Abstract
During corticogenesis, the regulation of neuronal migration is crucial for the functional organization of the neocortex. Glutamatergic neurons are major excitatory components of the mammalian neocortex. In order to elucidate the specific molecular mechanisms underlying their development, we used single-cell microarray analysis to screen for mouse genes that are highly expressed in developing glutamatergic neurons. We identified dpy-19-like 1 (Dpy19l1), a homolog of C. elegans dpy-19, which encodes a putative multi-transmembrane protein shown to regulate directed migration of Q neuroblasts in C. elegans. At embryonic stages Dpy19l1 is highly expressed in glutamatergic neurons in the mouse cerebral cortex, whereas in the subpallium, where GABAergic neurons are generated, expression was below detectable levels. Downregulation of Dpy19l1 mediated by shRNA resulted in defective radial migration of glutamatergic neurons in vivo, which was restored by the expression of shRNA-insensitive Dpy19l1. Many Dpy19l1-knockdown cells were aberrantly arrested in the intermediate zone and the deep layer and, additionally, some extended single long processes towards the pial surface. Furthermore, we observed defective radial migration of bipolar cells in Dpy19l1-knockdown brains. Despite these migration defects, these cells correctly expressed Cux1, which is a marker for upper layer neurons, suggesting that Dpy19l1 knockdown results in migration defects but does not affect cell type specification. These results indicate that Dpy19l1 is required for the proper radial migration of glutamatergic neurons, and suggest an evolutionarily conserved role for the Dpy19 family in neuronal migration.
Keywords: dpy-19, Neuronal migration, Corticogenesis, Excitatory neuron, Mouse
INTRODUCTION
The mammalian cerebral cortex is the center for higher functions of the brain. The cortical area has been evolutionarily expanded, acquiring a distinct six-layer structure (Preuss and Kaas, 1999). During early cortical development, neuronal migration plays an important role in functional organization of the cortex. Several genetic malformations, such as lissencephaly and subcortical band heterotopia (SBH), are caused by deficient neuronal migration (Ross and Walsh, 2001; Kato and Dobyns, 2003; Guerrini and Marini, 2006). For example, mutations in the LIS1 (PAFAH1B1 – Human Gene Nomenclature Committee) gene or in the X-linked gene doublecortin (DCX) lead to classical lissencephaly in humans, which has been shown to be caused by neuronal migration defects (Reiner et al., 1993; des Portes et al., 1998; Gleeson et al., 1998).
During corticogenesis, glutamatergic neurons are generated from progenitors called radial glia and intermediate progenitors in the ventricular/subventricular zone of the dorsal pallium. These neurons then migrate radially into the cortical plate in an inside-out pattern (Rakic, 1972; Miyata et al., 2001; Noctor et al., 2001; Tamamaki et al., 2001; Nadarajah and Parnavelas, 2002). By contrast, most GABAergic interneurons arise from the ganglionic eminence, then migrate tangentially and settle in the cerebral cortex (Anderson et al., 1997; Tamamaki et al., 1997; Marín and Rubenstein, 2003). The radial migration of glutamatergic neurons is strictly controlled by coordinated extracellular and intracellular molecules to establish the distinct layers of the cerebral cortex (Hatten, 2002). For example, the secreted protein reelin is well known to act as a guidance cue for the radial migration of cortical neurons (Rice and Curran, 2001), and cyclin-dependent kinase 5 (CDK5) and the cytoskeletal regulator RhoA are also involved in cortical radial migration (Gilmore et al., 1998; Hand et al., 2005). Neuronal polarization is also important for neuronal migration (Reiner and Sapir, 2009). Knockdown of the polarity kinases Par1/Mark2 and Par4/Lkb1 (Stk11) disrupts radial migration by affecting centrosome positioning and microtubule dynamics in vivo (Asada et al., 2007; Sapir et al., 2008). However, much less is known about the regulation of cerebral cortex-specific neuronal migration during development.
To elucidate the molecular mechanisms specific to glutamatergic neuron development, single-cell microarray analysis was used to screen for genes expressed strongly and/or specifically within glutamatergic neurons. From this screen we identified Dpy19l1 as being prominently expressed in glutamatergic neuronal lineage cells in the developing mouse cerebral cortex. DPY-19, a multi-transmembrane protein, was first identified in C. elegans. Mutation in any of the dpy genes causes the ‘dumpy’ phenotype in C. elegans, which is characterized by short body length (Brenner, 1974). In C. elegans, Q neuroblasts (QL and QR) are generated in bilaterally symmetrical positions and then undergo asymmetrical migration posteriorly and anteriorly, respectively. In dpy-19 mutants, the polarization of Q neuroblasts becomes randomized, resulting in defective migration, suggesting involvement of dpy-19 in the polarization and migration of neuroblasts (Honigberg and Kenyon, 2000). Four dpy-19-like genes (Dpy19l1-4) encoding putative multi-transmembrane proteins have been identified in both the human and mouse genomes (Carson et al., 2006). The Dpy19l1 gene encodes a protein of 746 amino acids; however, its expression and function during development of the mammalian cerebral cortex have yet to be determined.
In this study we investigated the role of Dpy19l1 in mouse corticogenesis. Dpy19l1 knockdown mediated by short hairpin RNA (shRNA) resulted in defective radial migration of glutamatergic neurons in vivo, which could be rescued by the expression of shRNA-insensitive Dpy19l1. This migration defect was partly caused by the abnormal migration of bipolar cells in Dpy19l1-knockdown brains. Dpy19l1-knockdown cells correctly expressed the layer-specific marker Cux1 despite their aberrant positioning. Two to 3 weeks after electroporation, most of the downregulated cells in the intermediate zone showed an abnormal rounded or multipolar morphology, whereas many of the cells arrested in the deep layer were of the characteristic pyramidal morphology. Our study identifies the significance of the transmembrane protein Dpy19l1 in the proper migration of glutamatergic neurons during mammalian corticogenesis.
MATERIALS AND METHODS
Animals
Timed-pregnant ICR and C57BL/6 mice were obtained from Japan SLC. NEX/Neurod6-Cre (NEX-Cre) mice were kindly provided by Dr K. A. Nave (Schwab et al., 2000). Genotyping of NEX-Cre and GAD67-GFP (GAD67 is also known as Gad1) knock-in mice was as described previously (Schwab et al., 2000; Tamamaki et al., 2003). NEX-Cre and GAD67-GFP heterozygous embryos were obtained by time-mating C57BL/6 wild-type female mice with NEX-Cre homozygous and GAD67-GFP heterozygous male mice, respectively. Noon on the day of vaginal plug was considered embryonic day (E) 0.5. All procedures were approved by the Animal Research Committee of Kumamoto University.
Plasmids
shRNA sequences against Dpy19l1 were as follows (5′ to 3′): Dpy19l1 sh647, GGACTCAGTCCGATTGAGA; Dpy19l1 sh1769, GGTTCAGCAAACCTACAAA; Dpy19l1 sh2069, GAGTCATGGTGCATAAGAA; and the scrambled sequence Dpy19l1-control shRNA, ACAGCTAGGCTCGGATATG. Each shRNA oligonucleotide was inserted into pSilencer 1.0-U6 (Ambion). All plasmids contained a 9 nt hairpin loop sequence (5′-TTCAAGAGA-3′). These constructs were co-transfected with a reporter plasmid by in utero electroporation as described below.
To generate the Dpy19l1-EGFP fusion construct, both the EcoRI-BspHI Dpy19l1 5′ fragment (I.M.A.G.E. cDNA clone ID 6856095, Invitrogen) and the BspHI-ApaI 3′ fragment (PCR-amplified fragment) were cloned into the EcoRI and ApaI sites of pEGFP-N1 (Takara Clontech).
To generate the Dpy19l1 shRNA-insensitive construct (Dpy19l1-i), a mutated 3′ fragment was synthesized. Both the EcoRI-BspHI Dpy19l1 5′ fragment and the BspHI-NotI mutated 3′ fragment were cloned into the EcoRI and NotI sites of the pCAG-RB vector (a pCAG-GS vector containing a modified multiple cloning site).
