jaime Kaminer, Tzvia Pinkhasov, Alice S. Powers, and Michael Ryan for their valuable comments on earlier drafts of this manuscript.
Footnotes
Supported by National Eye Institute Grant EY07391 (CE) and by the Stony Brook University School of Medicine Dean's Office (SD).
Disclosure: S. Dolgonos, None; H. Ayyala, None; C. Evinger, None
References
- 1. Aslam TM, Haider D, Murray IJ. Principles of disability glare measurement: an ophthalmological perspective. Acta Ophthalmol Scand. 2007;85:354–360 [DOI] [PubMed] [Google Scholar]
- 2. Nahar NK, Sheedy JE, Hayes J, Tai YC. Objective measurements of lower-level visual stress. Optom Vis Sci. 2007;84:620–629 [DOI] [PubMed] [Google Scholar]
- 3. Sheedy JE, Hayes JN, Engle J. Is all asthenopia the same? Optom Vis Sci. 2003;80:732–739 [DOI] [PubMed] [Google Scholar]
- 4. Sheedy JE, Truong SD, Hayes JR. What are the visual benefits of eyelid squinting? Optom Vis Sci. 2003;80:740–744 [DOI] [PubMed] [Google Scholar]
- 5. Murray IJ, Plainis S, Carden D. The ocular stress monitor: a new device for measuring discomfort glare. Lighting Res Technol. 2002;34:231–239 [Google Scholar]
- 6. Nelson JD, Helms H, Fiscella R, Southwell Y, Hirsch JD. A new look at dry eye disease and its treatment. Adv Ther. 2000;17:84–93 [DOI] [PubMed] [Google Scholar]
- 7. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500, discussion 500 [DOI] [PubMed] [Google Scholar]
- 8. Lowder CY, Char DH. Uveitis: a review. West J Med. 1984;140:421–432 [PMC free article] [PubMed] [Google Scholar]
- 9. Tabbara KF, Sharara N. Dry eye syndrome. Drugs Today (Barc). 1998;34:447–453 [DOI] [PubMed] [Google Scholar]
- 10. Adams WH, Digre KB, Patel BC, Anderson RL, Warner JE, Katz BJ. The evaluation of light sensitivity in benign essential blepharospasm. Am J Ophthalmol. 2006;142:82–87 [DOI] [PubMed] [Google Scholar]
- 11. Emoto H, Suzuki Y, Wakakura M, et al. Photophobia in essential blepharospasm—a positron emission tomographic study. Mov Disord. 2010;25:433–439 [DOI] [PubMed] [Google Scholar]
- 12. Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF International Workshop. Neurology. 2008;71:1275–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chronicle EP, Mulleners WM. Visual system dysfunction in migraine: a review of clinical and psychophysical findings. Cephalalgia. 1996;16:525–535, discussion 523 [DOI] [PubMed] [Google Scholar]
- 14. Drummond PD. A quantitative assessment of photophobia in migraine and tension headache. Headache. 1986;26:465–469 [DOI] [PubMed] [Google Scholar]
- 15. Evans RW, Digre KB. Light sensitivity in migraineurs. Headache. 2003;43:917–920 [DOI] [PubMed] [Google Scholar]
- 16. Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37:492–495 [DOI] [PubMed] [Google Scholar]
- 17. Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733–741 [DOI] [PubMed] [Google Scholar]
- 18. Kowacs PA, Piovesan EJ, Werneck LC, et al. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 2001;21:184–188 [DOI] [PubMed] [Google Scholar]
- 19. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jan JE, Groenveld M, Anderson DP. Photophobia and cortical visual impairment. Dev Med Child Neurol. 1993;35:473–477 [DOI] [PubMed] [Google Scholar]
- 21. Fine PG, Digre KB. A controlled trial of regional sympatholysis in the treatment of photo-oculodynia syndrome. J Neuroophthalmol. 1995;15:90–94 [PubMed] [Google Scholar]
- 22. Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amini A, Digre K, Couldwell WT. Photophobia in a blind patient: an alternate visual pathway: case report. J Neurosurg. 2006;105:765–768 [DOI] [PubMed] [Google Scholar]
- 24. Custer PL, Reistad CE. Enucleation of blind, painful eyes. Ophthal Plast Reconstr Surg. 2000;16:326–329 [DOI] [PubMed] [Google Scholar]
- 25. Henriquez VM, Evinger C. Modification of cornea-evoked reflex blinks in rats. Exp Brain Res. 2005;163:445–456 [DOI] [PubMed] [Google Scholar]
- 26. Dauvergne C, Evinger C. Experiential modification of the trigeminal reflex blink circuit. J Neurosci. 2007;27:10414–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evinger C, Basso MA, Manning KA, Sibony PA, Pellegrini JJ, Horn AK. A role for the basal ganglia in nicotinic modulation of the blink reflex. Exp Brain Res. 1993;92:507–515 [DOI] [PubMed] [Google Scholar]
- 28. Cruccu G, Agostino R, Berardelli A, Manfredi M. Excitability of the corneal reflex in man. Neurosci Lett. 1986;63:320–324 [DOI] [PubMed] [Google Scholar]
- 29. Henriquez VM, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res. 2007;179:691–702 [DOI] [PubMed] [Google Scholar]
- 30. Pellegrini JJ, Horn AK, Evinger C. The trigeminally evoked blink reflex, I: neuronal circuits. Exp Brain Res. 1995;107:166–180 [DOI] [PubMed] [Google Scholar]
