Abstract
The aim of this study was to evaluate the glycemic index and peak incremental indices of six popular fruits in Taiwan, comparing healthy subjects (n = 20) and patients with Type 2 diabetes (n = 17). The six kinds of fruits tested were grapes, Asian pears, guavas, golden kiwifruit, lychees and bananas. Glycemic index values were tested according to the standard glycemic index testing protocol. The glycemic index and peak incremental indices were calculated according to published formulas. In Type 2 diabetes subjects, the glycemic index values of grapes, Asian pears, guavas, golden kiwifruit, lychees and bananas were 49.0 ± 4.5, 25.9 ± 2.9, 32.8 ± 5.2, 47.0 ± 6.5, 60.0 ± 8.0 and 41.3 ± 3.5. In healthy subjects, the glycemic index values were 49.1 ± 7.3, 18.0 ± 5.4, 31.1 ± 5.1, 47.3 ± 12.1, 47.9 ± 6.8 and 35.1 ± 5.6. There was no significant difference in glycemic index values between healthy and Type 2 diabetes subjects. There was also no significant difference in PII when comparing healthy subjects and subjects with Type 2 diabetes. In conclusion, glycemic index and peak incremental indices in healthy subjects can be approximately the same for Type 2 diabetes.
Keywords: glycemic index, peak incremental indices, diabetes
Introduction
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin activity, or both.(1) The incidence of Type 2 diabetes is increasing dramatically.(2) The growing prevalence of Type 2 diabetes is associated with the obesity epidemic, and diagnosis is occurring at increasingly younger ages.(3) The major goal of Type 2 diabetes management is to better regulate the blood sugar and prevent or slow the development of complications, such as retinopathy, nephropathy, neuropathy and CVD.(4) Numerous studies have demonstrated dietary intervention is a critical factor in Type 2 diabetes management. Some researchers have recommended low-glycemic index (GI) diets as a strategy for improving diabetes.(5–7)
The GI of foods is defined as the carbohydrates in different foods measured according to time-integrated effect on postprandial glycemia.(8) Foods with high GI are more rapidly digested and absorbed, so they result in higher postprandial blood glucose levels per unit of carbohydrate and a faster rise in blood glucose levels than do foods with lower GI.(9) Research on GI has shown that different carbohydrate foods produce significantly different glycemic responses and postprandial glycemic excursions.(10) This is important in the context of diabetes management, because excessive rise in postprandial glycemia puts patients with diabetes at greater risk of developing complications.(11)
Similarly, peak incremental indices (PII) are also used to evaluate postprandial glycemia and the glycemic effects of different foods.(12) Jennie et al.(13) studied the use of low GI diets for the management of diabetes. They found that low GI diets improved overall glycemic control in individuals with diabetes, as assessed by GI and PII. Nalysnyk et al.(14) demonstrated that better daily control of blood glucose excursions, especially in the postprandial period, could be a predictor of the rate of diabetic complications, independent of HbA1c levels, in patients with Type 2 diabetes. In addition, the American Diabetes Association has suggested that the use of low-GI foods may reduce postprandial hyperglycemia.(15)
Fruits are a food rich in carbohydrates. The carbohydrates in fruit are glucose, fructose and sucrose. According to Atkinson’s study(16) the GI values of glucose, fructose and sucrose are 103, 15 and 65, respectively. Several studies have shown that the different amount and percentage of monosaccharides, disaccharides and dietary fiber in fruit will affect its GI value.(17–18)
Although GI and PII are two widely-used methods to evaluate the postprandial glycemia and glycemic effects of different foods, little research has been done on whether there is any difference between the GI values and PII in healthy subjects and Type 2 diabetes patients. This study aimed to measure and compare the GI and PII of six kinds of fruit in healthy subjects and patients with Type 2 diabetes in Taiwan.
