Abstract
Crocetin is a natural carotenoid compound of gardenia fruits and saffron, which has various effects in biological systems. In this study, we investigated the antioxidant effects of crocetin on reactive oxygen species such as hydroxyl radical using in vitro X-band electron spin resonance and spin trapping. Crocetin significantly inhibited hydroxyl radical generation compared with the control. Moreover, we performed electron spin resonance computed tomography ex vivo with the L-band electron spin resonance imaging system and determined the electron spin resonance signal decay rate in the isolated brain of stroke-prone spontaneously hypertensive rats, a high-oxidative stress model. Crocetin significantly reduced oxidative stress in the isolated brain by acting as a scavenger of reactive oxygen species, especially hydroxyl radical, as demonstrated by in vitro and ex vivo electron spin resonance analysis. The distribution of crocetin was also determined in the plasma and the brain of stroke-prone spontaneously hypertensive rats using high-performance liquid chromatography. After oral administration, crocetin was detected at high levels in the plasma and the brain. Our results suggest that crocetin may participate in the prevention of reactive oxygen species-induced disease due to a reduction of oxidative stress induced by reactive oxygen species in the brain.
Keywords: crocetin, antioxidant, oxidative stress, brain, electron spin resonance (ESR)
Introduction
Reactive oxygen species (ROS) such as the superoxide (O2•−) and/or hydroxyl radical (HO•) have been implicated in the pathogenesis of various types of brain dysfunction including ischemia-reperfusion injury,(1) Alzheimer’s disease,(2) aging,(3) and other neurodegenerative disease.(4) Among the organs that can be affected by ROS-induced diseases, the brain is particularly susceptible to the effects of aging and oxidative stress.(5) The brain protective properties of several carotenoids are well known.(6–9) It has recently been reported that antioxidant carotenoids such as β-carotene and lycopene reduce ischemia-reperfusion injury of the brain via their antioxidant properties.(10,11)
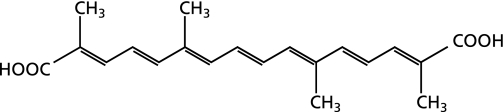
Crocetin is a natural carotenoid compound found in the stigmas of saffron (Crocus sativus L.) and the fruits of Gardenia jasminoides Ellis. This yellow compound has been used as an important spice and natural food colorant in various parts of the world.(12,13) In addition, saffron and gardenia fruits have been used as traditional medicine and crocetin is one of the major active compounds of these herbal medicines. Crocetin is an amphiphilic low-molecular weight carotenoid compound, as shown in Fig. 1. Extensive research on crocetin has indicated that it inhibits tumor promotion,(14) is hepatoprotective,(15) has neuroprotective potential,(16) exerts anti-inflammatory effects,(17) and is beneficial in cardiac diseases.(18) In a recent clinical studies, crocetin showed positive effects on asthenopia(19) and attenuating effects on physical fatigue.(20) Antioxidant potential of crocetin may be contributing to these pharmacological actions. However, there are almost no reports on a direct ROS scavenging effect of crocetin.
Fig. 1.
Chemical structure of crocetin.
We previously reported on the use of an electron spin resonance (ESR)-based technique for the detection of free radical reactions in biological systems.(21–26) Nitroxyl radicals are very useful as spin probes for measuring ROS distribution, oxygen concentration, and redox metabolism by in vivo ESR in biological systems.(21–26) It has been reported that the nitroxyl radical, referred to as a ’nitroxyl spin probe’, loses its ESR signal by rapidly reacting with HO• (k>109 M−1 s−1),(27,28) O2•− (k = 104–105 M−1 s−1) in the presence of thiols or NAD(P)H,(28) and other radicals such as alkyl (k = 107–109 M−1 s−1)(29) and lipid peroxyl radicals.(30) The signal decay rate of the nitroxyl spin probe provides evidence of ROS generation and changes in the redox status of biological systems.(31,32)
The stroke-prone spontaneously hypertensive rat (SHRSP) is a genetic model of spontaneous hypertension, stroke, and endothelial dysfunction.(33,34) It has several characteristics of increased oxidative stress.(21,35–38) The blood brain barrier-permeable nitroxyl spin probe 3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (MC-PROXYL) has been used for ESR assessment of oxidative stress in the rodent brain.(21,37,38) In the present study, we used the ESR technique to investigate the ROS scavenging effect of crocetin and the decay rate constant of MC-PROXYL in the isolated brain of the SHRSPs. In addition, we investigated the absorption and distribution of crocetin in the plasma and the brain following oral administration in SHRSPs. The results showed that oral administration of crocetin to SHRSPs was capable of reducing ROS-mediated oxidative stress in the brain due to a direct ROS-scavenging effect.
