Abstract
Açai (Euterpe oleracea Mart.) has recently emerged as a promising source of natural antioxidants. Because increased oxidative stress and impaired antioxidant defense mechanisms are important factors in the development of diabetic complications and many health claims have been reported for açai, the present study was undertaken to evaluate the possible protective effects of açai on the production of reactive oxygen species by neutrophils and on the liver antioxidant defense system in control and streptozotocin-induced diabetic rats. Diet supplementation with 2% açai was found to increase mRNA levels for gamma-glutamylcysteine synthetase and glutathione peroxidase in liver tissue and to decrease reactive oxygen species production by neutrophils. Compared to control animals, diabetic rats exhibited lower levels of mRNA coding for Zn-superoxide dismutase, glutathione peroxidase and gamma-glutamylcysteine synthetase and higher levels of reactive oxygen species production by neutrophils, thiobarbituric acid-reactive substances and carbonyl proteins in hepatic tissues. Although açai supplementation was not effective in restore gene expression of antioxidant enzymes in diabetic rats, it showed a protective effect, decreasing thiobarbituric acid-reactive substances levels and increasing reduced glutathione content in the liver. These findings suggest that açai can modulate reactive oxygen species production by neutrophils and that it has a significant favorable effect on the liver antioxidant defense system under fisiological conditions of oxidative stress and partially revert deleterious effects of diabetes in the liver.
Keywords: açai, antioxidant enzymes expression, neutrophils, diabetes, rats
Introduction
Açai berry is the fruit of the Euterpe oleracea Mart. palm tree, a species that is native to the Amazon region. In recent years, açai has been the subject of much attention due to its high antioxidant capacity and its role as a ”functional food”.(1,2) Açai is currently one of the main export products of the Amazon region; it is widely distributed and commercialized as frozen pulp, juice or wine.(3) Biochemical studies have revealed that açai is rich in phytochemicals, especially polyphenols such as anthocyanins, proanthocyanidins and other flavonoids.(4) A variety of assays show that açai pulp has a high in vitro antioxidant capacity, especially against superoxide and peroxyl radicals; its antioxidant capacity is the highest of any fruit reported to date in the literature.(5)
Recent studies have shown that supplementation of the diet with açai pulp improved lipid and oxidative stress biomarker profiles in sera of hypercholesterolemic rats(6) and that it increased the longevity of flies fed a high-fat diet through the activation of transcription of genes coding for proteins that function in oxidative stress response pathways.(7) A clinical trial has shown that consumption of açai pulp and juice resulted in a significant increase in the plasma antioxidant capacity of healthy human volunteers.(8) Açai pulp also showed a protective effect against hydrogen peroxide-mediated damage to lipids and proteins in the cerebral cortex, hippocampus and cerebellum of rats(9) and against DNA damage induced by doxorubicin (DXR) in liver and kidney of mice.(3) Furthermore, açai extract showed antiproliferative and proapoptotic activity against C-6 rat brain carcinoma cells,(10) as well as anti-inflammatory properties demonstrated by its ability to inhibit the activity of cyclooxygenases (COX)-1 and COX-2 in cell culture.(5) Many of these effects are attributed to the polyphenol fraction of açai.
Diabetes mellitus is a chronic metabolic disorder that affects more than 170 million people worldwide.(11) Diabetes is characterized by hyperglycemia and insufficient insulin production or action and is associated with damage to and dysfunction of several body tissues, altering quality of life and longevity.(12) Oxidative stress plays a central role in the pathogenesis and development of the complications of diabetes. Disease progression is usually accompanied by increased production of reactive oxygen species and/or reduction in the efficiency of antioxidant defense systems.(13) The mechanisms involved in the increased oxidative stress associated with diabetic complications are partially known; they include activation of transcription factors, formation of advanced glycation end products and activation of protein kinase C.(14)
Many in vivo and in vitro studies have been conducted in conjunction with the search for new treatments for the control of diabetes. Currently used therapies include insulin and several synthetic anti-diabetic drugs used alone or in combination, and many of the available anti-diabetic drugs possess a number of adverse side effects.(15) The fact that management of diabetes without causing side effects is still a challenge has generated a growing interest in the use of natural antioxidants as a strategy to reduce the occurrence of diabetic complications. Dietary compounds such as polyphenols can play an important role in the improvement of antioxidant status because they are able to neutralize reactive oxygen species (ROS), acting as metal ion-chelating agents and enzyme modulators.(16) These compounds can therefore protect against complications of diabetes caused by increase in oxidative stress. However, the effect of açai on diabetes-induced oxidative stress is still unknown.
The aim of this study is to investigate in vivo the effects of açai supplementation on ROS production in neutrophils and on oxidant/antioxidant balance and regulation of gene expression of antioxidant enzymes in livers of control and diabetic rats, representing normal physiological conditions and conditions of potentially high oxidative stress.
Materials and Methods
Reagents and açai pulp
DPPH (2,2-diphenyl-1-picrylhydrazyl), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) and gallic acid were purchased from Sigma–Aldrich (St. Louis, MO). Pasteurized açai pulp (Euterpe oleracea Mart.) was obtained from Icefruit Comércio de Alimentos Ltda. (Tatuí, SP, Brazil). For the phytochemical and antioxidant capacity determination analyses, the pulp was centrifuged (2,000 g at 4°C for 15 min) to remove solid residues and lipid, and the supernatant was collected and filtered.
