Abstract
Hexanal, a secondary product of lipid oxidation, was identified as the major volatile aldehyde generated from lipid peroxidation in human milk. Hexanal was quantified in human milk using solid phase microextraction-gas chromatography/flame ionization detection that required correction for recovery based on the fat content of human milk. Alpha-tocopherol was the only tocopherol isomer in human milk found to be significantly correlated with hexanal (R = −0.374, p<0.05) and the total antioxidant capacity of human milk (ORACFl (R = 0.408, p<0.01)). Ascorbic acid content was negatively correlated (R = −0.403, p<0.05) with hexanal, but not to ORACFl in human milk. The effect of Holder pasteurization on oxidative status of human milk was determined using multiple parameters that included, hexanal level and malondialdehyde as markers of lipid oxidation, vitamins C and E content and antioxidant capacity (e.g. ORACFl). Pasteurization did not affect the oxidative status of milk as measured by hexanal level, ORACFl and malondialdehyde content. We conclude that hexanal is a sensitive and useful chemical indicator for assessing peroxidation reactions in human milk and that alpha tocopherol and ascorbic acid are two key antioxidant components in milk that contribute to protection against oxidation of milk lipids.
Keywords: human milk, hexanal, SPME, pasteurization, antioxidant capacity
Introduction
The benefits of breastfeeding to infant health have been partially attributed to the antioxidant activity of human milk.(1,2) The incidence of necrotizing enterocolitis, an ’oxygen radical disease’, for example, is reduced in breastfed infants.(3) Breastfeeding however is not always feasible and mothers can alternatively choose to obtain milk from milk banks. Milk banks are required to pasteurize breast milk to prevent disease transmission and reduce the risk of bacterial contamination.(4,5) Pasteurization utilizes heat that may compromise the oxidative status of milk.(6) For example, lipid oxidation reactions that occur in milk produce a variety of peroxidation products, some of which have been shown to contribute to the development of various ’oxygen radical disease states’ in infants.(3,7) Determining the extent of lipid oxidation as an indicator for oxidative status of human milk is therefore of strategic importance to ensure both safety and quality of mother’s milk.
Lipid peroxidation of human milk has been studied using numerous indices of lipid oxidation, such as the measurement of conjugated dienes, peroxide value and malondialdehyde formation.(8,9) These methods; however, can lack specificity due to interference with non-lipid components in the food matrix, or have variable reactivity that depends on assay conditions.(10) The presence of volatile carbonyl compounds in foods generated from the decomposition of lipid hydroperoxides is an established indicator of lipid oxidation products in some food systems, but has not previously been used to quantitate the onset of lipid oxidation in human milk. Factors that may impede the progression of lipid oxidation in human milk include the presence of antioxidant vitamins, E and C. While we have previously established that α-tocopherol (Toc), the predominant tocopherol isomer present in breast milk, is significantly related to the free radical scavenging capacity of human milk,(11) there is no information on how vitamin E and related tocopherol isomers correlate with specific peroxidation reaction products in human milk.
The objective of this study was to first develop a method for identifying and quantifying the volatile aldehyde content in human milk and then to determine the relative contribution of vitamins E and C in protecting against lipid oxidation in human milk. Furthermore, the effect of pasteurization on the oxidative status of milk was determined using volatile oxidation products as the primary indicator of lipid oxidation, and malonaldehyde content as well as total antioxidant capacity (TAC) using ORACFL.
Materials and Methods
Materials
All reagents were purchased from Sigma-Aldrich, Canada (Oakville, ON, Canada) unless otherwise mentioned. Mature human milk samples, defined as milk obtained two weeks after birth, were collected from ten apparent non-smoking, uniparous mothers over thirty different lactation dates. Participants were instructed to collect a milk sample expressed after five minutes of nursing (hindmilk). The milk was immediately frozen at −80°C upon arrival in the laboratory until analysis, which was analyzed in batches no later than 2 weeks after collection. All the donors gave informed consent to participate in the study. This study was reviewed and approved by the University of British Columbia Ethical Review Board Committee.
Holder pasteurization of milk samples
The Holder pasteurization technique (62.5°C at 30 min), as recommended by the Human Milk Bank Association of North America(4) was used in this study. Aliquots of milk samples were thawed and pasteurized shortly thereafter at 62.5°C for 30 min. Pasteurized milk was immediately chilled and analyzed.
