Abstract
Embolism and refilling of vessels was monitored directly by cryomicroscopy of field-grown corn (Zea mays L.) roots. To test the reliability of an earlier study showing embolism refilling in roots at negative leaf water potentials, embolisms were counted, and root water potentials (Ψroot) and osmotic potentials of exuded xylem sap from the same roots were measured by isopiestic psychrometry. All vessels were full at dawn (Ψroot −0.1 MPa). Embolisms were first seen in late metaxylem vessels at 8 am. Embolized late metaxylem vessels peaked at 50% at 10 am (Ψroot −0.1 MPa), fell to 44% by 12 pm (Ψroot −0.23 MPa), then dropped steadily to zero by early evening (Ψroot −0.28 MPa). Transpiration was highest (8.5 μg cm−2 s−1) between 12 and 2 pm when the percentage of vessels embolized was falling. Embolized vessels were refilled by liquid moving through their lateral walls. Xylem sap was very low in solutes. The mechanism of vessel refilling, when Ψroot is negative, requires further investigation. Daily embolism and refilling in roots of well-watered plants is a normal occurrence and may be a component of an important hydraulic signaling mechanism between roots and shoots.
Xylem vessels in the roots of corn (Zea mays) embolize and refill daily (McCully et al., 1998). At dawn, in plants growing in moist soil in the field, all vessels were sap filled, but by 1 to 2 h after sunrise embolisms began to form in the large, LMX vessels. The proportion of embolized, LMX vessels peaked in the middle of the day at values between 70% and 80%. The percentage declined beginning from mid- to late-afternoon, and reached 0% in most roots by sunset. At all times while embolisms were present some of the empty vessels appeared to be refilling, with liquid entering them from the side. The number of refilling vessels increased as the afternoon progressed. Ψleafs (as determined by pressure-chamber measurement) were less negative (−0.3 to −0.4 MPa) when root embolism began in the morning, and more negative (−0.5 to −1.2 MPa) when embolism was decreasing during the afternoon. Thus, vessels in the roots were refilling when the water potential in the leaves was −0.5 to −1.2 MPa.
The customary interpretation is that values for water potentials of plant tissues (determined either by pressure chamber or psychrometer) are also values for the tensions (negative pressures) in the sap-filled tracheary elements. Some drop in tension of xylem sap columns would be expected between the corn leaves and the roots. Nevertheless, in the corn plants measured by McCully et al. (1998), embolized vessels (at atmospheric pressure) in the roots were refilling, whereas intact sap columns in nearby vessels were apparently under considerable tension, as the plants were transpiring.
One possible explanation of these discordant data might be that the samples of roots, the embolisms of which were counted, were not representative of the whole population of roots on a plant. They might, for example, have different values of water potential. A second explanation might be that the Ψleaf were very different from those in the roots. To investigate these possibilities I have followed a daily course of embolism and refilling of root vessels, and simultaneously measured the water potentials and xylem sap osmotic potentials of the same roots. Ψleaf of the same plants were also recorded. As before, all measurements were made on corn plants growing in the field.
MATERIALS AND METHODS
Plant Material
Corn (Zea mays L. cv 41-417; Pick Seeds, Ltd., Lindsay, Ontario, Canada) plants were grown in a field plot at the Central Experimental Farm of Agriculture Canada in Ottawa. The soil was a light, sandy loam. Adequate moisture was maintained by occasional irrigation. Plants were studied during the 2nd week of August 1997 and were at the 10th leaf stage, just preflowering.
Individual nodal roots of tiers 1 to 4 (terminology of Hoppe et al. [1986]) were located within the soil close to the base of the stem by gentle probing with fingers. The soil surrounding an approximately 4-cm-long portion of each root close to its base was removed by hand to minimize disturbance to the root and its short, determinate laterals. The root portion was exposed for only a few seconds before being recovered with loose soil until freezing. The distal portion of this root remained in situ in undisturbed soil. These roots were fully mature with open LMX conduits for many centimeters distal to the region studied. Root diameters ranged from 0.4 to 1.3 mm. They were collected from dawn to dusk on several days.
Collection of Root Pieces, Xylem Sap, and Soil for Cryomicroscopy
The root portion loosely covered by soil was quickly exposed, then frozen in situ with specially constructed copper-jawed pliers cooled in LN2. The jaws of these pliers are 2.7 cm wide and come together parallel to each other and the spacing between the closed jaws is adjustable to accommodate roots of different diameters, so that during freezing rapid contact can be made with the roots without crushing them. The sap within the intact vessels was thus stabilized by freezing. Very fast cooling was achieved by pressing tissue to a polished copper surface at the temperature of LN2. Echlin (1992) gives the cooling rate of the surface layers as 80,000 to 100,000 K s−1, and somewhat deeper in the tissue as 25,000 K s−1. With the cryopliers such a heat sink is applied to both sides of the narrow roots. Thus, the vessel contents will freeze within a few milliseconds, allowing no time for movement of water out of the lumens. Any nucleating centers formed by the separation of nanometer-sized gas bubbles during the rapid freezing cannot induce embolisms until the vessel contents are thawed. (Even slow, natural freezing of the xylem sap in trees does not produce embolisms until the sap is thawed [Tyree and Sperry, 1989].) The corn roots remained deep-frozen through subsequent preparation and observation.