Phytochemical composition of açai pulp
The total polyphenol content of açai pulp was determined by the Folin–Ciocalteu method as described by George et al.(17) Briefly, 2.5 mL of Folin reagent diluted in distilled water (1:10) was added to 500 µL of the diluted sample or of a standard solution of gallic acid. The blank consisted of distilled water. After 2 min at room temperature, 2 mL of 7.5% sodium carbonate solution was added and mixed vigorously. After incubation at 50°C for 15 min, the mixture was placed in an ice bath. Absorbance at 760 nm relative to the blank was determined. All analyses were performed in triplicate. Total polyphenol content was expressed in milligrams of gallic acid equivalent (GAE) per 100 g of fresh pulp.
Total anthocyanin content was determined by the differential pH method.(18) Diluted samples were added to 0.025 M chloride buffer (pH 1.0) and 4.0 M sodium acetate buffer (pH 4.5). Absorbances were determined simultaneously as absorption maxima for the visible light spectrum and at 700 nm after incubation in the dark for 30 min at room temperature. Total anthocyanin content was expressed in milligrams of cyanidin-3-glucoside equivalent per 100 g of fresh pulp. A molar absorptivity of 26,900 M−1cm−1 and a molecular mass of 449.2 g/mol were used for cyanidin-3-glucoside.
DPPH-radical-scavenging activity
The DPPH Radical Scavenging Activity of açai pulp was determined using a modified method of Brand-Williams et al.(19) In short, 100 µL of different concentrations of açai pulp (0, 1, 2.5, 5 and 10%) and the standard antioxidant Trolox (0, 50, 75, 100, 125, 150, 175 and 200 mg/L) were added to 3.9 mL of 60 µM DPPH dissolved in 80% methanol. The mixture was homogenized and kept in the dark for 30 min at room temperature. The absorbance of the solution at 515 nm was determined. Methanol (80%) was used as a blank. Antioxidant activity was determined by the reduction in absorbance of the DPPH radical at 515 nm; the percentage inhibition was determined according to the formula below.
| % Scavenging activity = (1 – ASample 515/AControl 515) × 100 |
Animals and experimental design
Female Fisher rats, weighing on average 180 g, were obtained from the Experimental Nutrition Laboratory of the Federal University of Ouro Preto (UFOP). The animals were habituated in polypropylene boxes, maintained in an environment controlled for temperature, light and humidity and given food and water ad libitum. The Ethics Committee on Animal Use of the Federal University of Ouro Preto approved all animal procedures. The animals were divided into four groups, control (C), açai (A), diabetic (D), diabetic + açai (DA), according to the treatment received. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ) (35 mg/kg body weight) in 0.1 M of citrate buffer, pH 4.5. Rats in groups C and A were injected with vehicle only. The animals were considered diabetic if blood glucose was higher than 15 mM 72 h after STZ injection. Animals in groups C and D were fed a standard diet (AIN-93);(20) those in groups A and DA were given the standard diet with 2% (w/w) açai pulp added. After 30 days of treatment, the animals were fasted overnight, anesthetized with isofluorane and euthanized by exsanguination. The liver was removed, immersed in liquid nitrogen and immediately stored at −80°C for subsequent analysis.
Glucose, insulin and fructosamine plasma levels
Glucose, fructosamine and insulin levels were determined by the Kits Labtest (Lagoa Santa, MG, Brazil) and the Ultra-Sensitive Rat Insulin Elisa Kit (Crystal Chem, Downers Grove, IL), respectively.
ROS production by neutrophils
Isolation of neutrophils.
Blood was obtained by exsanguination of the brachial plexus and collected in heparinized tubes. Neutrophils were isolated using two different density gradients, Monopaque (d = 1.08) and Leucopaque (d = 1.12), in accordance with the procedures described by Bicalho et al.(21) with minor adjustments. The cell viability of each sample was greater than 95% as determined by the exclusion test with trypan blue.
Chemiluminescence assay.
To measure ROS production, chemiluminescence assays were carried out as described by Chaves et al.(22) For each assay, 1 × 106 neutrophils were incubated in Hank’s solution, pH 7.4, with 500 µl of luminol (10−4 M). Photon emission was determined each minute for 30 min using a luminometer (Lumat, LB 9507, Berthold, Germany). The values were expressed as relative units of light/min (RLU/min).
Antioxidant defenses and oxidative stress biomarkers in liver homogenate
Catalase (CAT) activity was determined according to Aebi,(23) whose method is based on the enzymatic decomposition of H2O2 observed spectrophotometrically at 240 nm for 5 min. Ten µL of homogenate supernatant was added to a cuvette containing 100 mM phosphate buffer (pH 7.2) and the reaction was initiated by the addition of 10 mM H2O2. Hydrogen peroxide decomposition was calculated using the molar absorption coefficient 39.4 M−1cm−1. The results were expressed as activity per milligram of protein. One unit of CAT is equivalent to the hydrolysis of 1 µmol of H2O2 per min.
