FGF-2 stimulates proliferation of human CECs through PI 3-kinase and its downstream target ERK1/2 pathways. This signal transduction downregulates p27 through its phosphorylation at both Ser10 and Thr187 sites mediated by KIS and Cdc25A, respectively.
Abstract
Purpose.
FGF-2 stimulates cell proliferation of rabbit corneal endothelial cells (rCECs) by degrading the cyclin-dependent kinase inhibitor p27Kip1 (p27) through its phosphorylation mechanism. The authors investigated whether the cell proliferation of human CECs (hCECs) is also induced by FGF-2 stimulation through the p27 phosphorylation pathway.
Methods.
Expression and activation of protein were analyzed by immunoblotting. Cell proliferation was measured by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay. Transfection of hCECs with small interference RNA (siRNA) was performed using a transfection reagent.
Results.
FGF-2 stimulated cell proliferation in hCECs; the FGF-2 action was completely blocked by pathway-specific inhibitors for PI 3-kinase (LY294002) and MEK1/2 (U0126), respectively. Using immunoblotting, the authors showed that FGF-2 induced phosphorylation of p27 at both serine 10 (Ser10) and threonine 187 (Thr187) sites. These effects were also completely blocked by LY294002 or U0126. The authors then determined cross-talk between PI 3-kinase and extracellular signal-regulated kinase (ERK)1/2; blocking of ERK1/2 activation by LY294002 indicated that in hCECs ERK1/2 works as a downstream effector to PI 3-kinase for cell proliferation induced by FGF-2, whereas the ERK1/2 pathway in rCECs is parallel to the PI 3-kinase pathway. However, the downstream mechanism involved in cell cycle progression in hCECs is identical to that of rCECs: phosphorylation of p27 at Ser10 was mediated by kinase-interacting stathmin (KIS), confirmed with siRNA to KIS, and phosphorylation of p27 at Thr187 was mediated by cell division cycle 25A (Cdc25A), confirmed using Cdc25A inhibitor.
Conclusions.
FGF-2 stimulates proliferation of hCECs through PI 3-kinase and its downstream target ERK1/2 pathways. This linear signal transduction significantly downregulates p27 through its phosphorylation at both Ser10 and Thr187 sites mediated by KIS and Cdc25A, respectively.
Corneal endothelium (CE) is the single layer of cells forming a boundary between the corneal stroma and anterior chamber. The major function of the corneal endothelial cells (CECs) is not only to maintain corneal transparency by regulating corneal hydration through their barrier and ionic pump functions, but also to facilitate the passage of nutrients from the aqueous humor to the cornea stroma.1–3 Human CECs (hCECs) are considered nonproliferative in vivo and are arrested at the G1 phase of the cell cycle throughout their lifespan.4,5 Therefore, corneal endothelial wound healing is predominantly maintained by cell migration and increase in cell size. This repair process differs from that of most cell types, in which both cell proliferation and migration are involved in the wound healing process. In contrast, in the nonregenerative (pathologic) wound healing process, CECs are transformed into mesenchymal cells that subsequently produce a fibrillar extracellular matrix (ECM) in the basement membrane environment. Thus, corneal fibrosis induces a significant pathophysiological problem; that is, it causes blindness by physically blocking light transmittance. One clinical example of corneal fibrosis observed in CE is the development of a retrocorneal fibrous membrane (RCFM) in Descemet's membrane. In RCFM, the contact-inhibited monolayer of CECs is lost, cell proliferation is markedly increased, and fibrillar ECM is deposited in the basement membrane.6,7 Our previous studies using a rabbit system demonstrated that fibroblast growth factor-2 (FGF-2) is the direct mediator for such endothelial mesenchymal transformation (EMT); FGF-2 signaling upregulates the steady state level of α1(I) collagen RNA by stabilizing the message and subsequently facilitates synthesis and secretion of type I collagen into the extracellular space; FGF-2 signaling induces a change in cell shape from a polygonal to a fibroblastic morphology and causes loss of the contact-inhibited phenotypes; and lastly, FGF-2 signaling directly regulates cell cycle progression through phosphorylation of p27Kip1 (p27) by the action of PI 3-kinase.8–12
The negative cell cycle regulators, such as p16INK4a, p21Cip, and p27, are all expressed in CECs of several species and are important for maintenance of the G1-arrested phenotype through inhibition of cell cycle progression.4,13,14 When cells are induced to express these negative regulators of G1/S transition, the cell cycle is sustained at the G1 phase and senescence phenotypes are increased in various cell types. In contrast, downregulation of their expression turns on cell cycle progression and induces cell proliferation.15–18 Especially in hCECs, reduction of negative cell cycle regulators by small interference RNA (siRNA) induced cell proliferation, resulting in an increase in the number of cells entering the cell cycle and in an increase in total cell numbers.14,19 For these reasons, studying the regulatory mechanism of these negative cell cycle regulators is important to understanding of cell proliferation pathways in hCECs.