Materials and Methods
Subjects
Twenty healthy non-diabetic people with a mean age of 21.9 years, and 17 patients with Type 2 diabetes and a mean age of 57.5 years, were enrolled in this study. For Type 2 diabetes patients, the exclusion criteria were as follows: HbA1c level higher than 7.5%; complications, such as nephropathy; and insulin-dependence. In addition, subjects were excluded if they were smokers, taking prescription medication, and dieting. The study was approved by the Institutional Review Board of Taipei Medical University Hospital and written informed consent was obtained from every subject. The characteristics of all subjects are listed in Table 1 and 2.
Table 1.
Characteristics of healthy subjects
| Females (n = 11) | Males (n = 9) | |
|---|---|---|
| Age (years) | 21.0 ± 1.1 | 21.9 ± 1.8 |
| Fasting blood glucose (mg/dl) | 85.6 ± 5.3 | 90.5 ± 1.3 |
| Height (cm) | 160.7 ± 5.0 | 176.3 ± 4.8 |
| Weight (kg) | 51 ± 6.5 | 69.2 ± 7.5 |
| BMI (kg/m2) | 19.7 ± 1.6 | 22.2 ± 2.2 |
| Body fat (%) | 26.2 ± 5.1 | 20.3 ± 5.8 |
Values are mean ± SD. BMI: body mass index.
Table 2.
Characteristics of Type 2 diabetes subjects
| Females (n = 9) | Males (n = 8) | |
|---|---|---|
| Diabetic duration (years) | 3.6 ± 3.4 | 5.4 ± 5.3 |
| HbA1c (%) | 6.7 ± 0.4 | 6.8 ± 0.6 |
| Age (years) | 52.9 ± 10.5 | 57.5 ± 6.4 |
| Fasting blood glucose (mg/dl) | 122.2 ± 31.2 | 121.8 ± 24.8 |
| Height (cm) | 156.7 ± 4.6 | 169.9 ± 3.3 |
| Weight (kg) | 58.5 ± 8.0 | 70.8 ± 5.8 |
| BMI (kg/m2) | 23.8 ± 2.1 | 24.6 ± 2.0 |
| Body fat (%) | 33.8 ± 4.0 | 23.6 ± 3.4 |
Values are mean ± SD. HbA1c: hemoglobin A1c, BMI: body mass index.
Following a study by Wu,(19) we choose two kinds of fruit: grapes (with a higher GI value: 49.1) and Asian pears (with a lower GI value: 18.0) as our test fruits. In addition, the cognition of fruits of type 2 diabetes patients will affect their choice for fruits. In this study, we wanted to know the type 2 diabetes patients’ attitudes toward various fruits in Taiwan. So, the 17 Type 2 diabetes patients were asked to fill out a questionnaire about their attitudes toward various fruits. This questionnaire listed most popular fruits in Taiwan, and the Type 2 diabetes subjects had to answer ”What are the fruits that Type 2 diabetes patients can not eat, in your opinion” (choose at least five kinds), and ”What are the fruits that Type 2 diabetes patients can eat, in your opinion” (choose at least five kinds). Based on the results of the questionnaire, we chose four kinds of fruit as the test fruits (two ”can not eat”, and two ”can eat” fruits). Thus, the GI and PII of a total of six fruits were included in this study for measurement of GI and PII.
The GI values of the six fruits were tested according to the standard GI testing protocol [http://www.saiglobal.com/shop/script/details.asp?DocN=AS0733779662AT]; the reference food was 50 ml 50% glucose solution (25 g glucose). Briefly, on each test day, all subjects consumed fruit containing 25 g carbohydrates with 100 ml water in 15 min. One edible portion of fruit that contained 25 g carbohydrates was calculated using the amount of carbohydrates listed for each in the Taiwanese Nutrient Database [http://www.doh.gov.tw/FoodAnalysis/ingredients.htm]. Venous blood was sampled in a heparin-containing tube at 0 (start of ingestion), 30, 45, 60, 90 and 120 min after ingestion. Blood samples were centrifuged (1400 × g for 10 min at 4°C) to obtain plasma. Blood sugar was measured using a commercial kit (glucose oxidase and peroxidase, Randox Lab-Ltd.). To calculate GI values, the area under curve (AUC) must be identified in advance. AUC refers to the area included between the baseline and incremental blood glucose points when connected by straight lines. The GI of a food is identified as(20): The area under the glycemic curve of the test food/area under the glycemic curve of glucose. For PII calculation, PII is defined as the ratio of the maximal increment of plasma glucose produced by tested fruits to that produced by glucose.(21) The formula is as follow: maximal increment produced by the test food/maximal increment produced by glucose (maximal increment means the difference between the peak point and the fasting point).
