Abstract
This study was to assess the resting energy expenditure of patients with esophageal cancer using indirect calorimetry. Eight male patients with esophageal cancer and eight male healthy controls were enrolled in this study. All patients underwent transthoracic esophagectomy with lymph nodes dissections. The resting energy expenditure was measured preoperatively, and on postoperative day 7 and 14 using indirect calorimetry. Preoperatively, the measured resting energy expenditure/body weight in these patients was significantly higher than that of the controls (23.3 ± 2.1 kcal/kg/day vs 20.4 ± 1.6 kcal/kg/day), whereas the measured/predicted energy expenditure from the Harris-Benedict equation ratio was 1.01 ± 0.09, which did not differ significantly from the control values. The measured resting energy expenditure/body weight was 27.3 ± 3.5 kcal/kg/day on postoperative day 7, and 23.7 ± 5.07 kcal/kg/day on postoperative day 14. Significant increases in the measured resting energy expenditure were observed on postoperative day 7, and the measured/predicted energy expenditure ratio was 1.17 ± 0.15. In conclusion, patients with operable esophageal cancers were almost normometabolic before surgery. On the other hand, the patients showed a hyper-metabolic status after esophagectomy. We recommended that nutritional management based on 33 kcal/body weight/day (calculated by the measured resting energy expenditure × active factor 1.2–1.3) may be optimal for patients undergoing esophagectomy.
Keywords: resting energy expenditure, indirect calorimetry, esophagectomy
Introduction
It is believed that the energy expenditure (mREE) is increased in cancer patients.(1) Typically, the mREE was found to be elevated in patients with lung or pancreatic cancers.(2) On the other hand, Dempsey suggested that the largest portion of esophageal cancer patients was normometabolic, whereas patients with pancreatic and hepatobiliary tumors tended to be hypometabolic, and patients with gastric cancer tended toward hypermetabolism.(3) However, it should be noted that malnourished cancer patients who were losing weight were enrolled in their studies.
In many patients, weight loss is observed in the terminal stages of cancer. There is usually a negative energy balance which results from a decrease in energy intake due to anorexia, and increased energy expenditure. This rise in energy expenditure is usually mild (100 to 300 kcal/day), but can cause a loss of body weight.(1) On the other hand, weight loss is the first symptom that prompts the patient to seek medical attention. However, it remains unclear whether the mREE is increased in patients with early stage cancer or operable cancer. Therefore, we compared the mREE in patients with operable esophageal cancer to that in healthy controls.
Furthermore, we also performed weekly indirect calorimetry on patients undergoing esophagectomy because an accurate assessment of their energy needs is required for patients undergoing surgery to fulfill their energy requirements, and to avoid complications associated with over feeding.(4,5)
Typically, the energy requirements in parenteral nutrition or enteral nutrition are determined by the predicted resting energy expenditure (pREE) calculated by the Harris-Benedict (HB) equation.(6) The value is multiplied by activity and stress factors to determine the total energy requirement.(7) Although there have been several studies reporting that the energy expenditure of patients undergoing surgery changes to a hyper-metabolic status, the finding of an increased mREE rate remains controversial.(8–11) Transthoracic esophagectomy accompanied by lymph nodes (LNs) dissection is one of the most invasive of all gastrointestinal procedures. Therefore, we evaluated the energy metabolism of patients undergoing esophagectomy, and determined the optimal energy requirements for nutritional management.
Subjects and Methods
Patients
Eight male patients and eight healthy controls were enrolled in this study. Patients with carcinomas of the thoracic esophagus underwent a right thoracotomy and transthoracic esophagectomy with LNs dissections. The patients were admitted to the department of Gastroenterology Unit of the Shiga University of Medical Science Hospital. The ethics committee of the Shiga University of Medical Science approved this study. Staging of the carcinomas after surgery indicated that one patient had stage I, two had stage II, four had stage III, and one had stage IV a tumors.
The patients were given 750 kcal/day of liquid diet supplemented with arginine, n-3 fatty acids, and RNA (IMPACT; Ajinomoto Pharma, Tokyo, Japan) in addition to a conventional diet for 5 days before the operation. The patients received 35 kcal/body weight (BW)/day as the daily amount of IMPACT plus a conventional diet.