While still held with the pliers, the frozen piece of the root was quickly severed from the rest of the root by running a scalpel along the sides of the jaws of the pliers, then transferred to a shallow container of LN2. One to three millimeters was cut from each end of the root piece under LN2 with wire cutters and discarded to ensure that the retained piece had been quickly frozen all along its length. The remaining piece was then transferred to a cryovial under LN2 and held in LN2 in a dewar for return to the lab and storage in a cryostore.
Psychrometry
Ψroot
Further excavation with fingers exposed 6 to 8 cm of the root distal to where the frozen piece had been removed. The top 2 to 3 cm of this piece was cut off and discarded. The rest of the piece was carefully severed at its base with a sharp blade, and the loose soil was knocked off by quick flicking and shaking. The piece was immediately placed in a cup of the psychrometer and sealed by attaching it to the heat sink of the psychrometer.
Root Exudate (Exudate Osmotic Potential)
The proximal end of the remaining root was exposed for about 2 cm by careful removal of soil. The distal portion (most of the length of the root) remained in situ in undisturbed soil. The cut end was trimmed straight with a sharp razor blade, washed with deionized water, and blotted dry with a Kimwipe. When exudate appeared on the cut face, about 5 to 10 μL was collected by capillarity into 25-μL micropipettes.
Ψsoil
Samples were collected from the vicinity of the root pieces taken for Ψroot measurements. These were immediately placed in the cup of the psychrometer and sealed by attaching to the small heat sink of the psychrometer.
Ψleaf
Leaf discs were removed from the uppermost expanded leaf in a region perpendicular to the incoming light, immediately placed in a cup of the psychrometer, and the cup was sealed by attaching to the small heat sink of the psychrometer.
Determination of Potentials and Transpiration
The soil and tissue sampling was completed within 10 s to avoid dehydration after removal from the bulk soil or plant. The sealed-cup/heat-sink assemblies were stored in a Styrofoam box in the shade until they were transported to the laboratory, usually within 30 min. In the laboratory the assemblies were placed in the large heat sink, and Ψsoil, Ψroot, and Ψleaf were measured isopiestically according to the method of Boyer (1995) in an isopiestic thermocouple psychrometer (Isopiestics Co., Lewes, DE). For root exudate, about 3 μL was loaded onto a psychrometer thermocouple and the osmotic potential was measured in the isopiestic psychrometer operated as an osmometer according to the method of Boyer (1995). Soil, root, and root- exudate measurements were completed within 15 min, and the leaf measurements within about 60 min.
Ψleaf were also measured in a plant water-status console (pressure chamber, model 3000, Soil Moisture Corp., Santa Barbara, CA). Readings were taken just before and during root collection. Rates of transpiration of the same plants were measured with a steady-state porometer (LI1600, Li-Cor, Lincoln, NE).
Cryoanalytical Microscopy
A 3- to 5-mm piece was cut from the mid-point of the frozen root piece under LN2 and cryoplaned with glass and diamond knives at −80°C to give a smooth, transverse face. The planed piece was transferred under LN2 to a cryotransfer system (model CT 1500, Oxford Instruments, Eynsham, Oxford, UK) and hence to the cold stage of the scanning electron microscope (model JSM 6400, Jeol). The specimen was lightly etched at −90°C, coated with Al, and observed at 15 kV. The numbers and percent of embolized xylem vessels were recorded. For details, see McCully et al. (1998) and references therein.
The frozen contents of full and refilling xylem elements were analyzed by x-ray microanalysis (Link eXL, Oxford Instruments) using the Be and thin-window modes. Peak-to-background ratios were converted to absolute values by reference to standards and with corrections made for local thickness of the Al coat. Details are given by Hopkins et al. (1991) and Huang et al. (1994).
RESULTS
Vessels filled with sap were easily distinguished from embolized vessels in the cryopreparations (Fig. 1, A and B). No vessels were embolized at dawn, but embolisms were present at 8 am and by 10 am over 50% of the LMX vessels were embolized. This percent fell during the rest of the day to reach zero at sunset (Fig. 2C).
