The authors demonstrated the critical role of NOX2 in neuronal cell death in the ischemic retina, consequently indicating that the inhibition of NOX2 may offer a novel therapeutic strategy for neuronal and vascular injury during ischemic retinopathy.
Abstract
Purpose.
The aim of this study was to determine whether NOX2, one of the homologs of NADPH oxidase, plays a role in neuronal cell death during retinal ischemia.
Methods.
Ischemia reperfusion (I/R) injury was generated in C57/BL6 and NOX2−/− mice by increasing the intraocular pressure (IOP) to 110 mm Hg for 40 minutes followed by reperfusion. Quantitative PCR and Western blot analysis were performed to measure NOX2 expression. Reactive oxygen species (ROS) formation was assessed by dihydroethidium imaging of superoxide formation and Western blot analysis for tyrosine nitration. TUNEL assay was performed to determine cell death at 3 days after I/R. Survival of neurons within the ganglion cell layer (GCL) was assessed at 7 days after I/R by confocal morphometric imaging of retinal wholemounts immunostained with NeuN antibody. Activation of mitogen-activated protein kinases and nuclear factor κB (NF-κΒ) was measured by Western blot analysis.
Results.
NOX2 mRNA and protein and ROS were significantly increased in wild-type I/R retinas. This effect was associated with a 60% decrease in the number of GCL neurons and a 10-fold increase in TUNEL-positive cells compared with the fellow sham control eyes. Phosphorylation of ERK and NF-κB was significantly increased in wild-type I/R retinas. Each of these effects was markedly attenuated in the NOX2−/− retina (P < 0.01).
Conclusions.
These data demonstrate that the deletion of NOX2 can reduce I/R-induced cell death and preserve retinal GCL neurons after I/R injury. The neuronal cell injury caused by I/R is associated with the activation of ERK and NF-κB signaling mechanisms.
Retinal ischemia is critically involved in the pathogenesis of several major vision-threatening diseases, including diabetic retinopathy, age-related macular degeneration, retinal vein occlusion, and retinopathy of prematurity. Although these retinopathies are diagnosed primarily by their vascular abnormalities, several clinical and experimental studies have demonstrated the presence of inflammation,1–3 glial activation,4–6 and neurodegeneration7,8 in the retina before the appearance of typical vascular pathology such as macular edema and vitreous neovascularization. These studies suggest that ischemia-induced inflammation and neurotoxicity may play a pathophysiological role in mediating elements of the vascular pathology.
Evidence of neuronal injury in diabetic animal models includes degeneration of the nerve fiber layer, loss of inner retinal neurons, and activation of caspases 3 and 9.9,10 A recent study of patients with type 1 diabetes using high-resolution optical coherence tomography (OCT) demonstrated thinning of the retinal ganglion cell layer (GCL) that was closely correlated with the duration of diabetes.11 This study showed damage of the inner nuclear layer even though the vascular signs of diabetic retinopathy were minimal. These findings suggest the need for better understanding of the molecular mechanisms of neuronal cell injury in ischemic retinopathy to evaluate their pathophysiologic relationship to the vascular complications. This may identify specific molecular targets for clinical intervention and neuroprotection earlier in the course of the disease.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) is an enzyme that catalyzes the production of superoxide (O2−) from oxygen and NADPH. Studies have shown that superoxide produced by NOX plays a critical role in diverse vascular diseases such as stroke,12,13 cardiovascular disease,14,15 and diabetic microvascular complications.16 Deletion of the NOX2 catalytic subunit, gp91phox, has been shown to reduce inflammation and signs of vascular injury in models of streptozotocin-induced diabetes17,18 and endotoxin-induced uveitis,19 and the inhibition of NOX was shown to reduce retinal neovascularization in a model of retinopathy of prematurity.20 In addition, studies of cerebral ischemia reperfusion (I/R) injury demonstrated that inhibition or deletion of NOX2 resulted in decreased tissue damage, suggesting a pivotal role of NOX2 in neurodegenerative changes associated with brain ischemia.21,22 We therefore hypothesized that NOX2 may play a key pathophysiological role in retinal neuronal cell injury after I/R, and we tested this in an established animal model. Our results demonstrate that neuronal injury in the inner retina induced by I/R is markedly reduced by pharmacologic inhibition of NOX2 and in NOX2 knockout mice. Our data further suggest that the protective effects of NOX2 inhibition may be mediated by blocking I/R-induced activation of the proapoptotic mediators NF-κΒ, p65, and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK), each of which is known to be activated by severe oxidative stress.23–25
Methods
Treatment of Animals
All procedures with animals were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the institutional animal care and use committee (Animal Welfare Assurance no. A3307–01). All surgery was performed under anesthesia, and all efforts were made to minimize suffering. Transient retinal ischemia was applied to wild-type C57BL6 (WT) and NOX2-deficent (NOX2−/−; Jackson Laboratories, Bar Harbor, ME) mice. Mice were anesthetized with tribromoethanol (Avertin, 0.5 g/kg, intraperitoneally), pupils were dilated with 1% tropicamide (Akorn, Lake Forest, IL), and topical anesthesia (1 drop of proparacaine hydrochloride (Akorn) was applied to cornea). The anterior chamber of the right eye was cannulated with a 30-gauge needle attached to a line infusing sterile saline. The intraocular pressure (IOP) was raised to 110 mm Hg by elevating the saline reservoir. Ischemia was confirmed by whitening of the anterior segment of the globe and blanching of the episcleral veins.26 After 40 minutes of ischemia, the needle was withdrawn, and reperfusion was confirmed by observation of the episcleral veins. The left eye underwent sham surgery, in which the needle was inserted into the anterior chamber without elevating the IOP. The mice were killed at various times after I/R, and their retinas were prepared as described below.
