Abstract
Hypocretins (orexins) are hypothalamic neuropeptides that play a crucial role in regulating sleep/wake states and autonomic functions including parasympathetic cardiac activity. We have recently demonstrated stimulation of the lateral paragigantocellular nucleus (LPGi), the nucleus which is thought to play a role in rapid eye movement (REM) sleep control, activates an inhibitory pathway to preganglionic cardiac vagal neurons in the nucleus ambiguus (NA). In this study we test the hypothesis that hypocretin-1 modulates the inhibitory neurotransmission to cardiac vagal neurons evoked by stimulation of the LPGi using whole-cell patch-clamp recordings in an in vitro brain slice preparation from rats. Activation of hypocretin-1 receptors produced a dose-dependent and long-term facilitation of GABAergic postsynaptic currents evoked by electrical stimulation of the LPGi. Hypoxia/hypercapnia diminished LPGi-evoked GABAergic current in cardiac vagal neurons and this inhibition by hypoxia/hypercapnia was prevented by pre-application of hypocretin-1. The action of hypocretin-1 was blocked by the hypocretin-1 receptor antagonist SB-334867. Facilitation of LPGi-evoked GABAergic current in cardiac vagal neurons under both normal condition and during hypoxia/hypercapnia could be the mechanism by which hypocretin-1 affects parasympathetic cardiac function and heart rate during REM sleep. Furthermore, our findings indicate a new potential mechanism that might be involved in the cardiac arrhythmias, bradycardia, and sudden cardiac death that can occur during sleep.
Keywords: hypocretin, rapid eye movement sleep, rostral ventral medulla, parasympathetic preganglionic neurons, bradycardia
The hypocretin-1 and hypocretin-2 peptides, also called orexin-A and orexin-B, are exclusively synthesized in perifornical area and lateral hypothalamus neurons (de Lecea et al., 1998; Sakurai et al., 1998). Hypocretin has been strongly implicated in sleep-wake control (Chemelli et al., 1999; Kilduff and Peyron, 2000). Evidence from human and animal studies indicates that hypocretin regulates/maintains both waking state and rapid eye movement (REM) sleep (Chemelli et al., 1999; Kilduff and Peyron, 2000; Thannickal et al., 2000; Kiyashchenko et al., 2002), whereas deficiency in hypocretin neurotransmission results in narcolepsy, a disorder characterized primarily by excessive daytime sleepiness and REM sleep dysregulation (Chemelli et al., 1999; Kilduff and Peyron, 2000). In addition, compelling evidence links hypocretin to regulation of cardiovascular function (Ciriello et al., 2003; Dergacheva et al., 2005). Electrical or chemical stimulation of the lateral hypothalamic area has been shown to increase both heart rate and blood pressure and also to excite neurons in the lateral paragigantocellular nucleus (LPGi) (Sun and Guyenet, 1986; Allen and Cechetto, 1993). Hypocretin fibers have been found in nuclei that are well known to be involved in cardiovascular regulation, including the LPGi (Peyron et al., 1998; Ciriello et al., 2003). Intracisternal or intrathecal administration of hypocretin-1 as well as microinjection of hypocretin-1 into the rostral ventral medulla and the commissural nucleus of the nucleus tractus solitarius complex increases mean arterial pressure and heart rate of rats (Chen et al., 2000; Antunes et al., 2001; Smith et al., 2002). Activation of hypocretin-1 receptors facilitates inhibitory and diminishes excitatory spontaneous neurotransmission to cardiac vagal neurons in the nucleus ambiguus (NA) (Dergacheva et al., 2005). Thus, hypocretin neurons and receptors have been proposed to provide a link between central mechanisms that regulate arousal and sleep-wakefulness states and central control of autonomic functions (Young et al., 2005).