Materials and Methods
Reagents
Crocetin was provided by Riken Vitamin Corporation Limited (Tokyo, Japan). Hydrogen peroxide (H2O2) was purchased from Wako Pure Chem. Ind. (Osaka, Japan). The ESR spin trapping studies, using 5-(2,2-dimethyl-1,3-propoxycyclophosphoryl)-5-methyl-1-pyrroline-N-oxide (CYPMPO, Radical Research, Tokyo, Japan), indicated production of HO•. Pentobarbital sodium was purchased from Kyoritsu Seiyaku Co. (Tokyo, Japan). MC-PROXYL was synthesized from 3-carboxy-2,2,5,5-tetramethyl-pyrrolidine-1-oxyl (carboxy-PROXYL; Tokyo Kasei, Tokyo, Japan) by a method described previously.(25,37) All other reagents were analytical grades.
In vitro ESR measurement
HO• was generated by ultraviolet (UV, emission: 310–400 nm, 5 sec; 40 mW; SUPERCURE-203S, RU-360, Radical Research, Tokyo, Japan) irradiation of H2O2 as described previously.(21,39,40) Crocetin was prepared in 10% alkaline buffer (50 mM Na2B4O7-50 mM Na2CO3, pH 10.0). Other solutions were prepared in ultra-pure water. ESR spin-trapping was conducted with an ROS-generating system containing CYPMPO.(41) ESR observations were performed with a JES-RE 3X, X-band spectrometer (JEOL, Tokyo, Japan) connected to a WIN-RAD ESR Data Analyzer (Radical Research, Tokyo, Japan) at the following instrument settings: microwave power, 8.00 mW; magnetic field, 335.6 ± 7.5 mT; field modulation width, 0.079 mT; receiver gain, 200; sweep time, 1 min; and time constant, 0.03 sec. All experiments were repeated a minimum of 3 times. For each experiment, the effects of the compounds were calculated and presented as the percentage of the mean control value (designated as 100%).
Animal and ex vivo ESR-CT imaging measurements
The procedures used in this study were in accordance with the guidelines of the US National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication NO. 85–23, revised 1985) and the protocols were approved by the Animal Care Committees (Yokosuka, Japan). Male SHRSPs (6-weeks old) were purchased from Japan SLC (Shizuoka, Japan). Animals were housed in a light-controlled room with a 12-h light/dark cycle and were allowed ad libitum access to food and water. Crocetin was suspended in 0.5% (w/v) carboxymethylcellulose-sodium (CMC-Na) solution at a concentration of 10 mg/ml (crocetin/CMC-Na). We have previously confirmed that crocetin arrived at the maximum blood concentrations 90 min after oral administration (data not shown). Crocetin (100 mg/kg) or 0.5% CMC-Na solution was administered orally 90 min prior to measurement by ex vivo ESR. ESR-computed tomography (CT) imaging of the isolated rat brain was performed as follows. The rats were anesthetized with 50 mg/kg (i.p.) pentobarbital and injected with 140 mmol/l MC-PROXYL solution (10 mg/kg) i.v. via the tail vein. The brain was isolated 30 sec after the treatment and subsequently analyzed using ex vivo L-band ESR imaging, as described previously.(26)
Ex vivo ESR-CT imaging system constructed in our laboratory and JEOL ESR application laboratory software were used.(25,26,37,38) This system consists of a commercially available electromagnet (modified JES-RE 3X, JEOL, Tokyo, Japan), a pair of field scan coils, power supplies, a personal computer, and a 1-GHz microwave unit containing a 4-window loop-gap resonator (28 × φ43 mm, the measurement position centered on bregma). The system is provided with 4 different coil sets; 3 for the gradients (0.9 mT/cm, max) and 1 for rapid scanning. The gradient field was controlled by a current stabilizer linked with a personal computer (Dell Precision PWS 380).
