Abstract
The tobacco industry markets potential reduced exposure products (PREPs) to smokers, including oral products that are intended to be used in situations where cigarettes cannot. For example, Ariva, marketed by Star Scientific, is a tablet made from compressed tobacco powder and is intended for “adult smokers in situations where they cannot or choose not to smoke.” No objective data are available regarding Ariva’s effects in smokers, including its nicotine delivery, cardiovascular profile, or subjective effects. In this single-session, clinical laboratory study, 10 overnight-abstinent cigarette smokers were administered one Ariva tablet, followed 90 min later by two Ariva tablets, followed 90 min later by three Ariva tablets. Participants allowed each dose to dissolve in their mouths according to package instructions. Blood was sampled, heart rate monitored, and subjective effects assessed regularly. Ariva delivered nicotine in a dose-dependent manner; mean (SD) nicotine levels increased from 2.4 ng/ml (0.9) at baseline, to 3.4 ng/ml (1.4) 45 min post–1 tablet, 7.3 ng/ml (4.0) 45 min post–2 tablets, and 9.7 ng/ml (4.4) 45 min post–3 tablets. Heart rate increased after tablet administration, independent of dose. The tablets also significantly decreased subjective ratings of craving and urge, and increased ratings of nausea. Based on this short-term laboratory evaluation, Ariva exposes users to nicotine and may suppress some symptoms of tobacco abstinence, though its nausea-inducing characteristics may limit initial acceptability.
Introduction
Potential reduced exposure products (PREPs) are marketed by the tobacco industry as a way for smokers to reduce toxicant exposure (Warner, 2002). So-called low-yield cigarettes are initial examples of these industry-sponsored efforts (Giovino et al., 1996). These products gained widespread acceptance, even though subsequent objective evaluation demonstrated that they failed to reduce smokers’ carbon monoxide (CO), nicotine, and carcinogen exposure (Stratton, Shetty, Wallace, & Bondurant, 2001; National Cancer Institute, 2001). Given this history, a need exists for objective pre-market evaluation of new PREPs (Hatsukami et al., 2005), including noncombustible, oral PREPs marketed to smokers.
Oral PREPs for smokers are becoming increasingly common in the United States and include tobacco pouches such as Swedish Match’s Exalt (“No smoking? No problem.”), U.S. Smokeless Tobacco Company’s Revel (“Tobacco satisfaction without smoking.”), R. J. Reynold’s Camel Snus (“Pleasure for whenever.”), and Philip Morris’s Taboka (“Tuck a Taboka instead…”), as well as at least one tobacco tablet: Star Scientific’s Ariva (“When you can’t smoke.”). Some of these products have been analyzed to determine tobacco-specific nitrosamine (TSNA) content, and the mean level of TSNAs in Ariva was lower than that found in Exalt, Revel, and smokeless tobacco products typically marketed in the United States (Stepanov, Jensen, Hatsukami, & Hecht, 2006). Another study examined Ariva’s effects in smokeless tobacco (SLT) users and found that one tablet delivered nicotine (Cmax = 2.7 ng/ml; 95% CI = 2.0–3.6) and reduced craving over a 30-min period (Kotlyar et al., 2007). However, nicotine exposure may differ in SLT users from that of smokers (Benowitz, Porchet, Sheiner, & Jocob, 1988), Ariva’s intended audience. Thus this study examined the nicotine delivery, cardiovascular profile, and subjective effects of Ariva in cigarette smokers.
Method
Participants
Five men and five women (n=10) completed this institutional review board–approved study. Participants were included if they were healthy, between the ages of 18 and 50 years (M=32.8, SD=8.5), provided a screening expired-air CO level of no more than 15 ppm (M=22.7, SD=10.0), and reported smoking at least 10 cigarettes/day (M=22.0, SD=3.5) for at least 1 year (M=7.6, SD=7.0). Exclusion criteria consisted of history of chronic health problems, current pregnancy or breastfeeding, history of or active cardiovascular disease, and regular use of prescription medication (other than vitamins or birth control).