Analysis of fat content and fatty acid composition
Milk fat was measured using a creamatocrit centrifuge (IEC Model MB centrifuge) for 5 min, and the fat content was determined with an IEC micro-capillary reader (International Equipment Company, Needham Heights, MA). Fatty acid analysis of individual milk samples was performed on milk fatty acid methyl esters (FAME), analyzed by GC equipped with a capillary column (30 m × 0.25 mm; 0.25 µm film thickness; liquid phase: J&W DB 23).(12)
Analysis of milk fat volatiles
Thawed milk samples, preconditioned to room temperature were vortexed to ensure homogeneity prior to transfer to a 10 ml vial containing 1.5 g of salt. Samples were equilibrated at 25°C with continuous stirring for 15 min using a micro stir bar. Headspace volatiles were then sampled using SPME fiber (100 µm PDMS, Supelco, Bellefonte, PA) for 20 min followed by injection in splitless mode to Agilent 6890A GC coupled with Agilent 5973 mass selective detector. Adsorbed volatiles were desorbed to a Hewlett Packard HP-5 column (30 m × 0.25 mm i.d., 0.25 µm film thickness) at initial column temperature of 40°C that was held for 2 min, followed by an increase to 60°C at 15°C/min and a further increase to 300°C at 40°C/min, then held for 6 min. The carrier gas was hydrogen set at a flow rate of 40 cm/sec. Injection and detector temperatures were 250°C and 300°C, respectively. GC/MS ionization voltage was set at 70 eV. Identification of volatiles was performed by comparison of mass spectra to those found in Wiley7/NIST05 library database.
To quantify hexanal in milk samples, volatiles were sampled as described above and identified in split less mode using an Agilent 6890A GC-Flame Ionization Detector. External standard curves for hexanal were constructed and 4-heptanone was used as the internal standard. The recoveries of hexanal were made by spiking the milk samples with 0.2 ppm of the aldehyde.
Analysis of malondialdehyde (MDA) in milk
MDA concentrations in raw and pasteurized milk samples were determined as according to the method of Nielsen et al.(13) Milk was mixed with 1% ortho-phosphoric acid, and a 42 mM solution of thiobarbituric acid (TBA). Upon vortexing, samples were heated at 100°C for 1 h and the reaction was stopped by cooling on ice for 10 min. Heated samples were transferred to micro-centrifuge tubes containing 300 µl of 2 N NaOH: MeOH (1:12, v/v) solution and centrifuged for 10 min at 10,000 rpm. The supernatant was filtered through a 0.2 µm Nylon membrane and 50 µl was injected onto HPLC (Agilent 1100, Palo Alto, CA) equipped with a Luna column (5 µm, 150 × 4.6 mm, Phenomenex, Torrance, CA). An isocratic mobile phase consisting of 48% methanol and 52% potassium phosphate buffer (50 mM, pH 6.0) was used to separate the MDA-TBA complex. MDA was quantified at 532 nm, based on external standard 1,1,3,3-Tetraethoxypropane (TEP); which stoichiometrically produced MDA upon acid hydrolysis.
Analysis of tocopherol isomers milk
Toc isomers in human milk were determined using HPLC (Agilent Technology 1100 series, Palo Alto, CA) with fluorescence detection as previously described.(11) Briefly, 500 µl of milk was diluted with 500 µl of water followed by the addition of 500 µl of ethanol to precipitate milk protein. Milk samples were extracted twice with 2 ml of hexane and extracts evaporated under a stream of nitrogen. The residue was reconstituted in 100 µl of methanol, and the sample was filtered through a 0.2 µm Nylon membrane into an amber HPLC vial for injection. Separation of tocopherol isomers was performed using a 3 µm Sphereclone column (150 mm × 4.6 mm, Phenomenex, Torrance, CA) with methanol as mobile phase (1 ml/min). Tocopherol isomers were identified using fluorescence detection (e.g. excitation wavelength = 292 nm; emission wavelength = 330 nm).