The total glutathione content of liver homogenates was determined using a kit (CS0260) from Sigma (St. Louis, MO). This assay uses a kinetic method based on the reduction of DTNB (5,5'-dithiobis-(2-nitrobenzoic acid) to TNB (5-thio-2-nitrobenzoic acid), which can be determined spectrophotometrically at 412 nm.
The level of thiobarbituric acid reactive substances (TBARS) was estimated by the method of Buege and Aust.(24) Liver homogenate supernatants were mixed with TCA (28% w/v in 0.25 N HCl), TBA (1% in 0.25 M acetic acid) and BHT (125 mM in ethanol), heated for 15 min at 95°C and then placed in an ice bath. Precipitated material was removed by centrifugation, and the absorbance of the sample at 535 nm was determined. The TBARS level was calculated using the molar absorption coefficient of MDA (154,000 M−1cm−1).
Carbonyl protein levels were determined according to the method described by Levine.(25) Each sample was precipitated with 10% (w/v) TCA. After centrifugation, the precipitate was treated with 10 mmol of DNPH in 2 N HCl, incubated in the dark for 30 min and then treated with 10% TCA. After centrifuging, the precipitate was washed twice with ethanol/ethyl acetate (1:1) and dissolved in 6% SDS. Absorbance was determined at 370 nm. The results were expressed in nmol of DNPH incorporated/mg of protein. The content of DNPH incorporated was calculated using the molar absorption coefficient of DNPH (22,000 M−1cm−1). Total protein content was determined according to the method described by Lowry et al.,(26) using bovine serum albumin (BSA) as a standard.
Real time quantitative RT-PCR assay
Total RNA was isolated from liver tissue of rats using the RNAgents Total RNA Isolation System (Promega Corporation, Madison, WI) according to the manufacturer’s instructions. cDNA was synthesized from 2 µg of total RNA with random primers using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), following the manufacturer’s recommendations. Real-time PCR was performed with the Power SYBR® Green PCR Master Mix reagent (Applied Biosystems, Foster City, CA) in a final reaction volume of 12 µL; the reaction included 1 µL of cDNA and 0.5 µL of each primer (forward and reverse, 10 µM). The primers for PCR were designed according to the published nucleotide sequences(27) for CAT, glutathione peroxidase (GPx), gamma-glutamylcysteine synthetase (γ-GCS), Zn-superoxide dismutase (SOD), Mn-SOD and GAPDG. The reactions were carried out under the following conditions: 50°C for 2 min, 95°C for 10 min and then 40 cycles of 95°C for 15 sec (denaturation) and 60°C for 1 min (primer annealing and product extension). The specificity of the products obtained was confirmed by analysis of dissociation curves of the amplified product. The data obtained were analyzed using the comparative CT method. Target gene expression was determined relative to the expression of the endogenous GAPDH gene. All analyses were performed in triplicate.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Data were subjected to the Kolmogorov–Smirnov test for normality. The Student’s t test was used for data with normal distributions. Differences were considered significant for p<0.05. All analyses were conducted using the software GraphPad Prism version 5.00 for Windows (San Diego, CA).
Results
Phytochemical composition and DPPH radical-scavenging activity
Table 1 shows the total polyphenol and anthocyanin content of açai pulp. The ability of four different concentrations of açai pulp to neutralize the DPPH radical was determined. The ability of a given sample to reduce the absorbance of DPPH is indicative of its capacity to neutralize free radicals. All concentrations tested showed a high radical neutralization capacity, similar to that of the standard antioxidant Trolox in the range of 50 to 200 mg/L.
Table 1.
Content of total phenolics and total anthocyanins and DPPH radical-scavenging activity of açai pulp
| Compounds | Concentration |
|---|---|
| Total phenolic (mg GAE/100 g) | 118.3 ± 0.96 |
| Total anthocyanins (mg/100 g) | 28.36 ± 0.69 |
| DPPH radical scavenging activity | Inhibition % |
| Açai (v/v) | |
| 1% | 20.91 ± 0.72 |
| 2.5% | 35.09 ± 0.54 |
| 5% | 54.71 ± 0.32 |
| 10% | 78.16 ± 0.94 |
| Trolox (mg/L) | |
| 50 | 19.57 ± 0.44 |
| 75 | 29.43 ± 0.11 |
| 100 | 38.04 ± 0.22 |
| 125 | 45.42 ± 0.55 |
| 150 | 55.36 ± 0.94 |
| 175 | 69.10 ± 1.09 |
| 200 | 78.34 ± 1.21 |
DPPH, 2,2-diphenyl-1-picrylhydrazyl; Trolox, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. Values are expressed as mean ± SD. All mean values are from triplicate determinations. Total phenolics are expressed in milligrams of gallic acid equivalents (GAE) per 100 g of açai pulp.
Glucose profile, body weight and liver weight
The results presented in Table 2 show that diabetic rats had reduced body weight, increased glucose and fructosamine levels and reduced insulin levels compared to control animals (Table 2). Glucose levels in diabetic rats were 4.4 times higher than in control animals, and insulin levels were 4.4 times lower. Dietary supplementation with 2% açai pulp did not affect the levels of glucose, insulin, fructosamine, or body weight in diabetic and control rats. There was no significant difference in liver weight in animals in the different groups.
Table 2.