Our previous data showed that FGF-2 regulates the cell cycling pathway of rabbit CECs (rCECs) through degradation of p27 by its phosphorylation mechanism.9,12,20 To be degraded, p27 must be phosphorylated at the threonine 187 (Thr187) and serine 10 (Ser10) sites. The cycle-dependent kinase 2 (Cdk2)-Cyclin E complex is responsible for phosphorylation of p27 at Thr187,12,21,22 whereas Ser10 site phosphorylation is mediated by kinase-interacting stathmin (KIS; a nuclear serine-threonine kinase) or protein kinase B (Akt).20,23,24 Our kinetic studies using rCECs12,20,25 showed that the phosphorylated p27 at Ser10 (pp27Ser10) mediated by KIS was detected and degraded by the Kip1 ubiquitination-promoting complex 1/2 (KPC1/2) ubiquitin-proteosomal machinery in the cytoplasm at the early G1 phase of the cell cycle. In contrast, phosphorylated p27 at Thr187 (pp27Thr187) mediated by Cdk2 activated through cell division cycle 25A (Cdc25A) was degraded by nuclear ubiquitin ligase complex in the nucleus at the late G1 phase. Our recent study identified that extracellular signal-regulated kinase 1/2 (ERK1/2) is involved in G1/S transition parallel to and independent of the PI 3-kinase/Rac1 pathway and that both the ERK1/2 and PI 3-kinase/Rac1 pathways participate in activation of KIS and activation of Cdk2 through Cdc25A in rCECs.20 However, the regulatory mechanism for degradation of p27, leading to the induction of cell proliferation, in hCECs has not yet been defined. In the present study, we investigated the correlation between human and rabbit CECs in cell proliferation pathways. In hCECs, FGF-2 stimulated cell proliferation through the ERK1/2 pathway, as a downstream regulator of PI 3-kinase, and phosphorylation of p27 is mediated by KIS for the Ser10 site and Cdc25A for the Thr187 site. The differences between human and rabbit CECs in the regulatory pathway of FGF-2-mediated cell proliferation lie in the cross talk between PI 3-kinase and ERK1/2 for the phosphorylation event of p27: hCECs employ a linear signal transduction involving PI 3-kinase-dependent ERK1/2 activation, while rCECs use parallel and independent ERK1/2 and PI 3-kianse pathways.
Materials and Methods
Materials
LY294002, U0126, 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT), monoclonal antibody against β-actin, and peroxidase conjugated secondary antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Anti-pp27Ser10 and anti-pp27Thr187 were obtained from Zymed Laboratories Inc. (South San Francisco, CA). Fluorescein isothiocyanate (FITC)-conjugated secondary antibody, FGF-2 and BN82002 were purchased from Calbiochem (San Diego, CA). Anti-p27 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Akt, phosphor-Akt (Ser473), phosphor-Akt (Thr308), Cdc25A, ERK1/2 antibodies, and anti-phospho-ERK1/2 antibody were purchased from Cell Signaling Technology (Danvers, MA). Anti-KIS antibody was obtained from Abgent (San Diego, CA).
