Abstract
Endoscopic submucosal dissection has made it possible to resect large lesions during a single operation. The present study was undertaken to compare the time taken for recovery from artificial ulcers after endoscopic submucosal dissection between an H2 Receptor Antagonist treatment group and a Proton Pump Inhibitor treatment group, focusing on analysis of the time course of reduction rate in ulcer area. The powerful acid suppression by Proton Pump Inhibitor may not be needed to treat Japanese post-endoscopic submucosal dissection ulcer which usually develops after early gastric carcinoma in the mucosa of low acid secretory capacity. The study involved 60 patients with 69 artificial ulcers following endoscopic submucosal dissection for the treatment of tumors remaining in the gastric mucosa. Of all lesions, 36 were allocated to the H2 Receptor Antagonist group and 33 to the Proton Pump Inhibitor group. Patients in both groups underwent endoscopy at 4 and 8 weeks after the start of administration. There were no significant differences between two groups and ulcer healing rates were similar in the two groups. The efficacy of H2 Receptor Antagonists in curing this type of ulcer can thus be expected to be comparable to that of Proton Pump Inhibitors.
Keywords: endoscopic submucosal dissection, H2 receptor antagonist, proton pump inhibitor, gastric ulcer healing
Introduction
Endoscopic therapy for early gastric carcinoma was initiated in the 1960s and various technologies were developed. Since the strip biopsy method developed by Tada et al.(1) and the ERHSE (Endoscopic Resection with local injection of Hypertonic Saline Epinephrine solution) method developed by Hirao et al.(2) became available in the 1980s, endoscopic mucosal resection (EMR) for endoscopic therapy has come into widespread use. Various techniques have also become well-developed but most have focused on treatment of intra-mucosal lesions of 2 cm or less and therefore post-EMR ulcers have, like peptic ulcers, been treated with H2 receptor antagonists (H2RA) or proton pump inhibitors (PPI).(3,4) The insulated tip electrosurgical (IT)-knife(5) developed in 1988 and the approach of Ono et al.(6) in 1999 allowed dissection of larger lesions exceeding 2 cm; this treatment approach was termed endoscopic submucosal dissection (ESD). ESD enables larger specimens to be obtained, resulting in a complete resection rate of more than 90% compared with conventional EMR methods.
Post-ESD ulcers have been treated with H2RA or PPI like other post-EMR ulcers. The size of an ulcer artificially induced by endoscopic therapies including ESD is generally shallower than a common peptic ulcer. A meta-analysis found that peptic ulcers are potentially curable earlier with PPIs than H2RAs due to the stronger inhibitory action on gastric acid secretion.(7) However, there is no well-documented report examining whether treatment of post-ESD ulcers and that of common peptic ulcers should be same or not.
Most early gastric carcinomas, as indications for endoscopic therapy, are classified as well-differentiated with severe atrophy in the background mucosa of the stomach and an inferred decrease in acid secretory capacity. In addition, it is reported that 70 to 80% of Japanese were infected with Helicobacter pylori (HP) and that the acid secretion potential was lower in Japanese people than in Western populations.(8) Due to the change of dietary habits to Western-style, the acid secretion in Japanese tends to increase, but it is still lower than in Western populations.(9) Therefore, the powerful acid suppression by PPI may not be needed to treat Japanese post-ESD ulcer which usually develops after early gastric carcinoma in the mucosa of low acid secretory capacity.
For ulcer healing after EMR, Yamaguchi et al.(3) evaluated the effectiveness of H2RA and PPI, and concluded that the healing results were similar for the two drugs. However, whether or not the treatment for peptic ulcer is also effective for post-ESD ulcer, and which drugs are suitable for post-ESD ulcers with larger area than post-EMR ulcers, have not yet been examined.
Therefore, we compared the rate of decrease in ulcer size over time in patients with post-ESD ulcers treated with H2RA or PPI to establish a regimen for ESD-induced ulcers assumed to have a similar depth of ulceration.