Heart rate and parasympathetic activity to the heart change dramatically during transition from non-REM sleep to REM sleep (Sei et al., 2002; Valladares et al., 2008). We have recently demonstrated stimulation of the LPGi, the nucleus which is thought to play a role in REM sleep control (Verret et al., 2005, 2006), evokes an inhibitory pathway to preganglionic cardiac vagal neurons in the NA (Dergacheva et al., 2010b). The LPGi-elicited inhibitory GABAergic pathway to cardiac vagal neurons likely constitutes a neurochemical mechanism underlying REM sleep-related reduction in parasympathetic cardiac activity (Dergacheva et al., 2010b). In the present study we extend this framework and propose that hypocretin-1 influences REM sleep-associated changes in parasympathetic cardiac activity by modulating GABAergic synaptic neurotransmission to cardiac vagal neurons from the LPGi. Because hypercapnia activates hypocretin neurons (Williams et al., 2007; Kuwaki, 2010) and hypoxia/hypercapnia significantly influences the activity of cardiac vagal neurons in the NA (Dergacheva et al., 2010a,b) we also examined the effects of hypocretin-1 and hypoxia/hypercapnia on the GABAergic pathway from the LPGi to cardiac vagal neurons.
EXPERIMENTAL PROCEDURES
All animal procedures were performed in compliance with the institutional guidelines at George Washington University and are in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and any possible discomfort.
To identify cardiac vagal neurons in vitro a two-stage procedure was used. In an initial surgery, Sprague–Dawley rats (postnatal days 2–3; Hilltop, Scottdale, PA, USA) were anesthetized with hypothermia and received a right thoracotomy. The heart was exposed, and 0.05 ml of 1–5% rhodamine (XRITC, Molecular Probes, Eugene, OR, USA) was injected into the pericardial sac to retrogradely label cardiac vagal neurons. On the day of experiment (2–4 days later), the animals were anesthetized with isoflurane and killed by rapid cervical dislocation. The brain was submerged in cold (4 °C) buffer composed of 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 5 mM glucose, and 10 mM HEPES and continually gassed with 100% O2. Using a dissection microscope the cerebellum was removed and the hindbrain was isolated. A single slice of the medulla (400 μm thickness) that included the NA and the LPGi was obtained and submerged in a recording chamber, which allowed perfusion (5–10 ml/min) of artificial cerebrospinal fluid at room temperature (25 °C) containing 125 mM NaCl, 3 mM KCl, 2 mM CaCl2, 26 mM NaHCO3, 5 mM glucose, and 5 mM HEPES equilibrated with 95% O2 and 5% CO2 (pH 7.4).
Individual cardiac vagal neurons in the NA were identified by the presence of the fluorescent tracer. These identified cardiac vagal neurons were then imaged with differential interference contrast optics, infrared illumination, and infrared-sensitive video detection cameras to gain better spatial resolution. Patch pipettes (2.5–3.5 MΩ) were filled with a solution consisting of 150 mM KCl, 2 mM MgCl2, 2 mM EGTA, 10 mM HEPES, and 2 mM Mg-ATP, pH 7.4. With this pipette solution, the chloride current induced by activation of GABA receptors was recorded as an inward current. Voltage clamp whole-cell recordings were made at a holding potential of −80 mV with an Axopatch 200B and pClamp 8 software (Axon Instruments, Union City, CA, USA).
GABAergic inhibitory postsynaptic currents were isolated by continuous focal application of strychnine (1 μM), D-2-amino-5-phosphonovalerate (50 μM), and 6-cyano-7-nitroquinoxaline-2,3-dione (50 μM) to block glycine, N-methyl-D-asparate, and non–N-methyl-D-asparate glutamatergic receptors, respectively. Drugs were focally released using a picrospritzer and pressure ejected from a patch pipette positioned within 30 μm of the patched cardiac vagal neuron. The maximum range of drug application was determined previously to be 100–120 μm downstream from the drug pipette and was considerably less behind the drug pipette (Wang et al., 2002). Continual focal drug applications were performed using a pneumatic picopump pressure system (WPI, Sara-sota, FL, USA). Hypocretin-1 (0.1 μM, 0.5 μM, and 1 μM) and the hypocretin-1 receptor antagonist SB-334867 (10 μM) were bath-applied. After the end of each experiment the GABAergic neurotransmission was abolished by focal application of gabazine (25 μM) to the patched cardiac vagal neuron. All drugs used in this study were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
In experiments that examined the role of hypoxia/hypercapnia in modulation of GABAergic response in cardiac vagal neurons, slices were exposed to hypoxia/hypercapnia by changing control artificial cerebrospinal fluid to an identical solution bubbled with 85% N2, 6% O2, and 9% CO2. After 1 h equilibration with 85% N2, 6% O2, and 9% CO2 the pH of the solution was 7.1. Slices were exposed to hypoxia/hypercapnia for 10 min and then slices were reoxygenated during 10 min by returning the perfusate to initial control artificial cerebrospinal fluid.
