Three patients with Leber congenital amaurosis caused by mutations in AIPL1 were found to have unusual residual electroretinograms characterized by slow insensitive scotopic responses.
Abstract
Purpose.
To describe in detail the clinical phenotype and electrophysiological features of three patients with Leber congenital amaurosis caused by mutations of AIPL1.
Methods.
Ophthalmologic examination, color fundus photography, detailed electrophysiological assessment, and screening of AIPL1 were undertaken in three subjects. One patient also underwent visual field testing and spectral domain-optical coherence tomography.
Results.
All three patients, two of whom were siblings, had histories consistent with Leber congenital amaurosis (severely reduced vision, poorly responsive pupils, and nystagmus presenting within the first year of life). However, each patient had recordable and similar electroretinograms (ERGs), which demonstrated absent cone-driven responses and slow insensitive scotopic responses. The first patient was found to have a homozygous Trp278 stop mutation in AIPL1, whereas the siblings were each found to have novel heterozygous mutations in AIPL1 (Leu17Pro and Lys214Asn).
Conclusions.
Patients with mutations in AIPL1 may present with Leber congenital amaurosis and residual ERGs characterized by slow insensitive scotopic responses. Such responses are likely seen only in very young patients and may not be seen with the typical filter settings recommended by the ISCEV standards because of low-pass filtering. Progressive loss of residual ERG activity in young LCA patients with AIPL1 mutations suggests that gene replacement therapy will likely have to be performed early.
Leber congenital amaurosis (LCA) is a severe congenital or early-onset inherited retinal dystrophy that classically presents with searching nystagmus, absence of normal pupil responses, flat electroretinograms (ERGs), minimal, if any, vision beyond infancy, and an initially normal fundus appearance, followed by the development of pigmentary changes over time. The term LCA has traditionally been used when these features present within the first 6 months of life, whereas a variety of terms such as juvenile retinitis pigmentosa,1 early childhood onset retinitis pigmentosa,2 or severe early childhood onset retinal dystrophy (SECORD)3 have been used to describe milder forms of the disease that present after 1 year. LCA and SECORD are genetically extremely heterogeneous, and are caused by >16 genes (AIPL1, CEP290, CRX, CRB1, GUCY2D, IMPDH1, IQCB1, LCA5, LRAT, MERTK, RD3, RDH12, RPGRIP1, RPE65, SPATA7, and TULP1). All except CRX and IMPDH1 exhibit autosomal recessive inheritance in which some de novo mutations result in an autosomal dominant trait.3–5
The gene AIPL1 encodes aryl hydrocarbon receptor interacting protein-like 1 (AIPL1), a 384-amino acid protein with three tetratricopeptide repeat motifs. AIPL1 has been suggested to play a role in photoreceptor development6 and protein farnesylation7 and as a chaperone for NUB1, Hsp70, Hsp90, and photoreceptor-specific phosphodiesterases (PDE6β).8–12 Although AIPL1 was initially thought to be expressed only in adult rod photoreceptors,13 its expression in adult rodent cones has now been demonstrated.14
Mutations in AIPL1 are estimated to account for approximately 5% to 10% of all cases of LCA.4,15 Patients usually have severe vision loss (ranging from 20/200 to LP), but milder forms that would fit the definition of a later onset rod-cone dystrophy have also been reported.2,16–19 Other typical features include poorly responsive pupils, nystagmus, hyperopia, and unrecordable ERGs. The fundus appearance in patients with AIPL1 mutations can appear normal early in the disease, but most patients eventually demonstrate a pigmentary retinopathy with a high prevalence of macular atrophy. One series demonstrated that cataracts and keratoconus were common in those with homozygous AIPL1 mutations.18 Spectral domain-optical coherence tomography (SD-OCT) has shown severe loss of outer retinal thickness in the macula, lamellar disorganization, and increased inner retinal thickness.16
Successful gene replacement therapy in AIPL1-deficient mouse models has raised the hope that such methods could be translated for human treatment.20,21 However, Jacobson et al.16 recently studied the feasibility of treatment in a series of patients with AIPL1 mutations and concluded that the severe photoreceptor degeneration seen might mean they were not good candidates for gene replacement therapy unless and that evidence would be needed in much younger patients of some photoreceptor preservation. We herein report three young patients with mutations in AIPL1 who presented with the clinical features of LCA but had residual electroretinograms characterized by slow insensitive scotopic responses and absent photopic responses. The presence of these residual ERG responses potentially indicated greater photoreceptor preservation than seen in older patients and suggested that these patients might be better candidates for gene replacement therapy than previously considered.
Patients and Methods
All three patients were examined by two of the authors (RGW, MEP) in the Ophthalmic Genetics Clinic at Casey Eye Institute (CEI). All consented to molecular testing either through the Carver Nonprofit Genetic Testing Laboratory in Iowa City or the School of Medicine, Denver Genetic Laboratories, at the University of Colorado. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the local Institutional Review Board.