Procedure
Participants completed a single laboratory session, lasting approximately 5 hr, where they received one, two, and three Ariva tablets in ascending dose order. Following verification of overnight tobacco abstinence (i.e., expired-air CO<10ppm), a catheter was inserted into a forearm vein and heart rate and blood pressure monitoring commenced. After 30 min, participants completed baseline subjective questionnaires related to nicotine/tobacco withdrawal and provided blood (10 ml) and expired-air samples. One Ariva tablet was then administered according to product packaging (i.e., allowed to dissolve in mouth, approximately 15 min duration). For the following 90 min period, blood samples (10 ml) were obtained and subjective measures administered every 5 min for the first 30 min, then at 45 min and 90 min after tablet administration. This same pattern of events (tablet administration followed by periodic subjective assessment and blood sampling) was repeated for two and three Ariva tablet doses at 90 min intervals. After this final assessment period, another CO measurement was taken, the catheter was removed, and participants were paid for session completion ($150).
Outcome measures
Plasma nicotine level
Blood samples were centrifuged, and the plasma was separated and stored at −70°C. The plasma was analyzed for nicotine using LC-MS/MS (a modified version of that reported by Naidong, Shou, Chen & Jiang, 2001; see Breland, Kleykamp, & Eissenberg, 2006, for details). This assay had a limit of quantification (LOQ) of 2.0 ng/ml.
Cardiovascular effects
During each session, heart rate was measured every 20 sec (and, for safety, blood pressure was measured every 5 min) by noninvasive computerized equipment (Noninvasive Patient Monitor model 507E, Criticare Systems, Waukesha, Wisconsin).
Participant-rated tobacco abstinence effects and direct effects of nicotine
Participants responded to two questionnaires, both consisting of visual analog scale (VAS) items. Each word or phrase is centered above a horizontal line that represents a scale from 0 to 100 points; the left anchor is “not at all” (0) and the right is “extremely” (100). Clicking a mouse-controlled cursor produces a vertical mark whose position on the line can be adjusted. The score is the distance between the vertical mark and the left anchor, expressed as a percentage of line length.
Similar to previous work in our laboratory (see Buchhalter, Acosta, Evans, Breland, & Eissenberg, 2005), this study used 11 VAS items to assess nicotine/tobacco withdrawal symptoms. These 11 VAS items (adapted from Hughes & Hatsukami, 1986) are sensitive to tobacco abstinence effects: urges to smoke, irritability/frustration/anger, anxious, difficulty concentrating, restlessness, hunger, impatient, craving a cigarette/nicotine, drowsiness, depression/feeling blue, and desire for sweets.
Direct effects of nicotine were assessed using 10 VAS items that included known nicotine effects (Gourlay, Forbes, Marriner, Pethica, & McNeil, 1995; Pullan et al., 1994): nauseous, dizzy, light-headed, nervous, sweaty, headache, excessive salivation, heart pounding, confused, and weak.
Data analyses
Plasma values below the LOQ (2.0 ng/ml) were replaced with 2.0 ng/ml. Heart rate data were averaged in 5-min bins for each Ariva administration period, yielding a pre-administration time point and 5, 10, 15, and 20 min post-administration time points. All other measures had a pre-administration time point (i.e., baseline) and time points at 5, 10, 15, 20, 25, 30, and 45 min post-administration. Data for all measures were entered into a two-factor within-subject analysis of variance (ANOVA): dose (3 levels; 1, 2, and 3 tablets) by time (5 levels for heart rate; 8 levels for plasma nicotine and subjective measures). Huynh-Feldt corrections were used to adjust for potential violations of the sphericity assumption (Huynh & Felt, 1976), and differences between means were examined using Tukey’s honestly significant difference (HSD; Keppel, 1991). Comparisons for which p values were less than .05 are reported as significant.