All patients who underwent esophagectomy also received a jejunostomy. Parenteral nutrition and enteral nutrition were commenced on postoperative day (POD) 2. Oral intake was initiated on POD7. The daily energy requirements were calculated by the mREEs × activity factors. All patients underwent the esophagectomy without major complications.
Indirect calorimetry (IC)
The mREEs and respiratory quotients (RQ) were measured preoperatively with the patients lying at rest and on POD7 and POD14. A computed open-circuit indirect calorimeter (AE-300S; Minato Medical Science Co., Osaka, Japan)(12,13) was used for the mREEs and RQ determinations. The measurements were performed in the morning after an overnight fast. However, the infusion of parenteral nutrition was maintained in these patients.
The mREE was calculated from the oxygen consumption (VO2) and carbon dioxide production (VCO2) by the abbreviated Weir equation(14):
| mREE = (3.94 × VO2 + 1.11 × VCO2) × 1.44 |
The measurement of the non protein RQ was calculated as RQ = VCO2/VO2. The mREEs were compared with the pREE calculated by the HB equation based on current body weight.(6)
| pREE = 66.47 + 13.75 × W + 5.0 × H – 6.75 × A |
where W is the weight in kg, H is the height in cm, and A is the age in years.
Statistical analysis
Differences between the groups were analyzed with the Kruskal-Wallis test. Correlations were investigated with Spearman rank correlation tests, and a p value <0.05 was considered to be statistically significant.
Results
Nutritional status of patients with esophageal cancer
Table 1 shows the preoperative nutritional parameters of the eight male patients. The mean ± SD of the total protein (TP), serum albumin (Alb), total lymphocyte counts (TCL), and total cholesterol (T-chol) levels were 6.2 ± 0.5 g/dl, 3.6 ± 0.3 g/dl, 1248.7 ± 540.7/mm3, and 182.4 ± 20.3 mg/dl, respectively. The mean prealbumin (PA), retinol-binding protein (RBP), and C-reactive protein (CRP) levels were 28.0 ± 8.9, 3.5 ± 1.1, and 0.45 ± 1.04 mg/dl, respectively.
Table 1.
Baseline characteristics of patients with operable esophageal cancer
| Stage of disease | |
|---|---|
| I | 1 |
| II | 2 |
| III | 4 |
| IVa | 1 |
| TP (g/dl) | 6.2 ± 0.5 |
| Alb (g/dl) | 3.6 ± 0.3 |
| TLC (/mm3) | 1248.7 ± 540.7 |
| T-chol (mg/dl) | 182.4 ± 20.3 |
| CRP (mg/dl) | 0.45 ± 1.04 |
| PA (mg/dl) | 28.0 ± 8.9 |
| RBP (mg/dl) | 3.5 ± 1.1 |
Values are mean ± SD or total number of patients. (n = 8)
Comparison of the REE and RQ in healthy controls and patients
The characteristics of the eight male patients before surgery and the eight male healthy controls are shown in Table 2. The mean age of the patients and controls did not differ significantly. In comparison with the healthy controls, the mean BW, body mass index (BMI), and pREE were significantly lower in the cancer patients.
Table 2.
Background of the patients and healthy controls
| Controls | Patients | p value | |
|---|---|---|---|
| Patient number | 8 | 8 | — |
| Male / Female | 8/0 | 8/0 | — |
| Age (y) | 58.0 ± 19.3 | 58.4 ± 4.3 | 0.96 |
| Height (cm) | 169.1 ± 5.7 | 167.4 ± 7.3 | 0.62 |
| Body weight (kg) | 70.7 ± 8.0 | 54.3 ± 7.1 | <0.001 |
| BMI (kg/m2) | 24.7 ± 1.7 | 19.3 ± 1.9 | <0.001 |
| pREE (kcal/day) | 1497.3 ± 222.4 | 1256.0 ± 148.7 | 0.02 |
| mREE (kcal/day) | 1438.3 ± 204.3 | 1257.0 ± 109.9 | 0.06 |
BMI, Body mass index. Values are mean ± SD or total number of patients.
