Abstract
Obesity and related adipocytokine disbalance increase the risk of hepatocellular carcinoma. To determine the impact of increased levels of leptin, an obesity-related adipocytokine, on the recurrence of hepatocellular carcinoma, we conducted a prospective case-series analysis. Eighty-five consecutive primary hepatocellular carcinoma patients at our hospital from January 2006 to December 2008 were analyzed. Serum leptin level significantly correlated with Body Mass Index, total body fat, and the amount of subcutaneous fat. They included 33 with stage I/II, who underwent curative treatment. The factors contributing to recurrence of hepatocellular carcinoma, including leptin, were subjected to univariate and multivariate analyses using the Cox proportional hazards model. Body Mass Index (p = 0.0062), total body fat (p = 0.0404), albumin (p = 0.0210), α-fetoprotein (p = 0.0365), and leptin (p = 0.0003) were significantly associated with the recurrence of hepatocellular carcinoma in univariate analysis. Multivariate analysis suggested that leptin (hazard ratio 1.25, 95% CI 1.07–1.49, p = 0.0035) was a sole independent predictor. Kaplan-Meier analysis showed that recurrence-free survival was lower in patients with greater serum leptin concentrations (>5 ng/mL, p = 0.0221). These results suggest that the serum leptin level is a useful biomarker for predicting the early recurrence of hepatocellular carcinoma.
Keywords: hepatocellular carcinoma, carcinogenesis, leptin, obesity, insulin resistance
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and is estimated to cause approximately 500,000 deaths annually.(1) HCC frequently develops and in many cases recurs in cirrhotic livers due to persistent hepatitis B virus (HBV) and hepatitis C virus (HCV) infection; this is strongly associated with poor prognosis for this particular malignancy.(2) Therefore, careful surveillance of high-risk groups for HCC is important to improve prognosis. Hence, there is a critical need to identify useful risk factors for the development of HCC. Infection with HBV and HCV, alcohol consumption, aflatoxin exposure, and immune-related hepatitis are accepted as significant risk factors for the development of primary HCC.(3) Male gender, the presence of cirrhosis, high α-fetoprotein (AFP), large tumor foci, multiplicity of tumors, pathologically high-grade atypia of tumor cells, and the presence of portal venous invasion of tumors also raise the risk for HCC recurrence.(4–8)
In addition to these factors, recent studies demonstrate that obesity(9) and related metabolic abnormalities—especially diabetes mellitus (DM) and insulin resistance(10,11)—are important risk factors for the development of HCC. For instance, insulin resistance significantly raises the risk of the recurrence of stage I HCC after curative treatment.(10) Several pathophysiological mechanisms linking obesity and HCC development have been proposed and include the emergence of insulin resistance and a state of chronic inflammation.(12,13) Adipocytokine disbalance might also be involved in obesity-related liver carcinogenesis.(14) Among the adipocytokines, it is well known that the serum levels of leptin, which regulate the homeostasis of glucose and lipid metabolism,(15) are elevated in obese individuals.(16) In addition, both in vitro and in vivo studies indicate that leptin might play a role in the development of several types of human malignancies, including HCC.(17–21) These findings suggest that the dysregulation of serum leptin levels may be a critical link between obesity and liver carcinogenesis. However, whether leptin is a significant biomarker for predicting the development and/or recurrence of HCC has not been explored.
In this study, we measured the serum leptin concentration in patients with HCC and examined whether it is correlated with obesity and insulin resistance. In addition, we designed a prospective case-series analysis to examine the recurrence-free survival in consecutive patients with stage I/II HCC, who received curative treatment by surgical resection or radiofrequency ablation (RFA), stratified by serum leptin concentrations.