The location of the LPGi was identified using stereotaxic coordinates (Paxinos, 1997) in addition to the location relative to fluorescently identified cardiac vagal neurons in the NA. The LPGi was stimulated with electrical stimuli of 1 ms duration using a stimulus isolator (A.M.P.I., Jerusalem, Israel). Stimulus intensity was 1.5 times of the minimum intensity that evoked a response in cardiac vagal neurons. Synaptic events were measured using pClamp 8 software (Molecular Devices, Sunnyvale, CA, USA). The responses to a series of 10 consecutive stimulations in each neuron were averaged in all series of experiments. The mean value from each neuron in the population was then averaged for the population of neurons to create a summary of results for each condition.
Results are presented as mean±SE and statistically compared using ANOVA with repeated measurement and Dunnett’s post-test. Only one experiment was performed per preparation.
RESULTS
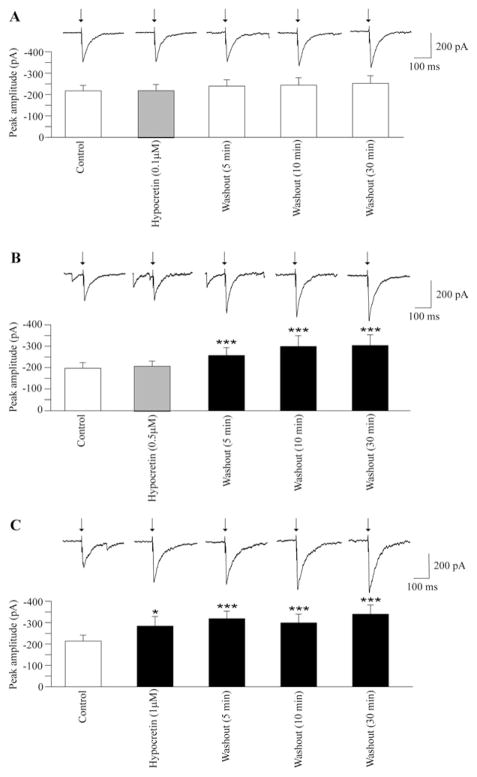
In agreement with previously published data (Dergacheva et al., 2010b), electrical stimulation of the LPGi evoked a GABAergic current in cardiac vagal neurons in the NA (see Figs. 1–3). Application of hypocretin-1 at a concentration of 0.1 μM did not significantly alter this GABAergic response (−217±26 pA vs. −220±29 pA, n=9, P>0.05, Fig. 1A). Washout of hypocretin-1 (0.1 μM) also did not change this LPGi-evoked GABAergic current (n=9, Fig. 1A). Similarly, at a concentration of 0.5 μM hypocretin-1 did not alter GABAergic current in cardiac vagal neurons (−197±27 pA vs. −209±24 pA, n=9, P>0.05, Fig. 1B). However, 5-min washout of hypocretin-1 (0.5 μM) significantly enhanced the LPGi-evoked GABAergic response (from −197±27 pA to 257±36 pA, n=9, P<0.001, Fig. 1B). This GABAergic response remained elevated for at least 30 min of hypocretin-1 washout (n=9, Fig. 1B). Application of hypocretin-1 at a concentration of 1 μM elicited a significant facilitation of LPGi-evoked GABAergic current in cardiac vagal neurons (from −214±28 pA to −284±45 pA, n=9, P<0.05, Fig. 1C). The LGPi-evoked GABAergic current remained elevated for at least 30 min of hypocretin-1 washout (n=9, Fig. 1C).
Fig. 1.