Analysis of total ascorbic acid in milk
Total ascorbic acid in milk was determined according to the method of Francis et al.(14) Samples were mixed with one ml of 100 mM DL-dithiothreitol by mechanical inversion for 30 sec in amber microcentrifuge tubes and treated with 400 µl of 0.56% metaphosphoric acid. The mixture was shaken for 30 sec, centrifuged at 10°C, 10,000 rpm and supernatants filtered prior to injection onto HPLC. Ascorbic acid was quantified using a Zorbax RX-C18 5 µm column (250 × 4.6 mm) at 45°C and a mobile phase of 0.1 M NaH2PO4 (pH 2.5) buffer at 1.5 ml/min and ascorbic acid was detected at 254 nm.(14)
Analysis of total antioxidant capacity (TAC)
TAC of human milk was determined using the Oxygen Radical Absorbance Capacity–ORACFL assay.(15) Values were expressed as µmole of Trolox Equivalent/mL of milk sample.
Statistical analysis
Creamatocrit and GC-FID measurements of milk samples were performed in duplicate, while ORACFL assay, MDA analysis, vitamins C and E concentrations in milk were performed in triplicate. A paired t test was performed to identify significant differences in lipid oxidation and antioxidant capacity between pasteurized and raw milk. The Pearson correlation coefficient between antioxidant components and end point measures of oxidative status in milk was determined using Graph-Pad Prism version 3.02 (La Jolla, CA) statistical software.
Results
Fat composition, total ascorbic acid and tocopherol isomers level
The fat content of the milk samples varied considerably (1.5–8.5%) between samples, collected from different mothers, with an overall average of 5.52% calculated for all samples (Table 1). Oleic acid was the predominant fatty acid (34.97 ± 5.21%), followed by linoleic acid (19.01 ± 5.12%) and palmitic acid (15.44 ± 1.39%) in mother’s milk. Linolenic acid constituted less than 2% (e.g. 1.48 ± 0.48%) of the total fatty acids. Alpha tocopherol was the dominant tocopherol isomer, followed by γ- and δ-tocopherol in respective order (Table 1). Ascorbic acid was also present in a wide range of concentrations (e.g. ND–67.95 µg/ml) in different individual mother’s milk samples.
Table 1.
Lipid profile and relevant antioxidant components of human milk samples analyzed in this study†
| Milk Parameter | Mean ± SD | Range |
|---|---|---|
| Fat (%) | 5.52 ± 1.85 | 1.5–8.5 |
| Mono-unsaturated (%) | 42.94 ± 6.38 | 29.92–50.49 |
| Oleic acid | 34.97 ± 5.21 | 26.26–41.78 |
| Poly-unsaturated (%) | 23.88 ± 5.36 | 19.02–33.48 |
| Linoleic acid | 19.01 ± 5.12 | 14.53–28.62 |
| Linolenic acid | 1.48 ± 0.48 | 0.84–2.23 |
| Antioxidant components (µg/mL) | ||
| α-tocopherol | 2.11 ± 0.66 | 1.21–4.62 |
| γ-tocopherol | 0.80 ± 0.28 | 0.26–1.43 |
| δ-tocopherol | 0.14 ± 0.07 | 0.05–0.33 |
| Total ascorbic acid | 21.70 ± 19.29 | 0–67.95 |
† Total number of milk samples (n = 30).
Identification of volatile aldehydes from human milk
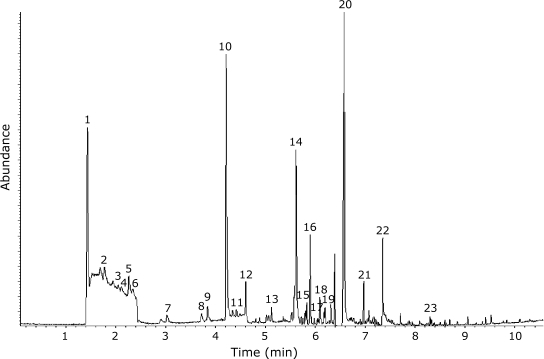
Alkanals identified in human milk included pentanal, hexanal, octanal, nonanal and 2-octenal, and dodecanal (Fig. 1); relative concentrations of each aldehyde varied in individual milk samples. Hexanal, was present in the highest abundance relative to other identified alkanals. Other non-alkanal volatiles identified in human milk included short chain hydrocarbons, chlorinated compounds such as 1-chloropentane and other benzene-containing compounds that were identified to be dichlorobenzene and cyclohexane (Fig. 1).
Fig. 1.