At the University of Iowa, 14 LCA-causing genes (AIPL1, CEP290, CRB1, CRX, GUCY2D, IQCB1, LCA5, LRAT, RD3, RDH12, RPE65, RPRIP1, SPATA7, and TULP1) were screened for disease-causing mutations using a multiplatform approach. Each patient's DNA was first screened with an allele-specific assay of 138 of the most common LCA-causing variations using assays (TaqMan; Applied Biosystems, Foster City, CA) analyzed with a Fluidigm (South San Francisco, CA) instrument. If no mutations were identified during the allele-specific phase, the coding regions of the LCA-causing genes were then sequenced in order of decreasing probability of mutation detection5 using automated bidirectional dideoxy nucleotide sequencing.
At the University of Denver, direct testing for mutations in the AIPL1, CABP4, CEP290, CRB1, CRX, GUCY2D, IMPDH1, IQCB1, LCA5, LRAT, OTX2, RD3, RDH12, RPE65, RPGRIP1, SPATA7, and TULP1 genes was performed by PCR amplification and DNA sequencing in two directions of all coding exons and exon/intron borders. A total of 190 PCR reactions covering 17 genes were simultaneously sequenced by Sanger dideoxy sequencing. Mutation analysis was performed with genetic analysis software (Mutation Surveyor; SoftGenetics, State College, PA). Codon 1 corresponds to the start ATG, and nucleotide 1 corresponds to the A. The reference sequence for the AIPL1 gene was NG_008474.1. PCR primers were designed by using the Primer3 program (http://frodo.wi.mit.edu/primer3/). PCR was performed using the PCR kits (KAPA2G Robust; KAPA Biosystems, Woburn, MA) according to the manufacturer's recommendations.
For each subject, a full history was taken and ophthalmologic examination was performed. Electrophysiological assessment included full-field electroretinograms (ERG) according to a previously described protocol22,23 that complied with the standards published by the International Society for Clinical Electrophysiology of Vision (ISCEV).24 All subjects underwent color fundus photography and electrophysiological assessment. One subject was examined with kinetic visual field testing using a projection perimeter (Octopus 101; Haag-Streit, Inc., Köniz, Switzerland). In addition, white-on-white full-field static perimetry was performed with the same perimeter using the GATE strategy,25 Goldmann stimulus size V, and a radially oriented, centrally condensed grid of either 119 or a 164 test points (Weleber et al., manuscript in preparation, 2011). One patient also underwent SD-OCT (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany).
Results
Table 1 summarizes the clinical findings for ease of comparison, including age at presentation, age at last review, visual acuities, and refractive errors.
Table 1.
Clinical Findings
| Patient | Sex | Age at Visit | AIPL1 Allele 1 | AIPL 1 Allele 2 | Distance VA | Near VA | Refractive Error | Kinetic Visual Field | Keratoconus | Cataract |
|---|---|---|---|---|---|---|---|---|---|---|
| Family 1 | ||||||||||
| Case 1 | F | 4 mo | p.Trp278X | p.Trp278X | CUSUM, F+F light | Unable | +6.00 + 1.50 × 90 OU | Unable | N | N |
| 10 mo | CUSUM, F+F light | Unable | +6.00 + 1.50 × 90 OU | Unable | N | N | ||||
| 1 4/12 y | CUSUM, F+F light | Unable | +6.00 + 1.50 × 90 OU | Unable | N | N | ||||
| 2 5/12 y | CUSM | Unable | +6.00 + 1.50 × 90 OU | Unable | N | N | ||||
| 3 5/12 y | CUSM | Unable | +5.50 + 1.00 × 90 OU | Unable | N | N | ||||
| Family 2 | ||||||||||
| Case 2 | M | 2 y | p.Leu17Pro | p.Lys214Asn | UCUSM | Unable | +4.75 + 1.50 × 100 | Unable | N | N |
| +5.25 + 1.00 × 80 | ||||||||||
| 3 10/12 y | F+F | Unable | +4.75 + 1.00 × 95 | Unable | N | N | ||||
| +5.00 + 1.00 × 5 | ||||||||||
| 4 5/12 y | F+F | Unable | +4.00 + 1.25 × 95 | Unable | N | N | ||||
| +4.00 + 1.50 × 85 | ||||||||||
| 6 1/12 y | HM OU | 20/400 OU | +5.00 + 1.00 × 90 OU | Unable | N | N | ||||
| 7 5/12 y | HM OU | 20/400 OU | +5.00 + 1.00 × 90 OU | Unable | N | N | ||||