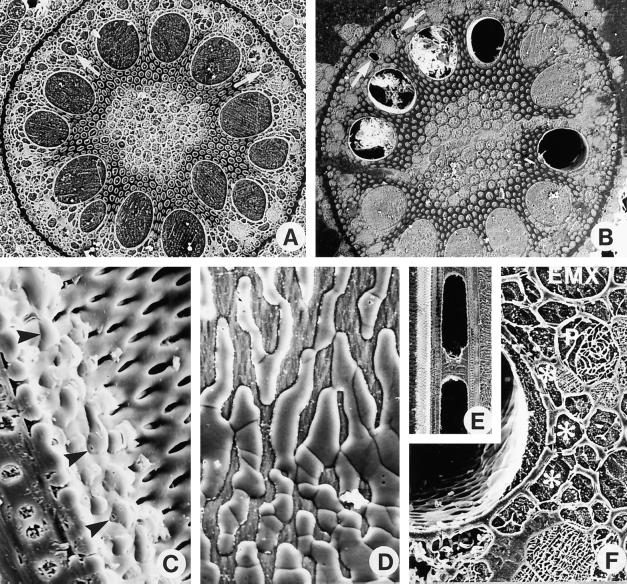
Figure 1.
Cryo-scanning electron microscopy images of field-grown corn roots. The roots were frozen while still attached to the plants, and the frozen pieces were cryoplaned, lightly etched at −90°C, coated with Al, and observed in the secondary electron mode. A, B, and F, Transverse faces. C, D, and E, Longitudinal faces. A, Root frozen at 6 h has no embolized xylem vessels. Magnification is ×112. B, At 8 h, 5 of the 12 LMX vessels were embolized in this root. Some frozen debris from the cryoplaning has fallen into three of the embolized vessels. Magnification is ×104. C, Root frozen at 10 h. The face of the block passes through an embolized LMX vessel that is refilling (as indicated by the convex shape of the frozen drops). Some pits in the lateral wall of this vessel (toward the right of the micrograph) are not yet flooded, but drops of xylem sap were entering the vessel lumen through pits closer to the left of the micrograph (arrowheads). The block face includes a section of part of the longitudinal wall of the vessel showing the bordered pits and the thin primary wall in the floor of each pit. Magnification is ×1600. D, A face view of the lumen side of the lateral wall of an LMX vessel in a root frozen at 14.5 h. Sap droplets have coalesced into rivulets, which obscure the underlying pits. Magnification is ×1680. E, Root frozen at 14.5 h. A column of water in the vessel is sandwiched between two bubbles of gas. Water movement to or from such a water mass must be radial. Magnification is ×112. F, Root frozen at 14 h showing an LMX vessel containing a lens of xylem sap on the side facing the endodermis. Asterisks indicate connecting vessels of a branch root. P, Sieve tube; EMX, early metaxylem vessel. Magnification is ×720.
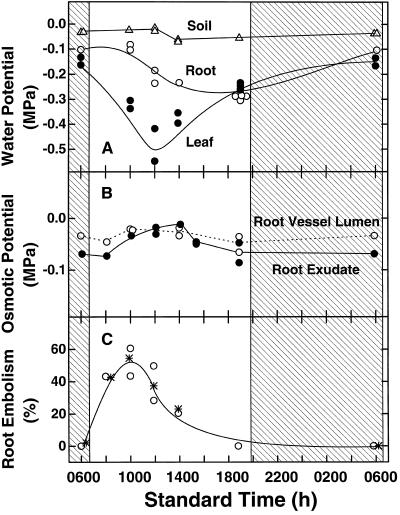
Figure 2.
A, Water potentials (measured isopiestically) of individual corn roots (○) collected at different times during the day, soil surrounding the same roots (▵), and leaves (•) of the plants from which the roots were sampled. Each symbol records a measurement from one root, soil, or leaf sample. B, Osmotic potential of the contents of the xylem lumens of the same individual roots used for the measurements of water potential in A. At each sample time the osmotic potential for the sap of each root was determined in two ways: (a) by isopiestic measurements of exuded sap (•), and (b) from in situ measurements of elemental concentrations in vessel lumens of frozen pieces of the same roots by cryoanalytical microscopy (○). C, Percent embolized LMX vessels in roots collected at the same times as those in A and B. Values (○) are for the same individual roots measured in A and B (see text for details). Values (*) are means (n = 2–4) for these and other roots from the same plants frozen at the same times.
During the day there were always some embolized vessels that were partly filled with sap. Such vessels may be emptying or refilling. At times when the number of vessels is increasing (am), partly filled vessels are likely to be emptying. At times when the number is decreasing, they are likely to be refilling. Vessels showing convex drops being pressed through pits (Fig. 1, C and D) may be scored unequivocally as refilling. This refilling most commonly began from the side of the vessels facing the endodermis, and was frequently associated with the connecting xylem of branch-root junctions. At the earliest stages of refilling convex, frozen drops of sap could be detected on the vessel lumen side of pits, separating the vessels from xylem parenchyma, or vessels of the connecting xylem (Fig. 1C). In other vessels the droplets had coalesced and spread out, obscuring all of the nearby pits (Fig. 1D). Figure 1E shows a longitudinal section of an embolized vessel in which a water column is isolated between two gas bubbles. Any movement of water to or from this column must be radial, not longitudinal. Figure 1F shows the more commonly seen accumulation of a lens-shaped (in transverse section) pool of sap on one side of a vessel. The low angle of contact of these spreading sap deposits indicates that the vessel lining is hydrophilic. Partially filled vessels were more plentiful from early afternoon onward. There was no evidence that vessels were refilling by sap moving basipetally from the distal regions of the roots.