The mREE in patients before surgery measured by IC was 1257.0 ± 109.9 kcal/day, and the pREE in these patients was 1256.0 ± 148.7 kcal/day. The mREE/pREE ratio in the patients before surgery was 1.01 ± 0.09, and there was a positive correlation between the pREE and mREE (p<0.05).
The mREE of the healthy controls was 1438.3 ± 204.3 kcal/day. Although no significant difference was noted between the mean mREE of the patients and the healthy controls, the mean mREE/BW of the patients before surgery was significantly higher than that of the controls (23.3 ± 2.1 kcal/kg/day vs 20.4 ± 1.6 kcal/kg/day) (Fig. 1a). The mREE/pREE ratio in the patients before surgery did not differ significantly from that in the controls (Fig. 1b).
Fig. 1.
Measured resting energy expenditure (mREE)/body weight (BW) in patients with esophageal cancer and healthy controls. (a) The mREE/BW of the patients was significantly higher than that of the healthy controls. Values represent means ± SD. *p<0.05 compared with healthy controls. (b) Measured resting energy expenditure (mREE)/predicted resting energy expenditure (pREE) in patients with esophageal cancer and healthy controls. There were no significant differences between the mREE/pREE of patients and healthy controls. Values represent means ± SD.
The RQ of the patients was almost the same as that of the controls (Fig. 2).
Fig. 2.
Respiratory quotients (RQ) in patients with esophageal cancer during the preoperative period. There were no significant differences between the RQ of the patients and healthy controls. Values represent means ± SD.
Changes in the REE and RQ of the patients during the perioperative period
The mREE/BW on POD7 (27.3 ± 3.5 kcal/kg/day) was significantly higher than the preoperative value. Thereafter, the mREE/BW on POD14 was almost the same as the preoperative period (Fig. 3a). The mean mREE/pREE was 1.17 ± 0.15 on POD7 and 1.05 ± 0.22 on POD14. (Fig. 3b). No statistically significant differences were observed in the RQ during the perioperative period (Fig. 4).
Fig. 3.
(a) Changes in the measured resting energy expenditure (mREE) of patients undergoing esophagectomy for esophageal carcinoma. The mREE of the patients was significantly increased on postoperative day 7. Values represent means ± SD. *p<0.05 compared with preoperative values. (b) Changes in the measured resting energy expenditure (mREE)/predicted resting energy expenditure (pREE) ratio of patients undergoing esophagectomy. The mREE/pREE ratio was significantly increased on postoperative day 7. Values represent means ± SD. *p<0.05 compared with preoperative values.
Fig. 4.
Changes in the respiratory quotients (RQ) in patients undergoing esophagectomy. There were no significant changes in the RQ during perioperative period. Values represent means ± SD.
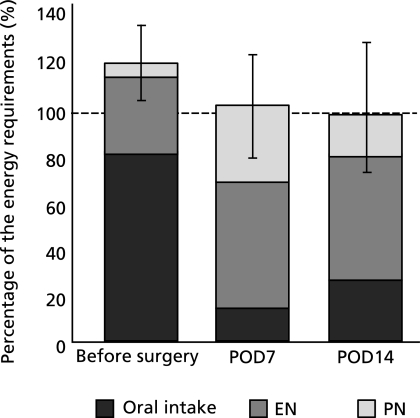
The mean energy requirements calculated by the mREEs × active factor 1.2–1.3 on POD7 and POD14 were 32.8 kcal/kg/day and 30.8 kcal/kg/day, respectively. As shown in Fig. 5, the mean nutritional intakes on POD7 and POD14, as percentages of the requirements, were 101.8% and 98.7% for energy, respectively. The oral intakes, as percentages of the requirements, were only 14.2% and 27.2% for energy, respectively.
Fig. 5.
Changes in nutritional intake, including enteral nutrition and parenteral nutrition, as a percentage of the energy requirements of patients undergoing oesophagectomy. The energy requirements of the patients were calculated by the mREE × stress factor 1.2–1.3. Values represent means ± SD.
Discussion
In this study, we showed that patients with operable esophageal cancers were almost normometabolic before surgery. However, we also demonstrated that the resting energy expenditure was significantly increased after surgery in patients undergoing a transthoracic esophagectomy with LNs dissections. In this study, we assessed the mREE and pREE in only male patients, because no female patients underwent transthoracic esophagectomies during this study period.