Apocynin Treatment for WT I/R
Apocynin (4-hydroxy-3-methoxy-acetophenone; Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline. Apocynin was injected intraperitoneally (10 mg/kg/d) 30 minutes before the surgery and again once daily until the end of the experiment.
Quantitative RT-PCR
Total RNA was isolated using a DNA-free RNA isolation kit (RNAqueous-4PCR Kit; Applied Biosystems, Austin, TX) according to the manufacturer's instructions. Total RNA was reverse transcribed with M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) to generate cDNA. Quantitative PCR was performed using a PCR system (StepOne; Applied Biosystems) with master mix (Power SYBR Green; Applied Biosystems). The fold difference between levels of various transcripts was calculated by the CT method using Hprt as the internal control. After PCR, a melting curve was constructed in the range of 60°C to 95°C to evaluate the specificity of the amplification products. Primer sequences are listed in Table 1.
Table 1.
Primers Used for q-PCR
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| NOX1 | CCAATGTGGGACAATGAGTTTTC | AACCCCCACCGCAGACTT |
| NOX2 | TCAAGACCATTGCAAGTGAACAC | TCAGGGCCACACAGGAAAA |
| NOX3 | TCTCCGGCTGCACAATGTC | CTGCCTGCCATTCAGCATAG |
| NOX4 | AGCATCTGCATCTGTCCTGAAC | ACTGTCCGGCACATAGGTAAAAG |
| p22phox | GGCTACTGCTGGACGTTTCAC | GCACACCTGCAGCGATAGAG |
| p47phox | CGGATGGCACAAAGGACAAT | ACCGCGGGCTGTGGTT |
| Hprt | GAAAGACTTGCTCGAGATGTCATG | CACACAGAGGGCCACAATGT |
Reactive Oxygen Species Formation
Superoxide production was evaluated in retinal frozen sections by the dihydroethidium (DHE) method, as described previously.20,27 Briefly, frozen sections were preincubated in NADPH (100 μM) or NADPH with PEG-SOD (400 U/mL) or apocynin (1 mM) for 20 minutes, followed by reaction with DHE (2 μM) for 20 minutes at 37°C. DHE is oxidized on reaction with superoxide to ethidium bromide, which binds to DNA in the nucleus and fluoresces red.28 DHE images from serial sections treated with or without inhibitors were obtained using an fluorescence microscope (AxioVision; Carl Zeiss, Thornwood, NY). DHE was excited at 488 nm with an emission spectrum of 610 nm. The images were analyzed for reaction intensity (Metamorph Image System; Molecular Devices, Downingtown, PA). Peroxynitrite (ONOO−) is a short-lived molecule at physiological pH, but it has been shown to nitrate protein tyrosine residues. Therefore, ONOO− formation was indirectly detected by Western blot analysis with a monoclonal anti–nitrotyrosine antibody (Cayman Chemical Co., Ann Arbor, MI).
Evaluation of Neuronal Cell Loss
Cell death was quantified by TUNEL (Millipore, Billerica, MA) assay using cryosections (10 μm) prepared from retinas collected 3 days after I/R, according to the manufacturer's protocol. Quantification of TUNEL-positive cells was performed manually on images extending from the optic disc to the ora serrata. A minimum of four sections (20 μm apart) per animal was used. Some frozen sections from each group were stained with hematoxylin eosin for evaluation of retinal morphology.
Surviving neurons within the GCL were quantified by confocal imaging of the GCL in retinal wholemount preparations double labeled with the neuronal cell marker NeuN and the vascular marker isolectin B4. Eyes were collected 7 days after I/R or sham surgery and were fixed overnight in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at 4°C. The retinas were dissected, washed with PBS, incubated in blocking solution (1% triton, 10% normal goat serum in PBS, 30 minutes), followed by labeling with anti-NeuN (Millipore) and Alexa fluor 594-isolectin B4 (Griffonia simplicifolia; Invitrogen). After washing with PBS, the retinas were incubated in anti-mouse IgG conjugated with FITC (1:400) for 1 hour at room temperature, washed in PBS, flatmounted, coverslipped, and imaged using a confocal microscope (LSM 510; Carl Zeiss). The GCL was identified by its proximity to the inner retinal vessels. Then five serial confocal images separated by 1 μm were taken of the NeuN-positive GCL neurons, and the images were merged to generate well focused images. Ten images were taken in the midperiphery of each retina using a 20× objective lens.29 The cells were counted using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) after image thresholding and manual exclusion of artifacts. The number of NeuN-positive cells in the GCL in I/R eyes was expressed as a ratio to the number of NeuN-positive cells in the contralateral sham surgery eye, as previously described.30
Immunohistochemistry
Eyes were enucleated, fixed in 4% PFA (overnight, 4°C), and washed in PBS, and retinas were isolated and cryoprotected in 30% sucrose. Cryostat sections (10 μm) were collected, permeabilized with 1% Triton (20 minutes), and blocked in 10% normal goat serum (1 hour). Sections were then incubated overnight in primary antibody GFAP at 4°C (1:100; Sigma-Aldrich) or p-ERK (1:200; Cell Signaling Technology, Beverly, MA). On the next day, they were incubated (1 hour) in fluorescein-conjugated secondary antibody (1:200; Molecular Probes), washed in PBS, and mounted with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA).