Effects of açai pulp on glucose profile, body and liver weight in control and diabetic rats
| Variables | Experimental Groups |
|||
|---|---|---|---|---|
| Control | Açai | Diabetic | Diabetic + Açai | |
| Body weight (g) | 199.24 ± 19.87 | 203.13 ± 25.33 | 130.00 ± 10.77*** | 126.25 ± 11.3*** |
| Glucose (mmol/L) | 5.36 ± 0.89 | 5.68 ± 0.94 | 23.52 ± 3.06*** | 24.67 ± 2.57*** |
| Plasma insulin (pmol/L) | 65.20 ± 45.80 | 88.21 ± 42.99 | 14.71 ± 4.31* | 12.54 ± 7.49** |
| Fructosamine (µmol/L) | 7.77 ± 1.38 | 8.35 ± 1.00 | 16.01 ± 2.81*** | 16.27 ± 2.39*** |
| Liver weight (g) | 4.83 ± 0.63 | 4.86 ± 0.75 | 4.94 ± 0.41 | 4.85 ± 0.41 |
Values are expressed as mean ± SD (n = 8). *Significant at p<0.05 with respect to control group. **Significant at p<0.01 with respect to control group. ***Significant at p<0.001 with respect to control group.
ROS production by neutrophils
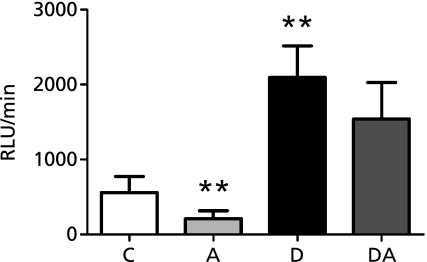
To investigate the antioxidant properties of açai pulp in vivo, ROS production was measured in neutrophils isolated from peripheral blood of experimental animals. Relative to neutrophils from control rats, neutrophils from diabetic rats showed a 3.7-fold increase in ROS production; however, ROS production in neutrophils of diabetic animals that received a diet supplemented with açai did not show a significant difference from the control. Addition of açai pulp to the diet of the non-diabetic animals caused an approximately 2.6-fold reduction in ROS production by neutrophils relative to the control group (Fig. 1).
Fig. 1.
Effects of açai supplementation on ROS production by neutrophils of control and diabetic rats. The data are expressed as mean ± SD (n = 8). C, control group; A, açai group; D, diabetic group; DA, diabetic + açai group. **Significant at p<0.01 with respect to control group.
Antioxidant defenses and biomarkers of oxidative stress
To evaluate the effects of dietary supplementation with açai pulp on hepatic antioxidant defenses, total glutathione level and CAT activity were determined. Açai pulp supplementation increased the total hepatic glutathione content approximately 1.6- and 1.7-fold in control and diabetic rats, respectively. CAT activity did not show significant differences between groups.
TBARS levels and carbonyl protein content are widely used as biomarkers of lipid peroxidation and oxidative modification in proteins, respectively.(28) Compared to control animals, diabetic rats showed an increase of 1.6-fold in TBARS levels and 2.1-fold in carbonyl protein levels. Adding 2% açai pulp to the diet significantly reduced TBARS levels in diabetic rats to levels similar to those observed in the controls. Supplementation with açai also reduced the levels of carbonyl protein 1.7-fold relative to the controls (Table 3).
Table 3.
Effects of açai pulp on antioxidant defenses and on biomarkers of oxidative stress in control and diabetic rats
| Variables | Experimental Groups |
|||
|---|---|---|---|---|
| Control | Açai | Diabetic | Diabetic + Açai | |
| Catalase (U/mg protein) | 102.62 ± 18.69 | 92.38 ± 10.06 | 91.66 ± 16.76 | 81.27 ± 20.02 |
| Glutathione (nmol/mL) | 31.82 ± 10.17 | 51.43 ± 8.08** | 41.96 ± 16.27 | 54.21 ± 10.27** |
| Carbonyl protein (nmol/mg protein) | 4.72 ± 1.98 | 2.75 ± 0.46* | 9.79 ± 2.14** | 8.31 ± 1.70* |
| TBARS (nmol/mg protein) | 0.34 ± 0.03 | 0.38 ± 0.06 | 0.56 ± 0.18* | 0.40 ± 0.07# |
TBARS, thiobarbituric acid-reactive substances. Values are expressed as mean ± SD (n = 8). *Significant at p<0.05 with respect to control group. **Significant at p<0.01 with respect to control group. #Significant at p<0.05 with respect to diabetic group.
Real-time reverse-transcriptase-polymerase chain reaction
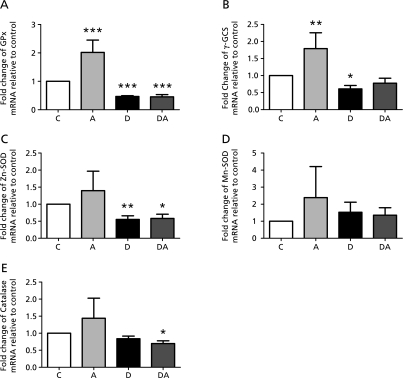
To investigate the molecular mechanism of the antioxidant effects of açai pulp on the liver, expression of the genes for the antioxidant enzymes γ-GCS, GPx, CAT, Zn-SOD and Mn-SOD was evaluated by qRT-PCR. The results in Fig. 2 show that adding açai pulp to the diet significantly increased the expression of γ-GCS and GPx, approximately 1.8- and 2-fold, respectively, relative to the control. Diabetic rats showed decreases in γ-GCS, GPx and Zn-SOD expression compared to control animals. The mRNA levels for γ-GCS in diabetic rats that received açai supplementation did not differ from those in controls, but CAT expression was significantly lower in these animals than in controls. The mRNA levels for Mn-SOD were not significantly different between groups (Fig. 2).