Volatiles identified from milk samples by SPME-GC/MS. Compounds identified are 1. CO2; 2. 2-methyl-2-propanol; 3. Cyclohexane; 4. Hexane; 5. 2-ethoxy-2-methyl-propane; 6. 4-methyl-1-heptanol; 7. Pentanal; 8. 1-chloropentane; 9. 4-methyl-heptane; 10. Hexanal; 11. 2,4-dimethyl-heptane; 12. 2,4-diethyl-1-heptene; 13. Heptanal; 14. Hexanoic acid; 15. Octanal; 16. Dichlorobenzene; 17. 3-ethyl-2-methyl-1,3-hexadiene; 18. 2-octenal; 19. Nonanal; 20. Octanoic acid; 21. 1,4-di-tert-butyl-benzene; 22. Decanoic acid; 23. Dodecanal.
Quantification of alkanals from milk samples
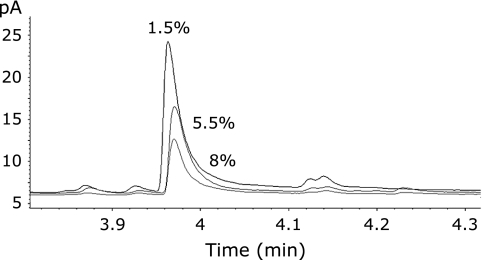
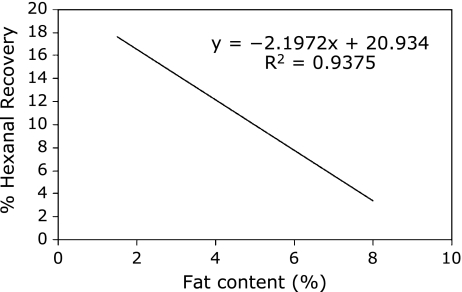
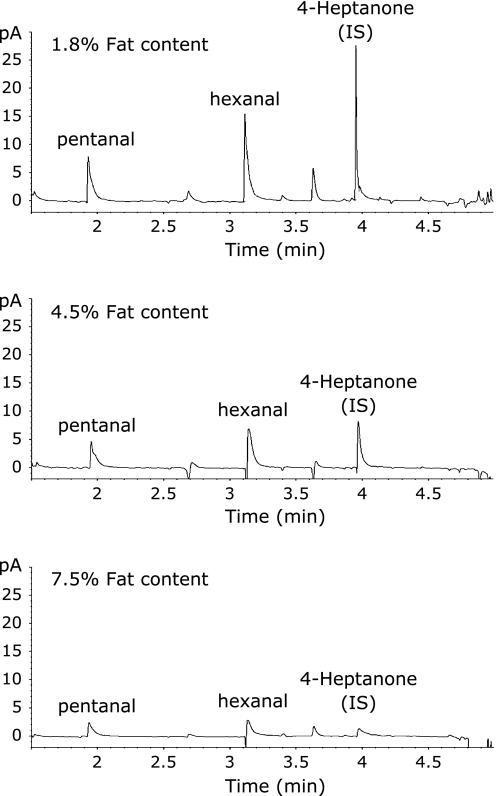
The use of 4-heptanone as internal standard for the quantification of alkanals from human milk was found to be unsuccessful, largely due to inconsistent recovery of 4-heptanone in all milk samples and poor relationship to the recovery of hexanal (Fig. 2). Preliminary experiments determined that the recovery of 4-heptanone from human milk was correlated with the fat content (R = −0.792, p<0.05), but not with the protein content (R = 0.2506, p>0.05). Similar incomplete recoveries of pentanal and hexanal when spiked at 0.2 ppm to milk were found in milk samples that had high fat content (Fig. 3). The inverse relationship between fat content and the recovery of the alkanals followed a linear trend for hexanal (R = −0.968, p<0.05, Fig. 4). To quantify hexanal therefore, we chose to determine the recovery rate of hexanal rather than the internal standard as the degree of recovery may differ depending on the properties of the aldehyde. Quantification of hexanal in mother’s milk was made from the area under the curve derived from the chromatogram corrected for the percent recovery of hexanal based on the fat content of milk using a linear regression (y = −0.21975x + 20.935) that equated the fat content of milk with the percent recovery of hexanal, where y is the % recovery of the hexanal, and × is the fat content (%). The average concentration of hexanal obtained from the external standard curve and corrected for recovery in all human milk samples was 0.285 ± 0.230 ppm. The limit of detection for hexanal using SPME-GC/FID was determined to be 0.14 ppt.
Fig. 2.
Results of 4-heptanone (internal standard) recovered from representative milk samples chosen with distinct fat contents. Milk samples were spiked with 0.2 ppm internal standard.