Materials and Methods
Patients
From January 2006 to December 2008, 85 primary HCC patients underwent initial treatment at our hospital. We measured visceral and subcutaneous fat volume using computed tomography (CT) scans at the umbilical level according to a previously reported technique (fatAnalyses and EV Insite R, PSP Corporation, Tokyo, Japan).(22) Tumor stage was defined according to the staging system of the Liver Cancer Study Group of Japan (LCSGJ).(23) HCC nodules were detected by imaging modalities including abdominal ultrasonography (US), dynamic CT, dynamic magnetic resonance imaging (MRI), and abdominal arteriography. The diagnosis of HCC was made from a typical hypervascular tumor stain on angiography and a typical dynamic-study finding of enhanced staining in the early phase and attenuation in the delayed phase.
Treatment, follow-up, and determination of recurrence
Fifteen patients were treated with surgical resection, 41 with RFA, 19 with transarterial chemoembolization (TACE), and 10 with transarterial infusion (TAI). Among them, we selected 33 curative cases that met the following criteria: tumor stage classified as I or II; and surgical resection or RFA conducted for the initial HCC treatment. In all 33 cases, therapeutic effects were judged to be curative using dynamic CT or MRI exhibiting a complete disappearance of the imaging characteristics of HCC described above.
Patients were thereafter followed up on a monthly outpatient basis using serum tumor markers every month, such as AFP and proteins induced by vitamin K absence or antagonists-II (PIVKA-II), and by abdominal US, dynamic CT scanning, or dynamic MRI every 3 months. Recurrent HCC was diagnosed, using the imaging modalities described earlier, by the appearance of other lesions differed from the primary lesions. The follow-up period was defined as the interval from the date of initial treatment until the date of diagnosis of recurrence or until April 2009 if HCC did not recur.
Statistical analysis
The Pearson product-moment correlation coefficient was used for measuring the linear correlation between 2 continuous variables. Recurrence-free survival was estimated using the Kaplan-Meier method, and differences between curves were examined with a log-rank test. Baseline characteristics were compared using Student’s t test for continuous variables or the χ2 test for categorical variables. There were 17 possible predictors for the recurrence of HCC after the initial curative treatment: sex, age, body mass index (BMI), total body fat, amounts of both visceral and subcutaneous fat, the presence of HCV-antibodies (HCV-Ab), Child-Pugh classification, serum albumin concentration, platelet count, homeostasis model assessment of insulin resistance (HOMA-IR = fasting plasma glucose (mg/dL) × fasting immunoreactive insulin (µU/mL)/405), hemoglobin A1c (HbA1c), serum tumor markers (AFP and PIVKA-II), initial treatment for HCC, tumor stage, and serum leptin concentration. Parameters that were significant as determined by univariate analysis were then subjected to multivariate analyses using the Cox proportional hazards model. Statistical significance was defined as p<0.05.
Results
Baseline characteristics and laboratory data of patients
The baseline characteristics and laboratory data of 85 patients (54 men and 31 women, median age 73 years) are shown in Table 1. The median follow-up period was 484 days (range, 14–1,429 days). Median BMI was 23.2 kg/m2, which was classified in the normal range according to the WHO classification of obesity (http://www.who.int/bmi). Median free plasma glucose (FPG), free immunoreactive insulin (FIRI), HOMA-IR, and HbA1c were 97 mg/dL, 8.115 µU/mL, 2.245, and 5.3%, respectively. The median serum leptin concentration was 5.0 ng/mL (range 1.4–26.6).
Table 1.