Fig. 2.
Effects of açai supplementation on mRNA expression of liver antioxidant enzymes in control and diabetic rats. (A) glutathione peroxidase, (B) gamma-glutamylcysteine synthetase, (C) Zn-superoxide dismutase, (D) Mn-superoxide dismutase, (E) Catalase. The data are expressed as mean ± SD (n = 6). C, control group; A, açai group; D, diabetic group; DA, diabetic + açai group. *Significant at p<0.05 with respect to control group, **Significant at p<0.01 with respect to control group, ***Significant at p<0.001 with respect to control group.
Discussion
ROS are continually formed as by-products of aerobic metabolism; these compounds are known to carry out physiological functions as well as to have deleterious effects.(29) Under physiological conditions, ROS are effectively neutralized by enzymes of the antioxidant defense system and glutathione (GSH)-related enzymes, including SOD, CAT, GPx and γ-GCS.
Overproduction of ROS results in oxidative stress, which can cause significant damage to cellular proteins, lipids and DNA. Oxidative stress has been associated with the development and progression of complications of diabetes.(13) Therefore, this study aimed to investigate the effects of açai supplementation on ROS production in neutrophils and on oxidant/antioxidant balance and gene expression of hepatic antioxidant defense system enzymes in control and diabetic rats.
The liver is the principal organ involved in oxidative and detoxification processes. In the initial stages of many diseases, oxidative stress biomarkers are elevated in the liver.(30) Experimental data show that the liver is subject to damage mediated by ROS in diabetes.(31,32) The results presented here show that an increase in hepatic oxidative stress in diabetic rats led to a reduction in the expression of mRNAs coding for the antioxidant enzymes Zn-SOD, GPx and γ-GCS. Because changes in the hepatic oxidant/antioxidant balance can affect the translocation of transcription factors sensitive to the redox state to the nucleus, the decrease in the mRNA levels of antioxidant enzymes that occurs in diabetes may be due to the oxidation of transcription factors that are responsible for initiating the transcription of antioxidant enzymes.(33)
SOD converts the superoxide anion into hydrogen peroxide, which is neutralized in the presence of water and molecular oxygen by the activity of CAT and GPx. The isoforms of SOD are located in different cell compartments. CuZn-SOD is found in the cytosol and in the nucleus, while Mn-SOD is the mitochondrial isoform. Our data corroborate recent studies that show decreased Zn-SOD mRNA and protein expression in the livers of diabetic rats,(33) mRNA expression for Mn-SOD did not change under the same conditions.(34) These results suggest that CuZn-SOD is more sensitive than Mn-SOD to oxidative stress caused by diabetes.
Several studies have shown decreased GPx activity in hepatic tissue of diabetic rats.(31,32,35) Nevertheless, the reported effects of oxidative stress caused by diabetes on the gene expression of GPx vary considerably. Matsunami et al.(36) found an increase in GPx mRNA expression in livers of diabetic rats. Another study reported no differences in the hepatic levels of mRNA for GPx in diabetic rats compared to controls.(34) These variations may be explained by differences in the experimental conditions, such as time since onset of diabetes and age of the experimental animals.
Açai supplementation was not effective in reverse these changes on gene expression in liver tissue of diabetic rats. Similarly, Sadi et al.(33) and Sadi and Guray(34) evaluated the effects of supplementation with the antioxidants vitamin C and alpha lipoic acid on gene expression of antioxidant enzymes in the STZ-induced diabetic rat liver tissues and also found no differences in both mRNA and protein expressions of Zn-SOD and GPx between diabetic control group and the diabetic supplemented groups.
The cellular redox environment is influenced by the production and removal of ROS.(37) The increase in ROS seen in diabetes can affect cellular signaling pathways and gene expression. Thus, even if exogenous antioxidants may provide benefit in attenuating oxidative stress, it becomes difficult to provide antioxidant in concentrations sufficient to completely restore physiologic redox status, since these cannot be regenerated enzymatically as glutathione. In this way, since patterns of gene expression have been altered by oxidative stress in diabetes, may not be possible to reverse this process and restore normal patterns of gene expression.(38) This may be the cause for which açai supplementation did not have a substantial effect on liver antioxidant enzymes gene expression in diabetic rats, and however, led to changes in the mRNA levels of antioxidant enzymes in control rats.