Stimulation of the LPGi evoked a GABAeric pathway and activation of postsynaptic GABA receptors in cardiac vagal neurons (A, B, C). Application of hypocretin-1 at a concentration of 0.1 μM did not alter the evoked GABAergic current as shown in a typical example (A, top) and in the summary data from nine neurons (A, bottom). Similarly, application of hypocretin-1 at a concentration of 0.5 μM did not change GABAergic current evoked by stimulation of the LPGi. However, during washout of hypocretin-1 (0.5 μM) the LPGi-evoked GABAergic current was significantly increased up to 30 min (a typical example is shown in B, top, and the summary data from nine neurons are illustrated in B, bottom). As shown in C, application of hypocretin-1 at a concentration of 1 μM significantly increased GABAergic current evoked by LPGi stimulation and the evoked GABAergic current remained elevated during the washout period up to 30 min (C, top, typical experiment, and C, bottom, summary data from nine neurons). In this and all subsequent figures: arrow indicates electrical stimulation. ■ with asterisks indicates statistically significant differences * P<0.05 and *** P<0.001.
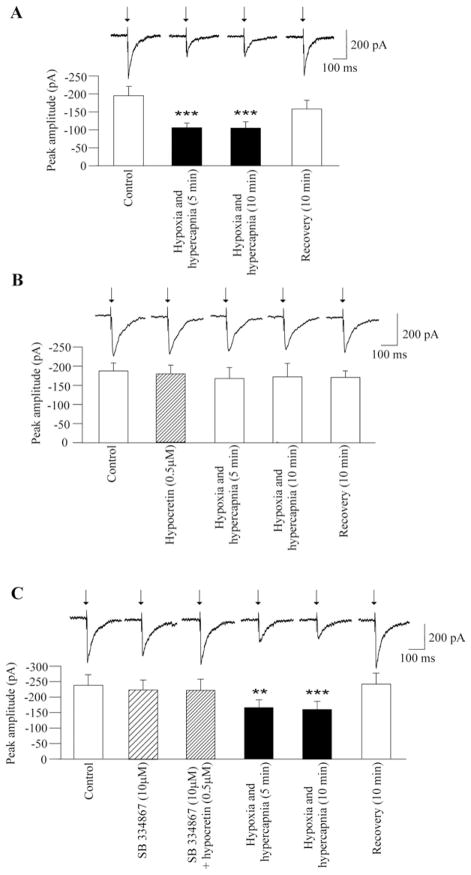
Fig. 3.
As shown in a typical experiment (A, top) and in the summary data from nine neurons (A, bottom) hypoxia/hypercapnia elicited a significant and reversible decrease in the peak amplitude of GABAergic current evoked by electrical stimulation of the LPGi. This inhibition in the evoked GABAergic response was prevented by pre-application of hypocretin-1 at a concentration of 0.5 μM, as demonstrated in a typical experiment (B, top) and in the summary data from nine neurons (B, bottom). However, in the presence of the hypocretin-1 receptor antagonist SB-334867 (10 μM) hypocretin-1 (0.5 μM) did not alter the hypoxia/hypercapnia-evoked inhibition in GABAergic response evoked by LPGi stimulation (C, top, typical experiment, and C, bottom, summary data from eight neurons). ■ with asterisks indicates statistically significant differences ** P<0.01 and *** P<0.001.
Since the temperature of the rat brain is higher than the room temperature (25 °C) at which most experiments for this study were performed we conducted six additional experiments at 37 °C. Hypocretin-1 at a concentration of 1 μM evoked a small but not significant facilitation of LPGi-evoked GABAergic current at 37 °C (−228±49 pA vs. −269±45 pA, n=6, P>0.05). However, similar to results obtained at 25 °C, 5-min washout of hypocretin-1 at 37 °C evoked a significant increase in the GABAergic current (from −228±49 pA to −349±69 pA, n=6, P<0.01) which remained elevated (−344±64 pA, n=6, P<0.05) for at least 30 min. Since the dynamics of the responses studied at 25 °C were similar to those at 37 °C, further experiments were conducted at room temperature (25 °C) which preserves cell health in vitro for longer durations than at 37 °C.