Baseline demographic and clinical characteristics
| Variable | Total patients (n = 85) |
|---|---|
| Sex (male/female) | 54/31 |
| Age (years) | 73 [36–87] |
| BMI (kg/m2) | 23.2 [17.5–30.7] |
| Total body fat (cm2) | 188.81 [12.93–501.8] |
| Amount of visceral fat (cm2) | 76.43 [3.82–359.83] |
| Amount of subcutaneous fat (cm2) | 105.66 [9.11–265.26] |
| Etiology (B/C/B + C/other)* | 8/55/2/20 |
| Child-Pugh classification (A/B/C) | 60/23/2 |
| Ascites on CT imaging (present/absent) | 7/78 |
| ALB (g/dL) | 3.5 [2.2–4.5] |
| PLT (×104/µL) | 11.7 [3.0–76.4] |
| FPG (mg/dL) | 97 [67–271] |
| FIRI (µU/mL) | 8.115 [1.21–90.2] |
| HOMA-IR | 2.245 [0.27–28.28] |
| HbA1c (%) | 5.3 [3.7–10.3] |
| Leptin (ng/mL) | 5.0 [1.4–26.6] |
| Stage (I/II/III/IVA/IVB) | 19/26/27/10/3 |
| Initial treatment for HCC (resection/RFA/TACE/TAI) | 15/41/19/10 |
| AFP (ng/mL) | 48 [0–222000] |
| PIVKA-II (mAU/mL) | 170 [7–474000] |
| Follow-up period (days) | 484 [14–1429] |
Values are median [range]. *B means positive for hepatitis B surface antigen and C means positive for hepatitis C virus antibody. AFP, α-fetoprotein; B, hepatitis B virus; BMI, body mass index; C, hepatitis C virus; FPG, fasting plasma glucose; FIRI, fasting immunoreactive insulin; HbA1c, hemoglobin A1c; HCC, hepatocellular carcinoma; HOMA-IR, homeostasis model assessment of insulin resistance; PIVKA-II, protein induced by vitamin K absence or antagonists-II; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TAI, transarterial infusion.
Association of the serum leptin concentration with obesity and insulin resistance
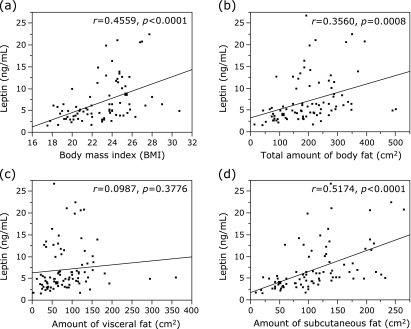
Four obesity-related factors were tested for possible association with the serum leptin concentration: BMI, total body fat, and the amounts of visceral and subcutaneous fat (Fig. 1). For BMI analysis, we excluded 7 patients with CT-detected ascites. The Pearson product-moment correlation coefficient and p values of BMI and the total body fat with serum leptin concentration were r = 0.4559 and p<0.0001, and r = 0.3560 and p = 0.0008, respectively; indicating that these 2 factors were significantly correlated with the serum leptin concentration. The amount of subcutaneous fat (r = 0.5174 and p<0.0001) was also strongly correlated with the serum leptin level, whereas the amount of visceral fat (r = 0.0987 and p = 0.3776) was not. In addition, no significant correlations were noted between the serum leptin concentration and insulin resistance-related factors, including FPG (r = −0.0816 and p = 0.4579), FIRI (r = 0.1049 and p = 0.3378), HOMA-IR (r = 0.0506 and p = 0.6385), and HbA1c (r = 0.0194 and p = 0.7820).
Fig. 1.
Correlation between the serum levels of leptin and (a) BMI, (b) total body fat, (c) amount of visceral fat, and (d) amount of subcutaneous fat in patients with HCC (n = 85). For BMI analysis, we excluded 7 patients with CT-detected ascites.
Possible risk factors for the recurrence of HCC
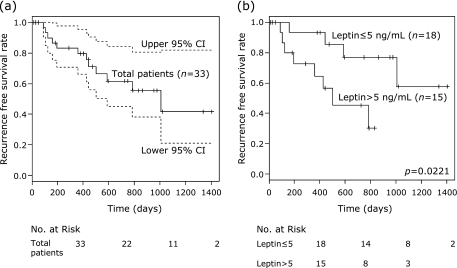
In all 33 curative cases of stage I/II HCC, 12 patients experienced recurrence in the liver, but none exhibited distant metastasis. The 1-year recurrence-free survival in the 33 patients was 79% (Fig. 2a). Fig. 2b shows Kaplan-Meier curves for recurrence-free survival divided into 2 subgroups on the basis of median serum leptin concentration (⩽5 or >5 ng/mL), which results in a significant difference (p = 0.0221).
