ABSTRACT
Purpose: The objective of this study was to examine the effects of intra-articular corticosteroid injection (ICI) on ipsilateral knee flexion/extension, ankle dorsiflexion/plantarflexion (DF/PF), and hip abduction/adduction (abd/add) during stance phase in people with an acute exacerbation of rheumatoid arthritis (RA) of the knee joint. The study also assessed the effects of ICI on spatiotemporal parameters of gait and functional status in this group.
Methods: Nine people with an exacerbation of RA of the knee were recruited. Kinematic and spatiotemporal gait parameters were obtained for each participant. Knee-related functional status was assessed using the Knee injury and Osteoarthritis Outcome Score (KOOS). Spatiotemporal gait parameters and joint angles (knee flexion, ankle DF/PF, hip abd/add) of the affected side were compared pre- and post-ICI.
Results: Data for eight people were available for analysis. Median values for knee flexion and ankle PF increased significantly following ICI. Gait parameters of cadence, velocity, bilateral stride length, bilateral step length, step width, double-support percentage, and step time on the affected side also showed improvement. Pain and knee-related functional status as measured by the KOOS showed improvement.
Conclusions: This study demonstrated a beneficial short-term effect of ICI on knee-joint movements, gait parameters, and knee-related functional status in people with acute exacerbation of RA of the knee.
Key Words: gait; intra-articular injections; biomechanics; arthritis, rheumatoid; knee
RÉSUMÉ
Objectif : L'objectif de cette étude est de se pencher sur les effets des injections intra-articulaires de corticostéroïdes (IIC) sur la flexion et l'extension du genou homolatéral, sur la flexion dorsale et plantaire de la cheville (FD/FP) et sur l'abduction/adduction (abd/add) de la hanche au cours de la phase d'appui chez les personnes avec exacerbation aiguë de la polyarthrite rhumatoïde du genou. L'étude a également évalué les effets des ICI sur les paramètres spatiotemporels de démarche et de statut fonctionnel chez ce groupe.
Méthode : Neuf personnes avec exacerbation de PR du genou ont été recrutées. Les paramètres cinématiques et spatiotemporels de la démarche ont été obtenus pour chaque participant. Le statut fonctionnel lié au genou a été évalué à l'aide de l'échelle KOOS (Knee injury and Osteoarthritis Outcome Score). Les paramètres spatiotemporels de la démarche et les angles des articulations (flexion du genou, FD/FP de la cheville, abd/add de la hanche) du côté affecté ont été comparés avant et après les injections.
Résultats : Les données pour huit personnes étaient disponibles pour analyse. Les valeurs médianes pour la flexion du genou et la FP de la cheville ont grandement augmenté à la suite des injections. Les paramètres de démarche tels que la cadence, la vélocité, la longueur de foulée bilatérale, la longueur de pas bilatérale, la largeur des pas, le pourcentage de double appui et la durée des pas pour le côté affecté se sont également améliorés. La douleur et le statut fonctionnel relatif au genou, tels que mesurés à l'échelle KOOS, se sont améliorés.
Conclusions : Cette étude a démontré les effets bénéfiques à court terme des IIC sur les mouvements de l'articulation du genou, les paramètres de la démarche et le statut fonctionnel relatif au genou chez les personnes avec exacerbation de PR du genou.
Mots clés : arthrite du genou, cinématique, démarche, GAITRite, injection intra-articulaire de corticostéroïdes, Vicon
Joint arthritis is increasing worldwide and becoming more prevalent in both developed and developing countries.1,2 Approximately one in six Canadians aged 15 years and over report having arthritis as a long-term health condition. Two-thirds of those with arthritis are women, and nearly three of every five people with arthritis are younger than 65 years of age. It is also estimated that 6 million Canadians will have some form of arthritis by 2026.3 In particular, rheumatoid arthritis (RA) is more prevalent in North America, Europe, and China across people of all ages.4–7 The costs related to RA are substantial and likely underestimated.8,9
Pain, local joint swelling, stiffness, and difficulties with activities of daily living (ADL) are the main symptoms of RA of the knee. Changes in lower-limb kinematics and deviations in gait pattern—specifically, altered excursion of movement in major lower-extremity joints and spatiotemporal parameters during walking10—have also been observed in this patient group.10–12 It has also been shown that people with RA of the knee experience functional difficulties and even premature mortality.13,14
Intra-articular corticosteroid injections (ICI) have traditionally been used in people with RA experiencing an acute exacerbation of knee pain and are considered beneficial in reducing pain and improving functional status (self-care activities, walking ability, and overall mobility).15,16 While no study has measured the effect of ICI on lower-limb joint kinematics in adults with RA of the knee, previous studies have indicated that ICI is beneficial in improving lower-limb joint movements and gait in people with other forms of arthritis. Shrader and colleagues17 examined the effect of ICI on joint kinematics using a three-dimensional (3D) motion-analysis system during gait and stair climbing in adults with osteoarthritis (OA) of the knee. Data were collected before and 15 minutes after ICI in the affected knee joint. No significant differences were observed in any of the joint angles at the hip, knee, or ankle during gait, but gait velocity and cadence increased significantly.17 In another study, changes in gait pattern were examined following ICI administered to lower-extremity joints in children with juvenile idiopathic arthritis (JIA). Lower-limb kinematics were measured during gait both before and 8–17 days after ICI. The study demonstrated a beneficial short-term effect of ICI on the kinematics of lower-extremity joints in children with JIA: knee and ankle joint movements, as well as gait velocity, improved significantly.18 Similarly, Tang and colleagues19 demonstrated that gait parameters such as velocity and cadence showed sustained improvement for at least 6 months following intra-articular hyaluronic injections in participants with knee OA.