The ESR-CT images were constructed on the basis of Lauterburg’s method,(42) known as a 3D zeugmatography. We applied linear magnetic field gradients along the x-, y-, and z-axes produced by the magnetic field gradient coils. For the 2D imaging, 36 projections alternating between gradient and non-gradient were acquired in 55 s. Each projection required 1,024 points of acquisition data for imaging. The ESR absorption spectra were obtained by integrating the derivative spectrum with the recorded gradient. The mid-field hyperfine line in the spectrum was separated from the triplet signal of the nitroxyl radicals. Each signal data set was convoluted with Shepp’s filter function into the Fourier domain before performing the inverse Fourier transformation to the spatial domain. The 2D imaging pictures of 512 × 512 points were obtained from 18 projections per gradient step at 10° in the spatial domain. Instrument settings for ESR detection of MC-PROXYL were as follows: microwave power, 20 mW; magnetic field, 31.0–34.0 ± 1.0 mT; field modulation width, 0.1 mT; receiver gain, 63–125; time constant, 0.01 sec; field intensity, 0.7 mT/cm.
Crocetin analysis in plasma and brain
Blood and brain was collected after crocetin administration orally 90 min later. After the collection of blood from common carotid artery, it was centrifuged at 1,500 g for 5 min at 4°C and plasma was separated. Brain was isolated after phosphate buffer saline perfused from the heart atrium. The samples were stored at −80°C. Plasma (100 µl) was mixed with 2.0 ml of methanol and centrifuged (3,000 rpm, 10 min). The supernatant was evaporated under nitrogen gas. We used the whole brain to analysis the crocetin distribution (control group: 1.44 ± 0.07 g wet weight, crocetin group; 1.67 ± 0.04 g wet weight) was homogenized in 2.0 ml of alkaline buffer and the homogenate was mixed with 6.0 ml of methanol/chloroform (1:1). The mixture was centrifuged (3,000 rpm, 10 min) and the supernatant was evaporated under nitrogen gas. The residue of plasma or brain was dissolved in 2.0 ml of alkaline buffer and loaded onto a solid-phase extraction cartridge (Oasis HLB Extraction Cartridge, Nihon Waters, Tokyo, Japan) pre-conditioned with methanol (2.0 ml) and alkaline buffer (2.0 ml). The cartridge was washed with water (2.0 ml) and hexane (2.0 ml). The analysis was eluted with methanol (2.0 ml) and the eluate was concentrated to dryness under nitrogen gas. The residue was reconstituted in 200 µl of methanol and filtered with a 0.45-µm Millipore filter for reversed-phase high performance liquid chromatography (HPLC) analysis. Crocetin was quantified by the HPLC method as described previously.(43) In recovery experiments, the recovery percentage of crocetin extracted from plasma and brain homogenate was determined to be 99% and 92%, respectively.
Statistical analysis
Results are expressed as mean ± SD. Student’s t test was used for comparisons between pairs of groups and Dunnet’s test was used for comparisons among 3 or more groups. Data were analyzed for statistical significance, and the significance level was set at p<0.05.
Results
Effects of crocetin on HO• generation by H2O2 with UV irradiation
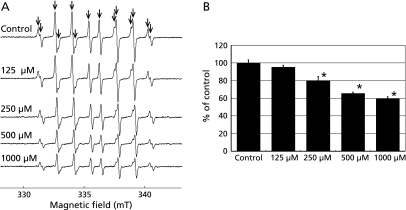
We investigated the effects of crocetin on HO•, which had been generated from H2O2 by UV irradiation, and by ESR spin trapping with CYPMPO. In agreement with our previous report,(41) we observed that H2O2 generated by UV irradiation in the presence of CYPMPO led to the formation of a characteristic CYPMPO-OH spin adduct spectrum with hyperfine splitting giving rise to 14 resolved peaks (Fig. 2A). The generation of HO• was not influenced by the 10% alkaline buffer (data not shown). As shown in Fig. 2B, CYPMPO-OH adduct formation was reduced in a dose-dependent manner by crocetin dissolved in 10% alkaline buffer (p<0.05). These data indicate that crocetin might be an effective HO• scavenger.
Fig. 2.
Effect of crocetin on HO• generation by H2O2 with UV irradiation. (A) ESR spin trapping measurement of HO• generated by H2O2 with UV irradiation for 5 sec with CYPMPO (5.0 mM) as the spin trap in the presence of crocetin (0, 125, 250, 500, 1,000 µM). (B) The effects of crocetin on HO• generation by H2O2 with UV irradiation. The signal intensity of the seventh peak of the spectrum was normalized as the relative height against the signal intensity of the control. Results are expressed as the percentage of the mean control value and are represented as mean ± SD. * significant difference (p<0.05) versus the corresponding control value.