In utero electroporation
Timed-pregnant mice were deeply anesthetized using pentobarbital (50 mg/kg). Plasmid DNA (1-2 μg/μl) was microinjected into the lateral ventricles of E13.5 or E14.5 forebrains and electroporated (five 50-msecond pulses of 30 V for E13.5 or 33 V for E14.5, with an interval of 950 mseconds; CUY21 electroporator, Nepagene). Embryos were dissected 24 hours to 3 weeks after electroporation, then single-cell microarray and histological analyses were carried out as described below. In order to obtain sparse labeling of GFP-positive cells, we used Cre-mediated recombination in which 10-50 ng/μl pCAG-Cre along with 2 μg/μl GFP reporter plasmids and either Dpy19l1 shRNA or control shRNA plasmid were electroporated at E14.5.
Single-cell microarray analysis
Single-cell microarray analysis was performed as described previously (Esumi et al., 2008). E13.5 NEX-Cre embryos (C57BL/6 background) were electroporated with pCAG-loxP-RFP-loxP-EGFP (Wu et al., 2005). After 24 hours, the cortices of NEX-Cre and GAD67-GFP knock-in mice were dissected and dissociated into single-cell suspensions by digestion with 0.25% trypsin and 0.01% DNaseI at 37°C for 5 minutes. GFP-positive cells were identified under a fluorescence microscope and picked up using the Quixell Automated Cell Selection and Transfer System (Stoelting).
Total RNA from a single cell was reverse transcribed into cDNA using the Super SMART PCR cDNA Synthesis Kit (Takara Clontech). We then assessed the quality of the Super SMART PCR products by capillary electrophoresis (Bioanalyzer 2100, Agilent Technologies) and quantitative real-time PCR (qPCR) analysis (ABI 7500, Applied Biosystems). The following PCR profile was used: 10 minutes at 95°C; 50 cycles of 15 seconds at 95°C, 1 minute at 60°C. Primers used in this study are listed in supplementary material Table S1.
The quality-checked products were subjected to one cycle of a T7 RNA polymerase reaction to produce biotinylated cRNA. The cRNA was subsequently fragmented and hybridized to a GeneChip Mouse Genome MOE430 2.0 array (Affymetrix), according to the manufacturer’s protocol. The microarray image data were processed with a GeneChip Scanner 3000 (Affymetrix). We performed per-chip normalization (normalized to the fiftieth percentile). Absolute calls (present, absent and marginal) were calculated with GCOS (Affymetrix) in the default setting. Further data analysis was carried out using GeneSpring software (Agilent).
RT-PCR
Total RNA from E14.5 or adult mouse cerebral cortex was extracted with TRIzol reagent (Invitrogen) following the manufacturer’s protocol. Single-stranded cDNAs were prepared using SuperScript reverse transcriptase III (Invitrogen) and amplified by PCR. Primers used for RT-PCR are listed in supplementary material Table S1.
Histology
Mouse embryos were fixed with 4% paraformaldehyde (PFA) in PBS for 60 minutes to overnight at 4°C, cryoprotected in 20% sucrose, then embedded in OCT compound (Sakura Finetechnical). Frozen sections were cut at 20 or 50 μm on a cryostat (CM1850, Leica) and then mounted onto MAS-coated glass slides (Matsunami). Immunohistochemical staining was performed as described (Watanabe et al., 2006). Primary antibodies used were: rat anti-GFP (1:2000, Nacalai Tesque), rabbit anti-GFP (1:1000, Invitrogen/Molecular Probes), mouse anti-beta III tubulin (1:1000, Covance), mouse anti-TAG-1 (Cntn2; 4D7, 1:5, DSHB), mouse RC2 (1:500, DSHB), mouse anti-BrdU (1:500, BD Pharmingen), rabbit anti-Cux1 (M-222, 1:50, Santa Cruz Biotechnology), rabbit anti-Ctip2 (1:200, Novus Biologicals), rabbit anti-calretinin (1:500, Swant), mouse anti-MAP2 (1:1000, Sigma), rabbit anti-Tbr1 [1:2000, a gift from Dr R. Hevner (Englund et al., 2005)] and rabbit anti-Tbr2 (1:2000, a gift from Dr R. Hevner). Rabbit anti-Dpy19l1 serum was generated against two mouse Dpy19l1 C-terminal peptides (SRKAPEDVKKELMKLKVC and VEDPDNAGKTPLC; 1:2000, MBL). For immunofluorescence, sections were labeled with species-specific secondary antibodies conjugated to Alexa Fluor 488 or 594 (Invitrogen) and counterstained with Hoechst 33342 (Sigma). Sections were processed by the ABC method (Vector Laboratories) following the manufacturer’s protocol. Detection with horseradish peroxidase was performed by incubation in 0.05% diaminobenzidine (DAB) and 0.015% hydrogen peroxide in PBS.
In situ hybridization (ISH) was performed as described (Watanabe et al., 2006). Mouse Dpy19l1 and Dpy19l3 cDNAs were generated by RT-PCR and used as probes. Primers for ISH are listed in supplementary material Table S1. DIG-labeled RNA hybrids were reacted with alkaline phosphatase-conjugated anti-DIG antibody (Roche). The reaction product was visualized by incubating the sections with nitroblue tetrazolium chloride (NBT; Roche) and 5-bromo-4-chloro-3-indolylphosphate (BCIP; Roche).
Images were taken with a digital camera (DP72, Olympus). Confocal images were captured with a confocal laser-scanning microscope (FV300, Olympus).
BrdU labeling
Pregnant mice were given two intraperitoneal injections of BrdU (50 μg/g body weight; Sigma) with a 30-minute interval. One hour after the first injection, embryos were isolated and fixed for immunostaining.
Time-lapse imaging
Slice culture was performed as described previously (Tabata and Nakajima, 2003) with minor modifications. Electroporation with shRNA and pCX-EGFP plasmids was performed at E14.5. After 3 days, embryonic brains were dissected and 250 μm coronal neocortical slices were prepared using a vibratome (Dosaka EM). The slices were placed on a Millicell-CM filter (Millipore), embedded in collagen gel and cultured in Neurobasal medium supplemented with B27, 2 mM Glutamax and penicillin-streptomycin (Invitrogen) at 37°C under 5% CO2. Seven optical z-sections at 5 μm intervals were captured at intervals of 30 minutes for 6-12 hours using the FV300.
Quantification
To quantify GFP-positive cells in the cortex at postnatal day (P) 2, we counted cells in eight areas formed by dividing the images of the cortical plate and intermediate zone of the lateral cerebral cortex into eight equal squares, and then analyzed the data from at least three embryos using Student’s t-test.
Neuron morphology was defined as having an apical dendrite and/or triangular morphology as observed in 50 μm sections.
RESULTS
Identification of genes specific to glutamatergic neuronal lineages
To study the molecular mechanisms underlying the development of cortical glutamatergic neurons, we performed single-cell microarray analysis to identify genes that are specifically expressed in glutamatergic neurons (Esumi et al., 2008). We labeled newly born glutamatergic neuron precursors by GFP expression utilizing NEX-Cre mice, and labeled GABAergic neuronal lineages using GAD67-GFP knock-in mice (Fig. 1A-D) (Schwab et al., 2000; Tamamaki et al., 2003). To label glutamatergic neurons, E13.5 NEX-Cre embryos were electroporated with a pCAG-loxP-RFP-loxP-EGFP construct; single GFP-positive cells were then selected after 24 hours (Fig. 1A,B).