Arthritis has been shown to affect ADL and can have a disabling impact on daily functions.20,21 Pain and functional status have been examined following intra-articular injections of corticosteroid22,23 and hyaluronic acid (HA)24 in people with RA of the knee. Bliddal and colleagues22 examined the effects of two different types of intra-articular injections in people with RA, using the Health Assessment Questionnaire (HAQ) and visual analogue scale (VAS) as measures of function and pain intensity. Their results showed no difference between the two groups. Chou and colleagues24 used the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) to examine the effects of intra-articular injection of HA in people with RA who also had OA of the knee. Injection of HA was beneficial in these people, as demonstrated by improvement in WOMAC scores.24
Patients with RA often seek physiotherapy treatment for improving their pain and function; individuals with RA of the knee and hand are those most likely to be seen at physiotherapy clinics. It is also common for physiotherapists to treat patients who have had ICI to the knee in the recent past or who receive ICI while attending physiotherapy. Therefore, it is important for physiotherapists to understand the changes in joint movements, gait parameters, and self-reported function in these patients following ICI. Because previous studies have largely focused on assessing the effects of ICI in patients with OA of the knee, little is known about the changes in kinematics and gait parameters in patients with RA of the knee. Patients with OA and RA may have some common clinical characteristics, but the underlying pathological mechanisms are different; therefore, the findings of previous studies focusing on the effects of ICI on patients with OA of the knee are not necessarily applicable to those with RA of the knee.
The purpose of this study was to examine the effects of ICI on the movement excursions for ipsilateral knee flexion/extension (flex/ext), ankle dorsiflexion/plantarflexion (DF/PF), and hip abduction/adduction (abd/add) during stance phase in people with acute exacerbation of RA of the knee. We also compared pre- and post-ICI spatiotemporal gait parameters such as cadence, velocity, bilateral stride length, bilateral step length, step width, bilateral step time, and percentage of the gait cycle in ipsilateral single and double support. This comparison provides a functional context for how these gait parameters relate to the lower-extremity kinematic data during stance phase. Lastly, we examined changes in pain intensity, measured by the VAS, and self-reported functional status, measured by the Knee injury and Osteoarthritis Outcome Score (KOOS), following ICI. We hypothesized that following ICI there would be improvement in functional status, movement excursions for the selected lower-extremity movements, and spatiotemporal parameters of gait.
METHODS
Participants
Formal sample-size calculations were not made; instead, a sample of convenience was used. Nine people with an exacerbation of RA of the knee were recruited for the study. The inclusion criteria were exacerbation of knee pain on only one side; identification by a rheumatologist as a candidate for ICI; ability to understand and follow study instructions; and willingness to attend the follow-up data-collection session. Participants with a previous history of neurological or other musculoskeletal condition that affected gait or caused muscle contracture or deformity in either the lower limbs or spine; those with exacerbation of RA in multiple lower-limb joints; and those who used an assistive device for ambulation were excluded from the study. A research nurse discussed the basic study details with each participant. Participants who agreed to be involved were referred for data collection, at which point the principal investigator (SM) obtained informed consent. Characteristics of the study population are illustrated in Table 1. The data for joint count and disease duration were only available for six participants. ICI was administered in the affected knee using a standardized approach: using sterile technique, 1 mL of 2% xylocaine and 1 mL of 40 mg/mL triamcinolone acetonide mixed together in a single syringe were delivered through a 25-gauge needle. The necessary ethics approval was obtained in advance of the study.
Table 1.
Patient Characteristics
| Parameter | Mean and (SD)* |
|---|---|
| Age, y; n=8 | 43.9 (12.9) |
| Sex (M/F) | 0/8 |
| Disease duration, y; n=6 | 8.7 (7.8) |
| Joint count; n=6 | |
| Swollen | 2.3 (2.2) |
| Tender | 3.2 (5.4) |
| Both | 2.7 (3.2) |
Unless otherwise indicated.
Study Protocol
Participants attended the laboratory for the primary data-collection session. They were asked to complete the KOOS questionnaire, basing their answers on their experience over the past week. Participants also reported pain intensity over the past 24 hours on a VAS by marking the point on the scale that best described their pain intensity. Reflective markers were attached bilaterally to the lower limbs and pelvis at predefined anatomic locations. Participants were familiarized with the gait-capture area by means of one practice trial. They were told to walk at their normal walking pace. For each trial, participants walked approximately 8 m. After each gait trial, a quick scan of the trial was performed to ensure that the trial was comprehensive and yielded sufficient data. Five successful gait trials were obtained for every participant in each session.
After this initial session, participants returned to the Rheumatology Clinic for ICI in the affected knee joint. A follow-up session was scheduled in the laboratory within 7 to 10 days. The same data-collection procedures were repeated at that time.
Instrumentation
Three-dimensional (3D) motion analysis is used to obtain kinematic data and often to assess changes in such data following an intervention.25,26 For our study, kinematic data were obtained using the Vicon 460 video motion-analysis system (Vicon, Oxford, UK) with six digital video cameras. The data were sampled at 120 Hz. Reflective markers were placed on the endpoints of each segment according to the Helen Hayes Model,27,28 and the coordinates were captured via Vicon's Plug-in-Gait software. 3D motion analysis has been shown to have high reliability and low error.29
Spatiotemporal parameters were obtained using a GAITRite system (CIR Systems Inc. Clifton, NJ). The GAITRite mat contains embedded sensors that record footfalls as a participant walks over the mat, processes these footfall patterns, and computes the spatial and temporal parameters of gait. The reliability of the GAITRite system has been assessed in previous studies,30,31 which have indicated excellent test–retest reliability (intra-class correlation coefficient [ICC] values between 0.84 and 0.97) for spatiotemporal gait parameters at self-selected walking speed.30 The GAITRite mat was positioned in the capture area of the Vicon system, and both systems were triggered simultaneously as participants entered the capture area and stopped as they left it.