Subjects and Methods
Subjects
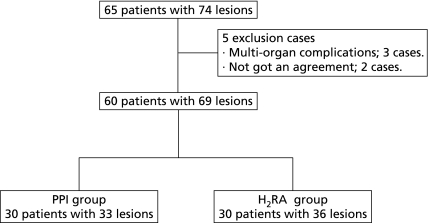
We conducted ESD for 65 patients with 74 gastric intra-mucosal tumors between January and June 2009 at the Osaka Medical College Hospital, Japan. We excluded patients with multi-organ complications and included 60 patients with 69 gastric tumors (51 early gastric carcinomas, 18 gastric adenomas) after giving written informed consent in this study. Subjects were randomly allocated into two groups (30 in each group) using the envelop method (Fig. 1).
Fig. 1.
Trial profile. The flow diagram of this study. (PPI group, H2RA group)
ESD procedure
The ESD procedure was performed with the approach of Ono et al.(6) and the actual steps are shown in Fig. 2. VIO300D (ERBE, Tubingen, Germany) was used as a high-frequency generator. A needle knife (KD-1L, Olympus Medical Systems Co. Ltd., Tokyo, Japan)(10) as the marking-tip device was used with a coagulation wave (Soft Coag 60W Effect 7) to mark dots approximately 5 mm outside the margin of the targeted lesion. Then, the mucosa around the lesion was adequately inflated by locally injecting 0.05% epinephrine dissolved in saline into the area between the muscularis propria and the mucosa. The lesion was pre-cut using the needle knife with the cut wave (Endo Cut I Effect 2 Duration 3 Interval 3) and incised circumferentially using an IT-knife 2(11) (KD-611L, Olympus Medical Systems Co. Ltd.) with the cut wave (Endo Cut Q Effect 2 Duration 4 Interval 3). The submucosal layer under the lesion was dissected using the same IT knife 2, usually with the coagulation wave (Swift Coag Effect 2, 60–70W), but occasionally with the cut wave (Endo Cut Q Effect 2 Duration 4 Interval 3) for the hard-to-dissect fibrous submucosal layer. The IT-knife 2 with the coagulation wave (Swift Coag Effect 3, 60–80W) was used to stop oozing blood during the procedure. In case of inadequate hemostasis, a soft coagulation wave (Soft Coag Effect 6, 80W) was applied to the bleeding site pinpointed and clamped with hemostatic forceps (FD-410LR, Olympus Medical Systems Co. Ltd.) to stop bleeding. The hemostatic forceps were also used for projectile bleeding; in the event of poor heat transfer of the soft coagulation wave to vessels, another hemostatic forceps with a larger contact area (Radial Jow 3 HOT Boston Scientific, Marlborough, Natick Massachusetts) and another setting for the higher-frequency coagulation wave (Forced Effect 2, 40W) with a coagulation output of approximately 1–2 s were used to stop the bleeding.
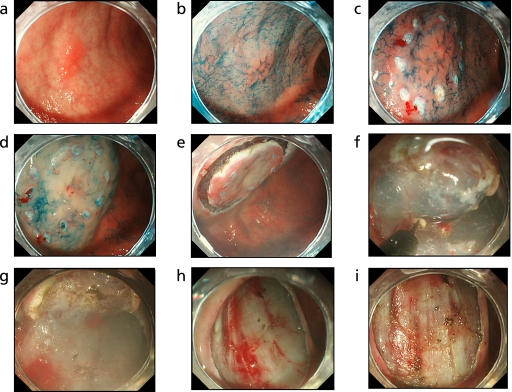
Fig. 2.
The ESD procedure and hemostasis. a: A IIa gastric tumor with the long axis of 10 mm at anterior pylorus. b: The lesion dyed with indigocarmine. c: Marking outer margin of the lesion by a coagulation wave. d: Inflation of the mucosa around the lesion by local injection of solution. e: The lesion after circumferential incision. f: Dissection of the lesion by IT-knife2. g: The lesion during dissection. h: An ulcer floor formed immediately after ESD. i: Hemostasis of vessels in the ulcer floor.