Fig. 3.
Regression analysis of the recovery of hexanal from human milk affected by fat content. Milk samples were spiked with 0.2 ppm hexanal for recovery purposes.
Fig. 4.
Recovery of pentanal, hexanal and 4-Heptanone (internal standard) in milk samples of varying fat content as determined using GC-FID. The peaks are obtained by subtracting the chromatogram obtained for milk spiked with 0.2 ppm of internal standard with the chromatogram of unspiked milk.
Relationship of antioxidant components to oxidative status of milk
To determine the possible contribution of specific antioxidant components present in human milk to protect against lipid oxidation, we determined the hexanal concentrations in milk, normalized for fat content, and then correlated this parameter with the concentration of individual tocopherol isomers or vitamin C. Amongst all tocopherol isomers, only α-tocopherol was significantly, and inversely, correlated with the concentration of hexanal in milk fat (Table 2). Ascorbic acid content in milk was also inversely related with hexanal concentration (R = –0.403, p<0.05; Table 2). No significant relationship was found between MDA content and any of the antioxidant components investigated in mother’s milk samples. ORACFL values, were significantly (p<0.05) correlated only with α-Toc levels (R = 0.408, p<0.01), with no significant relationship observed with total ascorbic acid concentration.
Table 2.
Correlation of lipid oxidation products with antioxidant parameters in human milk‡
| Lipid oxidation products | α-Toc | γ-Toc | δ Toc | vit. C | ORACFL |
|---|---|---|---|---|---|
| Hexanal | –0.374* | –0.026 | 0.143 | –0.403* | 0.169 |
| MDA | –0.304 | –0.161 | –0.001 | –0.525 | 0.556 |
*denotes significant correlation at p<0.05 (n = 30). ‡Hexanal and MDA variables were adjusted to the fat content of the respective milk samples.
Effect of pasteurization on oxidative status of milk
Holder pasteurization of human milk did not significantly affect the antioxidant capacity of human milk as determined by the ORACFL assay (Table 3). The average concentration of hexanal and MDA in all human milk samples was also not significantly altered by pasteurization (Table 3). However, higher hexanal concentrations were observed in two individual human milk samples following pasteurization that showed no effect on MDA content. Of particular interest was the observation that two milk samples which had relatively high concentrations of hexanal also contained extremely low vitamin C contents (<8.5 µg/ml).
Table 3.
Effect of pasteurization on oxidative status of human milk (n = 30)1
| Parameter | Fresh |
Pasteurized |
Difference‡ |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| ORACFL (µmole Trolox Eq./mL) | 3.12 ± 0.84 | 3.00 ± 0.75 | ns |
| Hexanal (ppm) | 0.285 ± 0.230 | 0.312 ± 0.213 | ns |
| Malondialdehyde (µM) | 0.26 ± 0.17 | 0.25 ± 0.16 | ns |
1 (n = 30). ‡ns = No significant difference.
Discussion
Hexanal is a decomposition product of linoleic acid(16) and in this study it was found to be the major aldehyde generated from lipid oxidation of human milk. Pentanal was also detected in mother’s milk, albeit in lesser quantity, and represents another peroxidation product derived from linoleic acid. Despite the fact that oleic acid is the main fatty acid in human milk, the generation of octanal and nonanal, which represents decomposition products of oleic acid were negligible in comparison to that found for hexanal. This finding can be attributed to the relatively greater susceptibility of linoleic acid to lipid oxidation, compared to oleic acid.(10)
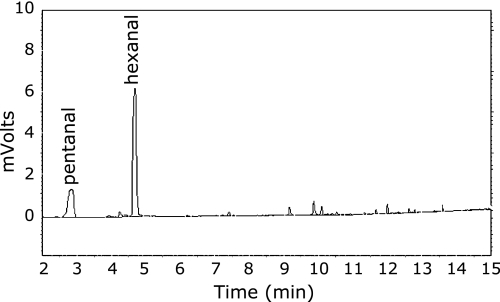
In a preliminary experiment, we determined that hexanal was the primary product generated in human milk samples following a forced peroxidation that involved treatment with extended heat exposure (50°C for 24 h) and measurement by static headspace GC (Fig. 5). Pentanal, another volatile generated from oxidation of linoleic acid was also present in forced peroxidized samples; however this occurred at lesser concentrations and with considerable variability that in fact compromised its use as a reliable indicator of lipid oxidation. Our parallel experiment established that hexanal was the primary product of hydroperoxide decomposition of human milk fat. Static headspace GC was found to be insufficiently sensitive to detect hexanal in fresh milk samples, which lead us to use SPME-GC/FID as the preferred method of analysis. For example, by comparison, the signal to noise ratio for hexanal in our SPME-GC/FID method was found to be approximately 50 times greater than that established using static headspace (data not shown). This SPME-GC/FID procedure proved to be a reliable and more sensitive method for the quantification of hexanal in both fresh and pasteurized human milk; producing reliable data that could be employed as a chemical indicator of lipid oxidation in human milk samples.