Average pain intensity over the previous 24 hours was measured using the VAS, a 100-mm vertical line with the anchors of “no pain” and “pain as bad as it can be,” which is considered a reliable and valid tool for measuring pain intensity.32,33
The KOOS was used to measure participants' functional status. The KOOS is a 42-item self-report questionnaire with five sub-scales: Pain, Other Symptoms, Functions in Daily Living, Sport and Recreation Function, and Knee-Related Quality of Life. A score of 100 indicates no problems; a score of 0 indicates extreme problems. The KOOS is an extension of the WOMAC, which has previously been used with this patient group,24 but has better utility in younger adults (<65 years of age) who are more active. Previous studies have concluded that the KOOS is a valid outcome tool for assessing pain, function, and quality of life in people with knee arthritis.34,35 Knee-joint girth on the affected side was also used to examine changes in swelling following ICI.
Data Analysis
Kinematic data were initially conditioned within the Vicon software. Gait trials were scanned manually to ensure that the data were continuous. Up to six missing data points (≤50 ms) were filled in using the Vicon software. Bilateral hip, knee, and ankle angles and bilateral ankle and heel marker trajectories were exported for analysis. Custom scripts using MatLab v. 7.1 (The MathWorks Inc., Natick, MA) separated each gait cycle into swing and stance phases using the z coordinates of ankle and heel trajectories. The number of stance phases varied between pre- and post-ICI sessions for each participant. Five to eight stance phases were extracted from the five walking trials for each participant for each session. Movement excursions for ipsilateral knee flex/ext, ankle DF/PF, and hip abd/add were normalized to the full gait cycle (0–100%). In addition, median values for ipsilateral knee flexion, ankle DF and PF, and hip abduction were obtained and compared for the group for the pre- and post-ICI sessions.
The GAITRite data included 10 gait cycles per session for each participant. Cadence, velocity, bilateral stride length, bilateral step length, step width, bilateral step time, double-support percentage, and ipsilateral single-support percentage were averaged and compared.
The VAS score was calculated by measuring the distance (mm) from 0 to the mark the participant made indicating pain intensity. Average scores for all participants were compared for pre- and post-ICI sessions. KOOS scores were normalized, and each sub-scale score was calculated separately for each participant. In order to obtain normalized scores for each sub-scale, the total score of the sub-scale was divided by the possible maximum score for that sub-scale.33 The normalized scores for each sub-scale were averaged and compared across participants. Similarly, average knee-joint girth (cm) was compared for pre- and post-ICI sessions.
Statistical Analysis
Median values for ipsilateral knee flexion, ankle DF and PF, and hip abduction, as well as spatiotemporal gait parameters, were compared for pre- and post-ICI sessions. The Wilcoxon signed-rank test was used for comparing the joint angles and spatiotemporal parameters; mean values for VAS, KOOS, and knee girth were compared using paired t-tests. The Wilcoxon signed-rank test was used for other variables that failed the test for normality. Sigma Stat v. 3.1 (Systat Software Inc., Chicago, IL) was used for statistical analyses. Differences were considered significant at p<0.05.
RESULTS
All nine participants recruited for the study were female. One participant was unable to return for the follow-up assessment; therefore, pre- and post-ICI data for eight participants were available for analysis and comparison.
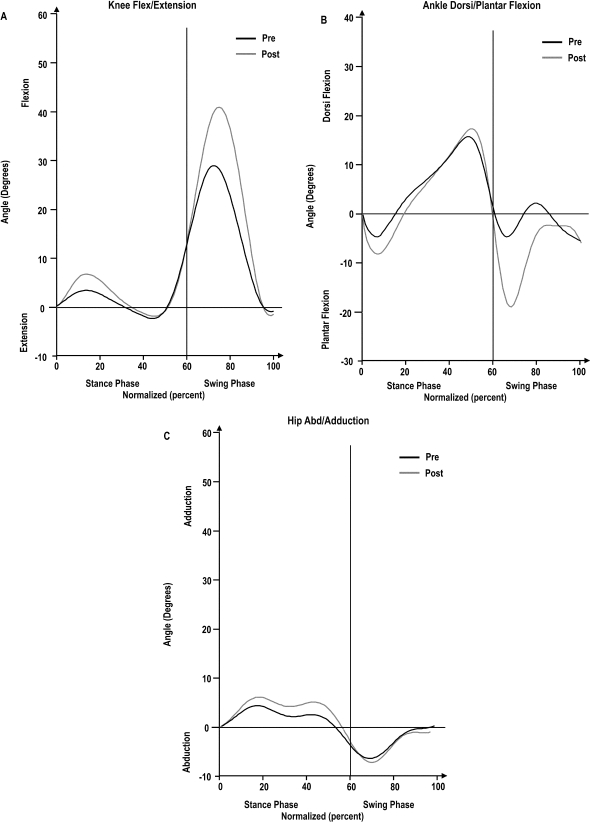
The knee-flexion angle at initial contact during stance decreased from 14° (SD 5.7°) to 11.4° (SD 5.9°) following ICI (p<0.05). Figure 1 shows knee flex/ext, ankle DF/PF, and hip abd/add over the full gait cycle for the group at pre- and post-ICI time points; Table 2 compares these joint angles. Median values for ankle DF and hip add did not change post ICI, whereas median values for knee flexion and ankle PF increased significantly post ICI, as illustrated in Table 2.
Figure 1.
Normalized gait cycle (0–100%) for knee flex/ext (A), ankle DF/PF (B), and hip abd/add (C) for all participants. Normalized percentages are represented on the x-axis; angles in degrees are represented on the y-axis. Pre-ICI gait cycles are represented by black lines, and post-ICI gait cycles by grey lines. The stance and swing phases are separated by the black line at 60% of the normalized curve.
