Abstract
Although the combustion of natural and synthetic products can yield cyanide, its toxic role in residential fires is unclear. This case concerns a woman aged over 50 years who presented comatose, pulseless and apnoeic after a domestic fire. Cardiopulmonary resuscitation and on-site administration of 2.5 g hydroxocobalamin as an antidote to cyanide resulted in a return of spontaneous circulation. On admission to the intensive care unit, the patient was treated with hyperbaric oxygen for suspected carbon monoxide poisoning. In a blood specimen collected at the scene before hydroxocobalamin administration, blood cyanide and carbon monoxide levels were 68 µmol/l and 10.9%. On admission to hospital, plasma lactate was at 4.6 mmol/l. Brain scans revealed lesions which were confirmed 2 months later, consistent with the haemorrhagic necrosis often seen after poisoning by cyanide. These data suggest that smoke inhalation in a residential fire may cause cyanide poisoning. This case provides clinical, biological, analytical and brain imaging data supporting the hypothesis of the toxic role of smoke-induced cyanide poisoning which may result in neurological sequelae.
Background
The toxic role of cyanide in humans exposed to residential fire smoke remains a matter of debate.1–4 Some investigators claim fatal cyanide exposure is uncommon, and that even when inhalation occurs, it is almost always accompanied by elevated or lethal levels of carbon monoxide.3 Potentially toxic blood cyanide concentrations have sometimes been reported in fatal and living human fire victims with non-lethal carboxyhaemoglobin concentrations,3 5–16 but analytical data that could sufficiently support or refute this claim are lacking in the vast majority of cases, and long-term follow-up data are even rarer.
To support the assumption that cyanide is a primary toxicant in fire smoke, we present the case of a near-fatal residential fire victim with data from comprehensive acute clinical, biological, analytical and therapeutic assessments, and long-term neurological follow-up.
Case presentation
A female non-smoker was found comatose, apnoeic and pulseless at the scene of a residential fire. Cardiopulmonary resuscitation was immediately performed by the emergency service and a medically-staffed ambulance arrived after 8 min. The patient had a Glasgow Coma Score (GCS) of 3. Endotracheal intubation was performed and the patient was mechanically ventilated with 100% oxygen. Because cyanide poisoning was suspected, the patient received an intravenous 2.5 g dose of hydroxocobalamin while being resuscitated at the scene of the fire. Her blood pressure subsequently rose to 120/80 mm Hg without any further requirement for fluid repletion or catecholamine treatment. No additional doses of hydroxocobalamin were administered during her treatment.
On admission to our toxicological intensive care unit, the patient’s GCS was 7 and she had intermediate and symmetric-reactive pupils. Deep tendon reflexes were brisk and symmetric; a Babinski sign was present on the left. The patient had soot deposits on her face and mouth but no cutaneous burns. A pink skin colour was noted and attributed to hydroxocobalamin. Her heart rate was 80 bpm, blood pressure 120/80 mm Hg, and she was mechanically ventilated. Her ECG was normal.
Investigations
Carboxyhaemoglobin levels and blood cyanide concentrations were measured before and after hydroxocobalamin administration and hyperbaric oxygen treatment. These were 10.9% and 68 µmol/l, respectively at the scene of the fire, and reduced to 0.9% and 3.8 µmol/l after 6 h. Plasma lactate concentration decreased from 4.6 mmol/l on admission to 2.7 mmol/l 4 h later. On admission, the anion gap at 17.7 mmol/l was mildly increased and completely explained by the corresponding plasma lactate. Serum creatinine concentration was 99 µmol/l which returned to 47 µmol/l after 48 h.
CT scans were carried out on day 2 and day 17 after exposure (figure 1a,d). MRI (0.5 T magnet) on day 7 (figure 1b,c) and day 60 confirmed the haemorrhagic necrosis observed earlier via CT (figure 1e). Cortical atrophy and ventricular enlargement were also observed.
Figure 1.