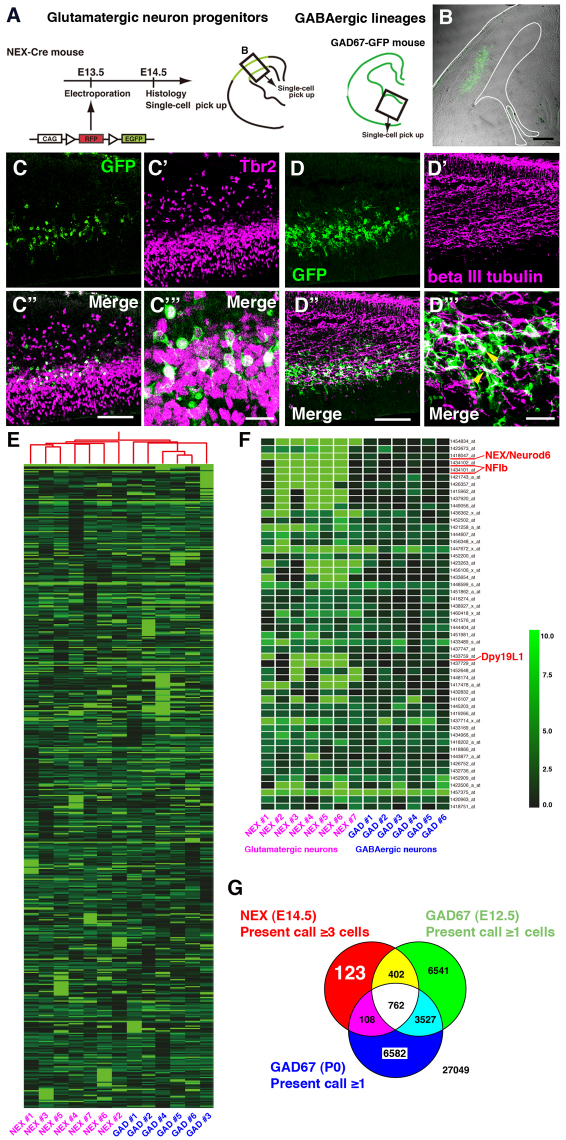
Fig. 1.
Subtraction screening of cortical glutamatergic neuron-specific genes by single-cell microarray. (A) The labeling procedure for glutamatergic or GABAergic neuron lineage cells. (B) Localization of GFP-positive cells in the NEX-Cre cerebral cortex 24 hours after electroporation with a reporter plasmid. The border of the section is outlined. (C-D″′) Immunohistochemistry of cortical sections of E14.5 NEX-Cre mouse embryos labeled with a GFP reporter showing overlay of Tbr2 (C-C″′) or beta III tubulin (D-D″′) and GFP. C″′ and D″′ show magnified images. Yellow arrowheads indicate cells positive for GFP and beta III tubulin. (E) Cluster analysis. Distinct gene expression profiles for glutamatergic and GABAergic neuron lineage cells were detected. (F) Expression levels of genes strongly expressed in glutamatergic neuronal lineage cells. (G) We selected 123 genes that were specifically expressed in glutamatergic neurons by filtering with present/absent calls. Scale bars: 200 μm in B; 100 μm in C-C″ ,D-D″ ; 20 μm in C″′,D″′.
Immunohistochemical analysis was used to examine the properties of GFP-positive cells in littermates. Many NEX-GFP-positive cells were also positive for Tbr2 (Eomes – Mouse Genome Informatics), a marker of intermediate/basal progenitors (Englund et al., 2005), and some also colabeled with the neuronal marker beta III tubulin, suggesting that most GFP-positive cells were glutamatergic neurons/progenitors (Fig. 1C-D″′) (Wu et al., 2005).
Additionally, we collected single GABAergic neurons from the medial ganglionic eminence of E12.5 GAD67-GFP mice (Fig. 1A) and performed single-cell microarray analysis on cells from both lineages: seven NEX-positive cells and six GAD67-positive cells were analyzed. Cluster analysis (including data from P0 GAD67-positive cells) (Esumi et al., 2008) revealed distinct gene expression profiles in the two lineages and identified 123 genes that were present only in NEX-positive cells (present call in at least three out of seven NEX-positive cells, absent call in all E12.5 and P0 GAD67-positive cells; Fig. 1E-G). We subsequently examined the expression pattern of ∼40 genes of interest at E14.5 by ISH, from which we identified several genes that were expressed in cortical glutamatergic neurons. Although some of these genes are expressed in the subpallium as well as the dorsal cortex, we were able to identify several genes that are expressed predominantly in glutamatergic neurons (supplementary material Fig. S1; data not shown). Among these genes, we focused on the Dpy19 family, as Dpy19l1 mRNA is highly expressed in the developing cerebral cortex (Fig. 2).
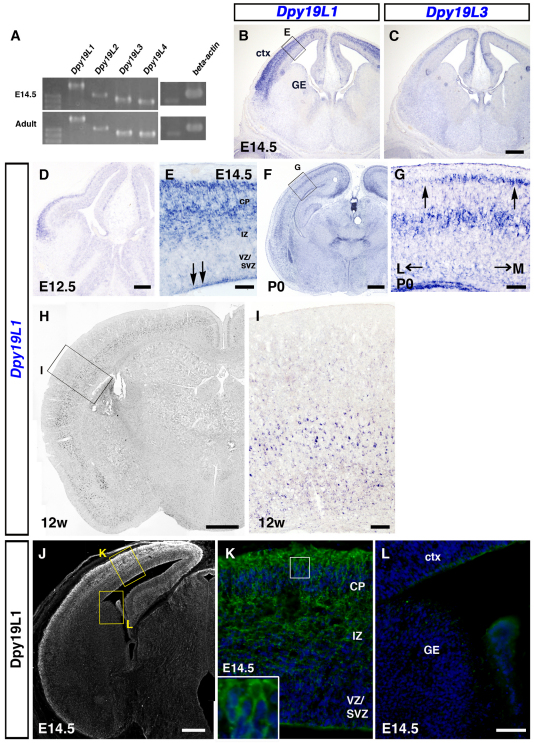
Fig. 2.
Expression analyses of the Dpy19 family in the developing cortex. (A) RT-PCR of Dpy19 family genes in E14.5 and adult mouse brains. beta-actin provides a control. (B-L) Expression of Dpy19l1 and Dpy19l3 mRNAs visualized by in situ hybridization (ISH) in the telencephalon. (B-E) At E12.5, expression of Dpy19l1 was observed in the dorsal telencephalon. At E14.5, Dpy19l1 mRNA was intensely expressed in the CP, the IZ and weakly in the VZ/SVZ of the cerebral cortex. Dpy19l3 mRNA showed an opposite gradient of expression. The boxed area in B is magnified in E. (F,G) Expression of Dpy19l1 was also observed in the P0 cortex, especially in the deep layer. The boxed area in F is magnified in G. (H,I) Dpy19l1 expression persists in the adult cerebral cortex at 12 weeks. The boxed area in H is magnified in I. (J-L) Distribution of Dpy19l1 protein at E14.5. In J, Dpy19l1 immunoreactivity is white. Dpy19l1 was mainly distributed in the IZ as well as the CP of the cortex. Inset in K shows a magnified view of the CP. Boxed areas in J are magnified in K and L. This section was counterstained with Hoechst 33342. CP, cortical plate; ctx, cerebral cortex; GE, ganglionic eminence; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone; L, lateral; M, medial. Scale bars: 300 μm in B,C; 200 μm in D,J; 50 μm in E,K,L; 500 μm in F; 100 μm in G,I; 1 mm in H.
Dpy19l1 is highly expressed in glutamatergic neurons of the developing cerebral cortex
The Dpy19 gene is evolutionarily conserved in C. elegans, rodents and human; four genes (Dpy19l1-l4) are found in mouse (Mouse Genome Informatics). We first examined the expression of Dpy19 family members in the mouse telencephalon by ISH and RT-PCR. At E14.5, strong expression of Dpy19l1 and Dpy19l3 were observed in the developing cerebral cortex (Fig. 2B,C), whereas Dpy19l2 and Dpy19l4 mRNA could not be detected with our ISH probes. However, mRNAs of all the Dpy19 family members were detected in both the E14.5 and adult cerebral cortex by RT-PCR (Fig. 2A) (Sugiura et al., 2001). Dpy19l2 mRNA was detected at relatively low levels at both stages. At E12.5, Dpy19l1 expression was detected in the cortical plate (CP) of the dorsal telencephalon (Fig. 2D). At E14.5, Dpy19l1 was expressed in a lateromedial gradient within the cerebral cortex, whereas Dpy19l3 showed an opposite expression gradient (Fig. 2B,C). At this stage, high levels of expression were observed in both the CP, where neurons terminate their migration, and the intermediate zone (IZ), through which neurons migrate to reach the CP, and weak expression was observed in the proliferative ventricular zone (VZ) and the subventricular zone (SVZ) (Fig. 2B,E). Additionally, the surface of the VZ showed intense expression of Dpy19l1 (Fig. 2E, arrows). By contrast, very low expression of Dpy19l1 was detected in the ganglionic eminence, the source of cortical GABAergic interneurons (Fig. 2B). We observed a very restricted expression of Dpy19l1 in other regions, such as the diencephalon and the dorsal midline of the brain stem (supplementary material Figs S2, S4). During perinatal stages, Dpy19l1 expression was apparent in the deep cortical layer and the surface of the VZ, whereas weaker expression was detected in the upper layer, particularly in the lateral regions of the cortex (Fig. 2F, arrows in 2G). At this stage, intense expression of Dpy19l1 mRNA could also be detected in regions other than the cerebral cortex (Fig. 2F). Dpy19l1 expression was also observed in the adult cerebral cortex, with stronger signals in the deep layer compared with the upper layer (Fig. 2H,I).