Statistics
The results were analyzed by analysis of variance (ANOVA) and student’s t test using SPSS. The results are presented as mean ± SD or mean ± SEM. A value of p<0.05 was considered significant.
Results
Table 1 and 2 showed the characteristics of healthy and type 2 diabetes subjects. Healthy non-diabetic people with a mean age of 21.9 years, and patients with Type 2 diabetes have a mean age of 57.5 years. In fasting blood glucose level aspect, the fasting blood glucose level in healthy non-diabetic people is 87.8 ± 5.0 mg/dl, significantly lower than that in patients with Type 2 diabetes (122.0 ± 27.5 mg/dl). There are no significantly differences of height, weight, BMI and body fat between two groups.
In the questions regarding Type 2 diabetes, the top five fruits patients identified as ”the fruits that Type 2 diabetes patients can not eat, in my opinion” were lychees, longan, custard apples, bananas and mangos. The top five fruits they identified as ”the fruits that Type 2 diabetes patients can eat, in my opinion” were guava, golden kiwifruit, tomatoes, apples and dragon fruit. Based on these results, we chose guavas, golden kiwifruit, lychees and bananas as our other four kinds of test fruits.
The GI value of six kinds of fruits: grapes, Asian pears, guavas, golden kiwifruit, lychees and bananas in all subjects are listed in Table 3. The GI value of grapes, Asian pears, guavas, golden kiwifruit, lychees and bananas in Type 2 diabetes subjects was 49.0 ± 4.5, 25.9 ± 2.9, 32.8 ± 5.2, 47.0 ± 6.5, 60.0 ± 8.0 and 41.3 ± 3.5, respectively. In healthy subjects, the GI value of these six fruits was 49.1 ± 7.3, 18.0 ± 5.4, 31.1 ± 5.1, 47.3 ± 12.1, 47.9 ± 6.8 and 35.1 ± 5.6, respectively. There was no significant difference between healthy subjects and those with Type 2 diabetes in the GI values of these six fruits.
Table 3.
Glycemic index of six fruits in all subjects
| Healthy subjects (n = 20) | Type 2 diabetes subjects (n = 17) | |
|---|---|---|
| Grapes | 49.1 ± 7.3 | 49.0 ± 4.5 |
| Asian pears | 18.0 ± 5.4 | 25.9 ± 2.9 |
| Guavas | 31.1 ± 5.1 | 32.8 ± 5.2 |
| Golden kiwifruit | 47.3 ± 12.1 | 47.0 ± 6.5 |
| Lychees | 47.9 ± 6.8 | 60.0 ± 8.0 |
| Bananas | 35.1 ± 5.6 | 41.3 ± 3.5 |
Values are mean ± SEM.