| 9 7/12 y | CF OD, HM OS | 20/400 OU | +7.50 + 1.25 × 90 OU | Unable | N | N | ||||
| 10 9/12 y | CF OD, HM OS | 20/400 OU | +7.50 + 1.25 × 90 OU | Unable | N | N | ||||
| 12 3/12 y | CF OU | 20/200M OD | +3.50 + 1.25 × 95 | Temporal Crescent to V4e target OD>OS, some macular sparing OD | N | N | ||||
| 20/400 OS | +3.50 + 1.50 × 85 | |||||||||
| 13 3/12 y | CF OU | 20/200 OU | +3.50 + 1.50 × 85 | Temporal Crescent to V4e target OD>OS, some macular sparing OD, some small areas to III4e target | N | N | ||||
| 14 4/12 y | CF OU | 20/200 OU | +4.25 + 1.50 × 90 | Temporal Crescent to V4e target OD>OS, some macular sparing OD | N | N | ||||
| +3.00 + 1.50 × 75 | ||||||||||
| Case 3 | M | 8 mo | p.Leu17Pro | p.Lys214Asn | CUSM | Uanble | +1.50 + 1.50 × 90 | Unable | N | N |
| +2.25 + 1.00 × 90 | ||||||||||
| 1 y | CUSUM | Unable | +2.25 + 1.00 × 90 | Unable | N | N | ||||
| 1 10/12 y | F+F | Unable | +1.00 + 1.25 × 105 | Unable | N | N | ||||
| 3 3/12 y | F+F | 20/600 | +1.50 + 1.25 × 105 | Unable | N | N | ||||
| +2.00 + 1.50 × 75 | ||||||||||
| 4 3/12 y | 20/800 w/BEO | NT | +2.00 + 1.50 × 75 | Unable | N | N | ||||
| 5 3/12 y | 20/500 w/BEO | NT | +2.00 + 1.50 × 75 | Unable | N | N | ||||
BEO, both eyes open; CF, count fingers visual acuity; CUSM, central, steady, unmaintained fixation; CUSUM, cenral, unsteady, unmaintained fixation; F+F, fix and follow; HM, hand motion vision; m, months, N, no; NT, not tested; OD, right eye; OS, left eye; OU, both eyes; VA, visual acuity.
Case 1
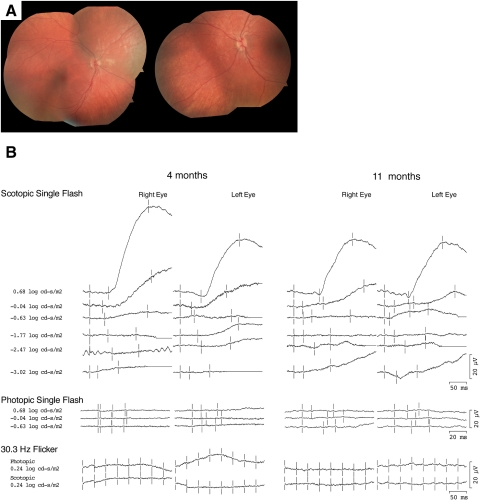
The case 1 patient, a Caucasian female born to nonconsanguineous parents, was noted to have nystagmus the day after birth. There was no family history of inherited retinal disease. Her parents indicated that initially she neither fixed nor followed objects but, beginning at 2 months of age, had begun to follow bright lights. Examination at CEI at 4 months of age disclosed fixation that was central, unsteady, and unmaintained (CUSUM), grossly intact extraocular movement, sluggish pupillary responses, normal intraocular pressure to palpation, and severe hyperopia with astigmatism. The anterior segment was unremarkable, and a dilated examination revealed a normal-appearing fundus (Fig. 1A).
Figure 1.
(A) Fundus photographs from the case 1 patient at 4 months of age demonstrating a normal-appearing fundus. (B) Full-field ERGs from the case 1 patient at 4 and 11 months of age. Photopic ERGs were unrecordable at both time points. Scotopic ERGs were unrecordable at dim intensities, but at higher intensities a slow positive waveform was elicited.
Full-field ERG using intravenous propofol sedation showed unrecordable dim scotopic responses. However, at the two highest intensities of the scotopic bright flashes, subnormal, very slow responses of unclear origin were present (Fig. 1B). For the brightest flash, the peak amplitudes for these slow insensitive, scotopic responses measured 0.6 log cd · s/m2 in right and left eyes were 71/46 μV (normal, 240–440 μV), and the peak implicit times were 168/170 ms (normal, 40–65 ms). Both single-flash photopic and 30-Hz flicker responses were indistinguishable from noise. Based on her examination and ERG results, a diagnosis of LCA was made.
At 10 months of age, her parents reported that though the nystagmus had dampened, she had begun to develop the oculodigital sign. Vision and fundus appearance were unchanged. Repeat sedated ERGs with propofol showed a pattern similar to those recorded at 4 months (Fig. 1B). Dim scotopic, single-flash photopic, and 30-Hz flicker responses were unrecordable. Bright-flash scotopic responses demonstrated a decrease in amplitude for the right eye and similar values for the left eye. The peak amplitudes in right and left eyes were 46/46 μV (normal values, 240–440 μV), and the peak implicit times were 168/167 ms (normal values, 40–65 ms). Repeat fundus examination during sedation was normal.