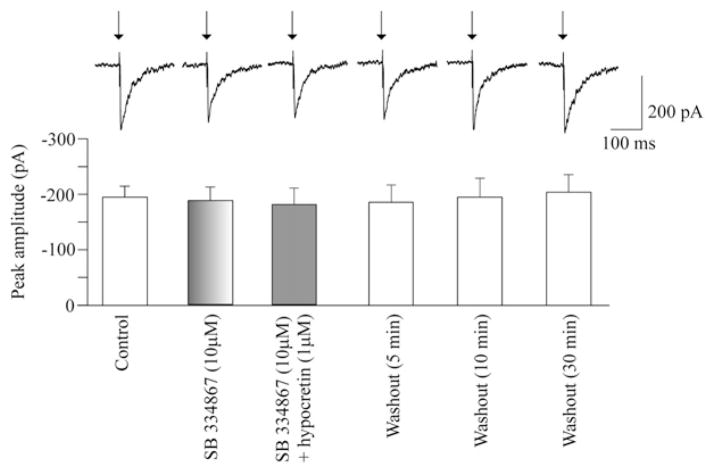
To test if the effect of hypocretin was mediated via hypocretin-1 receptors and eliminate a possibility of non-hypocretin-1 receptor activation, SB-334867, a hypocretin-1 receptor antagonist, was used prior to and during hypocretin-1 administration. Application of SB-334867 at a concentration of 10 μM by itself did not evoke any significant change in the LGPi-evoked GABAergic current in cardiac vagal neurons (−195±20 pA vs. −187±24 pA, n=8, P>0.05, Fig. 2). However, SB-334867 blocked the hypocretin-1-elicited facilitation of the LGPi-evoked GABAergic current since in the presence of SB-334867 (10 μM) hypocretin-1 (1 μM) failed to significantly alter the LGPi-evoked GABAergic current (−195±20 pA vs. −180±30 pA, n=8, P>0.05, Fig. 2). Similarly, the LPGi-evoked GABAergic current was not altered by washout of hypocretin-1 (1 μM) applied in the presence of SB-334867 (n=8, Fig. 2).
Fig. 2.
The hypocretin-1 receptor antagonist SB-334867 at a concentration of 10 μM did not alter GABAergic current evoked by stimulation of the LPGi. However, SB-334867 prevented the hypocretin-1-elicited facilitation of the evoked GABAergic current as in the presence of SB-334867 (10 μM) hypocretin-1 (1 μM) did not evoke any significant changes in the LPGi-evoked GABAergic neurotransmission. A typical experiment is shown on the top while the summary data from eight neurons are demonstrated in the bottom.
In agreement with previous studies (Dergacheva et al., 2010b), changing the perfusate from artificial cerebrospinal fluid equilibrated with 95% to identical O2–5% CO2 solution equilibrated with 9% CO2, 6% O2, and 85% N2 (hypoxia/hypercapnia) induced a significant decrease in the peak amplitude of the GABAergic response evoked by LPGi stimulation throughout hypoxia/hypercapnia (P<0.001, n=9, Fig. 3A). This inhibitory effect of hypoxia/hypercapnia was prevented by pre-application of hypocretin-1 at a concentration of 0.5 μM (P>0.05, Fig. 3B).
To confirm the effect of hypocretin during hypoxia/hypercapnia was mediated via hypocretin-1 receptors, SB-334867, a hypocretin-1 receptor antagonist, was applied prior to and during hypocretin-1 application. In the presence of SB-334867 (10 μM) hypocretin-1 (0.5 μM) failed to prevent the inhibition of the LPGi-evoked GABAergic current elicited by hypoxia/hypercapnia (n=9, Fig. 3C).
DISCUSSION
There are two major findings of this work: (1) Activation of hypocretin-1 receptors evokes a dose-dependent and long-term facilitation of LGPi-evoked GABAergic neurotransmission to cardiac vagal neurons, (2) Activation of hypocretin-1 receptors prevents the hypoxia/hypercapnia-dependent decrease of this synaptic pathway to cardiac vagal neurons.
The results from previous in vivo studies indicate that microinjection of hypocretin-1 into the rostral ventral medulla causes an increase in both mean arterial pressure and heart rate (Machado et al., 2002), whereas no effect is observed in vagotomized rats (Young et al., 2005). In addition, microinjection of hypocretin-1 into the rostral ventral medulla, including the LGPi, produces a tachycardia mediated in part by inhibition of parasympathetic activity to the heart (Ciriello et al., 2003). The findings from the present work are in accordance with these in vivo data and establish a cellular mechanism by which hypocretin-1 diminishes parasympathetic cardiac activity and thereby contributes to heart rate acceleration: hypocretin-1 facilitates the GABAergic pathway from the LGPi to cardiac vagal neurons in the NA.