Polyphenols are the main phytochemical components found in açai pulp, most notably flavonoids and anthocyanins. The predominant anthocyanins in açai are cyanidin-3-glucoside and cyanidin-3-rutenoside.(3,4,39,40) Others anthocyanins are found in minor amounts as cyanidin-3-sambubioside, peonidin-3-glucoside and peonidin-3-rutenoside. Major non-anthocyanin polyphenolic components found in acai include flavonoids such as homoorientin, orientin, isovitexin, quercetin and procyanidins, phenolic acids and lignans.(2,4,40,41)
Many dietary polyphenols have antioxidant activity, and this activity is generally attributed to their ability to directly neutralize pro-oxidant reactive species. Experimental data indicate that polyphenols can offer indirect protection against oxidative stress through the activation of gene transcription for enzymes that make up the endogenous antioxidant defense system.(42) However, the effect of açai consumption on the expression of mRNAs coding for antioxidant enzymes has not been investigated. This study demonstrated the induction of gene expression of the hepatic antioxidant enzymes γ-GCS and GPx by dietary supplementation with açai pulp. Our results suggest that açai plays a role in the up-regulation of the endogenous antioxidant defense system and has a potential protective effect against hepatic oxidative stress in vivo. In this regard, many reports show that modulation of antioxidant enzymes by flavonoids such as procyanidins and quercetin may be also important in their antioxidant effects in liver cells.(43,44)
The induction of expression of the antioxidant defense system enzymes by polyphenols obtained from the diet mainly results from activation mediated by the transcription factor Nrf2 through interaction with the antioxidant response element (ARE), which is found in the promoter region of many genes that are induced by changes in the redox state.(42)
In vivo experiments demonstrated the induction of mRNA expression of enzymes CuZnSOD, GPx and CAT, accompanied by an increase in Nrf2 protein levels in heart tissue of control rats following oral administration of phenolic acids for 14 days.(45)
Moreover, recent studies have reinforced the role of activation of the antioxidant response element by quercetin and other flavonoids in stimulating γ-GCS gene transcription in COS-1 and HepG2 cells.(46) This enzyme catalyzes the rate-limiting step in the synthesis of glutathione, the most prevalent endogenous cellular antioxidant.
Glutathione is an intracellular reducing agent that plays a central role in antioxidant defense by detoxifying ROS directly or by a mechanism catalyzed by GPx. Several studies have demonstrated induction of liver GSH by phytochemical agents.(47,48) Our results show that açai supplementation increases total hepatic glutathione levels in control and diabetic rats. The high glutathione content found in the liver after açai supplementation may reflect an increase in the antioxidant status of this tissue. Roig et al.(49) have demonstrated that the main detoxification pathway for lipid peroxidation products involves glutathione conjugation. Consequently, the increase in hepatic glutathione levels may have significantly contributed to the reduction of TBARS levels in diabetic rats receiving diets supplemented with açai. Açai supplementation also decreased hepatic carbonyl protein levels in control rats; this reduction may be associated with increased hepatic antioxidant status in these animals, evidenced by the induction of antioxidant enzyme expression in this organ.
Although diabetes increases cellular ROS levels by a variety of mechanisms, an important source of increased ROS in diabetes is the NADPH oxidase of neutrophils.(50) Activation of neutrophils leads to the production of ROS through oxidative metabolism; this involves activation of the NADPH oxidase enzyme complex, which catalyzes the reduction of oxygen to superoxide ion. It has been shown that the hyperglycemia associated with diabetes mellitus results in the activation of neutrophils(51) and that this activation contributes to an increase in oxidative stress that is partly responsible for diabetes complications. Our results corroborate this hypothesis in that neutrophils from diabetic rats showed a significant increase in the production of ROS, while neutrophils from diabetic rats supplemented with açai did not differ from controls in ROS production. Açai supplementation also reduced ROS production by neutrophils of control rats. Recent in vitro studies have demonstrated an inhibitory effect of açai on ROS production by neutrophils isolated from healthy humans.(2,4,52) Here, we show for the first time that açai supplementation significantly reduces ROS production in neutrophils in vivo. These data indicate a possible modulating effect of açai on ROS production in neutrophils.
In this study, the intraperitoneal administration of STZ effectively induced diabetes mellitus in rats. The STZ-induced diabetes experimental model displays most of the complications of diabetes that are mediated by oxidative stress.(13) As expected, diabetic rats showed significant increases in plasma glucose, reduction in insulin levels and increased levels of fructosamine, a biomarker used to determine the degree of protein glycosylation in diabetes. Diabetes induced by STZ also led to a sharp reduction in body weight. The weight loss typically associated with diabetes is due to increased muscle catabolism.(48) Although açai supplementation did not promote significant changes in the glucose profiles of diabetic rats, the results obtained in relation to oxidant/antioxidant balance indicate that açai consumption may play a protective role against diabetes complications associated with oxidative stress.
In conclusion, this study demonstrates that dietary supplementation with açai pulp not only acts as an antioxidant but also can modulate ROS production by neutrophils and improve the liver oxidant/antioxidant balance by the induction of mRNA expression of antioxidant enzymes under physiologic condition of oxidative stress. However, antioxidant properties of acai pulp in vivo appear to involve different mechanisms for the observed effects in different physiologic and pathologic condition of oxidative stress. Such differences may be due to changes in cellular signaling pathways and gene expression patterns related to diabetes.
Due to the complexity of the antioxidant defense system and involvement of multiple pathways in the increase ROS formation in diabetes, may be particularly difficult a dietary antioxidant reverse the adverse effects of oxidative stress. Although it may not be possible to completely reverse diabetic complications, açai attenuated oxidative stress in the liver through the reduction of TBARS and increased glutathione content.