Results
Statistical analyses (main effects and interactions) for all measures are displayed in Table 1 and discussed below.
Table 1.
Statistical analyses results for all outcome measures.
| Dosea p value |
Timeb p value |
Dose × Timec p value |
|
|---|---|---|---|
| Heart rate | >.573 | <.05 | >.321 |
| Plasma nicotine | <.001 | <.001 | <.001 |
| Hughes and Hatsukami | |||
| Anxious | >.691 | >.134 | >.097 |
| Craving | <.01 | <.01 | <.05 |
| Depression/feeling blue | <.05 | >.345 | >.522 |
| Difficulty concentrating | >.148 | >.144 | >.666 |
| Drowsy | >.205 | <.05 | >.418 |
| Hunger | >.250 | >.147 | >.462 |
| Impatient | >.811 | >.105 | >.205 |
| Irritability/frustration/anger | >.255 | <.05 | >.180 |
| Restless | >.442 | >.654 | >.610 |
| Desire for sweets | >.140 | >.595 | >.430 |
| Urges to smoke | <.01 | <.01 | <.05 |
| Direct effects of nicotine | |||
| Confused | >.390 | >.099 | >.583 |
| Dizzy | <.865 | <.149 | <.453 |
| Headache | >.886 | >.397 | >.773 |
| Heart pounding | >.463 | >.167 | >.261 |
| Lightheaded | >.831 | >.224 | >.471 |
| Nausea | <.05 | <.05 | >.567 |
| Nervous | >.667 | >.115 | >.622 |
| Salivation | >.886 | >.397 | >.773 |
| Sweaty | >.138 | >.300 | >.390 |
| Weak | >.413 | >.390 | >.271 |
Note.
df=(2, 18);
df=(7, 63);
df=(14, 126)
Nicotine delivery and cardiovascular response
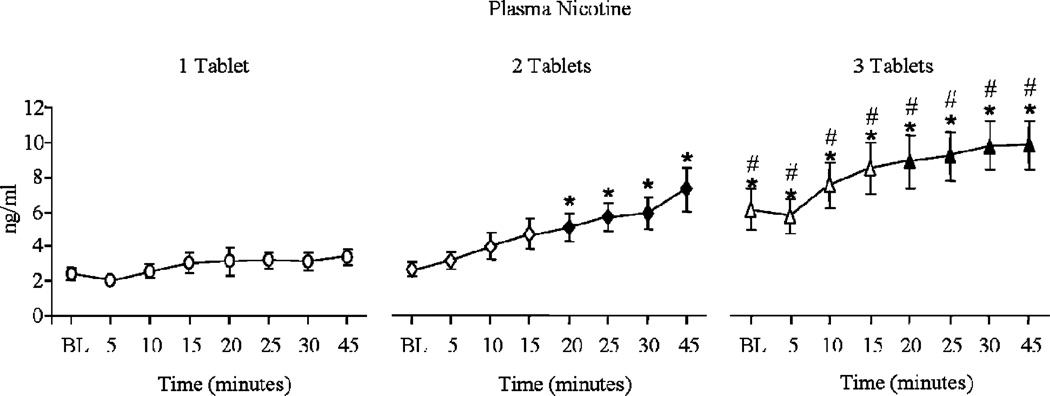
Table 1 shows that a significant dose by time interaction was observed for plasma nicotine; these data are displayed in Figure 1. For one tablet, mean (SD) plasma nicotine increased from a pre-administration level of 2.4 ng/ml (0.9) to 3.4 ng/ml (1.4) by 45 min post-administration. Subsequent tablets produced larger increases, with two tablets increasing plasma nicotine from 2.7 ng/ml (0.9) to 7.3 ng/ml (4.0), and three tablets increasing plasma nicotine from 6.0 ng/ml (3.7) to 9.7 ng/ml (4.4); p<.05, Tukey’s HSD. Heart rate also increased after tablet administration, though these increases were independent of dose. Collapsed across dose, mean (SD) heart rate was 67.5 bpm (8.1) at baseline, and then 70.2 bpm (7.2), 72.2 bpm (7.0), 71.7 bpm (6.8), and 70.3 bpm (7.0) at 5, 10, 15, and 20 min post-Ariva dose, respectively (ns, Tukey’s HSD).