Human Donor Corneas
Human donor corneas were purchased from National Disease Research Interchange (NDRI; Philadelphia, PA) and stored in corneal storage media (Optisol-GS; Bausch & Lomb, Rochester, NY) at 4°C. Donor confidentiality was maintained by NDRI and this laboratory in accordance with the tenets of the Declaration of Helsinki. We accepted corneas only if the donor history and condition of the corneas suggested no damage to the corneal endothelium and only with cell numbers larger than 2300 per mm2. The information about corneas and specific experiments used in this study is shown in Table 1.
Table 1.
Donor Information
| Donor Age (y) | Cells* (n) | Cause of Death | Experiment |
|---|---|---|---|
| 15 | 2645 | Anoxia | Western blot analysis |
| 18 | 3407 | Anoxia | Western blot analysis |
| 20 | 3440 | Head trauma | MTT assay |
| 20 | 2825 | Motor vehicle accident | Western blot analysis |
| 20 | 2584 | Motor vehicle accident | MTT assay |
| 22 | 3149 | Head trauma | MTT assay |
| 22 | 3030 | Motor vehicle accident | Western blot analysis |
| 27 | 3392 | Motor vehicle accident | Ex vivo staining |
| 32 | 2567 | Head trauma | siRNA/MTT assay |
| 37 | 3154 | Pulmonary embolism | siRNA/Western blot analysis |
| 40 | 3063 | Suicide by stabbing | Western blot analysis |
| 41 | 3262 | Gun shot wound | Western blot analysis |
| 44 | 2984 | Anoxia | Western blot analysis |
| 45 | 3053 | Intracranial hemorrhage | siRNA/Western blot analysis |
| 47 | 2800 | Cerebrovascular accident | siRNA/Western blot analysis |
| 53 | 2655 | Liver cancer | MTT assay |
| 57 | 2964 | Anoxic brain injury | siRNA/MTT assay |
| 58 | 2959 | Cardiac arrest | MTT assay |
| 58 | 3105 | Cardiac arrest | Western blot analysis |
| 59 | 2708 | Renal failure | Ex vivo staining |
| 59 | 2813 | Bilateral pneumonia | MTT assay |
| 63 | 2910 | Pulmonary embolism | Western blot analysis |
| 66 | 2638 | Ruptured aortic aneurysm | siRNA/Western blot analysis |
| 73 | 3442 | Failure to thrive | Western blot analysis |
| 75 | 2401 | Brain cancer | Western blot analysis |
All corneas were used within 3 d after preservation in corneal storage media (Optisol-GS; Bausch & Lomb) at 4°C.
Average number of CECs per mm2 of OD and OS.