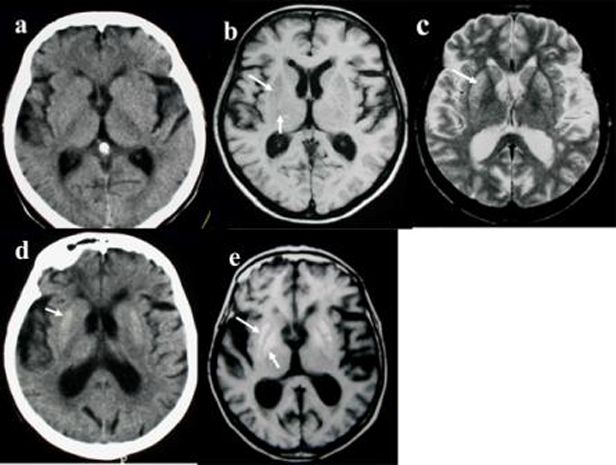
Serial non-contrast enhanced CT and MRI. (a) Day 2 after exposure. CT scan does not reveal any abnormality. Note cortical atrophy and ventricle enlargement. (b) – Day 7 after exposure. Axial T1 MR image reveals symmetrical sharply delimited and homogeneous hypointense lesions in the two putamini (long arrow), globi pallidi (short arrow) and caudate nuclei. (c) – Day 7 after exposure. Axial T2 MR image, at the same level, shows multiple punctuate foci of hyperintense signal in the same areas, most prominent on the antero-lateral part of the putamini (arrow). Note that (as on T1), especially on the right side, the globus pallidus interna and the globus pallidus externa are visualised as distinct structures, separated by a more hypointense zone. Cortical atrophy, ventricle enlargement and paraventricular and subcortical white matter patchy areas of hyperintensity are visible bilaterally. (d) – Day 17 after exposure. CT scan shows well-defined bilateral hyperdensity in the putamini, and at the posterior part of globus pallidi, (arrow), consistent with haemorrhage necrosis. Note more prominent cortical atrophy and ventricle enlargement. (e) – Day 60 after exposure. Axial T1 MR image reveals bilateral, homogeneous hypersignal intensity of both putamini (white arrow) and globi pallidi (black arrow).
Treatment
The patient was treated with an 1-h session of hyperbaric oxygen at 2.5 absolute atmospheres starting 2 h 30 min after presentation. The initial clinical course was marked by the development of pneumonia. The patient was extubated 10 days after admission.
Sedation was withdrawn on day 4. Her neurological course improved slowly, with gradual awakening. Spontaneous eye opening and appropriate reaction to painful stimuli occurred on day 8. The patient was transferred for rehabilitation on day 14. Thereafter, an extrapyramidal syndrome involving all four limbs developed, with plastic hypertonicity and flexion of the lower extremities. On day 30, choreo-athetotic movements of the upper extremities, head, and trunk appeared, and the patient was conscious but dysarthric. Her choreo-athetotic movements diminished and disappeared over the course of a month, although extrapyramidal hypertonia of all four limbs persisted.
Outcome and follow-up
Six months later, the extrapyramidal hypertonia of the four limbs was still present, incapacitating this left-handed patient. MRI scans revealed abnormalities, suggesting increased cerebral atrophy, in the white matter, putamini and globi pallidi, and these were still present 2 years later.
As the patient was oriented, cognitive functions were not further assessed by specific tests. During the next 2 years, the patient was followed up by a neurologist on an outpatient basis. The patient is now deceased.
Discussion
Over the last 50 years, the composition of household furniture in western countries has prompted debate about whether other gases, in addition to carbon monoxide, have a toxic role in victims of inhaled fire smoke.17–19 This debate is fuelled by the findings of several investigators who reported that some fire victims do not have lethal carboxyhaemoglobin levels from carbon monoxide poisoning.7 17 19 20 In the late sixties in New York, Zikria et al found that lethal carboxyhaemoglobin levels were present in only 24.3% of fire fatalities and may have been a significant factor in another 34.5%. This still leaves around 40% of victims whose deaths could not be explained by carbon monoxide poisoning alone.19 Fire fatalities with non-lethal carboxyhaemoglobin levels were also reported in Glasgow, Denmark and Paris,7 17 20 and there are numerous fatal and non-fatal cases where fire smoke victims have sublethal or subtoxic carboxyhaemoglobin levels, contrasting with toxic and even lethal blood cyanide concentrations.3 10–13 16
A number of animal and human studies have been designed to attempt to identify new gaseous fire smoke toxicants.5 The challenge is enormous however, as more than 150 volatile organic compounds have been detected in fire victims’ blood.17 In two animal models, the lethal potency of carbon monoxide and cyanide was consistently shown to be additive, although even now, carbon monoxide is still considered the lone killer with cyanide playing no toxic role.2 21–24
Closer examination of the supporting evidence for this hypothesis shows that the majority of the larger trials base their findings on forensic examination. Victims were found dead at the scene or died soon after, without clinical or biological data having been collected.8 9 14 19 25 This clearly precludes any assessment of the concentration-effect relationships of either carbon monoxide, cyanide or any other potential primary toxicant.