Figure 1.
Mean data (±1 SEM) for plasma nicotine for dose (1, 2, and 3 Ariva tablets) by time (pre-tablet and 5-, 10-, 15-, 20-, 25-, 30-, and 45-min post-tablet administration). Filled symbols indicate a significant difference from the pre-tablet value (baseline; BL) within that dose, asterisks (*) indicate a significant difference from one tablet at that time point, and number signs (#) indicate a significant difference from two tablets at that time point. All p values <.05, Tukey’s HSD post-hoc test.
Withdrawal suppression and direct effects of nicotine
Dose by time interactions (F values>4.1, p values<.05) were observed for the withdrawal related VAS items “urges to smoke” and “craving.” Specifically, mean scores for both measures decreased from pre- to post-tablet at each dose, and scores at all time points decreased as the number of tablets increased. For instance, mean (SD) scores for “urges to smoke” (the item with the largest interaction F value) decreased from 66.6 (29.1) at baseline to 23.1 (28.2) 45 min following one tablet, to 17.0 (26.7) 45 min following two tablets, and to 13.9 (26.2) 45 min following three tablets (p<.05, Tukey’s HSD). Table 1 also shows significant main effects of dose and time for several withdrawal measures. Generally, ratings decreased at each time point relative to pre-1 tablet values for items such as irritability/frustration/anger and drowsy. Collapsed across dose, for example, mean (SD) irritability ratings decreased from 16.5 (17.5) at baseline to 7.6 (9.9) by the end of the 45 min assessment period (ns, Tukey’s HSD).
For the direct effects of nicotine, significant main effects of dose and time were observed for nausea (F values>4.5, p values<05). Mean (SD) nausea ratings increased significantly following each Ariva dose, with scores typically peaking at the 10 min time points. From pre-administration to 10 min post-administration, for example, scores increased from 3.9 (8.7) to 25.6 (38.0) after one tablet, from 2.6 (4.3) to 31.8 (43.6) after two tablets, and from 5.7 (10.3) to 35.3 (44.2) after three tablets (ns, Tukey’s HSD). Indeed, increased ratings were observed from pre-to post-Ariva dose for most other items used to measure direct effects of nicotine (e.g., dizzy, confused, lightheaded, nervous), though these effects were less reliable.
Discussion
This pilot study is the first to examine, in cigarette smokers, the nicotine delivery, cardiovascular response, and subjective effects associated with use of an oral tobacco PREP (Ariva) marketed to cigarette smokers. The product delivered active doses of nicotine, especially when two or three tablets were used simultaneously (see Figure 1). In this study, one tablet did not increase plasma nicotine concentration reliably. However, in a study of SLT users (Kotlyar et al., 2007), one tablet produced significant increases in maximum nicotine concentration (Cmax). By way of comparison, a single cigarette can increase mean plasma nicotine concentration by 5–14 ng/ml (Benowitz et al., 1988; Breland et al., 2006). Thus, for smokers who typically receive larger doses of nicotine, administration of multiple tablets may be necessary to attain a cigarette-like nicotine dose.