Western Blot Analysis
Retinal homogenates were prepared using RIPA buffer (Millipore) containing protease and phosphatase inhibitors (Complete Mini and phosSTOP, respectively; Roche Applied Science, Indianapolis, IN). Proteins were separated on SDS-PAGE and transferred onto nitrocellulose membranes (Millipore), blocked in 5% milk or 3% BSA in TBST (Tris-buffered saline with 0.5% Tween-20). The membrane was incubated overnight (4°C) in primary antibodies diluted in blocking solution consisting of NOX2 (1:500; Abcam, Cambridge, MA), NF-κB p65 (1:1000; Cell Signaling Technology), p-P65 (Ser536, 1:1000, Cell Signaling Technology), ERK (1:5000; Cell Signaling Technology), p-ERK (Thr202/Tyr 204, 1:5000; Cell Signaling Technology), c-jun N-terminal kinase (JNK; 1:1000; Cell Signaling Technology), p-JNK (Thr183/Tyr185, 1:1000; Cell Signaling Technology), p38 MAPK (1:1000; Cell Signaling Technology), p-p38 MAPK (Thr180/Tyr182, 1:1000; Cell Signaling Technology), GFAP (1:1000; Sigma-Aldrich), and tubulin (1:10,000; Sigma-Aldrich). The next day, the membrane was washed in TBST, followed by horseradish peroxidase-conjugated secondary antibody (1:10,000 for tubulin, 1:5000 for ERK and p-ERK, 1:2000 for the other antibodies). Immunoreactive proteins were detected using the enhanced chemiluminescence system (GE Healthcare Bio-Science Corp., Piscataway, NJ).
Statistical Analysis
Results were expressed as mean ± SEM. Statistical analysis was performed with one-way or two-way ANOVA followed by Tukey test for multiple comparisons. In case of single comparison, the Student's t-test was applied. P ≤ 0.05 was considered statistically significant.
Results
Elevation of NOX2 NADPH Oxidase Expression and Activity during Retinal I/R Injury
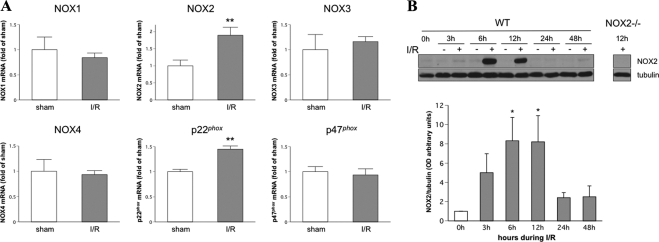
Studies have shown that oxidative stress is a key player in retinal neuronal injury in models of ischemia-reperfusion.31–33 NADPH oxidase is a major source of ROS in vascular cells. To understand the potential role of NADPH oxidase in neuronal injury in retinal I/R, the expression of different NOX isoforms and p47phox and p22phox subunits was analyzed at 6 hours after retinal ischemic injury. This analysis showed that the NOX2 catalytic membrane subunit of NADPH oxidase and the associated membrane subunit p22phox were significantly increased after retinal I/R. In contrast, there were no changes in the expression of the NOX1, NOX3, or NOX4 catalytic subunit isoforms or the p47phox cytosolic subunit (Fig. 1A). Correlated with the NOX2 mRNA increase, Western blot analysis also revealed a time-dependent increase in NOX2 protein after retinal I/R injury. NOX2 protein was increased as early as 3 hours and reached maximum levels at 6 and 12 hours. After 24 hours, NOX2 in the I/R retina returned to levels in the sham controls (0 hour). This result suggested that increases in NOX2 NADPH oxidase may be involved in retinal I/R injury.
Figure 1.
Expression of NOX2 and p22phox was increased during I/R. WT mice underwent I/R or sham surgery, and retinas were collected after 3, 6, 12, 24, and 48 hours. (A) The mRNA levels of different NOX subtypes, p47phox, and p22phox subunits were quantified at 6 hours after retinal ischemic injury by q-PCR and were normalized to WT sham surgeries (n = 4; **P < 0.01 vs. WT sham). (B) NOX2 protein levels were determined at different time points during I/R injury (n = 8; *P < 0.05 vs. 0 hour).
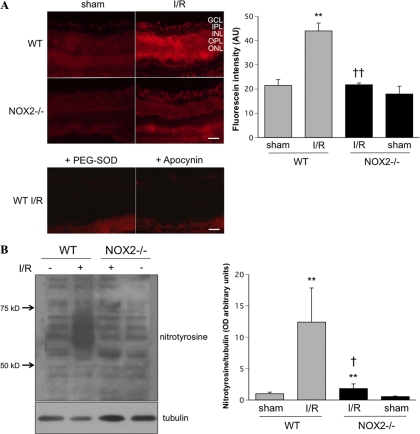
The rapid increase in NOX2 expression after I/R suggested a role for this protein in I/R-induced oxidative stress. We tested this by determining the effect of NOX2 deletion on the I/R-induced formation of superoxide and peroxynitrite. Superoxide formation was evaluated by DHE imaging. When incubated with substrate in snap-frozen tissues or living cells, DHE was oxidized by superoxide to form ethidium, which bound to DNA and fluoresced red. Figure 2A shows representative images and quantitative analysis of the DHE reaction. Imaging of the WT I/R retina showed increased DHE reaction in the GCL, inner nuclear layer, and outer nuclear layer. The DHE-superoxide reaction was largely prevented in the NOX2-deficient retina; ethidium staining was similar to that in WT sham retina. Furthermore, in control studies using sections from the WT I/R retina preincubated with the NADPH oxidase inhibitor apocynin or superoxide scavenger PEG-SOD, the DHE reaction was reduced to levels similar to those of the sham control retina, indicating specificity of the reaction. To further confirm the inhibitory effect of NOX2 deletion on ROS production during I/R, we assessed formation of the peroxynitrite biomarker nitrotyrosine by Western blot analysis (Fig. 2B). This analysis showed that nitrotyrosine immunoreactivity was dramatically increased in WT I/R retina, whereas NOX2 deletion blocked this effect.
Figure 2.