The data from the literature clearly support the assumption that toxic blood cyanide concentrations, with an accompanying non-toxic carboxyhaemoglobin concentration, may exist in victims of residential fires. In the present case, the concentrations of toxic gases were measured using blood specimens collected at the scene of the fire before any antidotal treatment. The blood cyanide concentration of 68 µmol/l is within toxic ranges while the carboxyhaemoglobin concentration of 10.9% is within non-toxic limits, showing that cyanide was the predominant toxicant. The rapid onset of coma, severe cardiovascular shock, lack of pulse and apnoea is quite common in severe cases of pure cyanide poisoning, and uncommon in severe cases of pure carbon monoxide poisoning with such short exposure durations.4 Our data therefore further support the hypothesis that a residential fire may cause near-fatal poisoning in the presence of toxic blood cyanide concentrations but non-toxic concentrations of carboxyhaemoglobin.
The 2.5 g dose of the cyanide antidote hydroxocobalamin used in this case also warrants comment. Fifty mg of hydrogen cyanide, an estimated LD50 of cyanide in humans, binds with 2.5 g of hydroxocobalamin in vitro.26 In fire victims, a fixed 5 g dose (equivalent to approximately 3.71 mmol) of hydroxocobalamin can wholly bind cyanide in blood cyanide concentrations up to 40 µmol/l.27 Recently, Hung et al reported a case of severe cyanide poisoning initially treated with a 2.5 g dose of hydroxocobalamin.28 This suboptimal dose resulted in partial reversal of lactic acidosis, and it was only after an additional 2.5 g dose was administered that complete recovery ensued. In the present case, owing to the initial blood cyanide level of 68 µmol/l, a 7.5 g dose of hydroxocobalamin should have been administered to neutralise cyanide on an equimolar basis. The delayed neurological sequelae observed in this patient support the assumption that in addition to hyperbaric oxygen, hydroxocobalamin doses of at least 5 g should be administered in adult fire victims with clinical and biological findings suggestive of severe cyanide poisoning.
The radiographic findings presented in our case are similar to scattered case reports of cyanide toxicity from other sources. The patient’s findings are not unique in terms of cyanide exposure, but to our knowledge, brain sequelae resulting from inhalation of cyanide produced by fire smoke have not been previously documented. The principal differences between the patterns of damage by cyanide and carbon monoxide seem to be the sparing of the hippocampi in cyanide intoxication29 and the early occurrence of haemorrhagic necrosis of the basal ganglia in cyanide poisoning.
The repeated imaging of our patient over 2 years follow-up did not show any abnormalities within the hippocampi, and supports the hypothesis that cyanide is a primary toxicant in residential fires.
Learning points.
-
▶
Toxic blood cyanide concentrations may sometimes be associated even with low carboxyhaemoglobin levels.
-
▶
Administering a 2.5g dose of hydroxocobalamin can initially reverse severe cardiovascular compromise but does not prevent the delayed onset of brain injury. Therefore, an initial dose not lower than 5 g is recommended for adults.
-
▶
In this case, the clinical, biological and toxicological assessments, the sustained reversal of cyanide-induced shock with hydroxocobalamin, and the long-term neurological follow-up are all suggestive of cyanide- rather than carbon monoxide-toxicity.
-
▶
Emergency response teams and intensive care physicians should consider treatment for poisoning by cyanide as well as carbon monoxide in patients affected by smoke inhalation. The subset of victims requiring cyanide treatment needs clarification through further study.
Footnotes
Competing interests None.
Patient consent Not obtained.