Fig. 5.
Hexanal and pentanal generation by treatment of milk at 50°C for 24 h as detected using static headspace gas chromatography.
Quantification of alkanals from human milk samples however, poses a unique challenge that is not often encountered in many common food materials. In order to quantify hexanal from a food matrix, it is ideal that a standard curve is constructed in a matrix that has an identical composition to the test sample. In the specific case of mother milk samples, the matrix represents a complex biological material that can have a wide range of solute components and concentrations that vary depending on many factors, such as time of lactation, inherent differences between mothers, as well as dietary habits.(17) For example, the fat content of the human milk samples in this study ranged from 1.5% to 8.5%. We demonstrate herein, that the recovery of hexanal is highly dependent on the fat content of the milk, suggesting that to accurately quantify hexanal from human milk, a standard curve is required for different concentrations of fat content. Spiking human milk samples containing different fat content with the same amount of hexanal, enabled a more accurate recovery of hexanal to be determined. The relationship between fat content in milk and hexanal recovery was found to be adequately corrected by using a linear regression equation constructed between the fat content of milk and the recovery of hexanal in the same sample.
Other workers have indicated that the presence of primary lipid oxidation products such as peroxides, conjugated diene and TBARS in mother’s milk are not associated with vitamin E content.(9) Our finding shows that an inverse relationship does exist between lipid oxidation and the α-Toc isomer of vitamin E when using hexanal as the end-point measure of lipid oxidation. Amongst all Toc isomers, only α-Toc isomer was inversely correlated with TAC as measured by ORAC assay, which supports our previous finding.(11) An explanation for this could be due to the fact that this isomer is relatively more hydrophobic (e.g. partition coefficient α-Toc = 3.36 vs γ-Toc = 3.14), with three methylated group on the chromanol ring as opposed to two for γ-Toc.(18) Thus the relatively greater affinity of α-Toc with the non-polar phase enables superior localization with the interface where propagation of lipid oxidation occurs.
The finding that the ascorbic acid content of human milk was negatively correlated with the hexanal content indicates a potential role for vitamin C in suppressing lipid oxidation reactions in milk. However, unlike α-Toc, there was no significant relationship found between total vitamin C in human milk and TAC measured by the ORACFL assay. The affinity of ascorbic acid to scavenge peroxyl radicals that are generated in the ORACFL assay is only 30–50% that of the Toc analog Trolox.(19,20) Considering that antioxidant properties of ascorbic acid are basically attributed to the electron donation, or by scavenging of singlet oxygen, it is understandable that ascorbic acid would not compare with vitamin E to sequester the peroxyl radicals generated in the ORACFL assay. The role of ascorbic acid in human milk to prevent oxidation reactions works through its affinity to prevent the initiation of lipid oxidation by neutralization of singlet oxygen, or by regenerating the tocopheroxyl radical that can be produced during the lipid oxidation cascade to its native form.(21)
It is important to note that although α-Toc is significantly correlated with higher ORAC values and lower hexanal concentration, no significant relationship was found between ORAC value and the hexanal content. Our finding agrees with Turoli et al.(9) that the total antioxidant capacity of milk did not correspond to larger presence of peroxidation products. In mother’s milk, the TAC should refer to the sum of activities derived from active enzymatic antioxidant systems (e.g. catalase, glutathione peroxidase), non-enzymatic antioxidants such as vitamins E and C and the presence of other bioactive factors (e.g. lactoferrin, uric acid).(9,11,22) ORACFL assay, as the case with other free radical scavenging assays that have been used to measure antioxidant capacity of mother’s milk is limited to measuring the activity of components in milk that act as antioxidants by scavenging of peroxyl radicals. We caution therefore that the use of ORACFL for measuring antioxidant capacity is limited by its affinity to only characterize those components that scavenge free radicals, and not the net generation of lipid oxidation products. Hexanal in milk representing secondary products of lipid oxidation was found to be a sensitive measure for characterizing the oxidative status of breast milk.