I/R induced-ROS formation was blocked by NOX2 deletion. (A) DHE imaging of superoxide formation at 6 hours after I/R showed increased DHE reaction in WT I/R mice. This effect was blocked in the NOX2-deficient mice and when sections were pretreated with SOD (400 U/mL) or apocynin (1 mM) (n = 4; **P < 0.01 vs. sham; ††P < 0.01 vs. WT I/R). (B) Western blot analysis shows a marked increase of nitrotyrosine formation in the WT I/R retina compared with WT sham. Knocking out NOX2 blocks this I/R effect (n = 4; **P < 0.01 vs. sham; †P < 0.05 vs. WT I/R). Scale bar, 50 μm. GCL, ganglion cell layer; IPL, inner plexiform layer, INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
Protection of the Inner Retina from I/R Injury by Apocynin Treatment or NOX2 Deletion
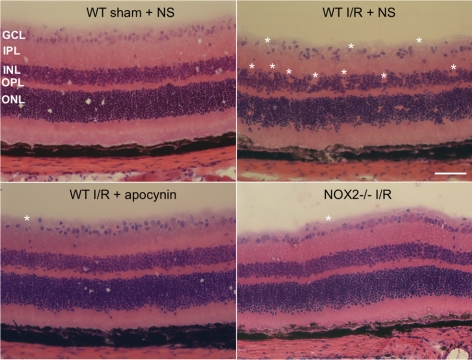
Thus, both the expression and the activity of NOX2 NADPH oxidase are increased by I/R injury. To evaluate the potential involvement of NOX2 activity in I/R-induced tissue damage, we analyzed the effects on retinal morphology of NOX2 deletion and apocynin treatment. Apocynin blocked the activity of NADPH oxidase by preventing assembly of the NADPH oxidase cytosolic subunits (p40phox, p47phox, and p67phox).34,35 As illustrated in Figure 3, the WT I/R retina treated with vehicle showed marked distortion of the inner retina and an apparent decrease in the number of GCL neurons at 7 days after retinal ischemic injury compared with the WT sham retina treated with vehicle. By contrast, the retinal morphology in the apocynin-treated WT I/R and NOX2−/− I/R retinas was similar to that in the WT sham retina.
Figure 3.
Apocynin treatment and NOX2 deletion preserve retinal morphology during I/R. Imaging of hematoxylin and eosin–stained retinal sections from I/R mice shows loss of the cells in the GCL and inner nuclear layer (*areas of distortion/missing cells) at 7 days after I/R in WT mice. This effect of I/R is markedly reduced in WT mice treated with apocynin and in NOX2−/− mice. Scale bar, 50 μm. NS, normal saline; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
Reduction in Retinal Cell Death, Improved Neuronal Survival, and Reduced Glial Activation by NOX2 Deletion
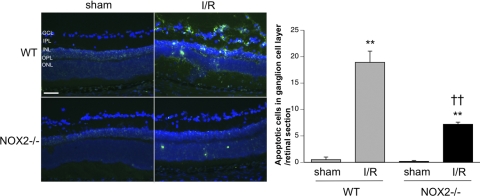
The improved retinal morphology in the apocynin-treated or NOX2−/− I/R mice suggested that NOX2 plays an important role in retinal injury during I/R. To test this, we used TUNEL labeling to assess retinal cell death in the WT and NOX2−/− I/R retinas. Figure 4A shows that TUNEL-positive cells were abundant in the WT I/R retina at 3 days after I/R injury and were localized mainly in the GCL and the inner nuclear layer. TUNEL-positive cells in the outer nuclear layer were relatively sparse compared with the inner retina, which is consistent with the reported sensitivity of the inner retinal layers to I/R injury.36 In contrast with the WT I/R retina, TUNEL-positive cells in the NOX2−/− I/R mice were few. Figure 4B summarizes the results of the quantitative analysis of these data. The number of TUNEL-positive cells in the GCL of the WT I/R retina was nearly 20-fold greater than that in the sham controls (P < 0.01). This increase was largely blunted in the NOX2−/− I/R retina and was reduced to roughly one-third of the levels observed in the WT I/R retinas.
Figure 4.
NOX2 deletion reduces neuronal death after I/R. TUNEL labeling of retinal sections 3 days after ischemic injury shows that cells with fragmented DNA are increased in the WT I/R retina. NOX2 deletion decreases the number of TUNEL-positive cells (**P < 0.01 vs. sham; ††P < 0.01 vs. WT I/R). Scale bar, 50 μm. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
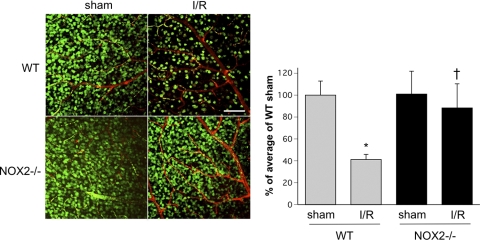
Loss of neurons within the GCL is a hallmark of retinal I/R injury.36 To assess the involvement of NOX2 in this aspect of the pathology, we next determined the effects of NOX2 deletion on the survival of GCL neurons. For this we performed confocal image analysis to quantify the number of NeuN-positive cells within the GCL in flatmounted retinas at 7 days after I/R. This analysis showed that the density of NeuN-positive GCL neurons in the WT I/R retina was markedly reduced compared with the contralateral sham control retina, whereas the density of GCL neurons in the NOX2−/− I/R retina was nearly normal (Fig. 5). Quantitation of the surviving GCL neurons in the WT I/R retina showed a 42% decrease relative to the sham control compared with only 14% in the NOX2−/− I/R retina. This indicated that the deletion of NOX2 significantly decreased the loss of GCL neurons caused by I/R (P < 0.01).
Figure 5.