Isolation and Growth of Human Corneal Endothelial Cells
Isolation and establishment of hCECs were performed according to previously published protocols14,26 with some technical modifications. Briefly, corneas were removed from the corneal storage media (Optisol-GS; Bausch & Lomb) and washed several times with reduced serum media (OptiMEM-I; Gibco-BRL, Grand Island, NY) containing 50 μg/mL gentamicin. The Descemet's membrane and endothelium complex was dissected in small pieces and then incubated overnight in reduced serum media (OptiMEM-I; Gibco-BRL) supplemented with 8% fetal bovine serum (FBS; Omega Scientific, Tarzana, CA) overnight to stabilize the cells before culture. After centrifugation, the Descemet's membrane and endothelium complex was treated with 0.2% collagenase type II and 0.05% hyaluronidase (Worthington Biochemical, Lakewood, NJ) for 90 minutes at 37°C. After centrifugation, the primary cells were resuspended in culture medium: reduced serum media (OptiMEM-I; Gibco-BRL) supplemented with 8% FBS, 5 ng/mL epidermal growth factor (Upstate Biotechnologies, Lake Placid, NY), 20 ng/mL nerve growth factor (Biomedical Technologies, Stoughton, MA), 100 μg/mL bovine pituitary extract (Biomedical Technologies), 20 μg/mL ascorbic acid (Sigma-Aldrich), 200 mg/mL calcium chloride, 0.08% chondroitin sulfate (Sigma-Aldrich), 50 μg/mL gentamicin, and antibiotic-antimycotic solution (Sigma-Aldrich) diluted 1:100 and then plated in precoated 24-well tissue culture plates with tissue culture reagent (FNC coating mix; Biological Research Faculty & Facility, Inc., Ijamsville, MD). For subculture, confluent primary cultures were treated with 0.05% trypsin and 5 mM EDTA in phosphate-buffered saline (PBS) for 5 minutes. Second passage CECs were used for all experiments. For serum starvation, culture medium was changed to Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL) and cultures were maintained for 24 hours. Heparin (10 μg/mL) was added to cell cultures treated with FGF-2 (10 ng/mL) because our previous study showed that the initiation of FGF-2 activity in CECs requires the addition of supplemental heparin.27 In some experiments, pharmacologic inhibitors were used in the presence of FGF-2 stimulation20: LY294002 (20 μM), U0126 (1 μM), or BN82002 (5 μM).
Cell Proliferation Assays
MTT assay was used to measure cell proliferation as previously described.20 Briefly, cells were seeded in 96-well tissue culture plates at a concentration 4 × 103 cells per well. When cells reached approximately 70% confluence, the medium was changed to DMEM for serum starvation and maintained for 24 hours. The serum-starved cells were then maintained for 24 hours in each culture condition. At the end of culture, the MTT (50 μg/mL) was added and the culture was maintained for an additional 2 hours at 37°C. The MTT-containing medium was discarded and 100 μL of undiluted dimethyl sulfoxide was added to the cells. After a 30-minute incubation at room temperature, absorbance of the converted dye was measured at a wavelength of 570 nm with background subtraction at 650 nm, using a spectrophotometric plate reader (Benchmark Plus Microplate Spectrophotometer, Bio-Rad Laboratories, Inc., Hercules, CA).
Immunostaining of p27 in Ex Vivo Cornea and Confocal Microscopy
Human whole corneas obtained from NDRI were cut in quarters. These corneal pieces were placed endothelial-side-up in individual wells of a 24-well tissue culture plate. The corneal pieces were incubated in DMEM with or without FGF-2 for 24 hours at 37°C. These corneal pieces were fixed for 10 minutes in 4% paraformaldehyde and rinsed three times with PBS. They were then permeabilized with 1.0% Triton X-100 (Sigma-Aldrich) in PBS for 10 minutes at room temperature, and nonspecific staining sites were blocked with bovine serum albumin (Sigma-Aldrich). The subsequent incubation was carried out with 2% bovine serum albumin in PBS, and all washes were carried out in PBS containing 0.1% Triton-X-100 at room temperature. Cells were incubated with anti-p27 antibody (1:200 dilution) for 2 hours at 37°C and then with FITC-conjugated secondary antibody (1:200 dilution) for 2 hours at 37°C in the dark. After extensive washing, the slides were mounted in a drop of mounting medium (Vectashield; Vector Laboratories Inc., Burlingame, CA) containing DAPI. Control experiments, performed in parallel with the omission of the primary antibodies, did not show the activity. Antibody-labeled and DAPI-stained cells were examined using a laser scanning confocal microscope (Zeiss LSM-510; Zeiss, Thornwood, NY). Image analysis was performed using the standard system operating software provided with the laser scanning confocal microscope. We counted the number of p27-stained cells per ten viewing fields at magnification ×400 and determined the percentage of labeled nuclei in a total of over 300 nuclei using four coverslips per experimental condition.