At 16 months of age, the nystagmus had dampened further, and her fixation had improved to central, unsteady, and maintained (CUSM). Dilated fundus examination revealed fine mottling of the RPE throughout the fundus, including the macula and posterior pole. At her most recent examination at 3 years of age, her parents reported that she was able to identify large pictures at 3 to 6 inches distance, recognize faces from several feet away, and identify colored lights. Her vision remained CUSM. The fundus appearance was unchanged apart from the new finding of waxy pallor to the discs in each eye.
Molecular testing of known LCA genes was performed, and the patient was found to be positive for a homozygous mutation, Trp278Stop, in the AIPL1 gene. Testing of the parents confirmed that each was heterozygous for the mutation.
Case 2
The case 2 patient is a Vietnamese male born to nonconsanguineous parents. At the time, there was no family history of inherited retinal diseases. Nystagmus was present at birth, and poor vision was observed at 3 months of age. He was first evaluated at 7 months of age by an ophthalmologist in Vietnam who told the family that he had a bilateral pigmentary degeneration. He presented to the CEI at 2 years of age, and, on evaluation, his vision measured UCUSM and retinoscopy disclosed significant hyperopia with astigmatism. His parents reported that he could only see objects up close and frequently bumped into walls. He was noted to have roving nystagmus, sluggish pupils, and photophobia. Intraocular pressures were normal to palpation, and extraocular movements were grossly intact. The anterior segment was unremarkable, and a dilated fundus examination revealed vascular attenuation but no pigmentary changes.
As in case 1, a full-field ERG using intravenous propofol sedation recorded at 2 years of age showed unrecordable dim scotopic responses and scotopic bright flashes that invoked subnormal slow responses with delayed onset (Fig. 2B). For the brightest flash, these responses measured 0.6 log cd · s/m2, with peak amplitudes for the right and left eyes of 60/64 μV (normal, 240–440 μV) and peak implicit times of 201/196 ms (normal, 40–65 ms). Both single-flash photopic and 30-Hz flicker responses were indistinguishable from noise. A diagnosis of LCA was made based on his examination and ERG results.
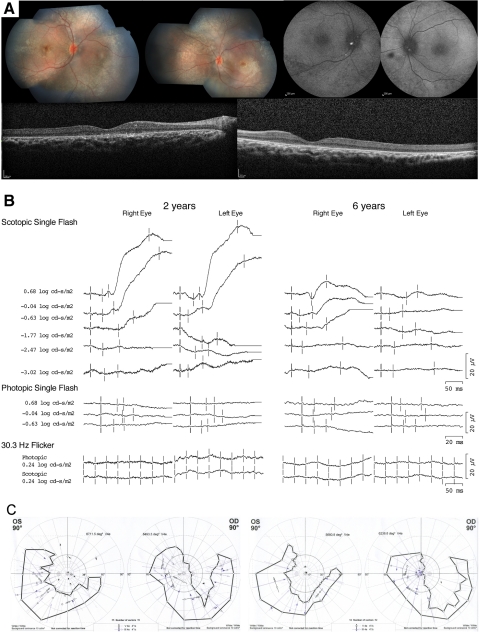
Figure 2.
(A) Fundus photographs from the case 2 patient at 10 years of age demonstrating optic nerve heads with 0.1 cup/disc ratio, attenuated retinal vessels, macular atrophy, and diffuse peripheral RPE mottling. Autofluorescence images taken at age 14 demonstrated relative decreases in signal centrally and punctate loss peripherally. SD-OCT images taken at 14 years of age (right and left eyes, respectively) demonstrating severe loss of outer retinal structures and a highly visible choroid suggestive of RPE atrophy. (B) Full-field ERGs from the case 2 patient at 2 and 6 years of age. Photopic ERGs were unrecordable at both time points. At 2 years of age, scotopic ERGs were unrecordable to dim flashes, but at higher intensities a slow positive waveform was elicited. At 6 years of age, the magnitude of the slow insensitive scotopic response had decreased significantly in amplitude. (C) Kinetic visual fields measured at 12 and 14 years of age (left, right), demonstrating severe loss of fields and a crescent area of preservation to the V4e target in each eye.
Between the ages of 3 and 5 years, the parents noted an improvement in vision. He became able to identify some colors, pick up smaller-sized objects, and navigate well. A repeat sedated ERG at 5 years and 3 months once again demonstrated unrecordable dim scotopic and cone-elicited responses. Bright scotopic flashes demonstrated subnormal slow responses with delayed onsets. At the brightest intensity in the right eye, the waveform had evolved to a more recognizable morphology with a small a-wave followed by a b-wave. A similar pattern was seen in the left eye, but amplitudes were relatively smaller (Fig. 2B). For the brightest flash measuring 0.6 log cd · s/m2, the peak amplitudes in right and left eyes were 14/6 μV (normal, 290–635 μV), and the peak implicit times were 101/106 ms (normal, 37–70 ms). Both single-flash photopic and 30-Hz flicker responses were indistinguishable from noise.