Comparison of the curves in Figure 2, A and C, shows that the percent of embolized vessels in the roots was rising rapidly and peaked, whereas values for Ψroot (about −0.1 MPa) had not changed from their high (least negative) value at dawn. Also, the percent embolism was rising, whereas values for Ψleaf (up to −0.2MPa) were still not greatly lower than at dawn, and the peak percentage of embolism was reached at 10 am, about 2 h before the lowest values for Ψleaf (mean about −0.5 MPa).
Values for both Ψleaf and Ψroot became lower during the day, those for Ψleaf dropping steadily from dawn to reach a mean of about −0.5 MPa by noon. The Ψleaf then rose during the afternoon to about −0.25 MPa by sunset (Fig. 2A). Balance pressures correspondingly rose to a peak between 12 and 2 pm (mean 0.42 MPa, range 0.32–0.58 MPa, n = 15). Peak transpiration rates were found from 12 to 2 pm with a mean of 8.5 μg cm−2 s−1 (range 6.9–11.8, n = 15). Rates before and after these times were lower. Ψsoil were close to 0 MPa throughout the experiment (Fig. 2A).
Surprisingly, Ψleaf rose to about 0.05 MPa higher than Ψroot by sunset. This difference was signicant (P < 0.01). By the following morning this difference was reversed, with Ψroot about 0.05 MPa higher than Ψleaf (Fig. 2A).
Values for the osmotic potential of the xylem exudate were somewhat more negative at dawn than later (Fig. 2B). They became less negative during the day to reach their highest value (about −0.03 MPa) in early afternoon. This time corresponded with the approximate time of highest rates of transpiration of the plants.
Potassium was the only element (including carbon) present in high enough concentration in the lumens of the full xylem vessels to be measurable by x-ray microanalysis. Even so, except at dawn, when [K] reached a mean of 12 mm (range 6–24, n = 38), the concentration was very close to or below the minimum needed for reliable quantification in the frozen solution (approximately 12 mm). To arrive at the values for [K] at the different sampling times, we arbitrarily set all values between 12 and 0 mm at 6 mm. These concentrations were then expressed as osmotic potential (Fig. 2B). The results can only be reliably interpreted to show that [K] of the solution is low, and that the values obtained confirm that the osmotic potentials measured for the xylem exudate are most negative during the night.
DISCUSSION
One of the referees of the present paper suggested that the embolisms may be produced by the freezing of the xylem columns, which are under transpirational stress, and suggested the following control experiment to check this. Roots were frozen as described in “Materials and Methods,” except that the initial freeze was followed after 5 s by a second freeze on the same root, with similar pliers upstream from the first freeze (still held in the first pliers). The comparison of embolized vessels in the two pieces showed that in the nine roots thus studied, six had identical percentage of embolized LMX conduits. The other roots ranged from 0.4% fewer to 17% more embolisms in the upstream piece. Thus, transpiration tension during freezing did not result in artifactual embolisms. This has also been shown earlier (Canny, 1997a) in sunflower petioles, which had the same amount of embolism, whether frozen intact or immediately after removal from the plant.
Recently, Pate and Canny (1999) have shown that gas volumes in xylem conduits in roots of Xanthorrhea preissii undergo daily changes. At any time, the volume of embolisms determined by direct measurement of gas in the sap aspirated from living roots was comparable to that determined by cryomicroscopy on the same root, thus further establishing the validity of the freezing technique.
The results of the present study are in accord with the earlier findings of McCully et al. (1998). The daily course of embolism formation and refilling was similar to that described for corn plants growing in Ottawa in the previous year, except that the maximum embolism was about 20% lower than that observed in 1996, and peak embolism was reached about 2 h earlier in the day. These values, however, were well within the range of variability seen in the much larger population of roots observed in the earlier study. In both seasons plants were growing under similar, well-watered conditions where Ψsoil were never far below zero.
In the previous study (McCully et al., 1998) Ψleaf was determined by pressure chamber. As expected from the findings of Boyer (1967) and later workers, and results in the present study, the values for Ψleaf determined by pressure chamber and psychrometer are in close agreement. Thus in both this study and that of McCully et al. (1998) root vessels were embolizing when Ψleaf was relatively high and refilling while Ψleaf was relatively low. Thus the doubts raised in the introduction about the reliability of the root sampling and about the relevance of the measurements of Ψleaf to the water status of the roots in the study of McCully et al. (1998) have been shown to be groundless.