Table 2.
Ipsilateral Joint Angles
| Median (25th–75th percentile) |
|||
|---|---|---|---|
| Joint angle, degrees | Pre-injection | Post-injection | p-value |
| Knee flexion | 4.4 (3.7–7.4) | 8.1 (6.2–10.5) | 0.016* |
| Ankle dorsiflexion | 10.2 (7.8–13) | 16.2 (8.4–18.7) | 0.148 |
| Ankle plantarflexion | 5.1 (3.4–6.5) | 9.5 (6.4–10.9) | 0.039* |
| Hip adduction | 4.4 (2.6–5.9) | 6.7 (3.2–8.5) | 0.109 |
Significant at p<0.05.
Table 3 summarizes the comparison of spatiotemporal parameters pre and post ICI; the results are described as median (25th–75th percentile). The spatial parameters that showed significant improvement were cadence, velocity, bilateral stride length, bilateral step length, and step width, which suggests that participants were able to ambulate faster and cover more distance in the same number of trials following ICI. With respect to temporal parameters, both ipsilateral step time and double-support percentage decreased significantly in the post-ICI session, while contralateral step time and ipsilateral single-support percentage remained unchanged. Percentage of time spent in single support (affected side) during the gait cycle also remained unchanged, which indicates that participants took less time to take steps on the affected side but that there was no significant change for the same parameter on the opposite side.
Table 3.
Spatiotemporal Gait Parameters
| Median (25th–75th percentile) |
|||
|---|---|---|---|
| Spatiotemporal parameter | Pre-injection | Post-injection | p-value |
| Cadence, steps/min | 101.5 (97.2–104.6) | 107.4 (103.1–108.8) | 0.008* |
| Velocity, cm/s | 79.3 (68.7–98.3) | 100.9 (87.4–116.6) | 0.008* |
| Ipsilateral single-support percentage | 33.9 (32.7–36.4) | 36 (34.8–37.7) | 0.100 |
| Ipsilateral double-support percentage | 30.2 (29.3–30.9) | 29.3 (28.3–29.8) | 0.008* |
| Ipsilateral stride length, cm | 93.8 (81.4–115. 5) | 114.9 (100.4–127.2) | 0.016* |
| Contralateral stride length, cm | 93.8 (81.9–115.5) | 114.9 (100.7–126.9) | 0.016* |
| Step width, cm | 13.3 (10.9–14.5) | 10.3 (8.4–12.3) | 0.016* |
| Ipsilateral step time, s | 0.59 (0.59–0.64) | 0.56 (0.54–0.58) | 0.016* |
| Contralateral step time, s | 0.58 (0.56–0.61) | 0.56 (0.55–0.57) | 0.195 |
| Contralateral step length, cm | 46.0 (39.4–56.5) | 58.8 (51.4–63.9) | 0.008* |
| Ipsilateral step length, cm | 49.4 (39.6–56.4) | 55.6 (48.6–63.1) | 0.016* |
Significant at p<0.05.
Table 4 illustrates the results for VAS, KOOS, and knee-girth measurement. VAS scores decreased, which suggests an improvement in pain levels following ICI, while the scores for the five sub-scales of the KOOS improved significantly following ICI. The change in knee girth following ICI was not statistically significant.
Table 4.
Pain, Knee Girth, and KOOS
| Mean and (SD)* |
|||
|---|---|---|---|
| Parameter | Pre-injection | Post-injection | p-value |
| KOOS Sub-scale | |||
| Pain (/100) | 32.9 (15.5) | 69.1 (23.9) | 0.005† |
| Symptoms (/100) | 33.0 (17.9) | 65.2 (23.9) | 0.01† |
| ADL (/100) | 32.9 (19.7) | 70.4 (23.5) | 0.002† |
| Sports and recreation (/100) | 11.4 (18.6) | 44.3 (23.2) | 0.005† |
| QOL (Median (25th–75th percentile)) | 12.5 (0–21.9) | 34.37 (9.4–53.1) | 0.047† |
| VAS (mm) | 65 (7.9) | 31.2 (9.2) | 0.003† |
| Knee girth (cm) | 11.6 (0.9) | 11.2 (0.6) | 0.14 |
Unless otherwise indicated.
Significant at p<0.05.
KOOS=Knee injury and Osteoarthritis Outcome Score (0=extreme problems, 100=no problems); ADL=Activities of daily living; QOL=quality of life; VAS=visual analogue scale (0=no pain, 100=pain as bad as it can be).
None of the eight participants demonstrated deterioration in any of the measured outcomes following ICI. All participants but one experienced significant improvements in the kinematic variables and spatiotemporal parameters during gait; for the remaining participant, the improvement in these outcomes was not significant.
DISCUSSION
The present study measured kinematic and functional status changes following ICI in people with acute exacerbation of RA of the knee. The results highlight the benefits of ICI for improving knee movement, gait pattern, and functional ability in these patients. To our knowledge, no previous study has examined the effects of ICI on lower-limb joint movements, gait, and knee-related functional status in people with RA.
Although we were able to recruit only nine participants to the study, we detected statistically significant differences in joint movement following ICI. Similar studies with larger participant populations that assessed the effects of ICI on patients with OA of the knee showed no changes in lower-extremity joint angles.17,36 Participants in these studies were over 60 years old on average and had advanced OA; it was therefore hypothesized that these participants might not exhibit significant changes in joint angles because of the presence of relatively advanced OA.36 Brostrom and colleagues18 recruited children with JIA, and their results indicated significant changes in knee- and ankle-joint movement following ICI. Therefore, it would appear that the homogeneity and younger age (43.9 years on average) of our participant population may have compensated for the small sample size.