Effects of crocetin on SHRSPs-induced oxidative stress in the brain
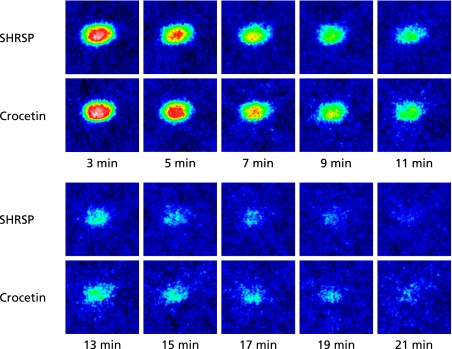
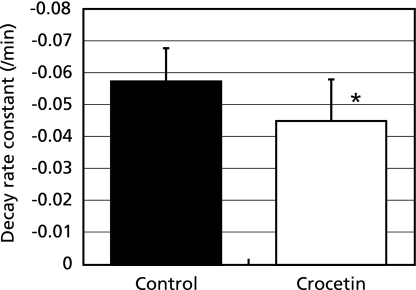
MC-PROXYL is a suitable spin probe for the study of free radical reactions in the brain by in vivo and ex vivo ESR detection.(26,38) The effect of crocetin on SHRSPs-induced oxidative stress in the brain was investigated using MC-PROXYL and the resulting spectra were analyzed with the ESR-CT imaging system. Administration of crocetin to SHRSPs significantly decreased the decay rate of the 2D ESR-CT image of MC-PROXYL in the isolated brain (Fig. 3). The signal decay rate of MC-PROXYL in this study was confirmed with preliminary data from a previous study usingESR-CT imaging with L-band ESR analysis. The signal decay rate constant of MC-PROXYL in brain of SHRSPs brain was significantly lower than that of the control (p<0.05) (Fig. 4).
Fig. 3.
ESR-CT imaging in isolated SHRSPs brain following crocetin administration. Effects of administration of crocetin on 2D ESR images (z–x plane) of MC-PROXYL distribution in isolated brain of SHRSPs. ESR was measured at 3, 5, 7, 9, 11, 13, 15, 17, 19 and 21 min after i.v. treatment with MC-PROXYL (isolated 30 sec after the treatment). The color scale shows 32 colors (white and 100 being the maximum ESR signal). The ESR images are reproduced in 32 colors with signals lower than 10% of the maximal signal intensity detected in all slices regarded as noise. Experimental conditions are described in Materials and Methods.
Fig. 4.
Effects of crocetin on oxidative stress in SHRSPs brain. SHRSPs were anesthetized with pentobarbital sodium (50 mg/kg, i.p.). The L-band ESR was used to determine the signal decay of MC-PROXYL in the isolated brain. Decay rate constants of MC-PROXYL after the administration; control (0.5% CMC-Na solution alone, open column), crocetin (suspended in 0.5% CMC-Na solution administration, closed column). The results are expressed as mean ± SD (n = 6). * significant difference (p<0.05) versus the corresponding value in the control group.
Crocetin analysis in plasma and brain
Crocetin was given to SHRSPs (n = 6) by oral administration at the same dose (100 mg/kg) used in the ESR experiments (Fig. 3 and 4). Plasma and brain were both collected 90 min after the administration of crocetin. The crocetin concentrations in plasma and brain measured by HPLC are shown in Table 1. These concentrations (about 0.14 mM in plasma and 2.43 nmol/g in brain) were significantly higher in the group that received crocetin as compared with the control group that did not receive crocetin.
Table 1.
Distribution of crocetin in plasma and brain
| Group | Concentration of crocetin | |
|---|---|---|
| Plasma | Control | nd |
| Crocetin | 0.14 ± 0.05* | |
| Brain | Control | nd |
| Crocetin | 2.43 ± 0.40* |
Plasma and brain crocetin concentrations (mean ± SD) after the administration of 100 mg/kg crocetin suspended in 0.5% CMC-Na solution (crocetin group) or 0.5% CMC-Na solution alone (control group). Crocetin was not detected (nd) in plasma and brain of the control group. Results are expressed as mmol/l (in plasma) or nmol/g (in brain) (n = 6, respectively). * significant difference (p<0.0001) versus the corresponding control value.