Neuronal cell density in the GCL is preserved in the NOX2−/− retina during I/R. Confocal imaging of flatmounted retina labeled with NeuN antibody at 7 days after I/R shows a prominent decrease in density of NeuN-positive cells in the GCL of the WT I/R retina compared with the WT sham retina. NOX2 deletion significantly decreased the loss of NeuN-positive GCL neurons after I/R (n = 4; *P < 0.05 vs. sham; †P < 0.05 vs. WT I/R). Scale bar, 100 μm.
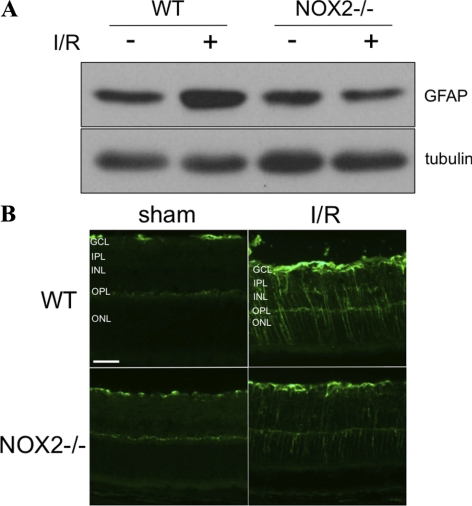
Glial cell activation is another prominent feature of retinal I/R injury, diabetic retinopathy, and other forms of ischemic retinopathy.4–6 To see whether NOX2 plays a role in this aspect of the retinal injury, we examined the expression of glial fibrillary acidic protein (GFAP), which is known to increase in activated glia. As is shown in Figure 6A, total levels of GFAP protein were markedly increased in the WT retina at 5 days after I/R compared with the WT sham control retina. By contrast, GFAP levels in the NOX2−/− I/R retina were similar to those in the WT and NOX2−/− sham retinas. Figure 6B shows that GFAP immunoreactivity in the WT I/R retina was localized to filamentous processes in the nerve fiber layer and radial processes extending from the nerve fiber layer to the outer limiting membrane, corresponding to the distribution of astrocytes and Müller cells, respectively. GFAP immunolabeling in the radial Muller cell processes of the NOX2−/− I/R retinas was much weaker than that in WT I/R retinas, suggesting that Müller cell activation was reduced.
Figure 6.
NOX2 deletion reduces glial activation during I/R injury. (A) Western blot analysis shows increases in GFAP at 5 days after ischemic injury that was reduced by NOX2 deletion. (B) Fluorescent microscopic imaging of retinal sections labeled with GFAP. Scale bar, 50 μm. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
Inhibition of ERK and NF-κB p65 Activation by NOX2 Deletion
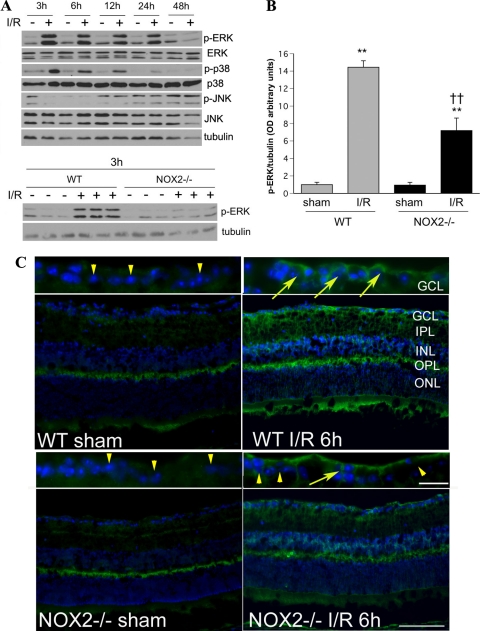
Activation of MAPKs, such as ERK, p38, and JNK has been reported during retinal I/R.37 MAPKs are redox sensitive, and inhibition of ERK and p38 has been shown to limit neurodegeneration.37 Thus, we performed Western blot analysis to see whether the protective effects of NOX2 deletion could involve effects on MAPK activation. This analysis showed that levels of phosphorylated ERK (p-ERK) were markedly increased as early as at 3 hours after I/R and declined to normal levels by 48 hours (Fig. 7A). The increase in p-ERK induced by I/R was significantly attenuated in the NOX2−/− retina (Fig. 7B; P < 0.01). Further analysis of p-ERK formation using immunolabeling of retinal cryosections showed that immunoreactivity for p-ERK was markedly increased in the GCL, inner nuclear layer, and both plexiform layers of the WT I/R mice and that the deletion of NOX2 partially blocked this effect (Fig. 7C). Western blot analysis of p-p38 formation in the WT I/R samples showed a pattern similar to that seen for p-ERK (Fig. 7A). Blots prepared from the NOX2−/− I/R samples showed a trend similar to that seen for p-ERK, but the results were variable and the group differences were not statistically significant (data not shown). Analysis of p-JNK showed no significant alterations in the I/R samples compared with the sham controls (Fig. 7A).
Figure 7.