Protein Preparation, Protein Assay, SDS-Polyacrylamide Gel Electrophoresis, Western Blotting Analysis, and Immunofluorescent Analysis
All details of methods and procedures have been presented previously.8,12,20 The following gel concentrations were used to separate proteins: 12.5% gel for p27, ERK1/2, and KIS, and 10% gel for Akt, actin, and Cdc25A. The following protein amounts were used to detect specific target proteins: 10 μg protein for positive control, such as ERK1/2, Akt, and actin; 50 μg protein for p27, KIS, Cdc25A, and phosphorylated form of Akt and ERK1/2; and 80 μg protein for the phosphorylated form of p27.
Small Interference RNA Transfection
Small interference RNAs (siRNAs) against human KIS were obtained from Ambion (Austin, TX) in deprotected and desalted form. The chemically synthesized, double-stranded siRNA with 21 nucleotides duplex RNA sequences (sense) targeting respective human KIS mRNA is listed: 5′-AAGCAGUUCUUGCCGCCAGGA-3′.24 Transient transfections of siRNA were performed according to previously published protocols using transfection reagent (Lipofectamine RNAiMAX; Invitrogen, Carlsbad, CA).20 Briefly, CECs were seeded on 24-well plates and maintained in culture until they reached 60% to 70% confluence. These cells were transiently transfected with 50 nM of the siRNA double-strand RNAs and 3 μL of transfection reagent (Lipofectamine RNAiMAX; Invitrogen) complex. After the 6-hour incubation, medium containing transfection reagent was removed, and the cells were maintained in DMEM with or without FGF-2 for an additional 24 hours. To verify specificity of the knockdown effect, we used a negative control siRNA (Silencer Select Negative Control; Ambion) with no known mammalian target as nonspecific siRNA. There was no cytotoxic effect of the transfection reagent (Lipofectamine RNAiMAX; Invitrogen), and the transfection efficiency was 50% to 60% under these transfection conditions. The transfection efficiency was detected using fluorescein-conjugated nonspecific siRNA by a fluorescence microscope.
Statistics
All experiments were performed at least three times, and the results are presented as the mean and SEM. Statistical differences were analyzed with the aid of commercially-available software (Excel 2003; Microsoft, Redmond, WA) using a paired Student's t-test as indicated in figure legends.
Results
Involvement of PI 3-Kinase and ERK1/2 in Cell Proliferation
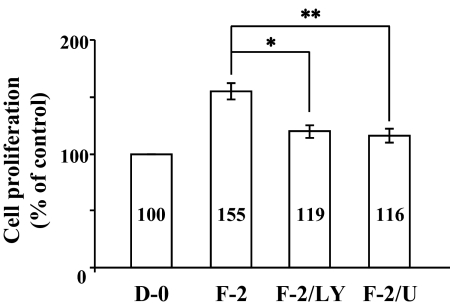
Our previous data using rCECs suggested that PI 3-kinase and ERK1/2 pathways are involved in cell proliferation stimulated by FGF-2.9,20 It is, therefore, natural to address whether FGF-2 induces cell proliferation in hCECs through PI 3-kinase and ERK1/2. To test this question, we performed cell proliferation assay using MTT. We first determined whether cell proliferation was induced in cells treated with FGF-2. When compared with the control value derived from the cells maintained in DMEM, FGF-2 showed a strong stimulatory effect on hCEC proliferation (Fig. 1) as observed in rCEC.9,20 In the cells pretreated with the pathway specific inhibitors LY294002 (PI 3-kinase inhibitor) and U0126 (inhibitor of MEK1/2, upstream kinase to phosphorylate ERK1/2) for 2 hours before stimulation with FGF-2 for 24 hours, cell proliferation triggered by FGF-2 was greatly blocked (Fig. 1). These findings suggest that PI 3-kinase and ERK1/2 are involved in the cell proliferation pathway in response to stimulation with FGF-2 in hCECs.