Fig. 2.
Kaplan-Meier curves for recurrence-free survival in (a) total patients and in (b) subgroups divided on the basis of the serum leptin concentration (≤5 or >5 ng/mL).
The Cox proportional hazards model was used to analyze risk factors for the recurrence of stage I/II HCC after curative treatments using the 17 variables listed in Table 2. BMI (hazard ratio 1.30, 95% CI 1.08–1.56, p = 0.0062), total body fat (hazard ratio 1.00, 95% CI 1.00–1.01, p = 0.0404), serum albumin concentration (hazard ratio 0.26, 95% CI 0.08–0.81, p = 0.0210), AFP (hazard ratio 0.99, 95% CI 0.99–0.99, p = 0.0365), and serum leptin concentration (hazard ratio 1.29, 95% CI 1.12–1.50, p = 0.0003) were identified as significant risk factors by univariate analysis. Multivariate analysis only identified serum leptin concentration (hazard ratio 1.25, 95% CI 1.07–1.49, p = 0.0035) as significant independent risk factor for the recurrence of HCC (Table 3).
Table 2.
Univariate analyses of possible risk factors for recurrence-free survival of HCC using the Cox proportional hazards model
| Variable | 95% CI |
|||
|---|---|---|---|---|
| HR* | lower | upper | p value | |
| Sex (male vs female) | 0.9 | 0.28 | 3.09 | 0.8726 |
| Age (years) | 0.96 | 0.89 | 1.03 | 0.277 |
| BMI (kg/m2) | 1.3 | 1.08 | 1.56 | 0.0062 |
| Total body fat (cm2) | 1 | 1 | 1.01 | 0.0404 |
| Amount of visceral fat (cm2) | 1 | 0.99 | 1.01 | 0.0909 |
| Amount of subcutaneous fat (cm2) | 1 | 0.99 | 1.01 | 0.0601 |
| The presence of HCV-Ab (yes vs no) | 0.42 | 0.12 | 1.98 | 0.2501 |
| Child-Pugh classification (B + C vs A) | 1.33 | 0.35 | 4.3 | 0.6482 |
| ALB (g/dL) | 0.26 | 0.08 | 0.81 | 0.021 |
| PLT (×104/µL) | 0.87 | 0.75 | 1.01 | 0.0714 |
| HOMA-IR | 1.03 | 0.94 | 1.1 | 0.4 |
| HbA1c (%) | 0.87 | 0.37 | 1.6 | 0.7108 |
| AFP (ng/mL) | 0.99 | 0.99 | 0.99 | 0.0365 |
| PIVKA-II (mAU/mL) | 0.99 | 0.99 | 1 | 0.7448 |
| Initial treatment for HCC (RFA vs resection) | 1.61 | 0.42 | 10.5 | 0.5128 |
| Stage (II vs I) | 1.08 | 0.32 | 3.78 | 0.89 |
| Leptin (ng/mL) | 1.29 | 1.12 | 1.5 | 0.0003 |
*HR represents the values with a unit increase in continuous variables. AFP, α-fetoprotein; BMI, body mass index; CI, confidence interval; HbA1c, hemoglobin A1c; HCC, hepatocellular carcinoma; HCV-Ab, hepatitis C virus antibody; HOMA-IR, homeostasis model assessment of insulin resistance; HR, hazard ratio; PIVKA-II, protein induced by vitamin K absence or antagonists-II; RFA, radiofrequency ablation.
Table 3.