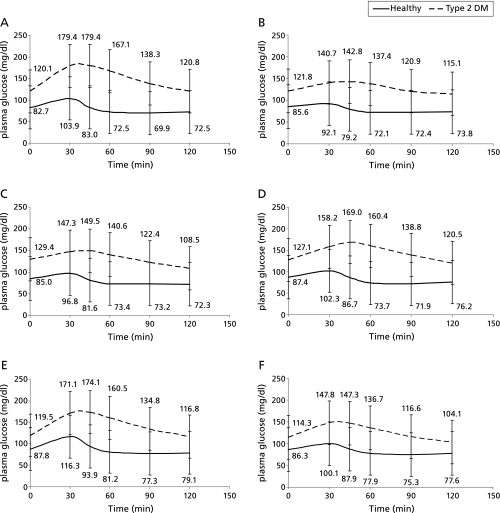
The postprandial glycemia curves for each fruit in healthy and Type 2 diabetes subjects are shown in Fig. 1. The peak points of postprandial glycemia were very similar in healthy and Type 2 diabetes subjects after 30 to 45 min. In healthy subjects, the blood sugar returned to fasting level within sixty minutes. However, in Type 2 diabetes subjects, the rate of reduction of postprandial glycemia was slower than in healthy subjects, and the blood sugar in most of the Type 2 diabetes patients returned to fasting level about within 120 min. The PII of healthy and Type 2 diabetes subjects are listed in Table 4. We found no significant difference between healthy and Type 2 diabetes subjects in the PII for these six fruits.
Fig. 1.
Postprandial glycemia curves of tested fruits in Type 2 diabetes subjects and healthy subjects. (A) grapes (B) Asian pears (C) guavas (D) golden kiwifruit (E) lychees (F) bananas.
Table 4.
The peak incremental index of six fruits in all subjects
| Healthy subjects (n = 20) | Type 2 diabetes subjects (n = 17) | |
|---|---|---|
| Grapes | 0.70 ± 0.09 | 0.89 ± 0.08 |
| Asian pears | 0.26 ± 0.06 | 0.35 ± 0.04 |
| Guavas | 0.44 ± 0.06 | 0.45 ± 0.05 |
| Golden kiwifruit | 0.56 ± 0.10 | 0.57 ± 0.06 |
| Lychees | 0.81 ± 0.08 | 0.93 ± 0.11 |
| Bananas | 0.43 ± 0.06 | 0.58 ± 0.04 |
Values are mean ± SEM.
Discussion
The weather in Taiwan is excellent for growing fruit, so fruit is a very popular part of the Taiwanese diet. Carbohydrates are major components of fruits, especially monosaccharides (glucose, fructose), disaccharides (sucrose) and dietary fiber. Atkinson et al.’s study measured the GI value of glucose, fructose and sucrose as 103, 15 and 65, respectively.(16) In addition, Nilsson et al.(22) showed that the foods with lower GI contained more dietary fiber and resistant starch. Trinidad et al.(23) found that there was a negative association between the dietary fiber level in food and its GI value. Thus, the different proportions of monosaccharides, disaccharides and dietary fiber in fruit will affect its GI values and postprandial glycemic effects. For Type 2 diabetes patients, chosing fruit correctly is important for the control of blood sugar. Thus, this study aimed to evaluate the GI and PII of six popular kinds of fruit consumed in Taiwan and to compare them in healthy and Type 2 diabetes subjects.
Foods with low GI produce lower postprandial blood sugar levels and a lower overall blood sugar response than do foods with a high GI.(24) The American Diabetes Association suggests that Type 2 diabetes patients consume more low GI foods to help optimize glycemic control.(25) Reduced insulin secretion and decreased insulin sensitivity are the major causes of Type 2 diabetes. Schulze et al.(26) showed that diets with higher GI and lower levels of dietary fiber are associated with greater risk of Type 2 diabetes. Two studies also found a positive correlation between GI in the diet and fasting blood sugar, fasting triglycerides and insulin, BMI, and HOMA (homeostasis model assessment) levels.(27,28) Weir et al.(29) proved that lifestyles including a high GI diet long term increase the volume of β-cells and the secretion of insulin. This results in the dysfunction of β-cells and decreases the sensitivity to insulin. Insulin resistance in turn results in higher level of postprandial blood sugar. According to a study by Lebovitz,(30) abnormal postprandial blood sugar levels are an early symptom of the development of Type 2 diabetes. Nalysnyk et al.(14) also found that postprandial blood sugar could be an independent indicator of the complications from Type 2 diabetes.