Figure 1.
Inhibitory effect of PI 3-kinase and ERK1/2 inhibitor on cell proliferation stimulated by FGF-2. Cell proliferation was determined by MTT assay. The serum-starved hCECs were pretreated with LY294002 for PI 3-kinase inhibition or U0126 for ERK1/2 inhibition for 2 hours and then maintained in DMEM with FGF-2 for 24 hours. At the end of incubation, MTT was added for 4 hours and then intracellular purple formazan, the MTT metabolic product by the action of dehydrogenase enzymes of metabolically-active cells, was quantified with a spectrophotometric plate reader at dual wavelengths of 570 and 650 nm. Data were normalized to cells maintained in DMEM without serum. D-0, DMEM without serum; F-2, FGF-2; LY, LY294002; U, U0126. The graphs represent the mean ± SEM from three independent experiments. (*P = 0.0009, paired t-test; **P = 0.0002, paired t-test).
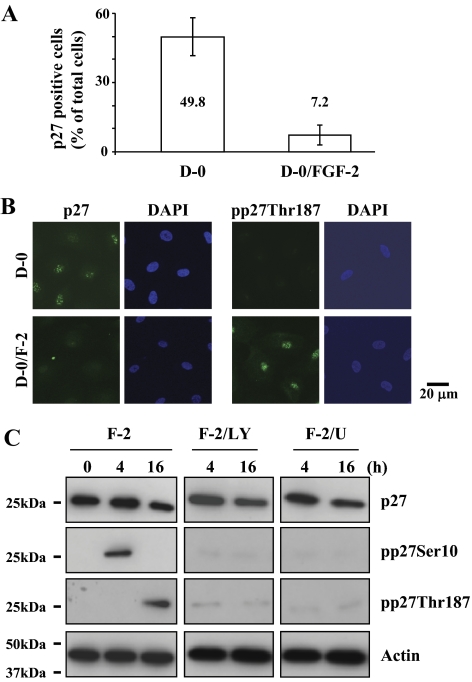
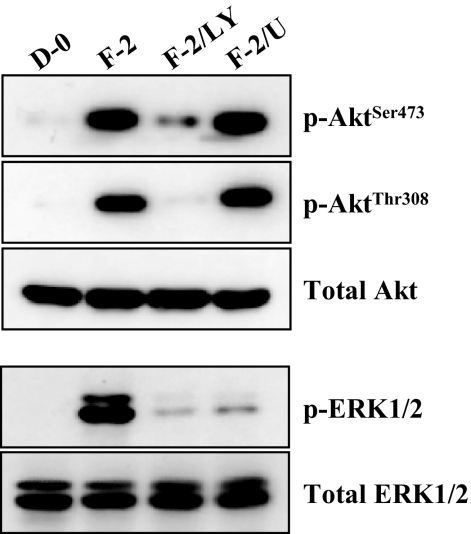
Because degradation of p27 through its phosphorylation by FGF-2-stimulation is a major signaling observed in the cell proliferation pathway in rCECs,10,12,25 we determined whether this is also true for FGF-2 to induce cell proliferation in hCECs. In ex vivo staining of corneal endothelium with anti-p27 antibody, 50% of cells maintained with DMEM were positive for p27 staining, whereas this positive staining was dramatically decreased in FGF-2-treated corneal endothelium (Fig. 2A). The differential phosphorylation was further confirmed using immunocytochemistry in vitro. When cells were stained with anti-pp27Thr187 antibody, positive staining of pp27Thr187 was only observed in the cells maintained in DMEM with FGF-2, but not in the cells maintained with D-0 (Fig. 2B). We then investigated whether PI 3-kinase and ERK1/2 participate in phosphorylation of p27 at both Ser10 and Thr187 sites in hCECs. Because our previous data with rCECs showed that phosphorylation of p27 at the Ser10 and Thr187 sites was maximized at 4 hours and 16 hours after FGF-2 stimulation, respectively,12 we chose two FGF-2 treatment time points: 4 hours and 16 hours. As expected, pp27Ser10 was detected at 4 hours, but not at 16 hours, whereas pp27Thr187 was detected at 16 hours but not at 4 hours in response to FGF-2 stimulation (Fig. 2C). The phosphorylation kinetics of p27 in hCECs are identical to those observed in rCECs. When pathway specific inhibitors were used, PI 3-kinase and ERK1/2 inhibition completely blocked phosphorylation of p27 at either the Ser10 or the Thr187 site induced by FGF-2 stimulation, suggesting that FGF-2 signaling also induced phosphorylation of p27 at both the Ser10 and Thr187 sites through PI 3-kinase and ERK1/2 