NOX2 deletion reduces the phosphorylation of ERK during I/R. (A) Western blot analysis shows a time-dependent increase in p-ERK and p-p38 in WT I/R retina, whereas p-JNK is not altered. (B) Densitometric analysis confirms a significant increase in p-ERK in the WT I/R retina and shows that this increase is markedly attenuated by NOX2 deletion (n = 3; **P < 0.01 vs. sham. ††P < 0.01 vs. WT I/R). (C) Immunohistochemical analysis of the WT I/R retina shows increases in p-ERK immunoreactivity in neurons within the GCL (arrows, inset; scale bar, 20 μm), the inner nuclear layer (INL), the inner plexiform layer (IPL), and the outer plexiform layer (OPL). In the NOX2−/− IR retina, p-ERK immunoreactivity is reduced compared with WT I/R samples. Many neurons in the GCL of NOX2−/− I/R and sham retinas are negative for p-ERK (arrowheads). Scale bar, 100 μm. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
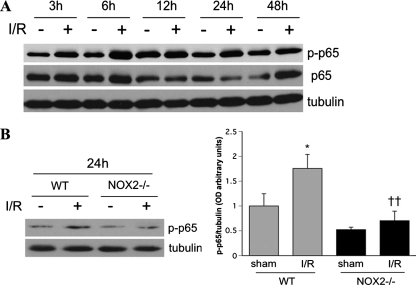
The transcription factor nuclear factor κB (NF-κB) has also been shown to be redox sensitive and to play a critical role in the signaling events that lead to neuronal cell death during I/R in both retina and brain.23,29 Therefore, we determined the effects of NOX2 deletion on the phosphorylation of NF-κB subunit p65, which has been shown to be activated during retinal I/R injury.38 This analysis confirmed a time-dependent increase in the phosphorylation of p65 (p-p65; Fig. 8A). The levels of p-p65 were increased by 3 hours after I/R and remain elevated for 24 hours. This increase in p-p65 was largely suppressed by NOX2 deletion (Fig. 8B; P < 0.01).
Figure 8.
NOX2 deletion reduces the phosphorylation of NF-κΒ (p65) during I/R. (A) Western blot analysis shows a time-dependent increase in phosphorylated NF-κβ (p-p65) at 3, 6, 12, 24, and 48 hours after ischemic injury I/R. (B) Densitometric analysis shows that NOX2 deletion suppresses p-p65 formation relative to the WT I/R samples (*P < 0.05 vs. sham; ††P < 0.01 vs. WT I/R).
Discussion
In the present study we examined the role of NOX2 in neuronal cell death in a model of retinal I/R injury. The results of this study showed that I/R injury of WT retinas induced the activation of ERK and NF-κB p65 that was correlated with retinal cell death, as indicated by the presence of numerous TUNEL-positive cells in the inner retina and a 60% decrease in neuronal cell density within the GCL. These effects were blocked by NOX2 deletion, demonstrating the role of the NOX2 NADPH oxidase in I/R-induced injury of the inner retina.
We previously reported that ROS generated by NOX2 play a key role in vascular inflammation and breakdown of the blood retinal barrier during diabetes.16,17 However, thus far no studies have elucidated the role of NOX2 in neuronal cell death and generation of ROS in ischemic retinal disease. Our q-PCR analysis clearly demonstrated that NOX2 and the membranous subunit p22phox were upregulated by I/R injury. Furthermore, our DHE imaging studies showed a prominent increase in superoxide formation in the I/R retina that was almost completely blocked by deletion of the NOX2 gene, indicating that NOX2 is a major source of oxidative stress in this model. Western blot analysis of the peroxynitrite biomarker nitrotyrosine showed numerous intensely reactive protein bands that were markedly increased by I/R. This effect was also largely attenuated in the NOX2-deficient retina, further confirming NOX2 as a source of ROS. Further work is needed to identify the protein species upregulated during I/R injury and to determine their specific involvement in neuronal cell injury. However, previous studies have shown that immunoreactivity for nitrotyrosine is significantly increased in neurons within the GCL at 6 hours after I/R, suggesting that peroxynitrite-induced tyrosine nitration could have a role in the neuronal response to ischemic injury.39
In the present study, numbers of TUNEL-positive cells were clearly increased 3 days after I/R surgery, implying the production and release of factors to promote cell death. Our experiments demonstrated that the deletion of NOX2 reduced TUNEL labeling and increased neuronal survival in the GCL, indicating that NOX2-derived ROS have an important role in the neuronal death process. A potential link between NADPH oxidase activity and neuronal cell loss has been described in previous studies, which showed that knocking out NOX2 or inhibiting NADPH oxidase with apocynin is protective against neuronal cell degeneration caused by I/R in the brain.21,22 To our knowledge, our study is the first to demonstrate that NOX2 deletion has an inhibitory effect on neuronal cell death during ischemic retinal disease. We did not identify specific types of GCL neurons damaged by the I/R, but it is likely that both ganglion and displaced amacrine cells are affected by the ischemic injury.
Glial activation is thought to be an indicator of retinal injury during stress and disease conditions such as I/R, hypoxia, and diabetes.4–6 Increased immunoreactivity for GFAP is a well-known marker for glial activation. Our data clearly show that I/R injury results in upregulation of GFAP in Müller cells and that deletion of NOX2 abrogates this effect. Wurm et al.40 have reported that I/R injury causes prominent activation of Müller cells. Moreover, their study demonstrated that the dysfunction of Müller cells plays an important role in retinal edema caused by I/R. Glial activation has also been described in models of diabetic retinopathy, and studies using retinas from diabetic patients and rats have shown that GFAP immunoreactivity is markedly increased in Müller cells.41,42 Although neither the previous work on diabetic retinopathy nor our present study on I/R has shown a direct relationship between Müller cell activation and ganglion cell death, it can be said that Müller cell activation is a feature of both I/R injury and DR. The present study is the first to demonstrate that the deletion of NOX2 decreases Müller cell activation in I/R, suggesting that inhibiting NOX2 may reduce retinal edema and limit injury of the inner retinal neurons.
Multiple pathways have been suggested to play a role in ganglion cell death induced by I/R, including apoptosis,36,43–45 necrosis,46,47 and a cell death hybrid process termed necroptosis .48 Our present study showed that NOX-dependent activation of ERK and NF-κB p65 is involved in this process. ERK is a classical MAPK and is involved in multiple processes, including proliferation,49 inflammation,50,51 and apoptosis.37,52 Roth et al.37 showed that inhibiting ERK during I/R in mice reduced neuronal cell death and blocked morphologic changes. Furthermore, ERK activation was reported to occur after I/R injury in the brain, indicating an important role of ERK activation in the ischemia-induced neuronal cell death. Although the mechanism underlying neuronal cell death after ERK activation is not completely understood, it is likely that ERK plays a key role in neuronal cell death in the ischemic retina.