Multivariate analyses of possible risk factors for recurrence-free survival of HCC using the Cox proportional hazards model
| Variable | 95% CI |
|||
|---|---|---|---|---|
| HR* | lower | upper | p value | |
| BMI (kg/m2) | 1.2 | 0.83 | 1.81 | 0.3278 |
| Total body fat (cm2) | 1 | 0.99 | 1.01 | 0.8003 |
| ALB (g/dL) | 0.54 | 0.12 | 2.28 | 0.4018 |
| AFP (ng/mL) | 0.99 | 0.99 | 1 | 0.1416 |
| Leptin (ng/mL) | 1.25 | 1.07 | 1.49 | 0.0035 |
*HR represents the values with a unit increase in continuous variables. AFP, α-fetoprotein; BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Table 4 shows the baseline characteristics and laboratory data of patients divided on the basis of the serum leptin concentration (⩽5 and >5 ng/mL). No significant differences were noted between the 2 subgroups, except the amount of subcutaneous fat (p = 0.0461).
Table 4.
Baseline demographic and clinical characteristics of patients classified on the basis of the serum leptin concentration
| Variable | Leptin ≤ 5 ng/mL (n = 18) | Leptin > 5 ng/mL (n = 15) | p value |
|---|---|---|---|
| Sex (male/female) | 13/5 | 6/9 | 0.0604 |
| Age (years) | 72.5 [59–87] | 70 [50–85] | 0.2565 |
| BMI (kg/m2) | 21.5 [17.8–30.7] | 24.5 [18.5–27.7] | 0.1111 |
| Total body fat (cm2) | 167.5 [73.9–490.9] | 207.3 [112.2–350.8] | 0.2591 |
| Amount of visceral fat (cm2) | 69.4 [19.9–294.4] | 98.9 [21.9–181.6] | 0.9479 |
| Amount of subcutaneous fat (cm2) | 90.2 [42.0–232.3] | 134.3 [79.6–242.5] | 0.0461 |
| Etiology (C/others) | 14/4 | 11/4 | 0.767 |
| Child-Pugh classification (A/B/C) | 15/3/0 | 10/5/0 | 0.2657 |
| ALB (g/dL) | 3.6 [2.6–4.2] | 3.3 [2.4–4.4] | 0.2708 |
| PLT (×104/µL) | 12.45 [7.7–26.1] | 9.5 [3.0–20.6] | 0.0895 |
| FPG (mg/dL) | 97.5 [83–271] | 105 [75–154] | 0.7424 |
| FIRI (µU/mL) | 6.05 [2.57–65.2] | 14.3 [7.3–27.4] | 0.3657 |
| HOMA-IR | 1.51 [0.53–24.8] | 3.41 [1.45–9.40] | 0.641 |
| HbA1c (%) | 5.3 [4.5–10.3] | 5.2 [3.7–6.8] | 0.3351 |
| Stage (I/II) | 7/11 | 9/6 | 0.2253 |
| Initial treatment for HCC (resection/RFA) | 6/12 | 2/13 | 0.1726 |
| AFP (ng/mL) | 8 [0–20500] | 28 [1–2530] | 0.1687 |
| PIVKA-II (mAU/mL) | 22.7 [8–201000] | 26 [7–29800] | 0.4385 |
Values are median [range]. AFP, α-fetoprotein; C, hepatitis C virus; FPG, fasting plasma glucose; FIRI, fasting immunoreactive insulin; HbA1c, hemoglobin A1c; HCC, hepatocellular carcinoma; HOMA-IR, homeostasis model assessment of insulin resistance; PIVKA-II, protein induced by vitamin K absence or antagonists-II; RFA, radiofrequency ablation.
Discussion
Leptin regulates body weight by signaling information to the brain regarding the availability of energy stored as fat; this negative feedback loop is disrupted in most obese individuals and results in a state known as leptin resistance.(16,24) Consistent with the results of previous studies,(16,24) the serum leptin concentration was significantly correlated with BMI and total body fat in the present study (Fig. 1 a and b). These parameters were also significant risk factors for the recurrence of HCC as determined by univariate analysis (Table 2); however, the serum leptin concentration was the most significant biomarker (p = 0.0003).