Discussion
Various ROS may be generated by essential metabolic processes or by environmental stress such as UV exposure. Although the ROS plays important roles to cell signaling, it also has a potential to cause significant cellular damage. The participation of ROS in the pathogenesis of many diseases including brain dysfunction has been suggested.(1–4) Thus, in order to prevent ROS-induced disease, supplementation of antioxidants such as vitamin C, vitamin E, and carotenoids has been proposed.(44,45)
Crocetin, a kind of carotenoid originally found in the dried stigma of saffron, has been used in the treatment of diversiform diseases for centuries.(46) It is well known that various carotenoids scavenge ROS such as HO• and O2•−.(6–8) The carotenoids β-carotene and lutein have also been reported to inhibit ROS generation.(47) In recent years, it has been suggested that crocetin might be an effective antioxidant to counter oxidative stress in a hemi-parkinsonian rat model.(16) However, the antioxidant activity of crocetin appeared to involve the activation of the endogenous antioxidant enzymatic activities such as superoxide dismutase (SOD) and glutathione peroxidase (GPx),(16,48) rather than a direct ROS scavenging effect. There are few reports of the scavenging activity for ROS of the crocetine. Tseng et al.(49) reported the scavenging activity against O2•− using xanthine/xanthine oxidase system. ESR is an invaluable technique that provides a direct means of measuring the antioxidant effects. In this in vitro X-band ESR study, crocetin reduced the generation of HO• from irradiation of H2O2 in a dose-dependent manner (Fig. 2). This study represents the first report of the scavenging effect of crocetin on HO•.
If we turn our attention to how our results may relate to the brain in vivo, it is critical to consider the relative concentration of the crocetin used in the present study. The concentration in rat brain of absorption of crocetin was about 2.43 nmol/g (Table 1), compared to the concentration of crocetin of 250 µM used in our in vitro experiments (Fig. 2). Indeed, it would be possibility that the scavenging effects of crocetin, much used in studies, may reach the brain. The redox potential of those unchanged crocetin and crocetin metabolites that reach the brain enables them to scavenge damaging radicals, but the endogenous brain antioxidants, especially ascorbate.(21) Regarding as the concentration of ROS generation on in vitro experiments, they would be much higher than in vivo situation. Brain tissue harvested from SHRSP is known to exhibit increased levels of oxidative stress.(21,35–38) Our present study has shown that the high concentration of crocetin treatment resulted in the recovery of normal levels of oxidative stress in the brain of SHRSP (Fig. 3 and 4). Other study revealed that crocetin would be helpful in preventing oxidative stress-induced neurologic disorder.(16,50) These results suggest the possibility that crocetin may show useful antioxidant activity for in vivo rodent model for human application. However, further studies will be required to examine the human application of crocetin upon oxidative stress-induced brain disease.
The involvement of O2•− in ischemia-reperfusion injury, stroke, and atherosclerosis is well known.(51) It is possible to generate HO• from O2•− via the Fenton reaction and/or Harber-Weiss reaction in biological systems.(52) These free radicals play an important role in brain damage after stroke. In addition to oxidizing macromolecules, leading to cell injury, oxidants are also involved in cell death/survival signaling pathways and cause mitochondrial dysfunction.(1) Various antioxidants have been investigated for the treatment of stroke.(9,35) Crocetin, like other carotenoids, has the potential to be an effective treatment for diseases related to ROS, such as stroke, ischemia-reperfusion injury, and atherosclerosis.
The SHRSP is a well-known model for atherosclerosis(53) and is useful for the study of oxidative stress caused by the generation of O2•− and HO• in the brain. Our research group previously reported the utility of quantitative ESR analysis with MC-PROXYL for the assessment of redox status under conditions of oxidative stress in SHRSPs brain.(21,37,38) Spontaneous ROS generation has been demonstrated in association with ischemia-reperfusion injury accompanying atherosclerosis in SHRSPs brain.(36,53) In this study, we used ex vivo ESR-CT imaging to demonstrate the ability of crocetin (at a dose of 100 mg/kg) to reduce ROS generation and decrease the decay rate constant of MC-PROXYL in SHRSPs brain (Fig. 3 and 4). We assessed the metabolic fate and the bioavailability of crocetin in the plasma and the brain of SHRSPs and found that the compound was detected in both within 90 min after oral administration (Table 1). This result suggested that orally administrated crocetin may cross the blood-brain barrier and distribute to the brain. Taken together, these results indicate that crocetin attenuates oxidative stress in the isolated brain of SHRSPs.