The product suppressed several symptoms of tobacco abstinence/withdrawal to varying degrees. The magnitude of the observed symptom suppression was higher than that observed in studies of some cigarette-like PREPs that also deliver minimal nicotine doses (e.g., Accord, see Breland, Buchhalter, Evans, & Eissenberg, 2002). These results suggest that oral tobacco products may provide some withdrawal relief to the abstinent smoker. However, this relief may be offset, at least with initial use, by the relatively high levels of nausea and other direct effects of the tablets noted here. Although withdrawal symptom suppression is likely a prerequisite of any successful PREP, inducing nausea, headache, dizziness, and other aversive effects is unlikely to be predictive of short-term product acceptability. Of course, these observations must be taken in the context of some study limitations, which include the absence of a positive control (i.e., smoking an own-brand cigarette) and the inability to measure carcinogen exposure in this short-term study. Methods are available for testing PREP users’ carcinogen exposure with rigorous experimental control (Breland et al., 2002; Breland et al., 2006), and these methods should be applied to oral PREPs for smokers.
Acknowledgments
This work was supported by U.S. Public Health Service grants R01 CA103827 and F31 DA018447. The authors thank Thomas Karnes and Randy James from the Bioanalytical Analysis Core Laboratories at Virginia Commonwealth University for analysis of plasma nicotine.
Footnotes
The authors have no other potential conflicts of interest to report.
Portions of these data were presented at the 13th annual meeting of the Society for Research on Nicotine and Tobacco, February 21–24, 2007.
Contributor Information
Melissa D. Blank, Department of Psychology, Virginia Commonwealth University, Richmond, VA
Cynthia Sams, Department of Psychology, Virginia Commonwealth University, Richmond, VA
Michael F. Weaver, Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA
Thomas Eissenberg, Department of Psychology and Institute for Drug and Alcohol Studies, Virginia Commonwealth University, Richmond, VA
References
- Benowitz NL, Porchet H, Sheiner L, Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology and Therapeutics. 1988;44:23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Breland AB, Buchhalter AR, Evans SE, Eissenberg T. Evaluating acute effects of potential reduced exposure products for smokers: Clinical laboratory methodology. Nicotine & Tobacco Research. 2002;4:S131–S140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8:727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: The role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Tomar SL, Reddy MN, Peddicord JP, Zhu B-P, Eriksen MP. The FTC cigarette test method for determining tar, nicotine, and carbon monoxide yields of U.S. cigarettes: Report of the NCI Expert Committee. Bethesda, MD: National Cancer Institute; 1996. Attitudes, knowledge, and beliefs about low-yield cigarettes among adolescents and adults. (Smoking and Tobacco Control Monograph No. 7) [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. British Medical Journal. 1995;311:363–366. doi: 10.1136/bmj.311.7001.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Giovino GA, Eissenberg T, Clark PI, Lawrence D, Leischow S. Methods to assess potential reduced exposure products. Nicotine & Tobacco Research. 2005;7:827–844. doi: 10.1080/14622200500266015. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in randomized block and split-plot designs. Journal of Educational Statistics. 1976;1:69–82. [Google Scholar]
- Keppel G. Design and analysis: A researcher’s handbook. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- Kotlyar M, Mendoza-Baumgart MI, Li ZZ, Pentel PR, Barnett BC, Feuer RM, Smith EA, Hatsukami DK. Nicotine pharmacokinetics and subjective effects of three smokeless tobacco products, moist snuff and nicotine lozenge. Tobacco Control. 2007;16:138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidong W, Shou W, Chen Y-L, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. Journal of Chromatography B. 2001;754:387–399. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, Author; 2001. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. (Smoking and Tobacco Control Monograph No. 13; NIH Publication No. 02-5074) [Google Scholar]
- Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell M, Feyerabend C, Thomas G, Sawe U. Transdermal nicotine for active ulcerative colitis. The New England Journal of Medicine. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine & Tobacco Research. 2006;8:309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S, editors. Clearing the smoke: Assessing the science base for tobacco harm reduction. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- Warner KE. Tobacco harm reduction: Promise and perils. Nicotine & Tobacco Research. 2002;4 Suppl. 2:S61–S71. doi: 10.1080/1462220021000032825. [DOI] [PubMed] [Google Scholar]