NF-κΒ is a redox-sensitive transcription factor that plays a critical role in neuronal cell death in ischemic injury in both brain and retina1,23,29,53–55 The function of NF-κΒ depends on the combination of its subunits p50, p65, and cRel; p65 activation has been shown to play a critical role in I/R-induced neuronal cell death54,55 Western blot analysis demonstrated that NF-κΒ p65 is activated from 3 hours to 48 hours after I/R injury. Furthermore, NOX2 deletion reduced the I/R-induced activation of NF-κΒ to control levels, indicating that ROS generated by NOX2 play a critical role in the activation of NF-κΒ during I/R. Our previous study showed that the inhibition of NOX by apocynin reduced the activation of NF-κΒ induced by TNF-α and resulted in a reduction of proinflammatory cytokine release by human umbilical endothelial cells.19 Of particular interest, previous studies showed that selective inactivation of NF-κΒ in astrocytes was able to prevent retinal ganglion cell death caused by I/R through a decrease in NOX2-mediated ROS and inflammation.29 Taken together these observations suggest that the generation of ROS by NOX2 plays a key role in neuronal cell death in the ischemic retina by a mechanism involving redox-mediated activation of NF-κΒ.
Although our present study clearly demonstrates a critical role of NOX2 in neuronal cell death in I/R, further work is needed to identify the specific cellular source or sources of NADPH oxidase activity responsible for the pathology. Studies have shown that the expression of NOX2 is upregulated in retinal endothelial cells in diabetic mice,16 in astrocytes during retinal I/R,29 and in neurons in a model of I/R injury in the brain.56 Thus, it seems likely that ROS produced by multiple cell types could be involved in this process.
In summary, our present study suggests that during acute ischemic injury of the retina, ROS derived from NOX2 NADPH oxidase cause ganglion cell death by a process involving the activation of ERK, MAPK and NF-κB. Inhibition of NOX2 NADPH oxidase may offer a novel therapeutic strategy for the treatment or prevention of neuronal and vascular injury during ischemic retinopathy.
Footnotes
Supported by Georgia Health Sciences University Vision Discovery Institute (SEB, RBC); National Eye Institute Grants R01 EY04618 (RBC) and R01 EY11766 (RBC, RWC); Veterans Administration MRA (RBC); National Heart, Lung, and Blood Institute Grant R01 HL70215 (RWC); American Heart Association Grant 11SDG4960005; and Juvenile Diabetes Research Foundation JDRF Grant 10-2009-575 (WZ).
Disclosure: H. Yokota, None; S.P. Narayanan, None; W. Zhang, None; H. Liu, None; M. Rojas, None; Z. Xu, None; T. Lemtalsi, None; T. Nagaoka, None; A. Yoshida, None; S.E. Brooks, None; R.W. Caldwell, None; R.B. Caldwell, None
References
- 1. Nagai N, Izumi-Nagai K, Oike Y, et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–4350 [DOI] [PubMed] [Google Scholar]
- 2. Esser P, Bresgen M, Fischbach R, Heimann K, Wiedemann P. Intercellular adhesion molecule-1 levels in plasma and vitreous from patients with vitreoretinal disorders. Ger J Ophthalmol. 1995;4:269–274 [PubMed] [Google Scholar]
- 3. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 4. Yoshida S, Yoshida A, Ishibashi T. Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol. 2004;242:409–413 [DOI] [PubMed] [Google Scholar]
- 5. Dyer MA, Cepko CL. Control of Muller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3:873–880 [DOI] [PubMed] [Google Scholar]
- 6. Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980 [PubMed] [Google Scholar]
- 7. Villarroel M, Ciudin A, Hernandez C, Simo R. Neurodegeneration: an early event of diabetic retinopathy. World J Diabetes. 2010;1:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Invest. 1998;102:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohr S, Xi X, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–1179 [DOI] [PubMed] [Google Scholar]
- 10. Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45:2760–2766 [DOI] [PubMed] [Google Scholar]
- 11. van Dijk HW, Verbraak FD, Kok PH, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:3660–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostrowski RP, Tang J, Zhang JH. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 2006;37:1314–1318 [DOI] [PubMed] [Google Scholar]
- 13. Didion SP, Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke. 2003;34:2038–2042 [DOI] [PubMed] [Google Scholar]
- 14. Qin F, Simeone M, Patel R. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic Biol Med. 2007;43:271–281 [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Zalewski A, Liu Y, et al. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–478 [DOI] [PubMed] [Google Scholar]
- 16. Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Shabrawey M, Rojas M, Sanders T, et al. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49:3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagaoka T, Kuo L, Ren Y, Yoshida A, Hein TW. C-reactive protein inhibits endothelium-dependent nitric oxide-mediated dilation of retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci. 2008;49:2053–2060 [DOI] [PubMed] [Google Scholar]
- 19. Zhang W, Rojas M, Lilly B, et al. NAD(P)H oxidase-dependent regulation of CCL2 production during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:3033–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167:599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen H, Kim GS, Okami N, Narasimhan P, Chan PH. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis. 2011;42:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim GS, Jung JE, Niizuma K, Chan PH. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci. 2009;29:14779–14789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crack PJ, Taylor JM, Ali U, Mansell A, Hertzog PJ. Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke. 2006;37:1533–1538 [DOI] [PubMed] [Google Scholar]