It has been shown that patients with RA have reduced movement in hip abduction during swing phase.10 However, no previous study has measured kinematic changes at the hip joint in the frontal plane during stance phase. Although our study found reduced hip abduction following ICI, the change was not significant. Step width was significantly reduced post ICI, however, which suggests that participants walked with a less “wide-based” gait after ICI and supports the finding of a reduction in ipsilateral hip-abduction angle. Though post-ICI kinematic changes for hip adduction on the affected side were not statistically significant, the possibility of an effect of ICI on hip adduction cannot be ruled out. Lower-limb kinematics vary depending on the severity of arthritis,37,38 and our sample size (n=8) may have been too small for us to detect these differences.
It has been shown that decreasing range of motion at the knee during stance is a strategy used by people with RA,10 JIA,18 and OA39,40 to stabilize the painful knee joint and reduce extensor moments, thus avoiding compressive forces. For this reason, we measured the excursion of joint movements during the stance phase in our study. The observed increase in knee-joint movement during the stance phase following ICI was likely due to the reduced pain intensity observed among participants. As reported above, the knee-flexion angle during initial contact of stance decreased significantly following ICI; however, knee flexion at heel strike following ICI was still greater than that reported in studies of people with knee OA.41,42 This indicates that participants in our study landed on the ground with the knee flexed even after ICI, a gait feature common in people with arthritis.
Abnormalities observed in spatiotemporal gait parameters pre ICI were likely due to pain and reduced movement excursion to guard the joint against pain during stance phase.43–45 Pain was reduced, while movement excursion increased, post ICI, leading to an increase in step length. Previous studies have shown that gait velocity is reduced in people with RA of the knee and is within the range of 60–100 cm/s when they walk at their self-selected speed.10,11,43 In our study, the average gait velocity increased significantly following ICI (from 79 cm/s to 102 cm/s) but was still slower than the self-selected gait velocity for control participants of similar age reported in other studies.10,11,43 Like participants in Brostrom and colleagues' study,18 our participants also increased their walking cadence. This result can be attributed to a reduced step time: participants took more steps per minute after ICI than before. Contralateral step length increased following ICI, which suggests that participants were able to spend more time in stance on the affected side, allowing the other leg to take a larger step. These changes in spatiotemporal gait parameters can be considered patient-relevant benefits of ICI. The ability to walk faster can be an important functional gain, facilitating increased independence in outdoor mobility.
Investigators have recently begun to focus on assessing gait symmetry rather than spatiotemporal parameters, especially in people with neurological disorders.46,47 It is hypothesized that symmetry in gait reflects better “control” and is associated with energy conservation during level walking.48 Though gait symmetry has mostly been assessed in people with neurological disorders, there is no reason that it cannot also be assessed in people with musculoskeletal disorders, such as those with RA or OA of the knee. Future studies are warranted to examine the effects of ICI on gait symmetry in people with RA or OA of the knee.
Participants in our study reported reduced pain intensity in the affected knee following ICI. On average, VAS scores were reduced by 34 mm. This finding is similar to those of a previous study, in which the intensity of knee pain decreased by 39 mm over a 1-week period,49 and represents a greater change than what others have observed.22 Bliddal and colleagues22 reported only a 22-mm change in the VAS; it should be noted, however, that the researchers combined the VAS scores for knee, wrist, and elbow joints, and thus the reported results do not reflect change in pain intensity for the knee specifically.
KOOS scores for our participants increased significantly following ICI, which suggests improvement in functional status. Previous studies have measured general function following intra-articular injections in knee arthritis using the WOMAC;50–52 however, we could find no study that used the KOOS to measure the effect of ICI in people with acute exacerbation of RA of the knee. The KOOS has been used for measuring functional status in other knee pathologies, such as anterior cruciate ligament injury,53,54 arthroscopic partial meniscectomy,55 patellar fractures,56 and total knee replacement.57 Given that a younger age group is reported in this study and in other studies,49,58 the KOOS may be more appropriate to measure the full spectrum of difficulties experienced by younger adults.34,35
There was no change in knee-joint swelling following ICI. It is likely that people with RA usually have swollen joints (as indicated by the swollen joint count in Table 1), and ICI may have little effect on chronic swelling. However, considering the improvement in overall function and activity level, the lack of reduction in knee swelling appears in no way to negate the benefits of ICI.
LIMITATIONS
The small and exclusively female participant group is the main limitation of the present study. Moreover, sample-size calculations were not performed, since this was an exploratory study. One reason that we could recruit only a small participant group may be that we sought individuals with exacerbation of their RA in one knee joint only, whereas most individuals with RA tend to experience exacerbation in multiple lower-extremity joints. The fact that all participants were female may be explained by the fact that the impact of RA, the overall pain experience, and the resulting work limitations are greater for women than for men.59–61 It is also known that 80% of people with RA are female.6,59 Previous studies that assessed the effects of ICI in people with RA49,58 and OA17,36 also reported greater recruitment of female participants. However, because the reports of these studies did not separate results by gender, we are unable to determine whether the effects of ICI on lower-limb kinematics and spatiotemporal parameters of gait are consistent across genders or whether gender affects these measurements in people with RA of the knee.
The participants were assessed at two time points, 7 to 10 days apart; the changes in lower-limb joint kinematics, gait characteristics, and functional status were not examined over a longer period. Moreover, there is a possibility that the kinematics of pelvis and lumbar spine would also change following ICI, but these were not analyzed in our study. Lastly, we did not measure changes in muscle-activation patterns and kinetics of the lower-extremity joints, which would have provided a more comprehensive picture of the effects of ICI in people presenting with an acute exacerbation of RA of the knee. Notwithstanding these limitations, however, the results of this study are expected to help physiotherapists understand the effect of ICI on knee-joint movements and gait parameters in people with RA of the knee.