In addition, we clearly showed for the first time that patients with greater serum leptin concentrations were susceptible to HCC recurrence (Fig. 2b); thus, increased serum leptin levels are a significant independent risk factor for the recurrence of this malignancy (Table 3). This finding indicates that increased serum leptin concentration, which might link obesity with liver carcinogenesis, is a preferable and useful biomarker for screening high-risk groups for the recurrence of HCC. We previously reported that a state of insulin resistance associated with obesity is an independent risk factor for the recurrence of HCC after curative treatment.(10) Furthermore, no significant correlations between serum leptin levels and insulin resistance-related factors were noted in the present study, suggesting these two conditions might be independent from each other in HCC patients. Therefore, a combination evaluation for both the serum leptin level and insulin resistance would be more effective for screening high-risk groups for HCC, and requires future confirmation.
Several studies report that leptin is a risk factor for carcinogenesis at various organ sites, including the liver.(17–21) Leptin can stimulate cellular proliferation in various types of cancer cells such as HCC cells.(19–21,25) In addition, when focusing on the liver, leptin is a potent profibrogenic cytokine and thus plays a key role in the progression of cirrhosis,(26) which is a precancerous condition of HCC. Indeed, increased serum leptin concentration has been documented in cirrhotic patients.(27,28) Moreover, increased leptin expression is associated with increased intratumor microvascular density. Consequently, it is hypothesized that leptin plays a stimulatory role in the development of HCC via neovascularization.(29) In addition to using leptin as a biomarker for the risk of HCC recurrence, the present findings suggest that targeting leptin might be an effective strategy for the prevention and treatment of HCC. Ribatti et al. state that anti-leptin antibodies reduce the angiogenic response in HCC biopsy specimens.(29) Decreases in serum leptin are also associated with the prevention of obesity-related liver tumorigenesis in obese and diabetic mice models.(14)
In conclusion, we report that patients with high serum leptin concentrations are susceptible to HCC recurrence in stage I/II cases curatively treated by surgical resection or RFA. Increased serum leptin concentration may be a useful biomarker for predicting the recurrence of HCC in high-risk patients.
Acknowledgments
This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (No. 22790638 to M. S. and No. 21590838 to H. M.) and by Grant-in-Aid for the 3rd Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan.
Abbreviations
- AFP
α-fetoprotein
- BMI
body mass index
- CT
computed tomography
- DM
diabetes mellitus
- FPG
fasting plasma glucose
- FIRI
fasting immunoreactive insulin
- HbA1c
hemoglobin A1c
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HOMA-IR
homeostasis model assessment of insulin resistance
- LCSGJ
Liver Cancer Study Group of Japan
- MRI
magnetic resonance imaging
- PIVKA-II
protein induced by vitamin K absence or antagonists-II
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
- TAI
transarterial infusion
- US
ultrasonography
References
- 1.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Toyama T, Hiramatsu N, Yakushijin T, et al. A new prognostic system for hepatocellular carcinoma including recurrent cases: a study of 861 patients in a single institution. J Clin Gastroenterol. 2008;42:317–322. doi: 10.1097/MCG.0b013e3180ebe790. [DOI] [PubMed] [Google Scholar]