Acknowledgments
This work was supported by FAPEMIG (Research Support Foundation of Minas Gerais State) – Process CDS-APQ-02832-09, Brazil. J.F.C.G and C.L.B.M were sponsored by a fellowship from CAPES.
We are grateful to Dr. José Augusto Nogueira-Machado for the density gradients and to the laboratories of the Research Center in Biological Sciences (NUPEB) of the Federal University of Ouro Preto for technical support.
Abbreviations
- A
açai group
- ARE
antioxidant response element
- BHT
butylated hydroxytoluene
- BSA
bovine serum albumin
- C
control group
- CT
threshold cycle
- COX
cyclooxygenase
- D
diabetic group
- DA
diabetic + açai group
- DNPH
2,4-dinitrophenylhydrazine
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- DTNB
5,5-dithiobis(2-nitrobenzoic acid)
- DXR
doxorubicin
- GAE
gallic acid equivalent
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
reduced glutathione
- GPx
glutathione peroxidase
- MDA
malondialdehyde
- Nrf2
NF-E2-related factor-2
- qPCR
quantitative reverse transcription polymerase chain reaction
- ROS
reactive oxygen species
- STZ
streptozotocin
- SOD
superoxide dismutase
- TBA
thiobarbituric acid
- TBARS
thiobarbituric acid reactive substances
- TCA
trichloroacetic acid
- γ-GCS
gamma-glutamylcysteine synthetase
References
- 1.Marcason W. What is the açaí berry and are there health benefits? J Am Diet Assoc. 2009;109:1968. doi: 10.1016/j.jada.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Kang J, Li Z, Wu T, Jensen GS, Schauss AG, Wu X. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.) Food Chem. 2010;122:610–617. [Google Scholar]
- 3.Ribeiro JC, Antunes LM, Aissa AF, et al. Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay. Mutat Res. 2010;695:22–28. doi: 10.1016/j.mrgentox.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Schauss AG, Wu X, Prior RL, et al. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleracea Mart. (acai) J Agric Food Chem. 2006;54:8598–8603. doi: 10.1021/jf060976g. [DOI] [PubMed] [Google Scholar]
- 5.Schauss AG, Wu X, Prior RL, et al. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (acai) J Agric Food Chem. 2006;54:8604–8610. doi: 10.1021/jf0609779. [DOI] [PubMed] [Google Scholar]
- 6.de Souza MO, Silva M, Silva ME, Oliveira RP, Pedrosa ML. Diet supplementation with acai (Euterpe oleracea Mart.) pulp improves biomarkers of oxidative stress and the serum lipid profile in rats. Nutrition. 2010;26:804–810. doi: 10.1016/j.nut.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Sun X, Seeberger J, Alberico T, et al. Açai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp. Gerontol. 2010;45:243–251. doi: 10.1016/j.exger.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, et al. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem. 2008;56:7796–7802. doi: 10.1021/jf8007037. [DOI] [PubMed] [Google Scholar]
- 9.Spada PD, Dani C, Bortolini GV, Funchal C, Henriques JA, Salvador M. Frozen fruit pulp of Euterpe oleraceae Mart. (Açai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J Med Food. 2009;12:1084–1088. doi: 10.1089/jmf.2008.0236. [DOI] [PubMed] [Google Scholar]
- 10.Hogan S, Chung H, Zhang L, et al. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010;118:208–214. [Google Scholar]
- 11.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 12.Khan A, Zaman G, Anderson RA. Bay leaves improve glucose and lipid profile of people with Type 2 diabetes. J Clin Biochem Nutr. 2009;44:52–56. doi: 10.3164/jcbn.08-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 14.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 15.Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TPA. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodovici M, Guglielmi F, Casalini C, Meoni M, Cheynier V, Dolara P. Antioxidant and radical scavenging properties in vitro of polyphenolic extracts from red wine. Eur J Nutr. 2001;40:74–77. doi: 10.1007/pl00007386. [DOI] [PubMed] [Google Scholar]
- 17.Georgé S, Brat P, Alter P, Amiot MJ. Rapid determination of polyphenols and vitamin C in plant-derived products. J Agric Food Chem. 2005;53:1370–1373. doi: 10.1021/jf048396b. [DOI] [PubMed] [Google Scholar]
- 18.Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE, Acree TE, An H, et al., editors. Current Protocols in Food Analytical Chemistry. New York: Wiley; 2001. pp. F1.2.1–F.2.13. [Google Scholar]
- 19.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. [Google Scholar]
- 20.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 21.Bicalho HM, Gontijo MC, Nogueira-Machado JA. A simple technique for simultaneous human leuckocytes separation. J Immunol. 1981;40:115–116. doi: 10.1016/0022-1759(81)90087-9. [DOI] [PubMed] [Google Scholar]
- 22.Chaves MM, Rocha-Vieira E, Pereira dos Reis A, et al. Increase of reactive oxygen (ROS) and nitrogen (RNS) species generated by phagocyting granulocytes related to age. Mech Ageing Dev. 2000;119:1–8. doi: 10.1016/s0047-6374(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 23.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 25.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Xiong Q, Xie P, Li H, et al. Acute effects of microcystins exposure on the transcription of antioxidant enzyme genes in three organs (liver, kidney, and testis) of male Wistar rats. J Biochem Mol Toxicol. 2010;24:361–367. doi: 10.1002/jbt.20347. [DOI] [PubMed] [Google Scholar]