To determine the cellular distribution of Dpy19l1 protein, we generated an antibody against the C-terminal region of Dpy19l1 and then used it for immunohistochemistry. The pattern of immunostaining was similar to that of ISH (supplementary material Fig. S2). Dpy19l1 protein was primarily localized in the IZ, the CP, the marginal zone (MZ) and the surface of the VZ in the E14.5 cerebral cortex, with weak expression detected in the VZ/SVZ (Fig. 2J,K; supplementary material Fig. S2A,B). Localization in the ganglionic eminence was barely detectable (Fig. 2J,L). Dpy19l1 protein also appeared to be localized in the axons of the CNS and peripheral tissues (supplementary material Figs S2, S3, arrows; data not shown).
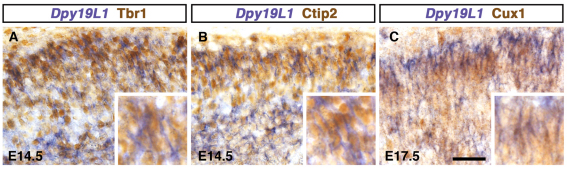
To confirm that Dpy19l1 is expressed in glutamatergic neurons, we examined the colocalization of Dpy19l1 mRNA with cortical subtype-specific markers. At E14.5, many Dpy19l1-expressing cells in the CP co-expressed Tbr1, a marker of layer VI neurons, or Ctip2 (Bcl11b – Mouse Genome Informatics), a marker of layer V neurons (Fig. 3A,B). At E17.5, Dpy19l1-positive cells expressed Cux1, a marker of upper layer neurons (layers II-IV), although Dpy19l1 expression in the upper CP was weaker at E17.5 than at E14.5 (Fig. 3C). These results suggest that Dpy19l1 is strongly expressed in glutamatergic neuronal lineage cells during corticogenesis.
Fig. 3.
Dpy19l1 is expressed in cortical glutamatergic neurons. (A-C) Immunostaining for Tbr1 (A), Ctip2 (B) and Cux1 (C) after ISH for Dpy19l1 in the developing mouse cerebral cortex at E14.5 (A,B) and E17.5 (C). Insets are magnified views. Dpy19l1-positive cells co-express different glutamatergic neuron markers: Tbr1, layer VI; Ctip2, layer V; Cux1, layers II-IV. Scale bar: 30 μm.
Dpy19l1 regulates the radial migration of cortical neurons
To study the function of Dpy19l1 during the development of glutamatergic neurons, we performed shRNA-mediated knockdown of Dpy19l1. Three shRNA constructs designed against Dpy19l1 and a scrambled shRNA plasmid as a control were tested. Co-transfection of COS-7 cells with any of the Dpy19l1 shRNAs along with the Dpy19l1-EGFP fusion plasmid specifically suppressed Dpy19l1-EGFP mRNA expression after 3 days, whereas co-transfection with the control shRNA did not (Fig. 4A).
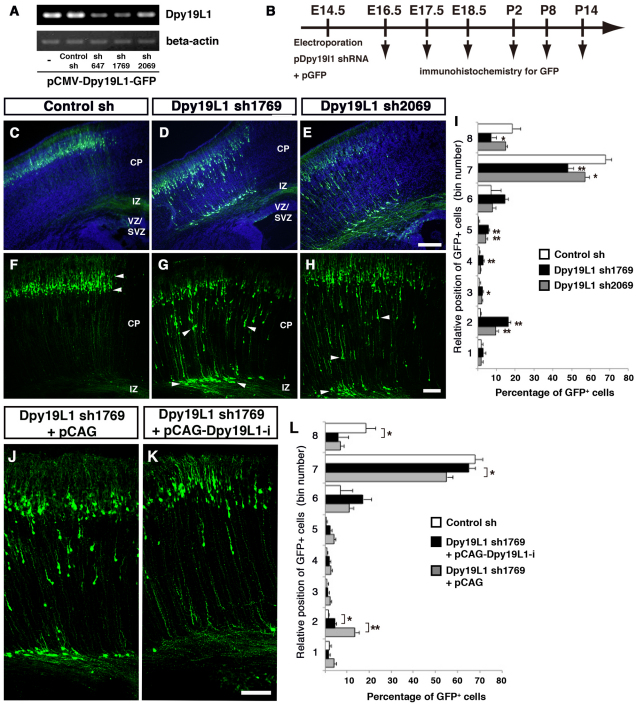
Fig. 4.
Dpy19l1 regulates radial migration of cortical neurons. (A) Knockdown experiment in COS-7 cells. Either control shRNA or one of three Dpy19l1 shRNA constructs along with a CMV-Dpy19l1-GFP fusion plasmid were co-transfected into COS-7 cells, and expression was examined by RT-PCR. Each shRNA construct downregulated Dpy19l1 expression. (B) Time line of these experiments. (C-I) Either control (C,F) or one of the Dpy19l1 shRNAs (sh1769 in D,G; sh2069 in E,H) was electroporated along with pCX-EGFP at E14.5. The distribution of cells with shRNA-mediated knockdown was analyzed at P2. Sections were counterstained with Hoechst 33342. (I) Quantification of GFP-positive cells in the P2 cerebral cortex. The percentage of GFP-positive cells is plotted as the mean ± s.e.m. (three embryos were analyzed per shRNA). *, P<0.05; **, P<0.01; versus control by t-test. Dpy19l1 downregulation disrupted the radial migration of cortical neurons. Many Dpy19l1-knockdown cells were aberrantly positioned in the IZ and the deep CP (arrowheads in G,H), whereas almost all cells reached the cortical upper layer in control brains (arrowheads in F). (J-L) Rescue experiments. Co-electroporation of pCAG control plasmid (J) or shRNA-insensitive pCAG-Dpy19l1 (Dpy19l1-i) expression plasmid (K) along with Dpy19l1 shRNA1769 and pCX-EGFP constructs was performed. (L) Quantification of GFP-positive cells at P2. The percentage of GFP-positive cells is plotted as the mean ± s.e.m. (three embryos for control shRNA, three for sh1769 + pCAG-Dpy19l1-i and six for sh1769 + pCAG). *, P<0.05; **, P<0.01; by t-test. The neuronal migration defect caused by Dpy19l1 knockdown was partially restored by re-expression of Dpy19l1-i. Scale bars: 200 μm in C-E; 100 μm in F-H,J,K.