In conclusion, the present study demonstrated that crocetin exhibits antioxidant properties by scavenging ROS, and that it may reduce oxidative stress induced by ROS generation in the isolated brain of SHRSPs. By extension, crocetin might be able to prevent ROS-related brain diseases such as stroke.
Abbreviations
- CMC-Na
carboxymethylcellulose-sodium
- CT
computed tomography
- CYPMPO
5-(2,2-dimethyl-1,3-propoxycyclophosphoryl)-5-methyl-1-pyrroline-N-oxide
- ESR
electron spin resonance
- GPx
glutathione peroxidase
- H2O2
hydrogen peroxide
- HO•
hydroxyl radical
- HPLC
high performance liquid chromatography
- MC-PROXYL
3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl
- O2•−
superoxide
- ROS
reactive oxygen species
- SHRSP
stroke-prone spontaneously hypertensive rat
- SOD
superoxide dismutase
- UV
ultraviolet
References
- 1.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 2.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 4..Evans PH. Free radicals in brain metabolism and pathology. Br Med Bull. 1993;49:577–587. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- 5.Kimoto-Kinoshita S, Nishida S, Tomura TT. Age-related change of antioxidant capacities in the cerebral cortex and hippocampus of stroke-prone spontaneously hypertensive rats. Neurosci Lett. 1999;273:41–44. doi: 10.1016/s0304-3940(99)00623-0. [DOI] [PubMed] [Google Scholar]
- 6.Andersen HR, Andersen O. Effects of dietary alpha-tocopherol and beta-carotene on lipid peroxidation induced by methyl mercuric chloride in mice. Pharmacol Toxicol. 1993;73:192–201. doi: 10.1111/j.1600-0773.1993.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 7.Bezerra Rde S, Abadie-Guedes R, Melo FR, Paiva AM, Amancio-Dos-Santos A, Guedes RC. Shrimp carotenoids protect the developing rat cerebral cortex against the effects of ethanol on cortical spreading depression. Neurosci Lett. 2005;391:51–55. doi: 10.1016/j.neulet.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 8.den Heijer T, Launer LJ, de Groot JC, et al. Serum carotenoids and cerebral white matter lesions: the Rotterdam scan study. J Am Geriatr Soc. 2001;49:642–646. doi: 10.1046/j.1532-5415.2001.49126.x. [DOI] [PubMed] [Google Scholar]
- 9.Kheir-Eldin AA, Motawi TK, Gad MZ, Abd-ElGawad HM. Protective effect of vitamin E, beta-carotene and N-acetylcysteine from the brain oxidative stress induced in rats by lipopolysaccharide. Int J Biochem Cell Biol. 2001;33:475–482. doi: 10.1016/s1357-2725(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 10.Hosseini F, Naseri MK, Badavi M, Ghaffari MA, Shahbazian H, Rashidi I. Effect of beta carotene on lipid peroxidation and antioxidant status following renal ischemia/reperfusion injury in rat. Scand J Clin Lab Invest. 2010;70:259–263. doi: 10.3109/00365511003777810. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao G, Fong TH, Tzu NH, Lin KH, Chou DS, Sheu JR. A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo. 2004;18:351–356. [PubMed] [Google Scholar]
- 12.Selim K, Tsimidou M, Biliaderis CG. Kinetic studies of degradation of saffron carotenoids encapsulated in amorphous polymer matrices. Food Chemistry. 2000;71:199–206. [Google Scholar]
- 13.Watanabe T, Terabe S. Analysis of natural food pigments by capillary electrophoresis. J Chromatogr A. 2000;880:311–322. doi: 10.1016/s0021-9673(00)00209-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang CJ, Lee MJ, Chang MC, Lin JK. Inhibition of tumor promotion in benzo[a]pyrene-initiated CD-1 mouse skin by crocetin. Carcinogenesis. 1995;16:187–191. doi: 10.1093/carcin/16.2.187. [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Hsu JD, Lin JK. Suppression of aflatoxin B1-induced hepatotoxic lesions by crocetin (a natural carotenoid) Carcinogenesis. 1991;12:1807–1810. doi: 10.1093/carcin/12.10.1807. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad AS, Ansari MA, Ahmad M, et al. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav. 2005;81:805–813. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen XC, Lu Y, Qian ZY. Effects of crocetin on the matrix metalloproteinases in cardiac hypertrophy induced by norepinephrine in rats. J Asian Nat Prod Res. 2006;8:201–208. doi: 10.1080/10286020412331286452. [DOI] [PubMed] [Google Scholar]