After dissection of the target lesion, all vessels in the floor of the ulcer were carefully treated using hemostatic forceps and with the coagulation wave (Soft Coag 80W Effect 6) for prophylaxis against post-operative hemorrhage.
Bleeding was defined as hematemesis or melena that required the endoscopic hemostasis and decreased the hemoglobin count by more than 2 g/dl, occurring from the time of treatment until cure of ESD-induced ulcer (Fig. 2).
Study protocol design (post-ESD treatment)
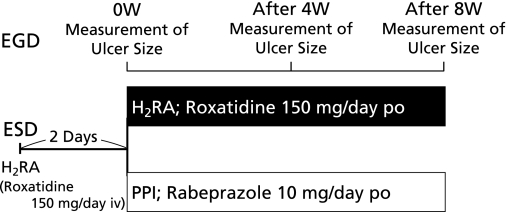
Patients were not fed for 2 days after ESD procedures and received roxatidine acetate hydrochloride, an intravenous H2RA given at a dosage of 150 mg/day during the fasting period. Then, both patient groups received an anti-ulcer drug orally for 8 weeks; the H2RA group received roxatidine 150 mg/day and the PPI group received rabeprazole 10 mg/day. Patients in both groups underwent upper gastrointestinal endoscopy at 4 and 8 weeks after the start of administration (Fig. 3).
Fig. 3.
Study protocol. Patients were not fed for 2 days after ESD procedures and received roxatidine acetate hydrochloride, an intravenous H2RA given at a dosage of 150 mg/day during the fasting period. Then, both patient groups received an anti-ulcer drug orally for 8 weeks; the H2RA group received roxatidine 150 mg/day and the PPI group received rabeprazole 10 mg/day. Patients in both groups underwent upper gastrointestinal endoscopy at 4 and 8 weeks after the start of administration.
Measurement of ulcer size
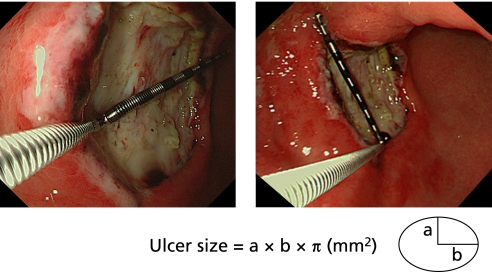
The ESD-induced ulcer size was assessed by area (mm2); long and short axes (mm) of an assumed ellipse were measured using an endoscope and measuring equipment and the ulcer size (long radius × short radius × π = ulcer size) was calculated (Fig. 4). The indication of EMR is gastric tumor with long axis less than 20 mm, with the maximum area = 10 mm radius × 10 mm radius × π = 314 mm2 of an assumed circle. The normal mucosa outside the tumor is resected in the procedure, resulted in ulcers with assumed larger maximum area with 400 mm2. Therefore, ulcers were classified into two groups, small ulcers of less than 400 mm2 and large ulcers of at least 400 mm2, using the ulcer size of Day 2 after ESD as the baseline value.
Fig. 4.
Measurement of ulcer size. The ESD-induced ulcer size was assessed by area; long and short axes (mm) of an assumed ellipse were measured using an endoscope and measuring equipment, and the ulcer size was calculated.
Ulcer sizes were measured at Weeks 4 and 8 after ESD to calculate the rate of decrease in ulcer size over time.
Statistical method
Analyses were performed with the SPSS 16.0 software package (SPSS Japan, Tokyo, Japan). Data were expressed as mean ± standard deviation and tested by chi-square test or t test. Statistical significance was defined as p<0.05 in a two-tailed test.