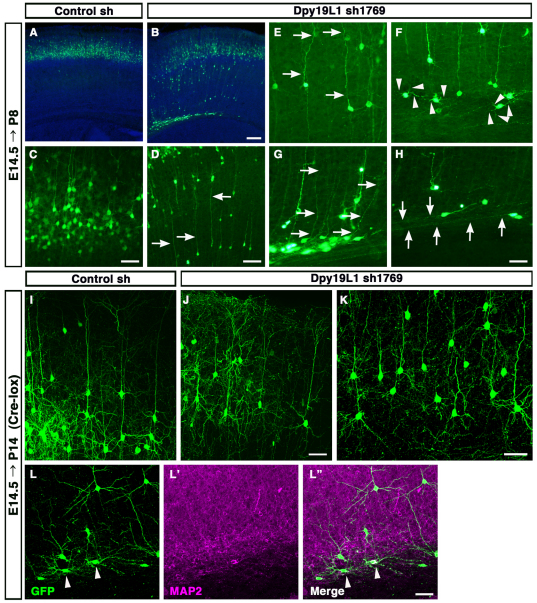
We next examined whether Dpy19l1 regulates the development of cortical glutamatergic neurons. The lateral cortices of E14.5 wild-type mice were electroporated with either a Dpy19l1 shRNA construct (sh1769 had a strong RNAi effect; sh2069 had a moderate effect; Fig. 4A) or control shRNA along with the pCX-EGFP plasmid. Two days to 3 weeks post-electroporation, the distribution of GFP-positive cells in the cerebral cortex was analyzed by GFP immunostaining (Fig. 4B). Neurons in the upper layers were labeled as a result of the experimental conditions. At P2, 7 days after electroporation, a time point at which most GFP-positive cells have terminated their migration, shRNA-mediated knockdown by two of the Dpy19l1 shRNAs (sh1769 and sh2069) resulted in cells with neuronal migration defects, whereas the control shRNA had no effect (Fig. 4C-I). A large number of Dpy19l1-knockdown GFP-positive cells remained in the IZ, and some were ectopically located in the deep cortical layer (arrowheads in Fig. 4G,H), whereas almost all GFP-labeled cells transfected with control shRNA had reached the upper layer (arrowheads in Fig. 4F). The migration defect was less severe in brains transfected with sh2069 than with sh1769 (Fig. 4D-I; see Discussion). Moreover, this migration defect could also be induced between P8 and P14 by downregulating Dpy19l1, suggesting that aberrant neural migration upon Dpy19l1 knockdown cannot be entirely attributed to developmental delay (Fig. 7A-D; supplementary material Fig. S7).
Fig. 7.
Morphological changes within migration-disrupted neurons in the Dpy19l1-knockdown brain. (A-H) Control (A,C) or Dpy19l1 shRNA (sh1769; B,D-H) was electroporated along with pCX-EGFP at E14.5. Dpy19l1-downregulated cells were analyzed at P8. (A,C) Almost all GFP-positive cells were found in the upper layer. (B,D-H) Dpy19l1-downregulated cells were stalled in the deeper CP and the IZ, although some cells in the IZ extended a long process towards the pial surface and an axon-like process towards the dorsal midline (arrows). Many of these cells showed a rounded or multipolar morphology in the IZ (arrowheads in F). Sections were counterstained with Hoechst 33342. (I-L″ ) E14.5 embryos were electroporated with control (I) or Dpy19l1 shRNA (sh1769, J-L″ ) together with Cre and GFP reporter plasmids and analyzed at P14. (L-L″ ) Double immunostaining of GFP and MAP2. Most Dpy19l1-downregulated cells in the deep layer assumed the characteristic pyramidal morphology, whereas many cells in the white matter showed abnormal morphologies (arrowheads). Scale bars: 200 μm in A,B; 100 μm in D; 50 μm in C,E-L″ .
To determine whether the migration defect caused by Dpy19l1 shRNAs is a direct result of Dpy19l1 deficiency, we performed a rescue experiment in which a construct was introduced that expressed shRNA-insensitive Dpy19l1 (Dpy19l1-i). We electroporated the control, pCAG or pCAG-Dpy19l1-i plasmid along with Dpy19l1 sh1769 and pCX-EGFP at E14.5 and observed the localization of GFP-positive cells at P2. Re-expression of Dpy19l1 in Dpy19l1-knockdown cells partially rescued the migration defect, whereas the injection of control pCAG and Dpy19l1 shRNA plasmids perturbed radial migration (Fig. 4J-L). These results suggest that Dpy19l1 is required for the proper radial migration of glutamatergic neurons during corticogenesis.
Disruption of radial migration of bipolar cells by Dpy19l1 downregulation
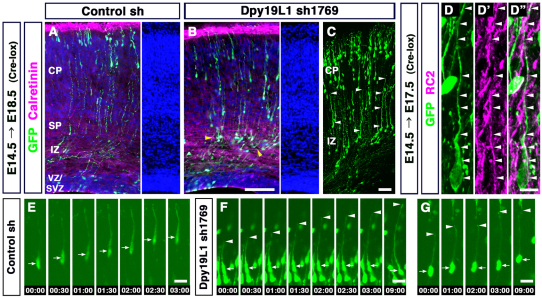
Prior to arriving at their terminal destination, glutamatergic neuron progenitors transit to a multipolar stage in the SVZ, transform into a bipolar shape in the IZ and then migrate into the CP while extending their axons (Tabata and Nakajima, 2003; Kriegstein and Noctor, 2004). To investigate which of these processes is affected by the downregulation of Dpy19l1, we performed morphological analyses of Dpy19l1-knockdown cells. Dpy19l1 shRNA-transfected cells were labeled using Cre-mediated GFP recombination in order to facilitate the morphological characterization of GFP-labeled cells (see Materials and methods). Four days post-electroporation (at E18.5), we observed that many Dpy19l1-knockdown cells did not pass through the subplate (SP) and instead were arrested within the upper IZ (Fig. 5A,B). Moreover, many of these cells assumed a bipolar morphology, although some of them extended a long process towards the pial surface (arrowheads in Fig. 5C). Some of the cells that were aberrantly stalled in the IZ were adjacent to the radial fibers of radial glial cells (arrowheads in Fig. 5D-D″ ).
Fig. 5.
Impaired neuronal migration of Dpy19l1-knockdown cells. (A-D″ ) Control (A) or Dpy19l1 shRNA (sh1769, B-D″ ) was electroporated along with Cre and GFP reporter plasmids at E14. The morphology of GFP-positive cells was analyzed at E18.5 (A-C) or E17.5 (D-D″ ). (A,B) The subplate (SP) was identified by immunostaining for calretinin (calbindin 2) and by nuclear staining (Hoechst 33342, blue). At E18.5, many Dpy19l1-dowregulated cells were abnormally stalled in the IZ (yellow arrowheads in B). Some cells in the IZ had single long processes extending towards the pial surface (arrowheads in C). (D-D″ ) Double immunostaining for GFP and RC2 (Ifaprc2), a marker of radial glial cells. (E-G) Time-lapse imaging of migrating cells expressing either control (E) or Dpy19l1 (F,G) shRNA. Time-lapse imaging was performed 3 days after electroporation. In Dpy19l1-knockdown brains, the leading process of bipolar cells in the IZ (F) and the CP (G) actively grew towards the pial surface, but the cell body remained in place. Arrowheads and arrows indicate the tip of the leading process and the cell body, respectively. Time is shown in hours:minutes. Scale bars: 100 μm in A,B; 50 μm in C; 10 μm in D-D″ ; 20 μm in E-G.
To examine this migration further we performed time-lapse imaging. E14.5 embryos were electroporated with shRNA, and cortical slices were cultured 3 days later. We observed that some bipolar cells in the IZ and the CP continued to extend a leading process towards the pial surface, but their cell body was almost immobile (Fig. 5F,G; supplementary material Movies 2-4). By contrast, many bipolar cells in the control brain translocated their cell soma during radial migration (Fig. 5E; supplementary material Movie 1). These results suggested that the defective migration in Dpy19l1-knockdown brains was caused, in part, by the abnormal migration of bipolar cells.
Normal cell type specification in Dpy19l1-knockdown cells
The impaired migration in Dpy19l1-knockdown brains could be the indirect result of abnormal specification of glutamatergic neuron progenitors. However, 2 days after electroporation, no significant change in the rate of BrdU incorporation was observed in the VZ of the Dpy19l1-knockdown cortex compared with the control brain (Fig. 6A-C). We next labeled GFP-positive cells with markers for cortical neurons/progenitors. In the E17.5 SVZ (3 days post-electroporation), the majority of Dpy19l1-knockdown/GFP-positive cells expressed Tbr2, a marker of intermediate/basal progenitors, similar to control shRNA-transfected cells (supplementary material Fig. S5). Furthermore, almost all GFP-positive cells expressed Cux1 in both control and Dpy19l1-knockdown brains at E17.5 (Fig. 6D; supplementary material Fig. S6) (Nieto et al., 2004). At P2, Dpy19l1-knockdown cells in the upper cortical layer expressed Cux1, as did control embryos (Fig. 6E,F). GFP-positive cells that were aberrantly stalled in the deep CP and the IZ also expressed Cux1 (Fig. 6F-H″ ). These results indicated that cells settling in aberrant positions maintained the ability to differentiate into layer-specific cortical neurons, even though knockdown of Dpy19l1 caused a defect in neuronal migration.