From age 6 to 10 years, there were no reported changes in vision. Visual acuity at distance averaged count fingers visual acuity (CF) for the right eye and hand motion vision (HM) for the left and 20/400 in each eye at near (1–2 inches). Dilated examination revealed optic nerve heads with 0.1 cup/disc ratios, attenuated retinal vessels, lack of foveal reflex in the macula, and an overall desaturated coloration of the retina with diffuse RPE mottling (Fig. 2A).
At 12 years of age, he reported that vision had improved slightly since his last clinic visit. Visual acuity at distance was CF in each eye. Visual acuity at near had improved in the right eye to 20/200 and remained 20/400 in the left. The retinal appearance had changed and now showed, in each eye, central macular atrophy and a few areas of perivascular pigment. Kinetic and static perimetry was obtained for the first time during this visit (Fig. 2C). Kinetic perimetry disclosed a U-shaped crescent to the V4e test target in both eyes, with slightly more preservation in the right eye. Static perimetry showed only a few limited areas of discernible sensitivity. During his most recent evaluation at age 13 years, vision was CF with eccentric fixation in each eye at distance (1 foot) and 20/200 at near in both eyes (1–2 inches). The fundus appearance and visual fields remained unchanged.
Molecular testing for the AIPL1 gene was initiated based on the similar ERG responses and AIPL1-positive testing in case 1. Testing disclosed compound heterozygosity for two novel mutations in the AIPL1 gene, Leu17Pro and Lys214Asn. Testing in parents confirmed that the mutations were in trans. Additionally, testing in the patient's unaffected sister confirmed that she had only the Leu17Pro mutation. The Leu17Pro variation was predicted to be probably damaging by PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), with a score of 0.995 (the highest score for a damaging variation is 1.0). The Lys214Asn variation was predicted to be probably damaging by PolyPhen-2, with a score of 1.0. The two variations are most likely pathogenic because the same two variations were also identified in the affected sibling (case 3).
Case 3
The case 3 patient, who is the 5-year-old brother of the patient in case 2, manifested nystagmus at birth. When first evaluated at CEI at the age of 8 months, he demonstrated good visual interest at near and would fix and follow a bright light. Vision was assessed as CUSM in each eye. Examination disclosed nystagmus, sluggish pupillary responses and mild hyperopia with astigmatism.
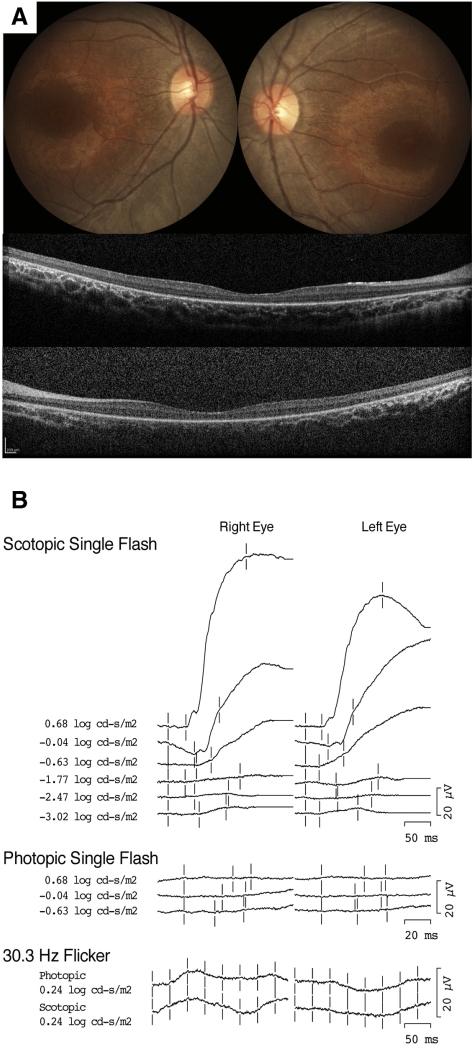
An ERG using intravenous propofol sedation at 11 months showed recordings similar to those of his brother (case 2) and to case 1. Dim scotopic responses were unrecordable, and scotopic bright flashes invoked subnormal slow responses with delayed onset (Fig. 3B). For the brightest flash measuring 0.6 log cd · s/m2, the peak amplitudes in the right and left eyes were 122/94 μV (normal, 240–440 μV), and the peak implicit times were 147/145 ms (normal, 40–65 ms). Fundus examination revealed a blunted foveal reflex, minimally attenuated retinal vessels, and mild pigment dispersion with traces of fine mottling. Both single-flash photopic and 30-Hz flicker responses were indistinguishable from noise. Fundus examination revealed a blunted foveal reflex, minimally attenuated retinal vessels, and mild pigment dispersion with traces of fine mottling. A diagnosis of LCA was made based on his examination and ERG results.