Results
Patient characteristics
The H2RA group (30 patients) and the PPI group (30 patients) were studied. The H2RA group consisted of 20 males and 10 females with a mean age of 68.7 ± 9.3 years, range 49 to 85, whereas the PPI group consisted of 21 males and 9 females with a mean age of 67.4 ± 7.8 years, range 47 to 80. HP IgG antibody levels in blood were measured (using ELISA kit: HM-CAP, Kyowa Medex, Tokyo, Japan) and the level of 1.8 U/ml or above was considered to be HP positive. The HP infection rate was 76.7% in the H2RA group and 80.0% in the PPI group. The H2RA group patients had ulcers (36 lesions) in the body (14 lesions), angulus (10) and antrum (12) of the stomach. The PPI group patients had ulcers (33 lesions) in the body (12 lesions), angulus (9) and antrum (12) of the stomach. There were no significant differences in extraneous factors between the two groups (Table 1).
Table 1.
Patient characteristics of the treatment groups (H2RA and PPI)
| Variables | H2RA | PPI |
|---|---|---|
| 2-sample t test (ns) | ||
| Patients (n) | 30 | 30 |
| Male | 20 | 21 |
| Female | 10 | 9 |
| Age: mean ± SD | 68.7 ± 9.3 | 67.4 ± 7.8 |
| (Range) | (49–85) | (47–80) |
| H. pylori positive | 23 | 24 |
| (%) | (76.7%) | (80.0%) |
| Lesions (n) | 36 | 33 |
| Site of lesions | ||
| Body | 14 | 12 |
| Angle | 10 | 9 |
| Antrum | 12 | 12 |
| Area of ulcer at 0W (mm2) | 519.2 ± 436.7 | 652.2 ± 514.4 |
The patient characteristics were similar in both groups, and no significant differences were observed. H2RA, H2 receptor antagonist; PPI, proton pump inhibitor.
Ulcer size after ESD
Ulcer sizes after ESD (baseline value) were 519.2 ± 436.7 in the H2RA group (36 lesions) and 652.2 ± 514.4 in the PPI group (33 lesions) and there was no significant difference between the two groups (Table 2).
Table 2.
Ulcer size at baseline (mm2)
| 0 W | 95% CI | |
|---|---|---|
| mean ± SD | ||
| 2-sample t test (ns) | ||
| H2RA (n = 36) | 519.2 ± 436.7 | (376.5–661.8) |
| PPI (n = 33) | 652.2 ± 514.4 | (476.7–827.7) |
Ulcer sizes after ESD (baseline value) were 519.2 ± 436.7 in the H2RA group (36 lesions) and 652.2 ± 514.4 in the PPI group (33 lesions) and there was no significant difference between the two groups. ESD, endoscopic submucosal dissection; H2RA, H2 receptor antagonist; PPI, proton pump inhibitor.
The rate of decrease in ulcer size
The rates of decrease in ulcer size were 92.9 ± 9.1% and 99.8 ± 0.7% at Week 4 and Week 8, respectively, in the H2RA group, and 89.0 ± 20.1% and 99.7 ± 1.1% in the PPI group, respectively. There were no significant differences between the two groups (Table 3).
Table 3.
The rate of decrease in ulcer size (all ulcers)
| Week 4 | Week 8 | |
|---|---|---|
| mean ± SD (95% CI) | ||
| 2-sample t test (ns) | ||
| H2RA | 92.9 ± 9.1% | 99.8 ± 0.7% |
| (n = 36) | (89.9–95.8) | (99.6–100) |
| PPI | 89.0 ± 20.1% | 99.7 ± 1.1% |
| (n = 33) | (82.1–95.9) | (99.3–100) |
The rates of decrease in ulcer size were 92.9 ± 9.1% and 99.8 ± 0.7% at Week 4 and Week 8, respectively, in the H2RA group, and 89.0 ± 20.1% and 99.7 ± 1.1% in the PPI group, respectively. There were no significant differences between the two groups. H2RA, H2 receptor antagonist; PPI, proton pump inhibitor.