As expected, the values for Ψroot were less negative than those for Ψleaf, at least during most of the day (maximum difference of about 0.3 MPa at 12 pm; Fig. 2A). The results show, however, that although Ψleaf (as determined by balance pressure or psychrometry) is a general guide to the water status of individual roots, there are subtle features that relate to root embolism that can only be detected if Ψroot is also measured. For example, the Ψroot was still at its least negative dawn value (−0.08 MPa), although Ψleaf of the same plant had become more negative than its dawn value, when the percentage of embolized vessels in the same roots was highest at 10 am (Fig. 2, A and C). Ψroot was becoming more negative while vessels were refilling and reached its most negative value (close to −0.3 MPa) by early evening, when all vessels had refilled (Fig. 2, A and C). Ψleaf rose quickly in the late afternoon and, surprisingly, was less negative than Ψroot when the latter was at its most negative value (coinciding with the disappearance of embolized vessels).
The formation of embolisms in the roots of well-watered corn plants in both this and the previous study occurred at water potentials that are less negative than has previously been reported for embolisms in other plants, including shoots of field-grown corn (Tyree et al., 1986), measured indirectly by monitoring acoustic emissions or changes in hydraulic conductivity. Most values were more negative than −1.0 MPa (for refs., see McCully et al., 1998). The least negative value (−0.5 MPa) was reported by Milburn (1993) for embolized banana stems. Byrne et al. (1977), using air flow through frozen roots to detect empty vessels, found embolisms in cotton roots when Ψleaf were below −0.9 MPa and root potentials should have been less negative. To our knowledge, no direct check has ever been made of the sensitivity of the indirect methods that have been used to detect embolisms, and recent studies suggest that they may underestimate the number of air-filled vessels and thus miss embolisms formed at high water potentials. In this regard, Tyree et al. (1986) note that it is uncertain whether acoustic emissions represent cavitation events in xylem vessels.
Canny (1997a, 1997b) was the first to visualize and quantify embolisms directly, as in the present study. In petioles of well-watered sunflowers, he observed that embolisms occurred at Ψleaf as negative as −0.15 MPa. These values are much closer to our directly measured values in roots, but still more negative. It appears that for some reason root vessels embolize at less-negative local water potentials than do shoots. This interpretation is supported by the results of Sperry and Ikeda (1997) and Mencuccini and Comstock (1997), who have reported that roots of Douglas fir and two desert shrubs, respectively, embolize before stems and leaves, presumably at a lower xylem tension. In contrast, however, Tsuda and Tyree (1997) found that the roots are the last organs to embolize in Acer saccharinum.
One possible reason that roots might embolize at low xylem tension is that they air-seed more readily than shoots. This seems unlikely, especially in the corn roots. The stele is sealed by the thick-walled endodermis, there are no air spaces close to the vessels (Fig. 1, A and B), and the branch-root junctions have a safety zone of single-celled vessels (McCully and Mallett, 1993; McCully et al., 1998), the end walls of which would trap any air bubbles coming from broken branch roots. The vessel pits, except where modified to link branch-root vessels as in Figure 1F, lie over living parenchyma cells and do not have access to air spaces. High-resolution electron microscopy of the linking pit membranes revealed no pores large enough to permit air seeding (McCully and Mallett, 1993).
Refilling of Embolized Vessels
The refilling of embolized xylem vessels during the day when Ψroot and water potentials of stems are negative and the plants are transpiring presents a paradox. Water will not move spontaneously from a space at any negative pressure into an empty vessel filled with air at atmospheric pressure. Yet, in the corn roots studied here, refilling was apparently occurring against an uphill step of as much as 0.3 MPa (Fig. 2, A and C). The positive pressure driving the refilling is clearly some form of “root pressure.”
Several mechanisms have been postulated by which embolized tracheary elements might be refilled when water potentials are negative. Edwards et al. (1994) have shown that a flow of degassed water can refill tracheids against a very small negative pressure (0.025 MPa) by dissolving the air. Borghetti et al. (1991) have shown that high surface tensions developed in narrow ends of tracheids can enlarge the sap space to a small extent, but these tensions could only refill the tracheid at water potentials near zero. Neither of these mechanisms, applicable to narrow tracheids, is feasible for the very large gas-filled vessels in our corn roots. Some of the possible refilling mechanisms have been discussed previously in respect to the corn root vessels (McCully et al., 1998) but the possibilities need to be explored further in light of the data from the present study.
Is the Refilling Osmotic?