CONCLUSION
The present study provides objective outcome measurements for a commonly used intervention in patients with RA of the knee. The findings demonstrate positive short-term effects of ICI on lower-extremity joint kinematics, spatiotemporal gait parameters, and function in people with an acute exacerbation of knee RA. Given the small sample recruited for the study, these results should be considered potential trends rather than definitive findings. Future studies exploring similar research objectives should recruit a greater number of participants and examine the long-term effects of ICI on both lower-extremity and spine kinematics. Electromyography assessment of muscle-contraction patterns and joint kinetics would provide a more comprehensive explanation of the benefits of ICI for people with RA of the knee.
Key Messages
What Is Already Known on This Topic
People suffering from rheumatoid arthritis (RA) of the knee experience pain, functional difficulties, and impaired kinematics and gait patterns. Intra-articular corticosteroid injection (ICI) is commonly used in treating exacerbation of RA of the knee to reduce pain and improve function in this patient group. Previous research has shown that ICI improves the kinematics of the lower limbs and spatiotemporal parameters of gait in people with osteoarthritis of the knee and juvenile idiopathic arthritis.
What This Study Adds
To our knowledge, this is the first study to examine the effect of ICI on movement excursion of the affected knee joint, spatiotemporal parameters of gait, pain intensity, and functional status in people with an exacerbation of knee RA symptoms. Our results are consistent with those of previous studies that measured the effect of ICI in other forms of arthritis and indicate that ICI is beneficial in improving kinematics of the affected knee joint, gait characteristics, and functional status. These findings contribute to a better understanding of the overall effects of ICI in people with RA of the knee and lay the groundwork for further research examining these effects.
Physiotherapy Canada 2011; 63(4);395–404; doi:10.3138/ptc.2010-26
References
- 1.Quintana JM, Arostegui I, Escobar A, et al. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168:1576–84. doi: 10.1001/archinte.168.14.1576. doi: 10.1001/archinte.168.14.1576. [DOI] [PubMed] [Google Scholar]
- 2.D'Ambrosia RD. Epidemiology of osteoarthritis. Orthopedics. 2005;28:s201–5. doi: 10.3928/0147-7447-20050202-04. [DOI] [PubMed] [Google Scholar]
- 3.Lagacé C, Perruccio A, DesMeules M, et al. The impact of arthritis on Canadians. In: Badley EM, DesMeules M, editors. Arthritis in Canada: an ongoing challenge. Ottawa: Public Health Agency of Canada; 2003. pp. 7–34. [Google Scholar]
- 4.Salaffi F, De AR, Grassi W. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study, I: the MAPPING study. Clin Exp Rheumatol. 2005;23:819–28. [PubMed] [Google Scholar]
- 5.Dai SM, Han XH, Zhao DB, et al. Prevalence of rheumatic symptoms, rheumatoid arthritis, ankylosing spondylitis, and gout in Shanghai, China: a COPCORD study. J Rheumatol. 2003;30:2245–51. [PubMed] [Google Scholar]
- 6.Carmona L, Villaverde V, Hernandez-Garcia C, et al. The prevalence of rheumatoid arthritis in the general population of Spain. Rheumatology. 2002;41:88–95. doi: 10.1093/rheumatology/41.1.88. doi: 10.1093/rheumatology/41.1.88. [DOI] [PubMed] [Google Scholar]
- 7.Alarcon GS. Epidemiology of rheumatoid arthritis. Rheum Dis Clin N Am. 1995;21:589–604. [PubMed] [Google Scholar]
- 8.Lundkvist J, Kastang F, Kobelt G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur J Health Econ. 2008;8:S49–60. doi: 10.1007/s10198-007-0088-8. doi: 10.1007/s10198-007-0088-8. [DOI] [PubMed] [Google Scholar]
- 9.Maetzel A, Li LC, Pencharz J, et al. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann Rheum Dis. 2004;63:395–401. doi: 10.1136/ard.2003.006031. doi: 10.1136/ard.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss RJ, Wretenberg P, Stark A, et al. Gait pattern in rheumatoid arthritis. Gait Posture. 2008;28:229–34. doi: 10.1016/j.gaitpost.2007.12.001. doi: 10.1016/j.gaitpost.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Sakauchi M, Narushima K, Sone H, et al. Kinematic approach to gait analysis in patients with rheumatoid arthritis involving the knee joint. Arthritis Rheum. 2001;45:35–41. doi: 10.1002/1529-0131(200102)45:1<35::AID-ANR81>3.0.CO;2-D. doi: 10.1002/1529-0131(200102)45:1<35::AID-ANR81>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Isacson J, Brostrom LA. Gait in rheumatoid arthritis: an electrogoniometric investigation. J Biomech. 1988;21:451–7. doi: 10.1016/0021-9290(88)90237-0. doi: 10.1016/0021-9290(88)90237-0. [DOI] [PubMed] [Google Scholar]
- 13.Tuominen R, Tuominen S, Suominen C, et al. Perceived functional disabilities among rheumatoid arthritis patients. Rheumatol Int. 2010;30:643–9. doi: 10.1007/s00296-009-1043-z. doi: 10.1007/s00296-009-1043-z. [DOI] [PubMed] [Google Scholar]
- 14.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22:1–12. doi: 10.2165/00019053-200422001-00002. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- 15.Wallen M, Gillies D. Intra-articular steroids and splints/rest for children with juvenile idiopathic arthritis and adults with rheumatoid arthritis. Cochrane Db Syst Rev. 2006:1. doi: 10.1002/14651858.CD002824.pub2. doi: 10.1002/14651858.CD002824.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundberg IE, Grundtman C, Larsson E, et al. Corticosteroids—from an idea to clinical use. Best Pract Res Cl Rh. 2004;18:7–19. doi: 10.1016/j.berh.2003.10.003. doi: 10.1016/j.berh.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Shrader MW, Draganich LF, Pottenger LA, et al. Effects of knee pain relief in osteoarthritis on gait and stair-stepping. Clin Orthop Relat R. 2004;421:188–93. doi: 10.1097/01.blo.0000119248.70353.a5. doi: 10.1097/01.blo.0000119248.70353.a5. [DOI] [PubMed] [Google Scholar]