Ulcers less than 400 mm2
The mean size of 19 ulcers which were less than 400 mm2 immediately after ESD was 263.6 ± 73.3 mm2 in the H2RA group and the rate of decrease in size was 96.1 ± 6.0% at Week 4 and 100% at Week 8. The mean size of 13 ulcers was 276.4 ± 51.6 mm2 in the PPI group and the rate of decrease in size was 94.2 ± 5.1% at Week 4 and 99.9 ± 0.2% at Week 8. There were no significant differences between the two groups (Table 4).
Table 4.
The rate of decrease in ulcer size (ulcers less than 400 mm2)
| Week 4 | Week 8 | |
|---|---|---|
| mean ± SD (95% CI) | ||
| 2-sample t test (ns) | ||
| H2RA | 96.1 ± 6.0% | 100% |
| (n = 19) | (93.4–98.7) | |
| PPI | 94.2 ± 5.1% | 99.9 ± 0.1% |
| (n = 13) | (91.4–97.0) | (99.9–100) |
The mean size of 19 ulcers which were less than 400 mm2 immediately after ESD was 263.6 ± 73.3 mm2 in the H2RA group and the rate of decrease in size was 96.1 ± 6.0% at Week 4 and 100% at Week 8. The mean size of 13 ulcers was 276.4 ± 51.6 mm2 in the PPI group and the rate of decrease in size was 94.2 ± 5.1% at Week 4 and 99.9 ± 0.2% at Week 8. There were no significant differences between the two groups. ESD, endoscopic submucosal dissection; H2RA, H2 receptor antagonist; PPI, proton pump inhibitor.
Ulcers of at least 400 mm2
The mean size of 17 ulcers exceeding 400 mm2 was 804.8 ± 496.8 mm2 in the H2RA group and the rate of decrease in size was 89.3 ± 10.7% at Week 4 and 99.7 ± 1.0% at Week 8. The mean size of 20 large ulcers was 896.5 ± 533.4 mm2 in the PPI group and the rate of decrease in size was 85.6 ± 25.2% at Week 4 and 99.5 ± 1.4% at Week 8. There were no significant differences between the two groups (Table 5).
Table 5.
The rate of decrease in ulcer size (ulcers at least 400 mm2)
| Week 4 | Week 8 | |
|---|---|---|
| mean ± SD (95% CI) | ||
| 2-sample t test (ns) | ||
| H2RA | 89.3 ± 10.7% | 99.7 ± 1.0% |
| (n = 17) | (84.2–94.4) | (99.2–100) |
| PPI | 85.6 ± 25.2% | 99.5 ± 1.4% |
| (n = 20) | (74.5–96.6) | (98.9–100) |
The mean size of 17 ulcers exceeding 400 mm2 was 804.8 ± 496.8 mm2 in the H2RA group and the rate of decrease in size was 89.3 ± 10.7% at Week 4 and 99.7 ± 1.0% at Week 8. The mean size of 20 large ulcers was 896.5 ± 533.4 mm2 in the PPI group and the rate of decrease in size was 85.6 ± 25.2% at Week 4 and 99.5 ± 1.4% at Week 8. There were no significant differences between the two groups. H2RA, H2 receptor antagonist; PPI, proton pump inhibitor.
Post-ESD complications
There were no patients with post-ESD bleeding in either group, from the time of treatment until cure of ESD-induced ulcer.
Discussion
ESD is becoming the standard treatment for gastric intra-mucosal tumors and an effective cure for post-ESD ulcers is anticipated to be an important future issue. Eriksson et al.(12) compared H2RA (ranitidine) and PPI (omeprazole) in 743 patients with gastric ulcers and the outcome as the rates of ulcer healing at Week 4 and Week 8. The results of the meta-analysis showed that the PPI group was superior in ulcer healing rate by 9.9% (95% CI, 3.0–16.8) (p = 0.005) at Week 4 and 6.7% (95% CI, 1.2–12.2) at Week 8 (p = 0.02). Another meta-analysis by Tunis et al.(7) in 1527 patients involved in 13 studies showed that the rate of ulcer healing was significantly higher in the PPI group with better outcomes at both Week 4 (risk ratio = 1.33, 95% CI, 1.19–1.49) and Week 8 (risk ratio = 1.12, 95% CI, 1.06–1.19) than in the H2RA group. The depth of these peptic ulcers was varied, ranging from erosion of level mucosa to ulceration beyond the muscularis propria, whereas the depth of ESD-induced ulcers remains at level sub mucosa.