Fig. 6.
Dpy19l1 knockdown does not affect neuronal differentiation. E14.5 mouse embryos were electroporated with control (A-A″ ,E) or Dpy19l1 shRNA (sh1769, B-B″ ,F-H″ ) and analyzed at E16.5 (A-B″ ) or P2 (E-H″ ). (A-B″ ) BrdU incorporation in VZ cells at E16.5. (C) Quantification of BrdU-labeled cells. The percentage of GFP and BrdU double-positive cells in the basal region of the VZ (brackets in A′,B′) is plotted as the mean ± s.e.m. (three embryos each for control and sh1769). (D) Quantification of Cux1-positive cells. The percentage of GFP and Cux1 double-positive cells in the E17.5 CP-IZ is plotted as the mean ± s.e.m. (three embryos each for control and sh1769). (E-H″ ) Double immunostaining of GFP and Cux1 at P2. In control embryos, most GFP-positive cells were immunolabeled by Cux1. In Dpy19l1-downregulated brains, Cux1 was expressed in GFP-positive cells that had stalled abnormally in the IZ and the CP, as well as in neurons that were correctly positioned in the upper cortex. Boxed areas in F are magnified in G-H″ . Scale bars: 20 μm in A-B″ ,G-H″ ; 100 μm in E,F.
Morphology of Dpy19l1-downregulated cells
To investigate if cells with defective migration can differentiate into mature neurons, we performed morphological analysis of shRNA-transfected cells. Two weeks after electroporation (at P8), most control neurons in the superficial layer had a triangular soma and a single apical dendrite (Fig. 7A,C). The majority of Dpy19l1-knockdown cells arrested in the deeper CP had a similar morphology, although they also had a longer process extending from the deeper layer towards the pial surface (arrows in Fig. 7D,E). Furthermore, most cells ectopically positioned within the IZ were either round in shape or exhibited a multipolar morphology (arrowheads in Fig. 7F). Some cells in the IZ had a long apical dendrite-like process extending towards the pial surface (arrows in Fig. 7G) and an axon-like structure extending towards the dorsal midline (arrows in Fig. 7H).
To accurately evaluate dendrite formation, we analyzed P14 embryos that had been labeled by Cre-mediated GFP recombination. In both control and Dpy19l1-knockdown embryos, many GFP-positive cells in the cortical upper layer assumed the characteristic pyramidal morphology, characterized by the extension of one apical dendrite and several basal dendrites, which were positive for the dendritic marker MAP2 (Mtap2 – Mouse Genome Informatics) (Fig. 7I,J; supplementary material Figs S7, S8). Many Dpy19l1-knockdown cells in the deep layer had a similar pyramidal morphology (116/150, 77.3%; Fig. 7K; supplementary material Fig. S7B-B″ ). However, most GFP-positive cells that were arrested in the white matter showed an abnormal rounded or multipolar morphology (70/80, 87.5%; Fig. 7L-L″ ; supplementary material Fig. S7B-D″ ).
DISCUSSION
In this study, we used single-cell microarray analysis to identify genes that are expressed specifically and/or strongly in cortical glutamatergic neurons. Among the candidate genes, we found that Dpy19l1 was prominently expressed in glutamatergic neurons of the developing cerebral cortex. Dpy19l1 knockdown studies showed that it is required for proper radial migration. However, Dpy19l1 downregulation did not affect cell type specification of cortical neurons. These results demonstrate that Dpy19l1 regulates radial migration of cortical glutamatergic neurons during development.
Identification of Dpy19l1 as a glutamatergic neuron-specific gene
Many studies have used genome-wide screens in an attempt to identify genes that regulate cell differentiation, migration and other developmental processes and that are specifically expressed in various cell types (Gray et al., 2004; Arlotta et al., 2005). Recently, Kawaguchi et al. reported the spatiotemporal heterogeneity of cortical progenitors using single-cell microarray analysis (Kawaguchi et al., 2008). In the present study, gene expression profiles were compared between glutamatergic and GABAergic neurons using the single-cell microarray method in order to identify genes that are expressed in developing glutamatergic neurons (Fig. 1) (Esumi et al., 2008). This type of single-cell microarray analysis is useful for detecting distinct expression patterns of each neuron/progenitor, although this technique is disadvantaged by its low sensitivity. With single-cell microarray analysis, however, we identified several genes, including Dpy19l1, that are prominently localized in glutamatergic neurons of the E14.5 telencephalon (Fig. 2; supplementary material Fig. S1; data not shown). We predict that our results will pave the way for the identification of other genes that regulate distinct developmental processes within each neuronal subtype.
Evolutionary conservation of Dpy19 genes
In C. elegans, DPY-19 is a putative multi-transmembrane protein that is required for the proper polarization and migration of Q neuroblasts (Honigberg and Kenyon, 2000). At least three Dpy19 genes are present in vertebrates (Carson et al., 2006). In the mammalian genome, four Dpy19 genes (Dpy19l1-4), encoding 8-11 predicted transmembrane proteins, have been identified, although the Dpy19 proteins lack any predicted functional domains. For the first time, we reveal that mammalian Dpy19l1 is crucial for directed neuronal migration during corticogenesis. Our results suggest a possible conserved role for the Dpy19 family during neuronal migration in both C. elegans and mammals. Interestingly, DPY19L3 has been reported to be associated with bipolar disorder by genome-wide association studies (Smith et al., 2009). More recently, DPY19L2 deletion has been found to cause human globozoospermia, which is a severe male infertility disorder resulting from round-headed spermatozoa that lack an acrosome (Harbuz et al., 2011; Koscinski et al., 2011). These observations are indicative of the importance of Dpy19 family members in development and disease.
Gradient expression of Dpy19l1 and Dpy19l3 in the developing cerebral cortex
We revealed that Dpy19l1 is mainly expressed in glutamatergic neurons, and not in GABAergic neurons, during embryonic development (Fig. 2). At E14.5, Dpy19l1 is expressed in a lateromedial gradient within the cerebral cortex, whereas Dpy19l3 is expressed in an opposite gradient (Fig. 2). Dpy19l1 expression also occurred in a rostral-to-caudal gradient (supplementary material Fig. S4). During early corticogenesis, when cortical neuron progenitors are initially specified, some transcription factors, such as Pax6, Emx2 and COUP-TFI (Nr2f1 – Mouse Genome Informatics), exhibit gradient expression in the VZ and regulate regional identity within the neocortex (Bishop et al., 2000; Hamasaki et al., 2004). Pax6 expression shows a rostral/lateralhigh to caudal/mediallow gradient, and caudal areas are expanded in Pax6 mutants (Bishop et al., 2000). This lateromedial gradient is similar to that of Dpy19l1, although Dpy19l1 is strongly expressed in postmitotic neurons (Fig. 2; supplementary material Fig. S4). It is possible that Dpy19l1 is a downstream target of transcription factors such as Pax6 and that they might regulate neuronal migration once cell fate is determined in the specific cortical areas.
Regulation of glutamatergic neuronal migration by Dpy19l1
A large number of Dpy19l1-knockdown cortical neurons were stalled deep within the CP and the IZ (Fig. 4). However, Dpy19l1-knockdown cells properly expressed the cell type-specific maker Cux1 (Fig. 6). These results suggest that the primary function of Dpy19l1 is the regulation of neuronal migration, but not neuronal specification. Furthermore, our results suggest that the efficiency of the shRNA is directly correlated with the severity of the migration defect (Fig. 4). Moreover, when we applied Dpy19l1 shRNA at reduced concentrations, the number of cells arrested in the IZ was decreased (data not shown). Thus, complete knockout of Dpy19l1 would likely result in a more severe migration defect in cortical neurons.