- 19.Kajita M, Umigai N, Nakano T, Amano H, Takeno R, Kajimoto O. Effect on asthenopia of high-crocetin-content Gardenia Jasminides Ellis extraction. Jpn J Vis Sci. 2007;28:77–84. (in Japanese) [Google Scholar]
- 20.Mizuma H, Tanaka M, Nozaki S, et al. Daily oral administration of crocetin attenuates physical fatigue in human subjects. Nutr Res. 2009;29:145–150. doi: 10.1016/j.nutres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Yoshino F, Takahashi SS, et al. Direct assessments of the antioxidant effects of propofol medium chain triglyceride/long chain triglyceride on the brain of stroke-prone spontaneously hypertensive rats using electron spin resonance spectroscopy. Anesthesiology. 2008;109:426–435. doi: 10.1097/ALN.0b013e318182a903. [DOI] [PubMed] [Google Scholar]
- 22.Lee C, Miura K, Liu X, Zweier JL. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:38965–38972. doi: 10.1074/jbc.M006341200. [DOI] [PubMed] [Google Scholar]
- 23.Lee CI, Liu X, Zweier JL. Regulation of xanthine oxidase by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:9369–9376. doi: 10.1074/jbc.275.13.9369. [DOI] [PubMed] [Google Scholar]
- 24.Lee MC, Shoji H, Komatsu T, Yoshino F, Ohmori Y, Zweier JL. Inhibition of superoxide generation from fMLP-stimulated leukocytes by high concentrations of nitric oxide or peroxynitrite: characterization by electron spin resonance spectroscopy. Redox Rep. 2002;7:271–275. doi: 10.1179/135100002125000776. [DOI] [PubMed] [Google Scholar]
- 25.Lee MC, Shoji H, Miyazaki H, et al. Measurement of oxidative stress in the rodent brain using computerized electron spin resonance tomography. Magn Reson Med Sci. 2003;2:79–84. doi: 10.2463/mrms.2.79. [DOI] [PubMed] [Google Scholar]
- 26.Yoshino F, Lee M-C-i, Kobayashi K, Hayashi Y, Aruoma OI. Assessment of the effect of fermented papaya preparation on oxidative damage in spontaneously hypertensive rat brain using electron spin resonance (ESR) imaging and L-band ESR spectroscopy. Journal of Functional Foods. 2009;1:375–380. [Google Scholar]
- 27.Asmus KD, Nigam S. Kinetics of nitroxyl radical reactions. A pulse-radiolysis conductivity study. Int J Radiat Biol Relat Stud Phys Chem Med. 1976;29:211–219. doi: 10.1080/09553007614550241. [DOI] [PubMed] [Google Scholar]
- 28.Takeshita K, Saito K, Ueda J, Anzai K, Ozawa T. Kinetic study on ESR signal decay of nitroxyl radicals, potent redox probes for in vivo ESR spectroscopy, caused by reactive oxygen species. Biochim Biophys Acta. 2002;1573:156–164. doi: 10.1016/s0304-4165(02)00420-8. [DOI] [PubMed] [Google Scholar]
- 29.J. Chateauneuf JL, K. U. Ingold. Absolute rate constants for the reactions of some carbon-centered radicals with 2,2,6,6-tetramethyl-1-piperidinoxyl. J Org Chem. 1988;53:1629–1632. [Google Scholar]
- 30.Takahashi M, Tsuchiya J, Niki E. Scavenging of radicals by vitamin E in the membranes as studied by spin labeling. J Am Chem Soc. 1989;111:6350–6353. [Google Scholar]
- 31.Gomi F, Utsumi H, Hamada A, Matsuo M. Aging retards spin clearance from mouse brain and food restriction prevents its age-dependent retardation. Life Sci. 1993;52:2027–2033. doi: 10.1016/0024-3205(93)90687-x. [DOI] [PubMed] [Google Scholar]
- 32.Miura Y, Ozawa T. Noninvasive study of radiation-induced oxidative damage using in vivo electron spin resonance. Free Radic Biol Med. 2000;28:854–859. doi: 10.1016/s0891-5849(00)00162-3. [DOI] [PubMed] [Google Scholar]
- 33.Coyle P. Dorsal cerebral collaterals of stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar Kyoto rats (WKY) Anat Rec. 1987;218:40–44. doi: 10.1002/ar.1092180108. [DOI] [PubMed] [Google Scholar]