The refilling of embolized vessels in trees has been postulated to be driven osmotically by salts secreted into the xylem lumens by the surrounding parenchyma (e.g. Grace, 1993; Salleo et al., 1996). This mechanism was rejected earlier as the basis for vessel refilling in the maize roots because of the low levels of solute detected in situ by microanalysis (McCully et al., 1998). The in situ microanalyses in the present study similarly showed solute levels in the partially and fully refilled vessels, which were too low to provide an osmotic uptake of water (compare Fig. 2, A–C). This very low level of solutes was confirmed for the xylem sap by the osmotic-potential measurements of the exuded sap of the same roots (Fig. 2B) obtained by the psychrometry method, which has been shown (Boyer, 1966, 1967) to be extremely accurate for such determinations. The measurements by psychrometry also confirm that total solute concentration in the xylem sap can be reasonably estimated from the microanalysis data even at concentrations near the limit of detection by the latter method.
The often quoted suggestion (Klepper, 1967; Kramer and Boyer, 1995) that there is a gradient of osmotic concentration (high at the tip and diluted toward the base) along the xylem lumens in the roots of a guttating plant so that the xylem sap is pressurized osmotically at the distal ends of the roots is not applicable to our observations, nor are those of McCully et al. (1998), where refilling, also near the base of the roots, was always observed to be through the lateral walls of the vessels and not basipetally from the root tips (Fig. 1, C–F). Moreover, Enns et al. (1998) have now measured, in guttating corn plants, the solute concentrations in mature vessel lumens from the base of roots to the still-living xylem elements closer to the tip, and shown there is no local high concentration of solutes, but a uniform low level.
The present study has not ruled out the possibility that such an osmotic mechanism, operating in the lateral roots, may be driving water into the vessels of the axile roots. Work is in progress to investigate this possibility.
Does the Phloem Play a Role in the Vessel Refilling?
Salleo et al. (1996) used girdling experiments and auxin application to determine if the phloem played a role in vessel refilling in laurel stems. They suggested that solutes released from the phloem may move along the walls of the ray cells, enter the vessels, and provide osmoticum to pull water in during refilling. Canny (1995) had already particularized the phloem as a tissue that could exert pressure to refill the vessels (see below). Whereas phloem tissue is in close juxtaposition to the xylem poles of the corn roots, particularly at branch-root junctions (see micrographs in McCully et al., 1998), the negligible concentrations of carbon detected by our light-element detector indicate that if sugar or amino acids were present, it was in very low amounts. This is corroborated by the low osmotic potential of the sap from the same roots, which was mostly accounted for by the measured [K] and the necessary balancing anions, and the very low concentration of total sugars (5.4 mm) in sap aspirated from field-grown corn roots (Canny and McCully, 1988).
Milburn (1996) has made the interesting suggestion that water for refilling of embolized vessels may be supplied locally from the phloem as photosynthate is removed and metabolized by nearby cells. Certainly, as originally recognized by Münch, some of the water released in this way must be recycled back to the shoot in the xylem. Milburn does not suggest a mechanism whereby this water could be forced into the embolized vessels.
Is Refilling by Reverse Osmosis?
Canny (1997a, 1997b) has found patterns of embolism formation and refilling similar to the present findings in the petioles of sunflowers. Embolisms formed early in the day when Ψleaf was around −0. 2 MPa, and were refilled during peak transpiration in the afternoon when Ψleaf was −0. 6 MPa. He has proposed that embolized vessels are refilled by sap squeezed out of the petiole parenchyma cells by reverse osmosis, driven by the positive tissue pressure of the petiole, this tissue pressure being generated by high solute concentration in some cells, which then squeeze water out of xylem parenchyma into the vessels at sufficient positive pressure to dissolve the air and refill the vessels. One source of the extra water could be the phloem, as proposed by Milburn (1996). The recent identification of aquaporins in plant cell membranes and specifically in xylem parenchyma cells (Chrispeels and Maurel, 1994; Barrieu et al., 1998; Schäffner, 1998) suggests that the distribution and/or frequency of these pores could control water flow from the xylem parenchyma to the vessel lumens.
In answer to the paradox of water movement into space at positive pressure when the measured tissue water potential is negative, Canny (1995, 1997b) has also proposed an alternative interpretation of water potential. Under circumstances where tissue pressure is high, water-potential is a measure of the amount of tissue pressure applied to squeeze water into the vessels, and not a measure of the negative pressure within the xylem sap columns, as is the conventional interpretation (see discussion in Canny, 1997b). In his view, the interpretation of water-potential measurements as indirect measures of negative pressures in the vessel water columns is mistaken. In the only published comparisons of water potentials with measurements of xylem tensions determined directly by xylem pressure probe (Balling and Zimmermann, 1990; Zimmermann et al., 1994), the actual tension in the xylem was often considerably overestimated by the indirect method. Recently, Schneider et al. (1997) and Zhu et al. (1995) have measured directly very low tensions (+0.024 MPa absolute, equivalent to a water potential of −0.076 MPa) in the root xylem of wheat and corn. The indirect methods used in the present study (pressure chamber and psychrometer) may have overestimated the tension in the intact xylem columns. However, positive pressures, not tensions, would still be required to refill the embolized vessels.