Defects in radial migration have also been observed in several mouse mutants and shRNA-transfected brains in which the cytoskeletal machinery has been modified, although there are differences in the severity of the migration defects (LoTurco and Bai, 2006). For example, Cdk5 loss-of-function in mice and Dcx knockdown in the rat neocortex result in severe migration defects in which a large number of cortical neurons are confined to the IZ (Gilmore et al., 1998; Bai et al., 2003). Lis1 heterozygous mice also show abnormalities in cortical neuronal migration (Hirotsune et al., 1998). A small percentage of Lis1 shRNA-transfected cells assumed a bipolar morphology and reached the IZ. Although the cell body was immobile, the leading process continued to grow (Tsai et al., 2005). Interestingly, we observed a phenotype similar to that of the Lis1 knockdown in the brains of Dpy19l1 knockdowns (Fig. 5). In C. elegans, dpy-19 is involved in the polarization of neuroblasts (Honigberg and Kenyon, 2000). In the mammalian brain, knockdown of the polarity kinases Mark2 and Lkb1 causes the arrest of neuronal migration in the IZ (Asada et al., 2007; Sapir et al., 2008). Although, at present, we do not know what signaling pathways interact with Dpy19 proteins to cause the defective migration, it is possible that Dpy19l1 participates in some of the aforementioned pathways to regulate neuronal migration during corticogenesis. It will be intriguing to investigate the possible interactions between Dpy19l1 and known migration regulators during corticogenesis. We are also in the process of designing a proteomic screen to identify binding partners of the Dpy19 family.
In addition to the proteins mentioned above, extracellular diffusible factors and cell adhesion molecules are also important for proper neuronal migration. Reelin and Sema3A have been shown to guide the radial migration of cortical neurons (Rice and Curran, 2001; Chen et al., 2008). Moreover, a recent report has shown that inhibition of the endocytic pathways also causes aberrant migration via disruption of N-cadherin trafficking (Kawauchi et al., 2010). Although dpy-19 and unc-40 (dcc), the receptor for unc-6 (netrin), are suggested to function in a shared signaling pathway in Q neuroblast migration in C. elegans (Honigberg and Kenyon, 2000), we did not observe any apparent defects in cortical layer formation in Dcc mutant mice (data not shown), suggesting that Dcc signaling is dispensable in the radial migration of mammalian cortical neurons. However, it is possible that Dpy19l1 mediates extracellular signals and/or is a component of the cell adhesion machinery, as Dpy19 family members are putative multi-transmembrane proteins.
Dendrite and axon formation in Dpy19l1-knockdown neurons
We observed that Dpy19l1-knockdown cells were localized in abnormal positions within the deep CP (Fig. 5). These cells assumed the characteristic pyramidal morphology but failed to correctly integrate into the upper layer (Figs 5, 6). Furthermore, the majority of Dpy19l1-knockdown cells in the IZ showed an abnormal rounded or multipolar morphology (Fig. 7; supplementary material Fig. S7). These results suggest that Dpy19l1 is involved in dendrite formation in addition to regulating neuronal migration. However, we cannot exclude the possibility that the morphological defects in the IZ are a reflection of the effects of the extracellular environment.
Since Dpy19l1 protein was localized in cortical axons (Fig. 2; supplementary material Fig. S3), it is possible that Dpy19l1 regulates axonal growth and/or pathfinding. We compared the axonal growth of cortical neurons in control and Dpy19l1 shRNA-electroporated neurons at P2. Many axons of Dpy19l1-knockdown neurons crossed the dorsal midline, similar to what is observed in control brains (supplementary material Fig. S9). Migrating neurons in the IZ started to extend their axons towards the dorsal midline in Dpy19l1 knockdowns (data not shown). However, we cannot exclude the possibility that the Dpy19l1 protein remaining after shRNA knockdown might still have a role in directing proper axonal projections. Additional complete loss-of-function studies using Dpy19l1 knockout mice will be required to clarify the role of Dpy19l1 in axonal projections.
Conclusions
The mammalian neocortex has been evolutionarily expanded to acquire higher functions. However, the developmental mechanisms specific to this region of the brain are poorly understood (Martínez-Cerdeño et al., 2006). Here, we identify Dpy19l1 as a gene expressed strongly in cortical glutamatergic neurons and as a regulator of radial neuronal migration. In mammals, the four members of the Dpy19 family are known to be associated with various diseases (Smith et al., 2009; Harbuz et al., 2011; Koscinski et al., 2011). Future study of Dpy19 family members might lead to further elucidation of the developmental mechanisms and functional organization of the mammalian neocortex.
Supplementary Material
Acknowledgments
We thank Dr Klaus-Armin Nave for generously providing NEX-Cre mice and Dr Marc Tessier-Lavigne for kindly providing Dcc-deficient mice; Dr Robert Hevner for the gift of anti-Tbr1 and anti-Tbr2 antibodies; Dr Jun-ichi Miyazaki for the pCAG-GS vector; the Developmental Studies Hybridoma Bank (DSHB) at the University of Iowa for monoclonal antibodies 4D7 (anti-TAG-1) and RC2; Drs Takeshi Kawauchi, Koji Oishi, Hitoshi Gotoh, Kenji Shimamura and Yohei Shinmyo for helpful comments; and Mr Noriyoshi Usui, Ms Mari Miyamoto, Dr Takashi Seki, Mr Shingo Usuki and members of Gene Technology Center at Kumamoto University for their contributions.
Footnotes
Funding
This work was supported by a Grant-in-Aid for Scientific Research [19700297 to K.W., 23590237 to H.T. and 20300121 to N.T.]; Scientific Research on Priority Area – Advanced Brain Science Project [18021030 to N.T.] from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan [to N.T.]; the Global Center of Excellence (COE) Program in Kumamoto University (Cell Fate Regulation Research and Education Unit); the Takeda Science Foundation [to K.W. and H.T.]; and the Naito Foundation [to K.W.]. Deposited in PMC for immediate release.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.068155/-/DC1
References
- Anderson S. A., Eisenstat D. D., Shi L., Rubenstein J. L. (1997). Interneuron migration from basal forebrain to cerebral cortex: dependence on Dlx genes. Science 278, 474–476 [DOI] [PubMed] [Google Scholar]
- Arlotta P., Molyneaux B. J., Chen J., Inoue J., Kominami R., Macklis J. D. (2005). Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207–221 [DOI] [PubMed] [Google Scholar]
- Asada N., Sanada K., Fukada Y. (2007). LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J. Neurosci. 27, 11769–11775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Ramos R. L., Ackman J. B., Thomas A. M., Lee R. V., LoTurco J. J. (2003). RNAi reveals doublecortin is required for radial migration in rat cerebral cortex. Nat. Neurosci. 6, 1277–1283 [DOI] [PubMed] [Google Scholar]
- Bishop K. M., Goudreau G., O’Leary D. D. M. (2000). Regulation of area identity in the mammalian cerebral cortex by Emx2 and Pax6. Science 288, 344–349 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson A. R., Cheung J., Scherer S. W. (2006). Duplication and relocation of the functional DPY19L2 gene within low copy repeats. BMC Genomics 7, 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Sima J., Jin M., Zheng W., Wang K. Y., Xue X. J., Ding Y. Q., Yuan X. B. (2008). Semaphorin-3A guides radial migration of cortical neurons during development. Nat. Neurosci. 11, 36–44 [DOI] [PubMed] [Google Scholar]
- des Portes V., Pinard J. M., Billuart P., Vinet M. C., Koulakoff A., Carrié A., Gelot A., Dupuis E., Motte J., Berwald-Netter Y., et al. (1998). A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell 92, 51–61 [DOI] [PubMed] [Google Scholar]
- Englund C., Fink A., Lau C., Pham D., Daza R. A., Bulfone A., Kowalczyk T., Hevner R. F. (2005). Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing cerebral cortex. J. Neurosci. 25, 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi S., Wu S. X., Yanagawa Y., Obata K., Sugimoto Y., Tamamaki N. (2008). Method for single-cell microarray analysis and application to gene-expression profiling of GABAergic neuron progenitors. Neurosci. Res. 60, 439–451 [DOI] [PubMed] [Google Scholar]