- 34.Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H2455–H2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606–611. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 36.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004;109:2357–2362. doi: 10.1161/01.CIR.0000128695.49900.12. [DOI] [PubMed] [Google Scholar]
- 37.Lee MC, Shoji H, Miyazaki H, et al. Assessment of oxidative stress in the spontaneously hypertensive rat brain using electron spin resonance (ESR) imaging and in vivo L-Band ESR. Hypertens Res. 2004;27:485–492. doi: 10.1291/hypres.27.485. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki H, Shoji H, Lee MC. Measurement of oxidative stress in stroke-prone spontaneously hypertensive rat brain using in vivo electron spin resonance spectroscopy. Redox Rep. 2002;7:260–265. doi: 10.1179/135100002125000758. [DOI] [PubMed] [Google Scholar]
- 39.Ogasawara Y, Namai T, Yoshino F, Lee MC, Ishii K. Sialic acid is an essential moiety of mucin as a hydroxyl radical scavenger. FEBS Lett. 2007;581:2473–2477. doi: 10.1016/j.febslet.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai K, Sasabe H, Koga T, Konishi T. Mechanism of hydroxyl radical scavenging by rebamipide: identification of mono-hydroxylated rebamipide as a major reaction product. Free Radic Res. 2004;38:487–494. doi: 10.1080/1071576042000209808. [DOI] [PubMed] [Google Scholar]
- 41.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Radic Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 42.Lauterbur PC. Progress in n.m.r. zeugmatography imaging. Philos Trans R Soc Lond B Biol Sci. 1980;289:483–487. doi: 10.1098/rstb.1980.0066. [DOI] [PubMed] [Google Scholar]
- 43.Umigai N, Murakami K, Ulit MV, et al. The parmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine. 2010 doi: 10.1016/j.phymed.2010.10.019. In press, [DOI] [PubMed] [Google Scholar]
- 44.Riccioni G, Bucciarelli T, Mancini B, Di Ilio C, Capra V, D’Orazio N. The role of the antioxidant vitamin supplementation in the prevention of cardiovascular diseases. Expert Opin Investig Drugs. 2007;16:25–32. doi: 10.1517/13543784.16.1.25. [DOI] [PubMed] [Google Scholar]
- 45.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 46.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med (Maywood) 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 47.Iannone A, Rota C, Bergamini S, Tomasi A, Canfield LM. Antioxidant activity of carotenoids: an electron-spin resonance study on beta-carotene and lutein interaction with free radicals generated in a chemical system. J Biochem Mol Toxicol. 1998;12:299–304. doi: 10.1002/(sici)1099-0461(1998)12:5<299::aid-jbt6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 48.Shen XC, Qian ZY. Effects of crocetin on antioxidant enzymatic activities in cardiac hypertrophy induced by norepinephrine in rats. Pharmazie. 2006;61:348–352. [PubMed] [Google Scholar]
- 49.Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995;97:61–67. doi: 10.1016/0304-3835(95)03964-x. [DOI] [PubMed] [Google Scholar]
- 50.Abe K, Sugiura M, Shoyama Y, Saito H. Crocin antagonizes ethanol inhibition of NMDA receptor-mediated responses in rat hippocampal neurons. Brain Res. 1998;787:132–138. doi: 10.1016/s0006-8993(97)01505-9. [DOI] [PubMed] [Google Scholar]
- 51.Maier CM, Chan PH. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- 52.Motohashi N, Mori I. Superoxide-dependent formation of hydroxyl radical catalyzed by transferrin. FEBS Lett. 1983;157:197–199. doi: 10.1016/0014-5793(83)81144-2. [DOI] [PubMed] [Google Scholar]
- 53.Yamori Y, Murakami S, Nara Y, Ikeda K. Stroke-prone SHR and arteriolipidosis-prone SHR as models for atherosclerosis: their mechanisms and application for nutritional and pharmacological studies. Clin Exp Pharmacol Physiol Suppl. 1995;22:S244–S245. doi: 10.1111/j.1440-1681.1995.tb02901.x. [DOI] [PubMed] [Google Scholar]