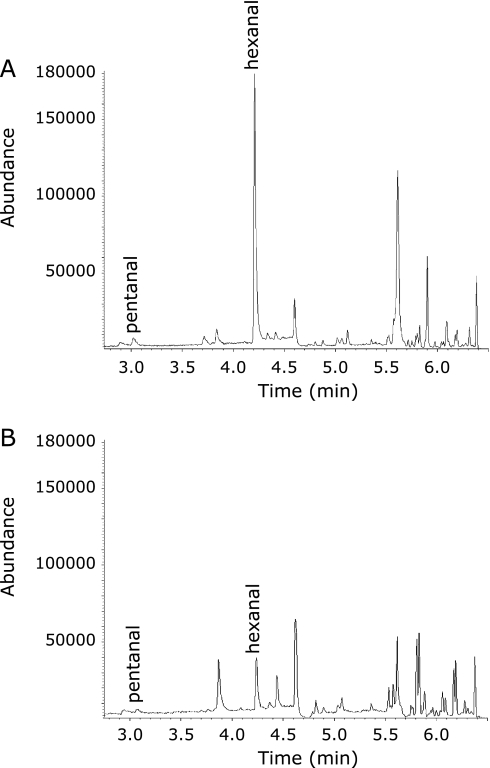
The Holder pasteurization method is commonly performed in human milk banks and involves heating milk at 62.5°C for 30 min.(4) We determined the effect of Holder pasteurization on the formation of hexanal as an index of lipid oxidation, as well as determining ORACFL values for assessing changes in antioxidant capacity. Malondialdehyde concentration was also measured level in human milk by TBA assay as one of the most commonly used end-point measure of lipid oxidation.(23) Collectively, pasteurization did not affect the oxidative status of human milk, as measured by all parameters including the ORACFL values, MDA and hexanal concentrations. Pasteurization as currently practiced, in general preserves the oxidative state of human milk. Of particular interest however, was the observation made on two individual milk samples that showed an increase in hexanal level after pasteurization and a corresponding low concentration of vitamin C (0–8.5 µg/ml) (Fig. 6). Concomitant decreases in ORACFL values were also observed in these milk samples, demonstrating that the increased generation of hexanal was relevant at identifying ongoing lipid peroxidation in samples that also had reduced antioxidant capacity. The marked increase in lipid oxidation in these particular milk samples that had undergone pasteurization was not detected when malondialdehyde was used as the end-point measure of peroxidation (Table 3). The fact that MDA artifacts can be formed during the heating step involving the TBA assay(23) further states that relying on TBARS as a measure of lipid oxidation will produce greater inaccuracy than measuring hexanal content of mother’s milk.
Fig. 6.
The aldehyde profile of human milk containing low vitamin C content (A) and high vitamin C content (B).
Conclusion
Hexanal was found to be a reliable indicator of lipid oxidation in human milk. A novel approach to quantify hexanal from human milk that required adjusting for the varying fat content is reported. Alpha- tocopherol was the only tocopherol isomer that was correlated with controlling lipid oxidation. At the same time, only α-Toc and vitamin C were correlated in preventing hexanal formation in human milk in individual milk samples. Pasteurization of human milk only affected milk that contained extremely low levels of vitamin C.
Acknowledgment
This work was supported by the FNH Vitamin Class Action Research Fund. We are most grateful to Lufiani Lina Madilao for technical assistance used for the SPME-GC/FID work. The authors also acknowledge Minh Dieu Huynh for the static headspace gas chromatography analysis and Katie Du for her laboratory assistance.