Yamaguchi et al.(3) evaluated the bleeding rate after EMR and the effects on the healing of post-EMR ulcers of in 57 patients who were randomly assigned to H2RA or PPI. Neither the bleeding rates (H2RA 18% versus PPI 14%) nor the size of post-EMR ulcers at 1, 30, 60 days were significantly different between the two groups.
Regarding PPI treatment duration, Lee et al.(4) assigned patients to treatment with PPI for 7 days (n = 26) or 28 days (n = 34) after EMR. At 4 weeks after EMR, there were no significant differences between the two groups in terms of ulcer reduction ratio or stage.
There are neither well-documented reports examining whether treatment for post-ESD ulcers and peptic ulcers produce similar outcomes nor those evaluating whether healing of post-ESD ulcers is different from post-EMR ulcers. Therefore, we compared the effects of H2RA and PPI on ulcer healing in patients with ESD-induced ulcers. We also classified ESD-induced ulcers into 2 groups of less than 400 mm2 and at least 400 mm2 and compared ulcer healing by size.
We compared the rate of decrease in ulcer size at 4 and 8 weeks after treatment initiation. There were no significant differences between the H2RA and PPI groups and ulcer healing rates were similar in the two groups. Post-ESD ulcer sizes were examined after stratifying ulcers into two ulcer size groups, less than 400 mm2 and at least 400 mm2 at baseline, and the analysis indicated no significant difference in the rate of decrease in ulcer size between the two groups at 4 and 8 weeks after the start of treatment. Furthermore, most of the patients in both groups showed cure at Week 8.
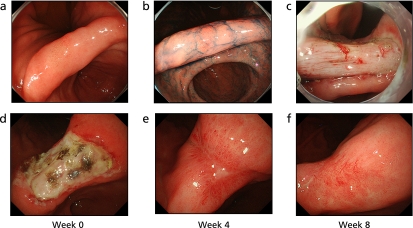
Kim et al.(13) reported that the safety and efficacy profiles of PPI and a newly developed acid-pump antagonist are similar for the treatment of ESD-induced ulcers. Kakushima et al.(14) indicated that even larger post-ESD ulcers are potentially curable with PPI treatment in 8 weeks. On the other hand, this study demonstrated roxatidine acetate hydrochloride, a H2RA, to also be as effective for post-ESD ulcers as PPIs (Fig. 5).
Fig. 5.
A case treated with H2RA (Roxatidine). a: IIa early gastric cancer at the lesser curvature of gastric angle. b: The lesion was dyed with indigocarmine. c: Immediately after ESD, an ulcer floor was formed. d: Two days after ESD, there was white moss in the ulcer floor. e: Four weeks after ESD, the ulcer floor shrunk and was surrounded by regenerating epithelium. f: Eight weeks after ESD, the ulcer floor disappeared and turned into a scar.
There are some reports(15,16) that the combination therapy of PPI plus rebamipide, a mucosal-protective agent, promoted post-ESD ulcers. In this study, we compared the safety and efficacy profiles of H2RA and PPI. Roxatidine acetate hydrochloride, H2RA has an acid secretion inhibitory effect and a gastric mucus-increasing effect.(17,18) Thus, roxatidine was considered to have favorable effects due to its pharmacological properties on healing of well-differentiated gastric carcinoma in this study; severe atrophy of the background mucosa and a possible decrease in gastric acid secretary capacity, and even decreases in gastric mucus protective factors, may all be contributory.