- Gilmore E. C., Ohshima T., Goffinet A. M., Kulkarni A. B., Herrup K. (1998). Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J. Neurosci. 18, 6370–6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson J. G., Allen K. M., Fox J. W., Lamperti E. D., Berkovic S., Scheffer I., Cooper E. C., Dobyns W. B., Minnerath S. R., Ross M. E., Walsh C. A. (1998). Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92, 63–72 [DOI] [PubMed] [Google Scholar]
- Gray P. A., Fu H., Luo P., Zhao Q., Yu J., Ferrari A., Tenzen T., Yuk D. I., Tsung E. F., Cai Z., et al. (2004). Mouse brain organization revealed through direct genome-scale TF expression analysis. Science 306, 2255–2257 [DOI] [PubMed] [Google Scholar]
- Guerrini R., Marini C. (2006). Genetic malformations of cortical development. Exp. Brain Res. 173, 322–333 [DOI] [PubMed] [Google Scholar]
- Hamasaki T., Leingartner A., Ringstedt T., O’Leary D. D. M. (2004). EMX2 regulates sizes and positioning of the primary sensory and motor areas in cerebral cortex by direct specification of cortical progenitors. Neuron 43, 359–372 [DOI] [PubMed] [Google Scholar]
- Hand R., Bortone D., Mattar P., Nguyen L., Heng J. I., Guerrier S., Boutt E., Peters E., Barnes A. P., Parras C., et al. (2005). Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron 48, 45–62 [DOI] [PubMed] [Google Scholar]
- Harbuz R., Zouari R., Pierre V., Ben Khelifa M., Kharouf M., Coutton C., Merdassi G., Abada F., Escoffier J., Nikas Y., et al. (2011). A recurrent deletion of DPY19L2 causes infertility in man by blocking sperm head elongation and acrosome formation. Am. J. Hum. Genet. 88, 351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten M. E. (2002). New directions in neuronal migration. Science 297, 1660–1663 [DOI] [PubMed] [Google Scholar]
- Hirotsune S., Fleck M. W., Gambello M. J., Bix G. J., Chen A., Clark G. D., Ledbetter D. H., McBain C. J., Wynshaw-Boris A. (1998). Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat. Genet. 19, 333–339 [DOI] [PubMed] [Google Scholar]
- Honigberg L., Kenyon C. (2000). Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY19 proteins in C. elegans. Development 127, 4655–4668 [DOI] [PubMed] [Google Scholar]
- Kato M., Dobyns W. B. (2003). Lissencephaly and the molecular basis of neuronal migration. Hum. Mol. Genet. 12, R89–R96 [DOI] [PubMed] [Google Scholar]
- Kawaguchi A., Ikawa T., Kasukawa T., Ueda H. R., Kurimoto K., Saitou M., Matsuzaki F. (2008). Single-cell gene profiling defines differential progenitor subclasses in mammalian neurogenesis. Development 135, 3113–3124 [DOI] [PubMed] [Google Scholar]
- Kawauchi T., Sekine K., Shikanai M., Chihama K., Tomita K., Kubo K., Nakajima K., Nabeshima Y., Hoshino M. (2010). Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron 67, 588–602 [DOI] [PubMed] [Google Scholar]
- Koscinski I., Elinati E., Fossard C., Redin C., Muller J., Velez de la Calle J., Schmitt F., Ben Khelifa M., Ray P. F., Kilani Z., et al. (2011). DPY19L2 deletion as a major cause of globozoospermia. Am. J. Hum. Genet. 88, 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A. R., Noctor S. C. (2004). Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 27, 392–399 [DOI] [PubMed] [Google Scholar]
- LoTurco J. J., Bai J. (2006). The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 29, 407–413 [DOI] [PubMed] [Google Scholar]
- Marín O., Rubenstein J. L. (2003). Cell migration in the forebrain. Annu. Rev. Neurosci. 26, 441–483 [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeño V., Noctor S. C., Kriegstein A. R. (2006). The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb. Cortex 16 Suppl 1, i152–i161 [DOI] [PubMed] [Google Scholar]
- Miyata T., Kawaguchi A., Okano H., Ogawa M. (2001). Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727–741 [DOI] [PubMed] [Google Scholar]
- Nadarajah B., Parnavelas J. G. (2002). Modes of neuronal migration in the developing cerebral cortex. Nat. Rev. Neurosci. 3, 423–432 [DOI] [PubMed] [Google Scholar]
- Nieto M., Monuki E. S., Tang H., Imitola J., Haubst N., Khoury S. J., Cunningham J., Gotz M., Walsh C. A. (2004). Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J. Comp. Neurol. 479, 168–180 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Flint A. C., Weissman T. A., Dammerman R. S., Kriegstein A. R. (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 [DOI] [PubMed] [Google Scholar]
- Preuss T. M., Kaas J. H. (1999). Human brain evolution. In Fundamental Neuroscience (ed. Zigmond M. J., Bloom F. E., Landis S. C., Roberts J. L., Squire L. R.), pp. 1283–1311 San Diego: Academic Press; [Google Scholar]
- Rakic P. (1972). Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145, 61–83 [DOI] [PubMed] [Google Scholar]
- Reiner O., Sapir T. (2009). Polarity regulation in migrating neurons in the cortex. Mol. Neurobiol. 40, 1–14 [DOI] [PubMed] [Google Scholar]
- Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W. B., Caskey C. T., Ledbetter D. H. (1993). Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature 364, 717–721 [DOI] [PubMed] [Google Scholar]
- Rice D. S., Curran T. (2001). Role of the reelin signaling pathway in central nervous system development. Annu. Rev. Neurosci. 24, 1005–1039 [DOI] [PubMed] [Google Scholar]
- Ross M. E., Walsh C. A. (2001). Human brain malformations and their lessons for neuronal migration. Annu. Rev. Neurosci. 24, 1041–1070 [DOI] [PubMed] [Google Scholar]
- Sapir T., Sapoznik S., Levy T., Finkelshtein D., Shmueli A., Timm T., Mandelkow E. M., Reiner O. (2008). Accurate balance of the polarity kinase MARK2/Par-1 is required for proper cortical neuronal migration. J. Neurosci. 28, 5710–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M. H., Bartholomae A., Heimrich B., Feldmeyer D., Druffel-Augustin S., Goebbels S., Naya F. J., Zhao S., Frotscher M., Tsai M. J., Nave K. A. (2000). Neuronal basic helix-loop-helix proteins (NEX and BETA2/Neuro D) regulate terminal granule cell differentiation in the hippocampus. J. Neurosci. 20, 3714–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. N., Bloss C. S., Badner J. A., Barrett T., Belmonte P. L., Berrettini W., Byerley W., Coryell W., Craig D., Edenberg H. J., et al. (2009). Genome-wide association study of bipolar disorder in European American and African American individuals. Mol. Psychiatry 14, 755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura N., Patel R. G., Corriveau R. A. (2001). N-methyl-D-aspartate receptors regulate a group of transiently expressed genes in the developing brain. J. Biol. Chem. 27, 14257–14263 [DOI] [PubMed] [Google Scholar]
- Tabata H., Nakajima K. (2003). Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J. Neurosci. 23, 9996–10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N., Fujimori K. E., Takauji R. (1997). Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J. Neurosci. 17, 8313–8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N., Nakamura K., Okamoto K., Kaneko T. (2001). Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci. Res. 41, 51–60 [DOI] [PubMed] [Google Scholar]
- Tamamaki N., Yanagawa Y., Tomioka R., Miyazaki J., Obata K., Kaneko T. (2003). Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 467, 60–79 [DOI] [PubMed] [Google Scholar]
- Tsai J. W., Chen Y., Kriegstein A. R., Vallee R. B. (2005). LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J. Cell Biol. 170, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Tamamaki N., Furuta T., Ackerman S. L., Ikenaka K., Ono K. (2006). Dorsally derived netrin 1 provides an inhibitory cue and elaborates the ‘waiting period’ for primary sensory axons in the developing spinal cord. Development 133, 1379–1387 [DOI] [PubMed] [Google Scholar]
- Wu S. X., Goebbels S., Nakamura K., Nakamura K., Kometani K., Minato N., Kaneko T., Nave K. A., Tamamaki N. (2005). Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 102, 17172–17177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.