References
- 1.Ballantyne B. In: Ballantyne B, Marrs T, eds. Hydrogen Cyanide as a Product of Combustion and a Fector in Morbidity and Mortality from Fires. Bristol: Wright; 1987:248–91 [Google Scholar]
- 2.Barillo DJ. Diagnosis and treatment of cyanide toxicity. J Burn Care Res 2009;30:148–52 [DOI] [PubMed] [Google Scholar]
- 3.Barillo DJ, Goode R, Esch V. Cyanide poisoning in victims of fire: analysis of 364 cases and review of the literature. J Burn Care Rehabil 1994;15:46–57 [DOI] [PubMed] [Google Scholar]
- 4.Baud FJ. Cyanide: critical issues in diagnosis and treatment. Hum Exp Toxicol 2007;26:191–201 [DOI] [PubMed] [Google Scholar]
- 5.Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol 2002;32:259–89 [DOI] [PubMed] [Google Scholar]
- 6.Anderson RA, Harland WA. Fire deaths in the Glasgow area: III. The role of hydrogen cyanide. Med Sci Law 1982;22:35–40 [DOI] [PubMed] [Google Scholar]
- 7.Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med 1991;325:1761–6 [DOI] [PubMed] [Google Scholar]
- 8.Birky MM, Clarke FB. Inhalation of toxic products from fires. Bull N Y Acad Med 1981;57:997–1013 [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CJ, Campbell D, Reid WH. Blood carboxyhaemoglobin and cyanide levels in fire survivors. Lancet 1981;1:1332–5 [DOI] [PubMed] [Google Scholar]
- 10.Ferrari LA, Arado MG, Giannuzzi L, et al. Hydrogen cyanide and carbon monoxide in blood of convicted dead in a polyurethane combustion: a proposition for the data analysis. Forensic Sci Int 2001;121:140–3 [DOI] [PubMed] [Google Scholar]
- 11.Hart GB, Strauss MB, Lennon PA, et al. Treatment of smoke inhalation by hyperbaric oxygen. J Emerg Med 1985;3:211–5 [DOI] [PubMed] [Google Scholar]
- 12.Lundquist P, Rammer L, Sörbo B. The role of hydrogen cyanide and carbon monoxide in fire casualties: a prospective study. Forensic Sci Int 1989;43:9–14 [DOI] [PubMed] [Google Scholar]
- 13.Silverman SH, Purdue GF, Hunt JL, et al. Cyanide toxicity in burned patients. J Trauma 1988;28:171–6 [DOI] [PubMed] [Google Scholar]
- 14.Symington IS, Anderson RA, Thomson I, et al. Cyanide exposure in fires. Lancet 1978;2:91–2 [DOI] [PubMed] [Google Scholar]
- 15.Yeoh MJ, Braitberg G. Carbon monoxide and cyanide poisoning in fire related deaths in Victoria, Australia. J Toxicol Clin Toxicol 2004;42:855–63 [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Adachi J, Watabiki T, et al. A study on house fire victims: age, carboxyhemoglobin, hydrogen cyanide and hemolysis. Forensic Sci Int 1991;52:13–20 [DOI] [PubMed] [Google Scholar]
- 17.Anderson RC. In: Curry AS, ed. Fire Gases. London: MacMillan; 1986:289–317 [Google Scholar]
- 18.Bowes PC. Casualties attributed to toxic gas and smoke at fires: a survey of statistics. Med Sci Law 1976;16:104–10 [DOI] [PubMed] [Google Scholar]
- 19.Zikria BA, Weston GC, Chodoff M, et al. Smoke and carbon monoxide poisoning in fire victims. J Trauma 1972;12:641–5 [DOI] [PubMed] [Google Scholar]
- 20.Teige B, Lundevall J, Fleischer E. Carboxyhemoglobin concentrations in fire victims and in cases of fatal carbon monoxide poisoning. Z Rechtsmed 1977;80:17–21 [DOI] [PubMed] [Google Scholar]
- 21.Levin BC. The development of a new small-scale smoke toxicity test method and its comparison with real-scale fire tests. Toxicol Lett 1992;64-65 Spec No:257–64 [DOI] [PubMed] [Google Scholar]
- 22.Levin BC. New research avenues in toxicology: 7-gas N-Gas Model, toxicant suppressants, and genetic toxicology. Toxicology 1996;115:89–106 [DOI] [PubMed] [Google Scholar]
- 23.Purser DA. The evolution of toxic effluents in fires and the assessment of toxic hazard. Toxicol Lett 1992;64-65 Spec No:247–55 [DOI] [PubMed] [Google Scholar]
- 24.Purser DA. In: Penney DG, ed. Interactions Among Carbon Monoxide, Hydrogen Cyanide, Low Oxygen Hypoxia, Carbon Dioxide, and Inhaled Irritant Gases. New York: CRC Press; 2000:157–91 [Google Scholar]
- 25.Wetherell HR. The occurrence of cyanide in the blood of fire victims. J Forensic Sci 1966;11:167–73 [PubMed] [Google Scholar]
- 26.Evans CL. Cobalt compounds as antidotes for hydrocyanic acid. Br J Pharmacol Chemother 1964;23:455–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houeto P, Hoffman JR, Imbert M, et al. Relation of blood cyanide to plasma cyanocobalamin concentration after a fixed dose of hydroxocobalamin in cyanide poisoning. Lancet 1995;346:605–8 [DOI] [PubMed] [Google Scholar]
- 28.Hung DZ, Tsan YT, Yu YJ, et al. Cyanide poisoning in Taiwan. Lancet 2009;374:1212. [DOI] [PubMed] [Google Scholar]
- 29.Borgohain R, Singh AK, Radhakrishna H, et al. Delayed onset generalised dystonia after cyanide poisoning. Clin Neurol Neurosurg 1995;97:213–5 [DOI] [PubMed] [Google Scholar]