- 18.Brostrom E, Hagelberg S, Haglund-Akerlind Y. Effect of joint injections in children with juvenile idiopathic arthritis: evaluation by 3D-gait analysis. Acta Paediatr. 2004;93:906–10. doi: 10.1111/j.1651-2227.2004.tb02688.x. [PubMed] [Google Scholar]
- 19.Tang SF, Chen CP, Chen MJ, et al. Changes in sagittal ground reaction forces after intra-articular hyaluronate injections for knee osteoarthritis. Arch Phys Med Rehab. 2004;85:951–5. doi: 10.1016/j.apmr.2003.08.095. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Chang RW, Dunlop DD. Population impact of arthritis on disability in older adults. Arthritis Rheum. 2006;55:248–55. doi: 10.1002/art.21842. doi: 10.1002/art.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado GM, Barreto SM, Passos VM, et al. Health status indicators among community-dwelling elders with arthritis: the Bambui health and aging study. J Rheumatol. 2006;33:342–7. [PubMed] [Google Scholar]
- 22.Bliddal H, Terslev L, Qvistgaard E, et al. A randomized, controlled study of a single intra-articular injection of etanercept or glucocorticosteroids in patients with rheumatoid arthritis. Scand J Rheumatol. 2006;35:341–5. doi: 10.1080/03009740600844530. doi: 10.1080/03009740600844530. [DOI] [PubMed] [Google Scholar]
- 23.Eberhard BA, Sison MC, Gottlieb BS, et al. Comparison of the intraarticular effectiveness of triamcinolone hexacetonide and triamcinolone acetonide in treatment of juvenile rheumatoid arthritis. J Rheumatol. 2004;31:2507–12. [PubMed] [Google Scholar]
- 24.Chou CL, Li HW, Lee SH, et al. Effect of intra-articular injection of hyaluronic acid in rheumatoid arthritis patients with knee osteoarthritis. J Chin Med Assoc. 2008;71:411–5. doi: 10.1016/S1726-4901(08)70092-3. doi: 10.1016/S1726-4901(08)70092-3. [DOI] [PubMed] [Google Scholar]
- 25.Liebensteiner MC, Herten A, Gstoettner M, et al. Correlation between objective gait parameters and subjective score measurements before and after total knee arthroplasty. Knee. 2008;15:461–6. doi: 10.1016/j.knee.2008.07.001. doi: 10.1016/j.knee.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Simon SR. Quantification of human motion: gait analysis-benefits and limitations to its application to clinical problems. J Biomech. 2004;37:1869–80. doi: 10.1016/j.jbiomech.2004.02.047. doi: 10.1016/j.jbiomech.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Ounpuu S, Gage JR, Davis RB. Three-dimensional lower extremity joint kinetics in normal pediatric gait. J Pediatr Orthoped. 1991;11:341–9. [PubMed] [Google Scholar]
- 28.Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8:383–92. doi: 10.1002/jor.1100080310. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 29.Labbe DR, Hagemeister N, Tremblay M, et al. Reliability of a method for analyzing three-dimensional knee kinematics during gait. Gait Posture. 2008;28:170–4. doi: 10.1016/j.gaitpost.2007.11.002. doi: 10.1016/j.gaitpost.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17:68–74. doi: 10.1016/s0966-6362(02)00053-x. doi: 10.1016/S0966-6362(02)00053-X. [DOI] [PubMed] [Google Scholar]
- 31.Titianova EB, Mateev PS, Tarkka IM. Footprint analysis of gait using a pressure sensor system. J Electromyogr Kines. 2004;14:275–81. doi: 10.1016/S1050-6411(03)00077-4. doi: 10.1016/S1050-6411(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 32.Summers S. Evidence-based practice part 2: reliability and validity of selected acute pain instruments. J Perianesth Nurs. 2001;16:35–40. doi: 10.1053/jpan.2001.20657. doi: 10.1053/jpan.2001.20657. [DOI] [PubMed] [Google Scholar]
- 33.Tiplady B, Jackson SH, Maskrey VM, et al. Validity and sensitivity of visual analogue scales in young and older healthy subjects. Age Ageing. 1998;27:63–6. doi: 10.1093/ageing/27.1.63. doi: 10.1093/ageing/27.1.63. [DOI] [PubMed] [Google Scholar]
- 34.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Out. 2003;1:17. doi: 10.1186/1477-7525-1-17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roos EM, Roos HP, Lohmander LS, et al. Knee injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sport Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 36.Skwara A, Peterlein CD, Tibesku CO, et al. Changes of gait patterns and muscle activity after intraarticular treatment of patients with osteoarthritis of the knee: a prospective, randomised, doubleblind study. Knee. 2009;16:466–72. doi: 10.1016/j.knee.2009.03.003. doi: 10.1016/j.knee.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Astephen JL, Deluzio KJ, Caldwell GE, et al. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26:332–41. doi: 10.1002/jor.20496. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- 38.Astephen JL, Deluzio KJ, Caldwell GE, et al. Gait and neuromuscular pattern changes are associated with differences in knee osteoarthritis severity levels. J Biomech. 2008;41:868–76. doi: 10.1016/j.jbiomech.2007.10.016. doi: 10.1016/j.jbiomech.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Maly MR, Costigan PA, Olney SJ. Role of knee kinematics and kinetics on performance and disability in people with medial compartment knee osteoarthritis. Clin Biomech. 