- 24. Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, Pervaiz S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PloS One. 5:e9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Probin V, Wang Y, Zhou D. Busulfan-induced senescence is dependent on ROS production upstream of the MAPK pathway. Free Radic Biol Med. 2007;42:1858–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Da T, Verkman AS. Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Invest Ophthalmol Vis Sci. 2004;45:4477–4483 [DOI] [PubMed] [Google Scholar]
- 27. Rojas M, Zhang W, Lee DL, et al. Role of IL-6 in angiotensin II-induced retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2010;51:1709–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305 [DOI] [PubMed] [Google Scholar]
- 29. Dvoriantchikova G, Barakat D, Brambilla R, et al. Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury. Eur J Neurosci. 2009;30:175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leahy KM, Ornberg RL, Wang Y, et al. Quantitative ex vivo detection of rodent retinal ganglion cells by immunolabeling Brn-3b. Exp Eye Res. 2004;79:131–140 [DOI] [PubMed] [Google Scholar]
- 31. Szabo ME, Droy-Lefaix MT, Doly M, Carre C, Braquet P. Ischemia and reperfusion-induced histologic changes in the rat retina: demonstration of a free radical-mediated mechanism. Invest Ophthalmol Vis Sci. 1991;32:1471–1478 [PubMed] [Google Scholar]
- 32. Nayak MS, Kita M, Marmor MF. Protection of rabbit retina from ischemic injury by superoxide dismutase and catalase. Invest Ophthalmol Vis Sci. 1993;34:2018–2022 [PubMed] [Google Scholar]
- 33. Rios L, Cluzel J, Vennat JC, Menerath JM, Doly M. Comparison of intraocular treatment of DMTU and SOD following retinal ischemia in rats. J Ocul Pharmacol Ther. 1999;15:547–556 [DOI] [PubMed] [Google Scholar]
- 34. Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102 [DOI] [PubMed] [Google Scholar]
- 35. Johnson DK, Schillinger KJ, Kwait DM, et al. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium. 2002;9:191–203 [DOI] [PubMed] [Google Scholar]
- 36. Rosenbaum DM, Rosenbaum PS, Gupta H, et al. The role of the p53 protein in the selective vulnerability of the inner retina to transient ischemia. Invest Ophthalmol Vis Sci. 1998;39:2132–2139 [PubMed] [Google Scholar]
- 37. Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci. 2003;44:5383–5395 [DOI] [PubMed] [Google Scholar]
- 38. Chen YG, Zhang C, Chiang SK, Wu T, Tso MO. Increased nuclear factor-kappa B p65 immunoreactivity following retinal ischemia and reperfusion injury in mice. J Neurosci Res. 2003;72:125–131 [DOI] [PubMed] [Google Scholar]
- 39. Shibuki H, Katai N, Yodoi J, Uchida K, Yoshimura N. Lipid peroxidation and peroxynitrite in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2000;41:3607–3614 [PubMed] [Google Scholar]
- 40. Wurm A, Iandiev I, Uhlmann S, et al. Effects of ischemia-reperfusion on physiological properties of Muller glial cells in the porcine retina. Invest Ophthalmol Vis Sci. 2011;52:3360–3367 [DOI] [PubMed] [Google Scholar]
- 41. Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449 [DOI] [PubMed] [Google Scholar]
- 42. Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes: the Penn State Retina Research Group. Invest Ophthalmol Vis Sci. 2000;41:3561–3568 [PubMed] [Google Scholar]
- 43. Produit-Zengaffinen N, Pournaras CJ, Schorderet DF. Retinal ischemia-induced apoptosis is associated with alteration in Bax and Bcl-x(L) expression rather than modifications in Bak and Bcl-2. Mol Vis. 2009;15:2101–2110 [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–367 [DOI] [PubMed] [Google Scholar]
- 45. Lam TT, Abler AS, Tso MO. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Invest Ophthalmol Vis Sci. 1999;40:967–975 [PubMed] [Google Scholar]
- 46. Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Liposome-delivered ATP effectively protects the retina against ischemia-reperfusion injury. Mol Vis. 2010;16:2882–2890 [PMC free article] [PubMed] [Google Scholar]
- 47. Buchi ER. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study, I: ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992;55:605–613 [DOI] [PubMed] [Google Scholar]
- 48. Rosenbaum DM, Degterev A, David J, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88:1569–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E. Regulation of vascular endothelial growth factor expression by advanced glycation end products. J Biol Chem. 2001;276:43836–43841 [DOI] [PubMed] [Google Scholar]
- 50. Satofuka S, Ichihara A, Nagai N, et al. (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009;58:1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takeda M, Takamiya A, Yoshida A, Kiyama H. Extracellular signal-regulated kinase activation predominantly in Muller cells of retina with endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2002;43:907–911 [PubMed] [Google Scholar]
- 52. Zhou RH, Yan H, Wang BR, Kuang F, Duan XL, Xu Z. Role of extracellular signal-regulated kinase in glutamate-stimulated apoptosis of rat retinal ganglion cells. Curr Eye Res. 2007;32:233–239 [DOI] [PubMed] [Google Scholar]
- 53. Stephenson D, Yin T, Smalstig EB, et al. Transcription factor nuclear factor-kappa B is activated in neurons after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:592–603 [DOI] [PubMed] [Google Scholar]
- 54. Pizzi M, Goffi F, Boroni F, et al. Opposing roles for NF-kappa B/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1beta. J Biol Chem. 2002;277:20717–20723 [DOI] [PubMed] [Google Scholar]
- 55. Pizzi M, Sarnico I, Boroni F, Benetti A, Benarese M, Spano PF. Inhibition of IκBα phosphorylation prevents glutamate-induced NF-κB activation and neuronal cell death. Acta Neurochir Suppl. 2005;93:59–63 [DOI] [PubMed] [Google Scholar]
- 56. Zhang QG, Raz L, Wang R, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836 [DOI] [PMC free article] [PubMed] [Google Scholar]