in hCECs, as observed in rCECs. We then investigated whether the PI 3-kinase and ERK1/2 pathways are parallel and independent, similar to those defined in rCECs.20 When hCECs were stimulated with FGF-2 for 8 hours, phosphorylation of Akt and ERK1/2 was observed similar to that in rCECs20 (Fig. 3). In contrast, pretreatment of hCECs with PI 3-kinase inhibitor for 2 hours before FGF-2 stimulation blocked phosphorylation of Akt at Ser473 and Thr308 (the downstream product of activated PI 3-kinase) and of ERK1/2, whereas MEK1/2 inhibitor blocked the ERK1/2 activation but did not block Akt activation after FGF-2 stimulation. These findings indicate that ERK1/2 is the downstream effecter to the PI 3-kinase/Akt pathways triggered by FGF-2 in hCECs (Fig. 3). These results demonstrate that hCECs employ the signaling molecules similar to those of rCECs, but that there is a hierarchy in signal transduction in hCECs unlike in rCECs.
Figure 2.
Reduction of p27 level ex vivo and induction of its phosphorylation at both Ser10 and Thr187 sites by FGF-2 stimulation in vitro. (A) The corneal endothelium was incubated with or without FGF-2 for 24 hours and then immunostained with anti-p27 antibody. The total DAPI-stained and p27-positive cells were counted in one microscopic field under high magnification (×400). The percentage of p27-positive CECs was significantly greater in the corneal pieces incubated in mitogen-deprived medium (D-0) than in those treated with FGF-2. (B) The serum-starved hCECs were treated with or without FGF-2 for 16 hours and maintained in D-0 for up to 24 hours. The cultured cells were fixed and labeled with anti-p27 or anti-pp27Thr187 antibody (FITC) and DAPI, respectively. (C) The serum-starved hCECs were pretreated with PI 3-kinase inhibitor or MEK1/2 inhibitor for 2 hours and then maintained in DMEM with FGF-2 for the designated time. At the end of treatment, cells were lysed and then immunoblotted with the respective antibody. Actin was used to control protein concentration on immunoblot analysis. The results represent data obtained in three independent experiments.
Figure 3.
Activation of ERK1/2 by FGF-2 stimulation through PI 3-kinase. The serum-starved hCECs were pretreated with PI 3-kinase inhibitor or MEK1/2 inhibitor for 2 hours and then maintained in DMEM with FGF-2 for 8 hours. At the end of treatment, cells were lysed and then immunoblotted with the designated antibody. ERK1/2 works as a downstream regulator to the PI 3-kinase/Akt pathways triggered by FGF-2 stimulation. Total Akt and ERK1/2 were used to control protein concentration on immunoblot analysis. The results represent data obtained in three independent experiments.