Figure 3.
(A) Fundus photographs from the case 3 patient at 3 years of age demonstrating slight temporal pallor of the optic nerve heads with 0.3 cup/disc ratio, mildly attenuated retinal vessels, and perifoveal atrophy (B) Full-field ERGs from the case 3 patient at 11 months of age. Photopic ERGs were unrecordable at both time points. Scotopic ERGs were unrecordable to dim flashes, but at higher intensities a slow positive waveform was elicited.
Between 1 and 3 years, his parents reported improvement of near vision and stated that he appeared to see better than his brother had at that age. Visual acuity was 20/600 with Allen pictures (8 inches). Fundus examination revealed parafoveal atrophy with an abnormal retinal sheen and mild foveal pigmentary changes in the macula of each eye (Fig. 3A). During his most recent examination at 4 years of age, his vision was stable. Acuity and fundus examination results were unchanged.
Molecular testing of AIPL1 revealed the same two novel mutations present in his brother. Interestingly, the case 3 patient demonstrated better vision than his older brother did at similar ages. He also had less hyperopia and a larger response on full-field ERG.
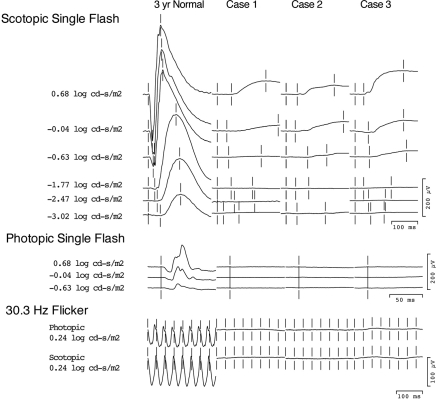
Figure 4 plots the ERG responses for the patients in cases 1, 2, and 3 on the same scale and compares them with those of a 3-year-old child with normal vision. All three patients presented with similar waveforms. The responses from our three patients were insensitive compared with normal scotopic responses, all requiring stimuli greater than 1.77 log cd/m2 to elicit. There is also a significant delay in these signals, with the time to peak measuring between 100 and 200 ms.
Figure 4.
Full-Field ERGs from the patients in cases 1 to 3 scaled to the same size and compared with traces of a 3-year-old with normal vision recorded under similar sedated conditions. Note the relatively small amplitude of the slow insensitive scotopic responses recorded from these three patients with AIPL1 mutations compared with normal b-wave amplitudes to similar stimuli.
Discussion
Gene therapy treatment for LCA patients with RPE65 mutations has opened a new era in the treatment of inherited retinal degenerations.26–30 However, such studies have also highlighted the need for careful functional testing to assess treatment outcomes. Often LCA patients have severe vision loss and poor fixation, making accurate visual acuity or visual field measurements impractical. Electroretinograms provide an objective measure of generalized retinal function, but many patients with LCA have extinguished ERGs, and it is unclear whether ERGs will be sensitive enough to detect changes after gene therapy.
We report three young children with LCA caused by mutations in AIPL1 who presented with similar residual electroretinograms, which we have termed slow insensitive scotopic responses. We have not seen similar responses in more than 70 other patients with LCA tested in our electrophysiology laboratory, suggesting that this pattern may be specific to patients with AIPL1 mutations. The presence of recordable ERGs in young AIPL1 patients is significant because there has been concern about whether these patients would be good candidates for gene replacement therapy given the severity of their photoreceptor loss.
The exact source of these slow insensitive scotopic responses remains unclear. Although the intensities needed to elicit these signals are above the cone threshold, the lack of recordable responses to photopic single flashes or 30-Hz flicker indicates severe cone dysfunction. It is more likely that diminished or desensitized rods drive these responses. Chromatic full-field sensitivity testing in other patients with AIPL1 mutations has also suggested that rods mediate remaining vision.16
It is likely that the slow insensitive scotopic response represents a small b-wave generated from residual rod photoreceptors. Patients with mutations in AIPL1 typically demonstrate macular atrophy, which could represent early cone death or fovea hypoplasia. Given that AIPL1 is thought to act as a molecular chaperone for PDE6β,10 the loss of AIPL1 function would result in low PDE6β levels and subsequently high cGMP levels. High cGMP levels would result in opening of the cGMP-gated ion channels, effectively desensitizing the remaining photoreceptors. In addition, the shape of this signal may be influence by inner retinal remodeling because patients with AIPL1 mutations have been shown, by imaging and histologic studies,16,31 to have thickened inner retinal layers. Whether the slow insensitive scotopic responses represent severely desensitized rod-driven b-waves or are secondary to inner retinal remodeling, the question arises as to why they have not been observed in other LCA patients with AIPL1.