- 28.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 29.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Stadler K, Jenei V, Bolcsházy G, Somogyi A, Jakus J. Increased nitric oxide levels as an early sign of premature aging in diabetes. Free Radic Biol Med. 2003;35:1240–1251. doi: 10.1016/s0891-5849(03)00499-4. [DOI] [PubMed] [Google Scholar]
- 31.Manna P, Das J, Ghosh J, Sil PC. Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IkappaBalpha/NF-kappaB, MAPKs, and mitochondria-dependent pathways: prophylactic role of arjunolic acid. Free Radic Biol Med. 2010;48:1465–1484. doi: 10.1016/j.freeradbiomed.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Shanmugama KR, Mallikarjuna K, Nishanth K, Kuo CH, Reddy KS. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011;124:1436–1442. [Google Scholar]
- 33.Sadi G, Yılmaz O, Güray T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu–Zn SOD and catalase. Mol Cell Biochem. 2008;309:109–116. doi: 10.1007/s11010-007-9648-6. [DOI] [PubMed] [Google Scholar]
- 34.Sadi G, Güray T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: effect of antioxidants. Mol Cell Biochem. 2009;327:127–134. doi: 10.1007/s11010-009-0050-4. [DOI] [PubMed] [Google Scholar]
- 35.Shukri R, Mohamed S, Mustapha NM. Cloves protect the heart, liver and lens of diabetic rats. Food Chem. 2010;122:1116–1121. [Google Scholar]
- 36.Matsunami T, Sato Y, Sato T, Ariga S, Shimomura T, Yukawa M. Oxidative stress and gene expression of antioxidant enzymes in the streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 2009;3:177–188. [PMC free article] [PubMed] [Google Scholar]
- 37.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 39.Pacheco-Palencia LA, Hawken P, Talcott ST. Phytochemical, antioxidant and pigment stability of acai (Euterpe oleracea Mart.) as affected by clarification, ascorbic acid fortification and storage. Food Research International. 2007;40:620–628. [Google Scholar]
- 40.Pacheco-Palencia LA, Duncan CE, Talcott ST. Phytochemical composition and thermal stability of two commercial acai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009;115:1199–1205. [Google Scholar]
- 41.Chin YW, Chai HB, Keller WJ, Kinghorn AD. Lignans and other constituents of the fruits of Euterpe oleracea (Açai) with antioxidant and cytoprotective activities. J Agric Food Chem. 2008;56:7759–7764. doi: 10.1021/jf801792n. [DOI] [PubMed] [Google Scholar]
- 42.Masella R, Benedetto RD, Varì R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Puiggros F, Llópiz N, Ardévol A, Bladé C, Arola L, Salvadó MJ. Grape seed procyanidins prevent oxidative injury by modulating the expression of antioxidant enzyme systems. J Agric Food Chem. 2005;53:6080–6086. doi: 10.1021/jf050343m. [DOI] [PubMed] [Google Scholar]
- 44.Crespo I, García-Mediavilla MV, Almar M, et al. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem Toxicol. 2008;46:1555–1569. doi: 10.1016/j.fct.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Yeh CT, Ching LC, Yen GC. Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J Nutr Biochem. 2009;20:163–171. doi: 10.1016/j.jnutbio.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Myhrstad MC, Carlsen H, Nordström O, Blomhoff R, Moskaug JØ. Flavonoids increases the intracellular glutathione level by transactivation of the g-glutamylcysteine synthetase catalytic subunit promoter. Free Radic Biol Med. 2002;32:386–393. doi: 10.1016/s0891-5849(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 47.Codoñer-Franch P, Muñiz P, Gasco E, Domingo JV, Valls-Belles V. Effect of a diet supplemented with α-Tocopherol and β-Carotene on ATP and antioxidant levels after hepatic ischemia-reperfusion. J Clin Biochem Nutr. 2008;43:13–18. doi: 10.3164/jcbn.2008038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravi K, Ramachandran B, Subramanian S. Protective effect of Eugenia jambolana seed kernel on tissue antioxidants in streptozotocin-induced diabetic rats. Biol Pharm Bull. 2004;27:1212–1217. doi: 10.1248/bpb.27.1212. [DOI] [PubMed] [Google Scholar]
- 49.Roig R, Cascón E, Arola L, Bladé C, Salvadó MJ. Procyanidins protect Fao cells against hydrogen peroxide-induced oxidative stress. Biochim Biophys Acta. 2002;1572:25–30. doi: 10.1016/s0304-4165(02)00273-8. [DOI] [PubMed] [Google Scholar]
- 50.Shurtz-Swirski R, Sela S, Herskovits AT, et al. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care. 2001;24:104–110. doi: 10.2337/diacare.24.1.104. [DOI] [PubMed] [Google Scholar]
- 51.Karima M, Kantarci A, Ohira T, et al. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol. 2005;78:862–870. doi: 10.1189/jlb.1004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Honzel D, Carter SG, Redman KA, Schauss AG, Endres JR, Jensen GS. Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J Agric Food Chem. 2008;56:8319–8325. doi: 10.1021/jf800401d. [DOI] [PubMed] [Google Scholar]