- 31. Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525 [DOI] [PubMed] [Google Scholar]
- 32. Acosta MC, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001;534:511–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolff H. Headache and Other Head Pain. New York: Oxford University Press; 1948:642 [Google Scholar]
- 34. Burke J, Hackley SA. Prepulse effects on the photic eyeblink reflex: evidence for startle-dazzle theory. Psychophysiology. 1997;34:276–284 [DOI] [PubMed] [Google Scholar]
- 35. Gruart A, Blazquez P, Delgado-Garcia JM. Kinematics of spontaneous, reflex, and conditioned eyelid movements in the alert cat. J Neurophysiol. 1995;74:226–248 [DOI] [PubMed] [Google Scholar]
- 36. Manning KA, Evinger C. Different forms of blinks and their two-stage control. Exp Brain Res. 1986;64:579–588 [DOI] [PubMed] [Google Scholar]
- 37. Tackmann W, Ettlin T, Barth R. Blink reflexes elicited by electrical, acoustic and visual stimuli, I: normal values and possible anatomical pathways. Eur Neurol. 1982;21:210–216 [DOI] [PubMed] [Google Scholar]
- 38. Stringham JM, Fuld K, Wenzel AJ. Action spectrum for photophobia. J Opt Soc Am A Opt Image Sci Vis. 2003;20:1852–1858 [DOI] [PubMed] [Google Scholar]
- 39. Stringham JM, Fuld K, Wenzel AJ. Spatial properties of photophobia. Invest Ophthalmol Vis Sci. 2004;45:3838–3848 [DOI] [PubMed] [Google Scholar]
- 40. Ponder E, Kennedy WP. On the act of blinking. Exp Physiol. 1927;18:89–110 [Google Scholar]
- 41. Lam YW, Wong A, Canli T, Brown TH. Conditioned enhancement of the early component of the rat eyeblink reflex. Neurobiol Learn Mem. 1996;66:212–220 [DOI] [PubMed] [Google Scholar]
- 42. Bangasser DA, Shors TJ. Acute stress impairs trace eye blink conditioning in females without altering the unconditioned response. Neurobiol Learn Mem. 2004;82:57–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beck KD, Servatius RJ. Stress and cytokine effects on learning: what does sex have to do with it? Integr Physiol Behav Sci. 2003;38:179–188 [DOI] [PubMed] [Google Scholar]
- 44. Vanagaite Vingen J, Stovner LJ. Photophobia and phonophobia in tension-type and cervicogenic headache. Cephalalgia. 1998;18:313–318 [DOI] [PubMed] [Google Scholar]
- 45. Kawasaki A, Purvin VA. Photophobia as the presenting visual symptom of chiasmal compression. J Neuroophthalmol. 2002; 22:3–8 [DOI] [PubMed] [Google Scholar]
- 46. Fitzgerald ME, Gamlin PD, Zagvazdin Y, Reiner A. Central neural circuits for the light-mediated reflexive control of choroidal blood flow in the pigeon eye: a laser Doppler study. Vis Neurosci. 1996;13:655–669 [DOI] [PubMed] [Google Scholar]
- 47. Fuchsjager-Mayrl G, Polska E, Malec M, Schmetterer L. Unilateral light-dark transitions affect choroidal blood flow in both eyes. Vision Res. 2001;41:2919–2924 [DOI] [PubMed] [Google Scholar]
- 48. Okamoto K, Bereiter DF, Tashiro A, Bereiter DA. Ocular surface-evoked Fos-like immunoreactivity is enhanced in trigeminal subnucleus caudalis by prior exposure to endotoxin. Neuroscience. 2009;159:787–794 [DOI] [PubMed] [Google Scholar]
- 49. Gutrecht JA, Lessell IM. Photophobia in trigeminal neuralgia. J Neuroophthalmol. 1994;14:122–123 [PubMed] [Google Scholar]
- 50. Drummond PD, Woodhouse A. Painful stimulation of the forehead increases photophobia in migraine sufferers. Cephalalgia. 1993;13:321–324 [DOI] [PubMed] [Google Scholar]
- 51. Yazulla S, Studholme KM. Vanilloid receptor like 1 (VRL1) immunoreactivity in mammalian retina: colocalization with somatostatin and purinergic P2X1 receptors. J Comp Neurol. 2004;474:407–418 [DOI] [PubMed] [Google Scholar]
- 52. Firth SI, Kaufman PL, De Jean BJ, Byers JM, Marshak DW. Innervation of the uvea by galanin and somatostatin immunoreactive axons in macaques and baboons. Exp Eye Res. 2002;75:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lehtosalo JI, Uusitalo H, Palkama A. Sensory supply of the anterior uvea: a light and electron microscope study. Exp Brain Res. 1984;55:562–569 [DOI] [PubMed] [Google Scholar]
- 54. Ruskell GL. Trigeminal innervation of the scleral spur in cynomolgus monkeys. J Anat. 1994;184(pt 3):511–518 [PMC free article] [PubMed] [Google Scholar]
- 55. Terenghi G, Polak JM, Ghatei MA, et al. Distribution and origin of calcitonin gene-related peptide (CGRP) immunoreactivity in the sensory innervation of the mammalian eye. J Comp Neurol. 1985;233:506–516 [DOI] [PubMed] [Google Scholar]
- 56. Uusitalo H, Krootila K, Palkama A. Calcitonin gene-related peptide (CGRP) immunoreactive sensory nerves in the human and guinea pig uvea and cornea. Exp Eye Res. 1989;48:467–475 [DOI] [PubMed] [Google Scholar]
- 57. Wang ZY, Alm P, Hakanson R. Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye. Neuroscience. 1995;69:297–308 [DOI] [PubMed] [Google Scholar]