Previously we have shown that activation of hypocretin-1 receptors facilitates spontaneous postsynaptic GABAergic neurotransmission to cardiac vagal neurons in the NA (Dergacheva et al., 2005). In the presence of tetrodotoxin (a selective blocker of Na+ channels) hypocretin-1 fails to evoke an increase in spontaneous GABAergic neurotransmission to cardiac vagal neurons (Dergacheva et al., 2005) indicating that hypocretin-1 likely acts on preceding GABAergic neurons to evoke action potential-dependent changes and increase the spontaneous GABAergic neurotransmission to cardiac vagal neurons. These preceding GABAergic neurons are likely located in the rostral ventral medulla, including the LPGi, as GABAergic current in cardiac vagal neurons evoked by activation of LGPi neurons is increased by hypocretin-1. Supporting this hypothesis, photo-excitation of individual GABAergic neurons in the rostral ventral medulla evokes GABAergic current in cardiac vagal neurons in the NA (Frank et al., 2009). Hypocretin-1, therefore, likely influences parasympathetic cardiac activity via excitation of GABAergic neurons located in the rostral ventral medulla, including the LGPi, which project to and inhibit cardiac vagal neurons within the NA.
The results from other work demonstrate that hypocretin-1 facilitates spontaneous GABAergic neurotransmission in cardiac vagal neurons in a concentration-dependent manner (Wang et al., 2005). Consistent with the previously published work the results from this study indicate that hypocretin-1 dose-dependently increases LGPi-evoked postsynaptic current in cardiac vagal neurons. Interestingly, at a comparatively low concentration (0.5 μM) hypocretin-1 does not alter LGPi-evoked GABAergic current in cardiac vagal neurons, but during washout of hypocretin-1 the LGPi-evoked GABAergic current is enhanced at least for 30 min. At a higher concentration (1 μM) hypocretin-1 elicits a significant facilitation of LGPi-evoked GABAergic current which remains elevated for at least 30 min after hypocretin-1 washout. Long-term effects of hypocretin have been shown in other studies. Hypocretin-1 induces long-term potentiation of excitatory neurotransmission to dopamine neurons in the ventral tegmental area (Borgland et al., 2006; Aston-Jones et al., 2010). In CA-1 field of hippocampal slices hypocretin-1 evokes long-term potentiation of synaptic neurotransmission in adult mice and long-term depression of synaptic neurotransmission in juvenile mice (Selbach et al., 2010). In addition, hypocretin is necessary for long-lasting augmentation of respiratory activity evoked by repetitive intermittent hypoxia (Terada et al., 2008; Toyama et al., 2009).
Hypocretin has been postulated to play a role in ventilatory chemosensitivity and stimulate breathing (Young et al., 2005; Deng et al., 2007). Hypocretin neuron firing is potently stimulated by CO2 and H+ (Williams et al., 2007) while hypercapnic chemoreflex is attenuated and sleep apnea is exaggerated in hypocretin knockout mice (Deng et al., 2007). Projections of hypocretin-containing neurons to the retrotrapezoid nucleus have been shown to contribute, via hypocretin-1 receptors, to the hypercapnic chemoreflex control (Dias et al., 2009). The results from this study indicate that hypocretin-1 receptors likely also play a role in parasympathetic-mediated heart rate responses to hypoxia/hypercapnia. Our data indicate that hypoxia/hypercapnia elicits a significant decrease in the GABAergic current evoked by LPGi stimulation, while activation of hypocretin-1 receptors prevents this hypoxia/hypercapnia-dependent inhibition. It is likely that release of hypocretin stimulated by hypercapnia and subsequent activation of hypocretin-1 receptors in the medulla oblongata serve to maintain GABAergic neurotransmission to cardiac vagal neurons to prevent excessive activation of cardiac vagal neurons and exaggerated parasympathetic activity to the heart during hypoxia/hypercapnia. Previously we have demonstrated that endogenous cholinergic activation, in particular activation of α7 subunit containing nicotinic receptors, is involved in hypoxia/hypercapnia-evoked inhibition of GABAergic neurotransmission to cardiac vagal neurons elicited by LGPi stimulation (Dergacheva et al., 2010b). The results from this study extend the framework presented in our previous study and suggest that there are two opposing modulatory mechanisms that could be activated by hypoxia/hypercapnia and influence the GABAergic pathway from LGPi to cardiac vagal neurons: activation of α7 nicotinic receptors diminishes the LGPi-evoked GABAergic pathway to cardiac vagal neurons while activation of hypocretin-1 receptors enhances this important pathway. An altered balance of these opposing inputs may play a role in apnea-associated bradyarrhythmias.