The possibility that daily embolism and refilling of vessels in roots forms part of a hydraulic signaling system between roots and shoots needs to be investigated, as does the mechanism of vessel refilling.
Paradox
The data of Figure 2 do not conform with the current understanding of plant water relations. The embolisms increase in the morning (Ψ root = −0.1 MPa), and decrease in the afternoon (Ψroot < −0.3 MPa). Either the cavitation threshold must be variable, or the refilling process must be more active later in the day. A possible solution to this paradox (seen also in other measurements of embolisms by this method) has been provided by Canny (1997b, 1998). He proposes that increasing tissue pressure around the xylem accounts for both the decreased vulnerability and the faster refilling.
CONCLUSIONS
(a) Embolism formation and refilling are normal, daily events in the roots of well-watered corn plants. Embolisms are absent at sunrise but form early in the day, before peak transpiration, in roots with high (i.e. little negative) water potentials. Embolized vessels refill while plants are still transpiring and in roots of low (i.e. more negative) water potentials.
(b) Refilling of vessels in the mature regions close to the base of the roots is not by sap pushed up from the distal end of the root, but by sap entering locally through the lateral walls. The mechanism of refilling is unclear and requires investigation. The low solute content of the refilling sap rules out the conventional osmotic-xylem-sap model for the generation of the necessary positive pressure in the refilling vessel.
(c) The plants have root systems that are overbuilt, so that embolism, even during peak transpiration times, does not seem to affect normal development.
ACKNOWLEDGMENTS
I thank Dr. J.S. Boyer for his full involvement in the planning and execution of these experiments with his own isopiestic psychrometer, and for the many discussions of how the results might be interpreted. I also thank Dr. Cheng Huang and Lewis Ling of the Carleton University Research Facility for Electron Microscopy for help with the cryomicroscopy, Jack Egerton for drawing Figure 2, and Adam Baker for making the plate for Figure 1.
Abbreviations:
- LMX
late metaxylem
- LN2
liquid nitrogen
- Ψleaf
leaf water potential(s)
- Ψroot
root water potential(s)
- Ψsoil
soil water potential(s)
Footnotes
This research was funded by an operating grant from the Natural Sciences and Engineering Research Council of Canada.
LITERATURE CITED
- Balling A, Zimmermann U. Comparative measurements of the xylem pressure of Nicotiana plants by means of the pressure bomb and pressure probe. Planta. 1990;182:325–338. doi: 10.1007/BF02411382. [DOI] [PubMed] [Google Scholar]
- Barrieu F, Chaumont F, Chrispeels MJ. High expression of the tonoplast aquaporin ZmTlP1 in epidermal and conducting tissues of maize. Plant Physiol. 1998;117:1153–1163. doi: 10.1104/pp.117.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghetti M, Edwards WRN, Grace J, Jarvis PG, Raschi A. The refilling of embolized xylem in Pinus sylvestris L. Plant Cell Environ. 1991;14:357–369. [Google Scholar]
- Boyer JS. Isopiestic technique: measurement of accurate leaf water potentials. Science. 1966;154:1459–1460. doi: 10.1126/science.154.3755.1459. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Leaf water potentials measured with a pressure chamber. Plant Physiol. 1967;42:133–137. doi: 10.1104/pp.42.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. Measuring the Water Status of Plants and Soil. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Byrne GF, Begg JE, Hansen GK. Cavitation and resistance to water flow in plant roots. Agric Meteor. 1977;18:21–25. [Google Scholar]
- Canny MJ. A new theory for the ascent of sap: cohesion supported by tissue pressure. Ann Bot. 1995;75:343–357. [Google Scholar]
- Canny MJ. Vessel contents of leaves after excision: a test of Scholander's assumption. Am J Bot. 1997a;84:1217–1222. [PubMed] [Google Scholar]
- Canny MJ. Vessel contents during transpiration: embolisms and refilling. Am J Bot. 1997b;84:1223–1230. [PubMed] [Google Scholar]
- Canny MJ. Applications of the compensating pressure theory of water transport. Am J Bot. 1998;85:897–901. [PubMed] [Google Scholar]
- Canny MJ, McCully ME. The xylem sap of maize roots: its collection, composition and formation. Aust J Plant Physiol. 1988;15:557–566. [Google Scholar]