Previous studies have suggested that the mREE may be elevated in patients with several types of tumors, such as lung and pancreatic cancers. However, the findings regarding gastrointestinal malignancy are controversial.(2,15,16) Dempsey compared the mREE to the pREE from the HB equation based on current BW, and suggested that most esophageal cancer patients were normometabolic. One third of their patients had liver metastases, and more than half of their patients had received TPN at the time of study.(3) However, few studies have reported the mREE in patients with early stages of cancer or operable cancer. This is the first report to assess the mREE in patients with operable esophageal cancers and compare it to healthy controls. We showed that the mREE measured by IC in patients with esophageal cancer was almost the same as the pREE, but it was significantly greater than that in the controls when expressed in relation to the BW. The higher mREE/BW of the patients was reflected in a significant reduction in their BMI when compared to the controls.
Weight loss is a common clinical finding in patients with cancer. Weight loss in cancer patients is frequently ascribed to a combination of reduced food intake and hypermetabolism. Recently, the mediators of anorexia and metabolic change in cancer patients have been postulated to include proinflammatory cytokines, neuroendocrine stress hormones and tumor-specific cachectic factors such as proteolysis-inducing factor (PIF).(2) However, the levels of serum albumin, prealbumin, retinol-binding protein, and C-reactive protein were within the normal range in our patients prior to surgery. In addition, all patients maintained adequate oral nutrition. These data implied that although weight loss is commonly associated with the terminal stages of cancer, weight loss also occurs in operable cancers, and weight loss is not exclusively caused by the mREE or food intake.
The pREE calculated by the HB equation has been widely used to evaluate the energy status of patients.(6) The total energy requirement is calculated by the pREE × activity factor × stress factor.(7,17) Theoretically, the pREE is expected to equal the mREE in healthy humans, and the mREE/pREE ratio is a marker for a hyper-metabolic status.(12,13,17)
The increased rates of mREE in patients undergoing abdominal surgery are controversial. Previously, it has been reported that the REE was significantly increased after abdominal surgery, and that TPN therapy consisting of 45 kcal/kg/day was optimal for these patients. This means that measurements of the energy requirement can be calculated by the pREE × 1.75. Rutten et al.(9) also reported that the pREE × 1.75–2.0 kcal/day should be optimal for TPN in patients undergoing major surgery. On the other hand, Federix et al.(10) showed a 10% increase in the mREE of patients undergoing gastrointestinal surgery without major complications.
An esophagectomy is one of the most invasive gastrointestinal procedures. Sato et al.(18,19) showed a 31% increase in the mREE in patients undergoing an esophagectomy. However, the increased rate in the mREE in our patients was lower than that from previous studies. The mREE of our patients had increased by 12% to 27.3 ± 3.5 kcal/kg/day after the esophagectomy. The mREE/pREE ratio, which reflects a stress factor, was elevated to 1.17 on POD7, and decreased to the preoperative level on POD14. Our results suggested that the stress factor for esophageal cancer surgery without major complications should be 1.2 through POD7. Similar results were obtained from our recent study, which found that patients who had undergone a pylorus preserving pancreaticoduodenostomy (PPPD) showed stress factors of 1.2.(20) Although one would be more likely to find stress-induced hypermetabolism on earlier postoperative days, the increase in the mREE of patients undergoing a transhiatal or transthoracic esophagectomy was almost the same as during the first seven days.(18)
Recently, perioperative management has been dramatically improved, with the goal of preventing infection, which causes hyper-metabolism in patients undergoing major surgery. Although all operations were performed by a single surgeon using the same surgical procedures in this study, it is possible that the magnitude of the surgical stress depends on the surgical technique of each surgeon. Thus, a stress factor of 1.2 in our patients may reflect the magnitude of the surgical stress induced by the esophagectomy. The measurement of the energy requirement is very critical for nutritional management in patients undergoing major surgery such as pancreatic or esophageal cancers, because over feeding has been shown to be associated with some clinical complications.(4,5)
In this study, a significant change in the RQ after esophagectomy was not observed. In previous reports, the RQ was reduced in patients after a liver resection.(21) This means that the energy substrate of the remnant liver is principally fatty acids rather than glucose after a liver resection. In patients undergoing an esophagectomy, however, glucose is mainly utilized after the surgery, as well as in patients undergoing PPPD.(20,22,23)
Recent studies suggest that specific nutritional compounds, including arginine, glutamine, n-3 fatty acids, and RNA have been identified as immunonutrition.(24,25) The ESPEN and SCCM/ASPEN guidelines support the use of immune enhancing diets in elective surgery patients during the preoperative period.(26,27) Immunonutrition has also been shown to be beneficial in subsets of Japanese patients undergoing esophagectomy.(28,29) In our study, the patients were given 750 kcal/day of IMPACT in addition to a conventional diet for 5 days before the operation, and all patients maintained oral nutrition satisfactorily. On the other hand, the oral intake was generally poor during the early postoperative period. Previous reports had estimated that the mean time required to achieve what the patients following esophagectomy considered to be a socially acceptable diet was 6 months.(30,31) In this regard, enteral nutrition and total parenteral nutrition were useful backups for patients with poor oral intake after esophagectomy. The oral intake must be closely monitored to give sufficient calories to meet the estimated nutritional requirements.