With few exceptions, the ERGs recordings in AIPL1 have been reported as flat or unrecordable.16,18,32,33 Sohocki et al.17 reported a single LCA patient with an Cys239Arg mutation in AIPL1 who had a residual 15-μV ERG response to a high-intensity flash under scotopic conditions but no response to 31-Hz flicker, suggesting a rod origin to the signal. One possible explanation for a lack of recordable ERGs in these patients is that most of the recordings were performed at older ages after severe photoreceptor loss had already occurred. No similar ERGs have been observed in the 35-year history of the ERG service at CEI. In the past 14 years, we have been recording sedated ERGs using propofol in children as young as 6 months of age without any serious adverse effects.34 Although we have found that propofol sedation decreases the b-wave amplitude by approximately a factor of 2, the quality of signals is superior to chloral hydrate because of better suppression of nystagmus and the ability to average a larger number of signals.35 Another possible explanation for our ability to detect these responses relates to the recording settings used in our laboratory. We set our low-pass filter at 0.1 Hz rather than the ISCEV standard of 0.3 Hz or 1 Hz used at other testing facilities. A low-pass filter of 0.1 Hz would be expected to enable better detection of lower frequency components that contribute to the b-wave.24
We have demonstrated recordable ERGs with similar waveforms in three young patients with LCA caused by mutations in AIPL1. Recordable ERGs at young ages suggests a greater degree of intact retinal function and provides an objective outcome measure for treatments. However, it should be noted that although these signals were detectable, even at 4 months of age, the amplitudes were still small, and there was no evidence of cone-driven function. Early gene therapy treatment may be able to rescue some rod function, but whether cones could be rescued remains questionable. Recent imaging studies suggest that extrafoveal photoreceptors might still be present, but macular loss was present even in the youngest patients studied.32 Future studies using imaging methods such as hand-held optical coherence tomography would be especially useful to determine whether there is evidence of persistent macular cone photoreceptors in the outer nuclear layer in infants with AIPL1 mutations.
Footnotes
Supported by National Institutes of Health/National Eye Institute Grant CDA 1K08EY021186-01 (MEP); NIH Grant RO1EY16822-07 (EMS); Foundation Fighting Blindness Center (RGW, NBS, EMS) and Grant CDA CD-CL-0808-0469-OHSU (MEP); Grousbeck Family Foundation (EMS, RGW); Howard Hughes Medical Institute (EMS); and an unrestricted grant from Research to Prevent Blindness (MEP, NBS, PWC, RGW).
Disclosure: M.E. Pennesi, None; N.B. Stover, None; E.M. Stone, None; P.-W. Chiang, None; R.G. Weleber, None
References
- 1. Foxman SG, Heckenlively JR, Bateman JB, Wirtschafter JD. Classification of congenital and early onset retinitis pigmentosa. Arch Ophthalmol. 1985;103:1502–1506 [DOI] [PubMed] [Google Scholar]
- 2. Walia S, Fishman GA, Jacobson SG, et al. Visual acuity in patients with Leber's congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010;117:1190–1198 [DOI] [PubMed] [Google Scholar]
- 3. Weleber RG, Francis P, Trzupek K. Leber congenital amaurosis. GeneReviews [Internet]. Seattle: University of Washington, Seattle; 1993July 27, 2004 [updated March 30, 2010] [Google Scholar]
- 4. den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419 [DOI] [PubMed] [Google Scholar]
- 5. Stone EM. Leber congenital amaurosis—a model for efficient genetic testing of heterogeneous disorders: LX Intravenous Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144:791–811 [DOI] [PubMed] [Google Scholar]
- 6. van der Spuy J, Kim JH, Yu YS, et al. The expression of the Leber congenital amaurosis protein AIPL1 coincides with rod and cone photoreceptor development. Invest Ophthalmol Vis Sci. 2003;44:5396–5403 [DOI] [PubMed] [Google Scholar]
- 7. Ramamurthy V, Roberts M, van den Akker F, Niemi G, Reh TA, Hurley JB. AIPL1, a protein implicated in Leber's congenital amaurosis, interacts with and aids in processing of farnesylated proteins. Proc Natl Acad Sci U S A. 2003;100:12630–12635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X, Bulgakov OV, Wen XH, et al. AIPL1, the protein that is defective in Leber congenital amaurosis, is essential for the biosynthesis of retinal rod cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 2004;101:13903–13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chapple JP, Grayson C, Hardcastle AJ, Saliba RS, van der Spuy J, Cheetham ME. Unfolding retinal dystrophies: a role for molecular chaperones? Trends Mol Med. 2001;7:414–421 [DOI] [PubMed] [Google Scholar]