- Chrispeels MJ, Maurel C. Aquaporins: the molecular basis of facilitated water movement through living plant cells? Plant Physiol. 1994;105:9–13. doi: 10.1104/pp.105.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echlin P (1992) Low Temperature Microscopy and Analysis. Plenum Press, New York
- Edwards WRN, Jarvis PG, Grace J, Moncrieff JB. Reversing cavitation in tracheids of Pinus sylvestris L. under negative water potentials. Plant Cell Environ. 1994;17:389–397. [Google Scholar]
- Enns LC, McCully ME, Canny MJ. Solute concentrations in xylem sap along vessels of maize primary roots at high root pressure. J Exp Bot. 1998;49:1539–1544. [Google Scholar]
- Grace J. Refilling of embolized xylem. In: Grace J, Raschi A, editors. Water Transport in Plants under Climatic Stress. Cambridge, UK: Cambridge University Press; 1993. pp. 52–62. [Google Scholar]
- Hopkins DM, Jackson AD, Oates K. The effect of aluminium coating on elemental standards in x-ray microanalysis. J Electron Microsc Tech. 1991;18:176–182. doi: 10.1002/jemt.1060180213. [DOI] [PubMed] [Google Scholar]
- Hoppe DC, McCully ME, Wenzel CL. The nodal roots of Zea: their development in relation to structural features of the stem. Can J Bot. 1986;64:2524–2537. [Google Scholar]
- Huang CX, Canny MJ, Oates K, McCully ME. Planing frozen hydrated plant specimens for SEM observations and EDX microanalysis. Microsc Res Tech. 1994;28:67–74. doi: 10.1002/jemt.1070280108. [DOI] [PubMed] [Google Scholar]
- Klepper B. Effects of osmotic pressure on exudation from corn roots. Aust J Biol Sci. 1967;20:723–735. [Google Scholar]
- Kramer PJ, Boyer JS. Water Relations of Plants and Soils. San Diego, CA: Academic Press; 1995. [Google Scholar]
- McCully ME, Huang CX, Ling LEC. Daily embolism and refilling of xylem vessels in the roots of field-grown maize. New Phytol. 1998;138:327–342. doi: 10.1046/j.1469-8137.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- McCully ME, Mallett J. The branch roots of Zea. 3. Vascular connections and bridges for nutrient recycling. Ann Bot. 1993;71:327–341. [Google Scholar]
- Mencuccini M, Comstock J. Vulnerability to cavitation in populations of two desert species, Hymenoclea salsola and Ambrosia dumosa, from different climatic regions. J Exp Bot. 1997;48:1323–1334. [Google Scholar]
- Milburn JA. Cavitation, a review: past, present and future. In: Grace J, Raschi A, editors. Water Transport in Plants under Climatic Stress. Cambridge, UK: Cambridge University Press; 1993. pp. 14–26. [Google Scholar]
- Milburn JA. Sap ascent in vascular plants: challengers to the cohesion theory ignore the significance of immature xylem and the recycling of Münch water. Ann Bot. 1996;78:399–407. [Google Scholar]
- Pate JS, Canny MJ (1999) Quantitation of vessel embolisms by direct observation: a comparison of two methods. New Phytol (in press)
- Salleo S, Lo Gullo MA, De Paoli D, Zippo M. Xylem recovery from cavitation-induced embolism in young plants of Lauris nobilis: a possible mechanism. New Phytol. 1996;132:47–56. doi: 10.1111/j.1469-8137.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Schäffner AR. Aquaporin function, structure, and expression: Are there more surprises to surface in water relations? Planta. 1998;204:131–139. doi: 10.1007/s004250050239. [DOI] [PubMed] [Google Scholar]
- Schneider H, Zhu JJ, Zimmermann U. Xylem and cell turgor pressure probe measurements in intact roots of glycophytes: transpiration induces a change in the radial and cellular reflection coefficients. Plant Cell Environ. 1997;20:221–229. [Google Scholar]
- Sperry JS, Ikeda T. Xylem cavitation in roots and stems of Douglas-fir and white fir. Tree Physiol. 1997;17:275–280. doi: 10.1093/treephys/17.4.275. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Tyree MT. Whole-plant hydraulic resistance and vulnerability segmentation in Acer saccharinum. Tree Physiol. 1997;17:351–357. doi: 10.1093/treephys/17.6.351. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Fiscus EL, Wullschleger SD, Dixon MA. Detection of xylem cavitation in corn under field conditions. Plant Physiol. 1986;82:597–599. doi: 10.1104/pp.82.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:19–38. [Google Scholar]
- Zhu JJ, Zimmermann U, Thürmer F, Haase E. Xylem pressure response in maize roots subjected to osmotic stress: determination of radial reflection coefficients by use of the xylem pressure probe. Plant Cell Environ. 1995;18:906–912. [Google Scholar]
- Zimmermann U, Meinzer FC, Benkert R, Zhu JJ, Schneider H, Goldstein G, Kuchenbrod E, Haase A. Xylem water transport: is the available evidence consistent with the cohesion theory? Plant Cell Environ. 1994;17:1169–1181. [Google Scholar]