Similar to GI values, PII has also been used to evaluate postprandial glycemia and glycemic effects of different foods in a study by Abdulrhman et al.(31) Using these methods, our study found that in healthy subjects, blood sugar returned to fasting level within sixty minutes. However, in Type 2 diabetes subjects, the rate of postprandial glycemia reduction is slower than in healthy subjects, and blood sugar in most of the diabetes patients returned to fasting level within about 120 min, but not for everyone. This situation could constitute a limitation affecting GI calculations. Because of their abnormal physiology, it is difficult for Type 2 diabetes patients to fast longer than 120 min during the GI testing protocol. We used AUC in the calculation of GI. However, if the blood sugar did not return to fasting level within 120 min, the AUC during the period from 90 to 120 min post-ingestion will be underestimated, which could affect the accuracy of GI measurement. In the contrast, PII is calculated based on the ratio of the maximal increment of plasma glucose produced by tested foods to that produced by reference foods, and is not based on the AUC, therefore calculation of PII is not subject to the same kind of limitation. We recommend that to reduce bias, GI and PII should be considered together when evaluating the postprandial glycemia and glycemic effect of different foods.
In this study, we found no significant difference in GI and PII of six kinds of fruits when consumed by healthy and Type 2 diabetes subjects. Abdulrhman et al.’s study compared the GI and PII of honey, sucrose and glucose in 20 patients with Type 1 diabetes and 10 healthy subjects.(31) In their study, they identified the GI and PII of glucose as 1. The GI of sucrose in healthy and Type 1 diabetes subjects was 1.32 and 1.19, respectively, while that of honey was 0.69 and 0.61. The PII of sucrose in healthy and Type 1 diabetes subjects was 1.25 and 1.10, respectively, while that of honey was 0.61 and 0.60. They concluded that the GI and PII of either sucrose or honey did not differ significantly between Type 1 diabetes patients and healthy subjects. They demonstrated that both GI and PII are approximately the same in diabetics and non-diabetics. A study by Samnata et al.(21) that measured the GI and PII of glucose, sucrose and honey in 12 healthy subjects, eight patients with insulin-dependent diabetes mellitus and six patients with non-insulin-dependent diabetes mellitus. They likewise found no remarkable difference between the healthy and diabetic patients in GI and PII. Similarly, our results showed, the GI and PII of six kinds of fruits are the same in healthy and Type 2 diabetes subjects. So we think that the GI and PII of foods in the healthy subjects could be applied to the diabetes patients.
In conclusion, it may be expected that both GI and PII will be approximately the same in both Type 2 diabetics and healthy subjects. So we think that the GI and PII of foods in the healthy subjects could be applied to the diabetes patients.
Acknowledgments
This paper was supported by the grant NSC 98-2320-B-038-021-MY3 from the National Science Council of Taiwan. We thank Dr. Hui-Wen Lin of Biostatistics and Research Consultation Center of Taipei Medical University for the assistance of the statistic consultation.