- 10. Kolandaivelu S, Huang J, Hurley JB, Ramamurthy V. AIPL1, a protein associated with childhood blindness, interacts with alpha-subunit of rod phosphodiesterase (PDE6) and is essential for its proper assembly. J Biol Chem. 2009;284:30853–30861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hidalgo-de-Quintana J, Evans RJ, Cheetham ME, van der Spuy J. The Leber congenital amaurosis protein AIPL1 functions as part of a chaperone heterocomplex. Invest Ophthalmol Vis Sci. 2008;49:2878–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akey DT, Zhu X, Dyer M, et al. The inherited blindness associated protein AIPL1 interacts with the cell cycle regulator protein NUB1. Hum Mol Genet. 2002;11:2723–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Spuy J, Chapple JP, Clark BJ, Luthert PJ, Sethi CS, Cheetham ME. The Leber congenital amaurosis gene product AIPL1 is localized exclusively in rod photoreceptors of the adult human retina. Hum Mol Genet. 2002;11:823–831 [DOI] [PubMed] [Google Scholar]
- 14. Kirschman LT, Kolandaivelu S, Frederick JM, et al. The Leber congenital amaurosis protein, AIPL1, is needed for the viability and functioning of cone photoreceptor cells. Hum Mol Genet. 2010;19:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sohocki MM, Perrault I, Leroy BP, et al. Prevalence of AIPL1 mutations in inherited retinal degenerative disease. Mol Genet Metab. 2000;70:142–150 [DOI] [PubMed] [Google Scholar]
- 16. Jacobson SG, Cideciyan AV, Aleman TS, et al. Human retinal disease from AIPL1 gene mutations: foveal cone loss with minimal macular photoreceptors and rod function remaining. Invest Ophthalmol Vis Sci. 2011;52:70–79 [DOI] [PubMed] [Google Scholar]
- 17. Sohocki MM, Bowne SJ, Sullivan LS, et al. Mutations in a new photoreceptor-pineal gene on 17p cause Leber congenital amaurosis. Nat Genet. 2000;24:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dharmaraj S, Leroy BP, Sohocki MM, et al. The phenotype of Leber congenital amaurosis in patients with AIPL1 mutations. Arch Ophthalmol. 2004;122:1029–1037 [DOI] [PubMed] [Google Scholar]
- 19. Galvin JA, Fishman GA, Stone EM, Koenekoop RK. Evaluation of genotype-phenotype associations in leber congenital amaurosis. Retina. 2005;25:919–929 [DOI] [PubMed] [Google Scholar]
- 20. Sun X, Pawlyk B, Xu X, et al. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 2010;17:117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan MH, Smith AJ, Pawlyk B, et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum Mol Genet. 2009;18:2099–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weleber RG, Gupta N, Trzupek KM, Wepner MS, Kurz DE, Milam AH. Electroretinographic and clinicopathologic correlations of retinal dysfunction in infantile neuronal ceroid lipofuscinosis (infantile Batten disease). Mol Genet Metab. 2004;83:128–137 [DOI] [PubMed] [Google Scholar]
- 23. Weleber RG. The effect of age on human cone and rod Ganzfeld electroretinograms. Invest Ophthalmol Vis Sci. 1981;20:392–399 [PubMed] [Google Scholar]
- 24. Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118:69–77 [DOI] [PubMed] [Google Scholar]
- 25. Schiefer U, Pascual JP, Edmunds B, et al. Comparison of the new perimetric GATE strategy with conventional full-threshold and SITA standard strategies. Invest Ophthalmol Vis Sci. 2009;50:488–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 27. Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Banin E, Bandah-Rozenfeld D, Obolensky A, et al. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: human gene therapy initiated in Israel. Hum Gene Ther. 2010;21:1749–1757 [DOI] [PubMed] [Google Scholar]
- 31. van der Spuy J, Munro PM, Luthert PJ, et al. Predominant rod photoreceptor degeneration in Leber congenital amaurosis. Mol Vis. 2005;11:542–553 [PubMed] [Google Scholar]
- 32. Testa F, Surace EM, Rossi S, et al. Evaluation of Italian patients with Leber congenital amaurosis due to AIPL1 mutations highlights the potential applicability of gene therapy. Invest Ophthalmol Vis Sci. 2011;52:5618–5624 [DOI] [PubMed] [Google Scholar]
- 33. Hanein S, Perrault I, Gerber S, et al. Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Hum Mutat. 2004;23:306–317 [DOI] [PubMed] [Google Scholar]
- 34. Lalwani K, Tompkins BD, Burnes K, Krahmer MR, Pennesi ME, Weleber RG. The ‘dark’ side of sedation: 12 years of office-based pediatric deep sedation for electroretinography in the dark. Paediatr Anaesth. 2011;21:65–71 [DOI] [PubMed] [Google Scholar]
- 35. Pennesi ME, Stover NB, Johnsen S, et al. Results from a Twelve Year Experience with Propofol for Sedated ERGs: International Society for Clinical Electrophysiology of Vision. Padua, Italy: Springer; 2009 [Google Scholar]