- 3.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koike Y, Shiratori Y, Sato S, et al. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus: an analysis of 236 consecutive patients with a single lesion. Hepatology. 2000;32:1216–1223. doi: 10.1053/jhep.2000.20237. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K, Saitoh S, Tsubota A, et al. Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma. Cancer. 1993;71:19–25. doi: 10.1002/1097-0142(19930101)71:1<19::aid-cncr2820710105>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Adachi E, Maeda T, Matsumata T, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology. 1995;108:768–775. doi: 10.1016/0016-5085(95)90450-6. [DOI] [PubMed] [Google Scholar]
- 7.Nagashima I, Hamada C, Naruse K, et al. Surgical resection for small hepatocellular carcinoma. Surgery. 1996;119:40–45. doi: 10.1016/s0039-6060(96)80211-x. [DOI] [PubMed] [Google Scholar]
- 8.Ishii H, Okada S, Nose H, et al. Predictive factors for recurrence after percutaneous ethanol injection for solitary hepatocellular carcinoma. Hepatogastroenterology. 1996;43:938–943. [PubMed] [Google Scholar]
- 9.Muto Y, Sato S, Watanabe A, et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204–214. doi: 10.1016/j.hepres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Imai K, Takai K, Nishigaki Y, et al. Insulin resistance raises the risk for recurrence of stage I hepatocellular carcinoma after curative radiofrequency ablation in hepatitis C virus-positive patients: a prospective, case series study. Hepatol Res. 2010;40:376–382. doi: 10.1111/j.1872-034X.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 12.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasa J, Shimizu M, Shiraki M, et al. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci. 2010;101:460–467. doi: 10.1111/j.1349-7006.2009.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda K, Okubo K, Shimomura I, Mizuno K, Matsuzawa Y, Matsubara K. Analysis of an expression profile of genes in the human adipose tissue. Gene. 1997;190:227–235. doi: 10.1016/s0378-1119(96)00730-5. [DOI] [PubMed] [Google Scholar]
- 16.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 17.Wang XJ, Yuan SL, Lu Q, et al. Potential involvement of leptin in carcinogenesis of hepatocellular carcinoma. World J Gastroenterol. 2004;10:2478–2481. doi: 10.3748/wjg.v10.i17.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stattin P, Palmqvist R, Söderberg S, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep. 2003;10:2015–2021. [PubMed] [Google Scholar]
- 19.Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 20.Somasundar P, Riggs D, Jackson B, Vona-Davis L, McFadden DW. Leptin stimulates esophageal adenocarcinoma growth by nonapoptotic mechanisms. Am J Surg. 2003;186:575–578. doi: 10.1016/j.amjsurg.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol. 1999;365:273–279. doi: 10.1016/s0014-2999(98)00884-x. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi J, Tadokoro N, Watanabe M, Shinomiya M. A novel method of measuring intra-abdominal fat volume using helical computed tomography. Int J Obes Relat Metab Disord. 2002;26:398–402. doi: 10.1038/sj.ijo.0801921. [DOI] [PubMed] [Google Scholar]
- 23.Liver Cancer Study Group of Japan The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg. 1989;19:98–129. doi: 10.1007/BF02471576. [DOI] [PubMed] [Google Scholar]
- 24.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, Chang YC, Liu CL, Liu TP, Chang KJ, Guo IC. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer. 2007;14:513–529. doi: 10.1677/ERC-06-0027. [DOI] [PubMed] [Google Scholar]
- 26.Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 27.Henriksen JH, Holst JJ, Møller S, Brinch K, Bendtsen F. Increased circulating leptin in alcoholic cirrhosis: relation to release and disposal. Hepatology. 1999;29:1818–1824. doi: 10.1002/hep.510290601. [DOI] [PubMed] [Google Scholar]
- 28.Ockenga J, Bischoff SC, Tillmann HL, et al. Elevated bound leptin correlates with energy expenditure in cirrhotics. Gastroenterology. 2000;119:1656–1662. doi: 10.1053/gast.2000.20256. [DOI] [PubMed] [Google Scholar]
- 29.Ribatti D, Belloni AS, Nico B, et al. Leptin-leptin receptor are involved in angiogenesis in human hepatocellular carcinoma. Peptides. 2008;29:1596–1602. doi: 10.1016/j.peptides.2008.05.011. [DOI] [PubMed] [Google Scholar]