REM sleep is characterized by an increased sympathetic and attenuated vagal tone when compared to non-REM sleep (Berlad et al., 1993). Release of hypocretin-1 in the brain is maximal during both wakefulness and REM sleep and minimal during non-REM sleep (Kiyashchenko et al., 2002). Hypocretin neurons are active during motor activity and pharmacologically-induced REM sleep (Torterolo et al., 2006). In addition, hypocretin neurons discharge during waking and REM sleep and remain virtually silent during non-REM sleep (Mileykovskiy et al., 2005; Takahashi et al., 2008). Furthermore, microinjection of either hypocretin-1 or -2 into the nucleus pontis oralis, the nucleus that plays a crucial role in generation of REM sleep, induces REM sleep (Xi et al., 2002).
In addition to hypocretin cell activation in the hypothalamus during REM sleep, neurons in the brainstem, including the LPGi, are also thought to be active during REM sleep. LPGi neurons increase their activity following REM sleep deprivation in rats (Verret et al., 2005, 2006). Similarly, neurons with an activity specific to REM sleep were identified in the cat LPGi (Sakai, 1988).
We have recently established likely neuroanatomical substrates and cellular mechanisms underlying REM sleep-related reduction in parasympathetic cardiac activity: activation of LPGi neurons elicits an inhibitory GABAergic pathway to cardiac vagal neurons in the NA (Dergacheva et al., 2010b). The results from this study extend the framework presented in our previous study (Dergacheva et al., 2010b) and suggest that release of hypocretin during REM sleep (Kiyashchenko et al., 2002) would facilitate the inhibition of cardiac vagal neurons evoked by activation of the LPGi and thereby contribute to REM-sleep related withdrawal of parasympathetic cardiac activity. Supporting this framework, hypocretin-1 like immunoreactivity has been found in the LPGi while microinjection of hypocretin-1 into the LGPi produces a tachycardia (Ciriello et al., 2003). In addition, hypocretin has been hypothesized to produce a relatively selective potentiation of GABA release during REM sleep (Kiyashchenko et al., 2002).
A reduction in hypocretin neurotransmission has been recently hypothesized to be implicated in sudden death during sleep (Parekh, 2009). In addition, abnormal parasympathetic cardiac activity has been implicated in diseases such as cardiac arrhythmia and sudden infant death syndrome (Mendelowitz, 1999). The results of the present study suggest that hypocretin-1 could control the parasympathetic activity to the heart via modulation of GABAergic pathway from the LPGi to cardiac vagal neurons in the NA. A diminished hypocretin-induced facilitation of inhibitory neurotransmission to cardiac vagal neurons might provoke disinhibition of cardiac vagal neurons and exaggerated parasympathetic activity to the heart which in turn could elicit bradycardia, asystole, and sudden cardiac death during sleep.
CONCLUSION
In conclusion, activation of hypocretin receptors facilitates an inhibitory GABAergic pathway to cardiac vagal neurons elicited by activation of LPGi neurons under normal conditions and prevents the inhibition of this GABAergic pathway to cardiac vagal neurons that normally occurs during hypoxia/hypercapnia. These cellular mechanisms might underline REM sleep-related reduction in parasympathetic cardiac activity while alteration in these important mechanisms in combination with other factors might play a role in sleep-related cardiac arrhythmias and sudden cardiac death.
Acknowledgments
This work was supported by NIH grants HL 49965,59895, and 72006 to D.M.