Bleeding is among the post-endoscopic therapy complications. Conventional EMR procedures dissecting relatively small carcinomas in a short time were considered to be less affected by electrocoagulation such that there would be no difference in post-EMR bleeding between the H2RA and the PPI treatment groups.(3) In contrast, ESD procedures using electrocautery applying an electric current to the target lesion may result in severe damage to the vessels, submucosal layer and the muscular layer on the floor of the ulcer. The incidence of postoperative bleeding was 3 to 5% with ESD procedures, higher than with EMR procedures. Isomoto et al.(19) reviewed that bleeding during ESD can be managed with coagulation forceps, and postoperative bleeding may be reduced with routine use of the PPIs. In addition, Uedo et al.(20) suggested, from the perspective of prophylaxis against bleeding, that PPIs might inhibit postoperative bleeding significantly better than H2RAs.
Specifically, since post-ESD bleeding was observed in 3.3% of patients with tumors measuring lesser than 2 cm in diameter and 31.6% of patients with tumors measuring 2 cm or more in diameter; they reported that the tumor size was one of the significant risk factors for post-ESD bleeding. We followed the procedures reported by Uedo et al.(20) to treat any visible vessels and adherent clots in the floor of the postoperative ulcer with hot biopsy forceps in the soft coagulation mode at 80-W current. Insufficient hemostasis was treated carefully with bigger hemostatic forceps (Radial Jow 3 HOT Boston Scientific, Marlborough, Natick, Massachusetts) or with a coagulation wave at a higher-power setting (Forced Effect 2, 40W).
Consequently, no post-ESD bleeding was observed in 69 lesions of patients receiving either H2RA or PPI treatment in this study and the percent decrease of the ulcer size was at least 90% in both groups at 8 weeks; there was no significant difference. Therefore, prophylactic hemostasis was considered to be useful to prevent post-ESD bleeding.
Takizawa et al.(21) also indicated that prophylactic coagulation of exposed blood vessels in the ulcer floor is essential for prevention of bleeding.
Kawano et al.(22) evaluated the possibility of reducing the dose of PPI for the treatment of ESD-induced ulcers and concluded that a reduced dose of PPI after 1 week of ESD was equivalent in treatment performance to the standard dose and cheaper. However, roxatidine acetate hydrochloride was considered to be a cost-effective drug due to its lower price than a reduced dose of PPIs.
Meanwhile, even though PPIs are useful for the treatment of peptic ulcers, interaction with clopidogrel used after coronary artery stenting is currently acknowledged as a problem. Both clopidogrel and PPIs are known to be metabolized by CYP2C19, therefore, PPIs may decrease the level of active metabolite of clopidogrel and attenuate the anti-platelet effect of clopidogrel. Moreover, Ho et al.(23) assessed the adverse outcomes in 8205 patients taking clopidogrel with or without PPIs after hospitalization for acute coronary syndrome, and reported that patients taking clopidogrel with PPIs had a significantly higher risk of death or rehospitalization for acute coronary syndrome compared with patients taking clopidogrel without PPI.
In conclusion, this study demonstrated that post-ESD ulcers of any size are potentially curable with either H2RA or PPI therapy in Japan, with outcomes being equivalent at approximately 8 weeks. H2RAs without effects on CYPs might be safer to treat post-ESD than PPIs because of the potential of drug interactions following concomitant administration of PPIs with other drugs.