Abbreviations
- AUC
area under curve
- CVD
cardiac vascular diseases
- GI
glycemic index
- HbA1c
glycosylated hemoglobin A1c
- HOMA
homeostasis model assessment
- PII
peak incremental indices
References
- 1.Ferland A, Brassard P, Lemieux S, et al. Impact of high-fat/low-carbohydrate, high-, low-glycaemic index or low-caloric meals on glucose regulation during aerobic exercise in type 2 diabetes. Diabet Med. 2009;26:589–595. doi: 10.1111/j.1464-5491.2009.02734.x. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Silink M.Childhood diabetes: a global perspective Horm Res 200257 Suppl. 11–5 [DOI] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmerón J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 6.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrates intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 8.Brand-Miller JC, Stockmann K, Atkinson F, Petocz P, Denyer G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a database of more than 1,000 foods. Am J Clin Nutr. 2009;89:97–105. doi: 10.3945/ajcn.2008.26354. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins DJ, Jenkins AL, Wolever TM, Collier GR, Rao AV, Thompson LU. Starchy foods and fiber: reduced rate of digestion and improved carbohydrate metabolism. Scand J Gastroenterol Suppl. 1987;129:132–141. doi: 10.3109/00365528709095867. [DOI] [PubMed] [Google Scholar]
- 10.FAO/WHO Expert consultation, carbohydrate in human nutrition. Report of Joint FAO/WHO expert consultation paper 66. Rome: FAO Food and Nutrition; 1998. [PubMed] [Google Scholar]
- 11.Nakagami T, the DECODA Study Group Hyperglycemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47:385–394. doi: 10.1007/s00125-004-1334-6. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 13.Brand-Miller J, Hayne S, Petocz P, Colaqiuri S. Low-glycemic index diets in the management of diabetes. Diabetes Care. 2003;26:2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 14.Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12:288–298. doi: 10.1111/j.1463-1326.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Bantle JP, Wylie-Rosett J, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riby JE, Fujisawa T, Kretchmer N. Fructose absorption. Am J Clin Nutr. 1993;58:748S–753S. doi: 10.1093/ajcn/58.5.748S. [DOI] [PubMed] [Google Scholar]
- 18.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugar stimulate fatty acid synthesis in adults. J Nutr. 2008;138:1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu PC. Factors affecting the glycemic index of fruits and its application in fruits choice of type 2 DM patients. Master’s Thesis. School of Nutrition and Health Sciences, Taipei Medical University; [Google Scholar]
- 20.Jenkins DJ, Jenkins AL. The glycemic index and the dietary treatment of hypertriglycemia and diabetes. J Am Coll Nutr. 1987;6:11–17. doi: 10.1080/07315724.1987.10720160. [DOI] [PubMed] [Google Scholar]
- 21.Samanta A, Burden AC, Jones GR. Plasma glucose response to glucose, sucrose and honey in patients with diabetes mellitus: an analysis of glycemic and peak incremental indices. Diabet Med. 1985;2:371–373. doi: 10.1111/j.1464-5491.1985.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson AC, Ostman EM, Granfeldt Y, Björck IM. Effect of cereal test breatfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr. 2008;87:645–654. doi: 10.1093/ajcn/87.3.645. [DOI] [PubMed] [Google Scholar]
- 23.Trinidad TP, Valdez DH, Loyola AS, et al. Glycaemic index of different coconut (Cocos nucifera)-flour products in normal and diabetic subjects. Br J Nutr. 2003;90:551–556. doi: 10.1079/bjn2003944. [DOI] [PubMed] [Google Scholar]
- 24.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25:202–212. doi: 10.2337/diacare.25.1.202. [DOI] [PubMed] [Google Scholar]
- 26.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 27.Murakami K, Sasaki S, Takahashi Y, et al. Dietary glycemic index and load in relation to metabolic risk factors in Japanese female farmers with traditional dietary habits. Am J Clin Nutr. 2006;83:1161–1169. doi: 10.1093/ajcn/83.5.1161. [DOI] [PubMed] [Google Scholar]
- 28.Du H, van der A DL, van Bakel MM, et al. Glycemic index and glycemic load in relation to food and nutrient intake and metabolic risk factors in a Dutch population. Am J Clin Nutr. 2008;87:655–661. doi: 10.1093/ajcn/87.3.655. [DOI] [PubMed] [Google Scholar]
- 29.Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50(Suppl. 1):S154–S159. doi: 10.2337/diabetes.50.2007.s154. [DOI] [PubMed] [Google Scholar]
- 30.Lebovitz HE. Postprandial hyperglycaemic state: importance and consequences. Diabetes Res Clin Pract. 1998;40(Suppl):S27–S28. [PubMed] [Google Scholar]
- 31.Abdulrhman M, EI-Hefnawy M, Hussein R, EI-Goud AA. The glycemic and peak incremental indices of honey, sucrose and glucose in patients with type 1 diabetes mellitus: effects on C-peptide level-a pilot study. Acta Diabetol. 2011;48:89–94. doi: 10.1007/s00592-009-0167-7. [DOI] [PubMed] [Google Scholar]