Abbreviations
- LPGi
lateral paragigantocellular nucleus
- NA
nucleus ambiguus
- REM
rapid eye movement sleep
References
- Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area. II. Ascending projections. J Comp Neurol. 1993;330:421–438. doi: 10.1002/cne.903300310. [DOI] [PubMed] [Google Scholar]
- Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1801–R1807. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlad SA, II, Ben-Haim S, Lavie P. Power spectrum analysis and heart rate variability in stage 4 and REM sleep: evidence for state-specific changes in autonomic dominance. J Sleep Res. 1993;2:88–90. doi: 10.1111/j.1365-2869.1993.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R692–R697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen KJ, Neff RA, Mendelowitz D. Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respir Physiol Neurobiol. 2010a;174:102–110. doi: 10.1016/j.resp.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Lovett-Barr MR, Jameson H, Mendelowitz D. The lateral paragigantocellular nucleus modulates parasympathetic cardiac neurons; a mechanism for rapid eye movement sleep-dependent changes in heart rate. J Neurophysiol. 2010b;104:685–694. doi: 10.1152/jn.00228.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Huang ZG, Bouairi E, Stephens C, Gorini C, Mendelowitz D. Hypocretin-1 (orexin-A) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. J Pharmacol Exp Ther. 2005;314:1322–1327. doi: 10.1124/jpet.105.086421. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photouncaging. J Neurophysiol. 2009;101:1755–1760. doi: 10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T. Hypothalamic modulation of breathing. Adv Exp Med Biol. 2010;669:243–247. doi: 10.1007/978-1-4419-5692-7_49. [DOI] [PubMed] [Google Scholar]
- Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept. 2002;104:75–81. doi: 10.1016/s0167-0115(01)00351-2. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh B. The mechanism of dead-in-bed syndrome and other sudden unexplained nocturnal deaths. Curr Diabetes Rev. 2009;5:210 –215. doi: 10.2174/157339909789804387. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K. Executive mechanisms of paradoxical sleep. Arch Ital Biol. 1988;126:239–257. [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sei H, Sano A, Ohno H, Yamabe K, Nishioka Y, Sone S, Morita Y. Age-related changes in control of blood pressure and heart rate during sleep in the rat. Sleep. 2002;25:279–285. doi: 10.1093/sleep/25.3.279. [DOI] [PubMed] [Google Scholar]
- Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson KS, Sergeeva OA, Haas HL. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol (Oxf) 2010;198:277–285. doi: 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002;950:261–267. doi: 10.1016/s0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]
- Sun MK, Guyenet PG. Hypothalamic glutamatergic input to medullary sympathoexcitatory neurons in rats. Am J Physiol. 1986;251:R798–R810. doi: 10.1152/ajpregu.1986.251.4.R798. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Sampogna S, Morales FR, Chase MH. MCH-containing neurons in the hypothalamus of the cat: searching for a role in the control of sleep and wakefulness. Brain Res. 2006;1119:101–114. doi: 10.1016/j.brainres.2006.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama S, Sakurai T, Tatsumi K, Kuwaki T. Attenuated phrenic long-term facilitation in orexin neuron-ablated mice. Respir Physiol Neurobiol. 2009;168:295–302. doi: 10.1016/j.resp.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Valladares EM, Eljammal SM, Motivala S, Ehlers CL, Irwin MR. Sex differences in cardiac sympathovagal balance and vagal tone during nocturnal sleep. Sleep Med. 2008;9:310–316. doi: 10.1016/j.sleep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Verret L, Leger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci. 2005;21:2488–2504. doi: 10.1111/j.1460-9568.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- Verret L, Fort P, Gervasoni D, Leger L, Luppi PH. Localization of the neurons active during paradoxical (REM) sleep and projecting to the locus coeruleus noradrenergic neurons in the rat. J Comp Neurol. 2006;495:573–586. doi: 10.1002/cne.20891. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Synaptic activation of hypoglossal respiratory motorneurons during inspiration in rats. Neurosci Lett. 2002;332:195–199. doi: 10.1016/s0304-3940(02)00957-6. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Chen YH, Li KY, Sun FY. Differential sensitivity of GABAergic and glycinergic inputs to orexin-A in preganglionic cardiac vagal neurons of newborn rats. Acta Pharmacol Sin. 2005;26:1442–1447. doi: 10.1111/j.1745-7254.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–2888. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]