Abbreviations
- EMR
endoscopic mucosal resection
- ERHSE
Endoscopic Resection with local injection of Hypertonic Saline Epinephrine solution
- ESD
endoscopic submucosal dissection
- H2RA
H2 receptor antagonist
- IT
insulated tip
- PPI
proton pump inhibitor
References
- 1.Tada M, Murakami A, Karita M, Yanani H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445–450. doi: 10.1055/s-2007-1010365. [DOI] [PubMed] [Google Scholar]
- 2.Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi Y, Katsumi N, Tauchi M, et al. A prospective randomized trial of either famotidine or omeprazole for the prevention of bleeding after endoscopic mucosal resection and the healing of endoscopic mucosal resection-induced ulceration. Aliment Pharmacol Ther. 2005;21(Suppl 2):111–115. doi: 10.1111/j.1365-2036.2005.02484.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee SY, Kim JJ, Lee JH, et al. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc. 2004;60:213–217. doi: 10.1016/s0016-5107(04)01683-9. [DOI] [PubMed] [Google Scholar]
- 5.Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221–226. doi: 10.1055/s-2001-12805. [DOI] [PubMed] [Google Scholar]
- 6.Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tunis SR, Sheinhait IA, Schmid CH, Bishop DJ, Ross SD. Lansoprazole compared with histamine2-receptor antagonists in healing gastric ulcers: a meta-analysis. Clin Ther. 1997;19:743–757. doi: 10.1016/s0149-2918(97)80098-7. [DOI] [PubMed] [Google Scholar]
- 8.Haruma K, Kamada T, Kawaguchi H, et al. Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol. 2000;15:277–283. doi: 10.1046/j.1440-1746.2000.02131.x. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita Y, Kawanami C, Kishi K, Nakata H, Seino Y, Chiba T. Helicobacter pylori independent chronological change in gastric acid secretion in the Japanese. Gut. 1997;41:452–458. doi: 10.1136/gut.41.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto H, Kawata H, Sunada K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690–694. doi: 10.1055/s-2003-41516. [DOI] [PubMed] [Google Scholar]
- 11.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54–58. [Google Scholar]
- 12.Eriksson S, Långström G, Rikner L, Carlsson R, Naesdal J. Omeprazole and H2-receptor antagonists in the acute treatment of duodenal ulcer, gastric ulcer and reflex oesophagitis: a meta-analysis. Eur J Gastroenterol Hepatol. 1995;7:467–475. [PubMed] [Google Scholar]
- 13.Kim YG, Jang BI, Kim TN. A matched case-control study of a novel acid-pump antagonist and proton-pump inhibitor for the treatment of latrogenic ulcers caused by endoscopic submucosal dissection. Gut Liver. 2010;4:25–30. doi: 10.5009/gnl.2010.4.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakushima N, Yahagi N, Fujishiro M, et al. The healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig Endosc. 2004;16:327–331. [Google Scholar]
- 15.Fujiwara S, Morita Y, Toyonaga T, et al. A randomized controlled trial of rebamipide plus rabeprazole for the healing of artificial ulcers after endoscopic submucosal dissection J Gastroenterol 2011. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Kato T, Araki H, Onogi F, et al. Clinical trial: rebamipide promotes gastric ulcer healing by proton pump inhibitor after endoscopic submucosal dissection—a randomized controlled study. J Gastroenterol. 2010;45:285–290. doi: 10.1007/s00535-009-0157-0. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa T, Ishihara K, Saigenji K, Hotta K. Structural requirements for roxatidine in the stimulant effect of rat gastric mucin synthesis and the participation of nitric oxide in this mechanism. Br J Pharmacol. 1997;122:1230–1236. doi: 10.1038/sj.bjp.0701488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito H, Sugiyama A, Sato T, et al. An influence on the human gastric surface mucous gel layer of roxatidine acetate hydrochloride. Ulcer Res. 2000;27:132–134. [Google Scholar]
- 19.Isomoto H, Yamaguchi N. Endoscopic submucosal dissection in the era of proton pump inhibitors. J Clin Biochem Nutr. 2009;44:205–211. doi: 10.3164/jcbn.SR09-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610–1616. doi: 10.1111/j.1572-0241.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- 21.Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy. 2008;40:179–183. doi: 10.1055/s-2007-995530. [DOI] [PubMed] [Google Scholar]
- 22.Kawano S, Okada H, Kawahara Y, et al. Proton pump inhibitor dose-related healing rate of artificial ulcers after endoscopic submucosal dissection: a prospective randomized controlled trial. Digestion. 2011;84:46–53. doi: 10.1159/000321660. [DOI] [PubMed] [Google Scholar]
- 23.Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]