In summary, patients with operable esophageal cancers were normometabolic as compared to healthy controls. The mREE of these patients was significantly increased after an esophagectomy. The mREE/pREE ratio was increased to 1.17 on POD7, but decreased to the preoperative level on POD14. Our results suggest that the daily energy requirements for patients undergoing esophagectomy are 31–33 kcal/BW/day (calculated by the mREE × activity factor 1.2–1.3), and that alternative nutritional therapies are needed to give sufficient calories to meet the daily energy requirements.
References
- 1.Bozzetti F, von Meyenfeldt MF. Nutritional support in cancer. In: Lubos Sobotka., editor. Basic in Clinical Nutrition Third Edition. Prague: Publishing House Galen; 2004. pp. 392–402. [Google Scholar]
- 2.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey DT, Feurer ID, Knox LS, Crosby LO, Buzby GP, Mullen JL. Energy expenditure in malnourished gastrointestinal cancer patients. Cancer. 1984;53:1265–1273. doi: 10.1002/1097-0142(19840315)53:6<1265::aid-cncr2820530609>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Sandstrome R, Drott C, Hyltander A, et al. The effect of postoperative intravenous feeding (TPN) on outcome following major surgery evaluated in randomized study. Ann Surg. 1993;217:185–195. doi: 10.1097/00000658-199302000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander JW, Gonce SJ, Miskell PW, Peck MD, Sax H. A new model for studying nutrition in peritonitis. The adverse effect of overfeeding. Ann Surg. 1989;209:334–340. doi: 10.1097/00000658-198903000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN J Parenter Enteral Nutr. 1979;3:452–456. doi: 10.1177/014860717900300609. [DOI] [PubMed] [Google Scholar]
- 8.Fredrix EW, Soeters PB, von Meyenfeldt MF, Saris WH. Resting energy expenditure in cancer patients before and after gastrointestinal surgery. JPEN J Parenter Enteral Nutr. 1991;15:604–607. doi: 10.1177/0148607191015006604. [DOI] [PubMed] [Google Scholar]
- 9.Rutten P, Blackburn GL, Flatt JP, Hallowell E, Cochran D. Determination of optimal hyperalimentation infusion rate. J Surg Res. 1975;18:477–483. doi: 10.1016/0022-4804(75)90121-3. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa M, Nishioka M, Hanaki N, et al. Postoperative metabolic and circulatory responses in patients that express SIRS after major digestive surgery. Hepatogastroenterology. 2006;53:228–233. [PubMed] [Google Scholar]
- 11.Gazzaniga AB, Polachek JR, Wilson AF, Day AT. Indirect calorimetry as a guide to caloric replacement during total parenteral nutrition. Am J Surg. 1978;136:128–133. doi: 10.1016/0002-9610(78)90212-x. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki M, Johtatsu T, Kurihara M, et al. Energy metabolism in Japanese patients with Crohn’s disease. J Clin Biochem Nutr. 2010;46:68–72. doi: 10.3164/jcbn.09-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki M, Johtatsu T, Kurihara M, et al. Energy expenditure in Japanese patients with severe or moderate ulcerative colitis. J Clin Biochem Nutr. 2010;47:32–36. doi: 10.3164/jcbn.10-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weir JB. New methods for calculating metabolic rate with special reference to protein matabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falconer JS, Fearson KC, Plester CE, Ross JA, Carter DC. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg. 1994;219:325–331. doi: 10.1097/00000658-199404000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argilés JM, Alvarez B, López-Soriano FJ. The metabolic basis of cancer cachexia. Med Res Rev. 1997;17:477–498. doi: 10.1002/(sici)1098-1128(199709)17:5<477::aid-med3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Barak N, Wall-Alonso E, Sitrin MD. Evaluation of stress factors and body weight adjustments currently used to estimate energy expenditure in hospitalized patients. JPEN J Parenter Enteral Nutr. 2002;26:231–238. doi: 10.1177/0148607102026004231. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Kusama A, Ohkawa A, et al. Resting energy expenditure in patients undergoing transhiatal or transthoracic oesophagectomy for carcinoma of the thoracic oesophagus. Br J Surg. 1993;80:1413–1415. doi: 10.1002/bjs.1800801119. [DOI] [PubMed] [Google Scholar]
- 19.Sato N, Oyamatsu M, Tsukada K, Suzuki T, Hatakeyama K, Muto T. Serial changes in contribution of substrates to energy expenditure after transthoracic esophagectomy for cancer. Nutrition. 1997;13:100–103. doi: 10.1016/s0899-9007(96)00382-6. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki M, Okamoto H, Johtatsu T, et al. Resting energy expenditure in patients undergoing pylorus preserving pancreatoduodenectomies for bile duct cancer or pancreatic tumors. J Clin Biochem Nutr. 2011;48:183–186. doi: 10.3164/jcbn.10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouchi K, Sakai K, Matsubara S, Mikuni J, Katayose Y, Matsuno S. Fuel utilization and glucose hyperalimentation after liver resection. Nutrition. 1994;10:411–414. [PubMed] [Google Scholar]
- 22.Kunisaki C, Shimada H, Nomura M, et al. Immunonutrition risk factors of respiratory complications after esophagectomy. Nutrition. 2004;20:364–367. doi: 10.1016/j.nut.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Sane S, Baba M, Kusano C, Shirao K, Yamada H, Aikou T. Influence of exogenous fat emulsion on pulmonary gas exchange after major surgery. World J Surg. 2002;26:297–302. doi: 10.1007/s00268-001-0221-2. [DOI] [PubMed] [Google Scholar]
- 24.Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30:1592–1604. doi: 10.1007/s00268-005-0657-x. [DOI] [PubMed] [Google Scholar]
- 25.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P, Canadian Critical Care Clinical Practice Guidlines Committee Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 26.ASPEN Board of Directors and the Clinical Guidelines Task Force. Guideline for use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26(Suppl 1):1SA–138SA. [PubMed] [Google Scholar]
- 27.Weimann A, Braga M, Harsanyi L, et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr. 2006;25:224–244. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi H, Ikeuchi S, Kawaguchi Y, et al. Clinical significance of perioperative immunonutrition for patients with esophageal cancer. World J Surg. 2007;31:2160–2167. doi: 10.1007/s00268-007-9219-8. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura M, Iwahashi M, Takifuji K, et al. Optimal dose of preoperative enteral immunonutrition for patients with esophageal cancer. Surg Today. 2009;39:855–860. doi: 10.1007/s00595-009-3967-z. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig DJ, Thirlby RC, Low DE. A prospective evaluation of dietary status and symptoms after near-total oesophagectomy without gastric emptying procedure. Am J Surg. 2001;181:454–458. doi: 10.1016/s0002-9610(01)00600-6. [DOI] [PubMed] [Google Scholar]
- 31.Ryan AM, Rowley SP, Healy LA, Flood PM, Ravi N, Reynolds JV. Post-oesophagectomy early enteral nutrition via a needle catheter jejunostomy: 8-year experience at a specialist unit. Clin Nutr. 2006;25:386–393. doi: 10.1016/j.clnu.2005.12.003. [DOI] [PubMed] [Google Scholar]