References
- 1.Shoji H, Koletzko B. Oxidative stress and antioxidant protection in the perinatal period. Curr Opin Clin Nutr Metab Care. 2007;10:324–328. doi: 10.1097/MCO.0b013e3280a94f6d. [DOI] [PubMed] [Google Scholar]
- 2.Ezaki S, Ito T, Suzuki K, Tamura M. Association between total antioxidant capacity in breast milk and postnatal age in days in premature infants. J Clin Biochem Nutr. 2008;42:133–137. doi: 10.3164/jcbn.2008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saugstad OD. Oxygen toxicity in the neonatal period. Acta Paediatr Scand. 1990;79:881–892. doi: 10.1111/j.1651-2227.1990.tb11348.x. [DOI] [PubMed] [Google Scholar]
- 4.Tully DB, Jones F, Tully MR. Donor milk: what’s in it and what’s not. J Hum Lact. 2001;17:152–155. doi: 10.1177/089033440101700212. [DOI] [PubMed] [Google Scholar]
- 5.Nutrition Committee, Canadian Paediatric Society. Statement on human milk banking. Can Med Assoc J. 1985;132:750–752. [PMC free article] [PubMed] [Google Scholar]
- 6.Silvestre D, Miranda M, Muriach M, Almansa I, Jareño E, Romero FJ. Antioxidant capacity of human milk: effect of thermal conditions for the pasteurization. Acta Paediatr. 2008;97:1070–1074. doi: 10.1111/j.1651-2227.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 7.van Zoeren-Grobben D, Moison RM, Ester VW, Berger HM. Lipid peroxidation in human milk and infant formula: effect of storage, tube feeding and exposure to phototherapy. Acta Paediatr. 1993;82:645–649. doi: 10.1111/j.1651-2227.1993.tb18032.x. [DOI] [PubMed] [Google Scholar]
- 8.Miranda M, Muriach M, Almansa I, et al. Oxidative status of human milk and its variations during cold storage. Biofactors. 2004;20:129–137. doi: 10.1002/biof.5520200302. [DOI] [PubMed] [Google Scholar]
- 9.Turoli D, Testolin G, Zanini R, Bellù R. Determination of oxidative status in breast and formula milk. Acta Paediat. 2004;93:1569–1574. doi: 10.1080/08035250410022495. [DOI] [PubMed] [Google Scholar]
- 10.Shahidi F, Wanasundara UN. Measurement of lipid oxidation and evaluation of antioxidant activity. In: Shahidi F, editor. Natural Antioxidants: Chemistry, Health Effects, and Applications. Vol. 24. Champaign, Illinois: AOCS Press; 1997. pp. 379–396. [Google Scholar]
- 11.Tijerina-Sáenz A, Innis SM, Kitts DD. Antioxidant capacity of human milk and its association with vitamins A and E and fatty acid composition. Acta Paediatr. 2009;98:1793–1798. doi: 10.1111/j.1651-2227.2009.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huynh MD, Kitts DD. Evaluating nutritional quality of pacific fish species from fatty acid signatures. Food Chem. 2009;114:912–918. [Google Scholar]
- 13.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]
- 14.Francis J, Rogers K, Brewer P, Dickton D, Pardini R. Comparative analysis of ascorbic acid in human milk and infant formula using varied milk delivery systems. Int Breastfeed J. 2008;3:19. doi: 10.1186/1746-4358-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tijerina-Sáenz A, Elisia I, Innis SM, Friel JK, Kitts DD. Use of ORAC to assess oxidative stability in human milk. J Food Compost Anal. 2009;22:694–698. [Google Scholar]
- 16.Belitz H-D, Grosch W, Schieberle P. Food Chem. Vol. Berlin: Springer-Verlag; 2009. pp. 203–207. [Google Scholar]
- 17.Jensen RG. Handbook of Milk Composition. San Diego, California: Academic Press; 1995. pp. 237–260. [Google Scholar]
- 18.Yoshida Y, Niki E. Antioxidant effects of alpha- and gamma-carboxyethyl-6-hydroxychromans. Biofactors. 2002;16:93–103. doi: 10.1002/biof.5520160305. [DOI] [PubMed] [Google Scholar]
- 19.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 20.Nenadis N, Lazaridou O, Tsimidou MZ. Use of reference compounds in antioxidant activity assessment. J Agric Food Chem. 2007;55:5452–5460. doi: 10.1021/jf070473q. [DOI] [PubMed] [Google Scholar]
- 21.Yuan YV, Kitts DD. Endogenous antioxidants: Role of antioxidant enzymes in biological systems. In: Shahidi F, editor. Natural Antioxidants: Chemistry, Health Effects, and Applications. Vol. 15. Champaign, Illinois: AOCS Press; 1997. pp. 258–270. [Google Scholar]
- 22.Aycicek A, Erel O, Kocyigit A, Selek S, Demirkol MR. Breast milk provides better antioxidant power than does formula. Nutrition. 2006;22:616–619. doi: 10.1016/j.nut.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]