2006;21:1051–9. doi: 10.1016/j.clinbiomech.2006.06.010. doi: 10.1016/j.clinbiomech.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman KR, Hughes C, Morrey BF, et al. Gait characteristics of patients with knee osteoarthritis. J Biomech. 2001;34:907–15. doi: 10.1016/s0021-9290(01)00036-7. doi: 10.1016/S0021-9290(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 41.Childs JD, Sparto PJ, Fitzgerald GK, et al. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech. 2004;19:44–9. doi: 10.1016/j.clinbiomech.2003.08.007. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Webster KE, Wittwer JE, Feller JA. Quantitative gait analysis after medial unicompartmental knee arthroplasty for osteoarthritis. J Arthroplasty. 2003;18:751–9. doi: 10.1016/s0883-5403(03)00152-9. doi: 10.1016/S0883-5403(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 43.Rome K, Dixon J, Gray M, et al. Evaluation of static and dynamic postural stability in established rheumatoid arthritis: exploratory study. Clin Biomech. 2009;24:524–6. doi: 10.1016/j.clinbiomech.2009.03.005. doi: 10.1016/j.clinbiomech.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Bejek Z, Paroczai R, Illyes A, et al. The influence of walking speed on gait parameters in healthy people and in patients with osteoarthritis. Knee Surg Sport Tr A. 2006;14:612–22. doi: 10.1007/s00167-005-0005-6. doi: 10.1007/s00167-005-0005-6. [DOI] [PubMed] [Google Scholar]
- 45.Gok H, Ergin S, Yavuzer G. Kinetic and kinematic characteristics of gait in patients with medial knee arthrosis. Acta Orthop Scand. 2002;73:647–52. doi: 10.1080/000164702321039606. doi: 10.3109/17453670209178029. [DOI] [PubMed] [Google Scholar]
- 46.Patterson KK, Gage WH, Brooks D, et al. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31:241–6. doi: 10.1016/j.gaitpost.2009.10.014. doi: 10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson's disease. Exp Brain Res. 2007;181:561–70. doi: 10.1007/s00221-007-0955-7. doi: 10.1007/s00221-007-0955-7. [DOI] [PubMed] [Google Scholar]
- 48.Macko RF, Smith GV, Dobrovolny CL, et al. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehab. 2001;82:879–84. doi: 10.1053/apmr.2001.23853. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 49.Konai MS, Vilar Furtado RN, Dos Santos MF, et al. Monoarticular corticosteroid injection versus systemic administration in the treatment of rheumatoid arthritis patients: a randomized double-blind controlled study. Clin Exp Rheumatol. 2009;27:214–21. [PubMed] [Google Scholar]
- 50.Juni P, Reichenbach S, Trelle S, et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 2007;56:3610–9. doi: 10.1002/art.23026. doi: 10.1002/art.23026. [DOI] [PubMed] [Google Scholar]
- 51.Turajane T, Tanavaree A, Labpiboonpong V, et al. Outcomes of intra-articular injection of sodium hyaluronate for the treatment of osteoarthritis of the knee. J Med Assoc Thailand. 2007;90:1845–52. [PubMed] [Google Scholar]
- 52.Ozturk C, Atamaz F, Hepguler S, et al. The safety and efficacy of intraarticular hyaluronan with/without corticosteroid in knee osteoarthritis: 1-year, single-blind, randomized study. Rheumatol Int. 2006;26:314–9. doi: 10.1007/s00296-005-0584-z. doi: 10.1007/s00296-005-0584-z. [DOI] [PubMed] [Google Scholar]
- 53.Swirtun LR, Renstrom P. Factors affecting outcome after anterior cruciate ligament injury: a prospective study with a six-year follow-up. Scand J Med Sci Sport. 2008;18:318–24. doi: 10.1111/j.1600-0838.2007.00696.x. doi: 10.1111/j.1600-0838.2007.00696.x. [DOI] [PubMed] [Google Scholar]
- 54.von Porat A, Henriksson M, Holmstrom E, et al. Knee kinematics and kinetics in former soccer players with a 16-year-old ACL injury—the effects of twelve weeks of knee-specific training. BMC Musculoskel Dis. 2007;8:35. doi: 10.1186/1471-2474-8-35. doi: 10.1186/1471-2474-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katz JN, Meredith DS, Lang P, et al. Associations among preoperative MRI features and functional status following arthroscopic partial meniscectomy. Osteoarthr Cartilage. 2006;14:418–22. doi: 10.1016/j.joca.2005.11.014. doi: 10.1016/j.joca.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Anand S, Hahnel JC, Giannoudis PV. Open patellar fractures: high energy injuries with a poor outcome? Injury. 2008;39:480–4. doi: 10.1016/j.injury.2007.10.032. doi: 10.1016/j.injury.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 57.Rastogi R, Davis AM, Chesworth BM. A cross-sectional look at patient concerns in the first six weeks following primary total knee arthroplasty. Health Qual Life Out. 2007;5:48. doi: 10.1186/1477-7525-5-48. doi: 10.1186/1477-7525-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11:R7. doi: 10.1186/ar2591. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puolakka K, Kautiainen H, Pekurinen M, et al. Monetary value of lost productivity over a five year follow up in early rheumatoid arthritis estimated on the basis of official register data on patients' sickness absence and gross income: experience from the FIN-RACo trial. Ann Rheum Dis. 2006;65:899–904. doi: 10.1136/ard.2005.045807. doi: 10.1136/ard.2005.045807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16:266–75. doi: 10.1046/j.1525-1497.2001.00229.x. doi: 10.1046/j.1525-1497.2001.016004266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furtado RN, Oliveira LM, Natour J. Polyarticular corticosteroid injection versus systemic administration in treatment of rheumatoid arthritis patients: a randomized controlled study. J Rheumatol. 2005;32:1691–8. [PubMed] [Google Scholar]