Involvement of PI 3-Kinase and ERK1/2 in Phosphorylation of p27 at Ser10 by KIS and at Thr187 by Cdc25A
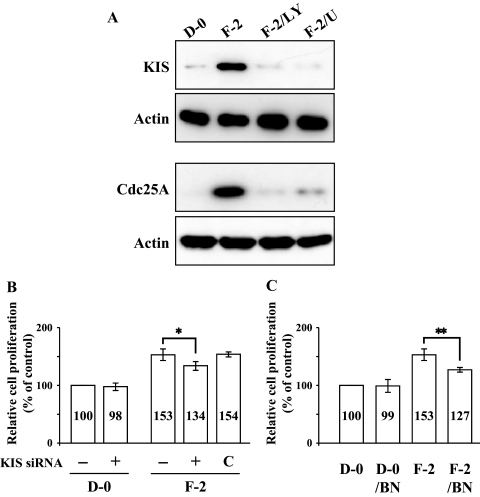
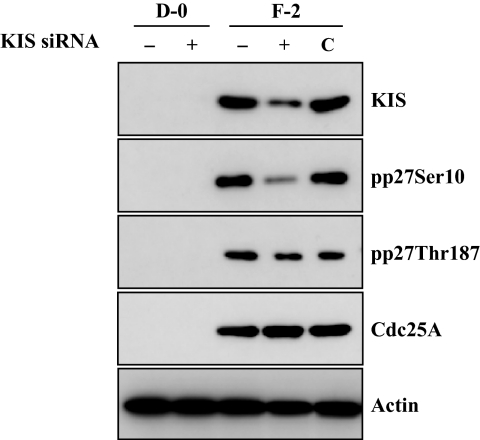
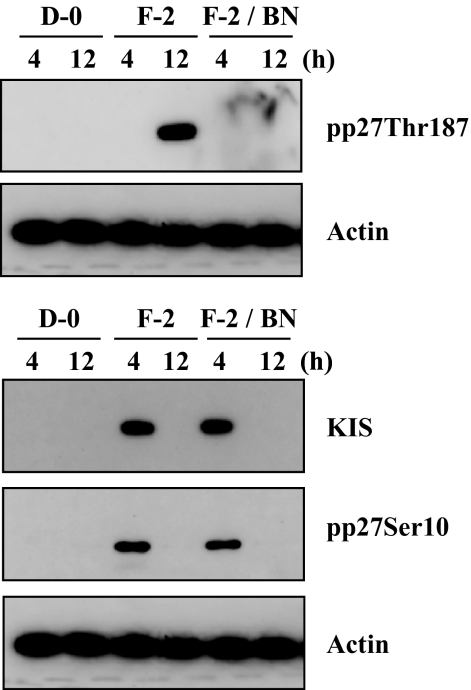
Our previous data demonstrated that KIS phosphorylates p27 at the Ser10 site and Cdk2 activated by Cdc25A phosphorylates p27 at Thr187 site in rCECs.20 We, therefore, defined a linking between the early signaling events (activation of PI 3-kinase and ERK1/2) and the final outcome (phosphorylation of p27) in hCECs. We first determined whether FGF-2 induced expression of KIS and Cdc25A in hCECs. The serum-starved hCECs were pretreated with PI 3-kinase inhibitor or MEK1/2 inhibitor for 2 hours and then maintained in DMEM with FGF-2 for 4 hours to detect KIS and for 12 hours to detect Cdc25A, according to the kinetics observed in rCECs.20 In the cells stimulated with FGF-2, protein levels of KIS and Cdc25A were greatly promoted, when compared with the corresponding levels in control cells maintained in DMEM. In contrast, when cells were pretreated with either PI 3-kinase or MEK1/2 inhibitor, the expression of both KIS and Cdc25A protein levels by FGF-2 stimulation was completely blocked (Fig. 4A). To further confirm whether KIS and/or Cdc25A were involved in the cell proliferation pathway triggered by FGF-2, gene knockdown of KIS gene by siRNA specific for KIS and blockade of Cdc25A by specific inhibitor (BN82002) were used. Cells were transfected for 6 hours with specific siRNA for KIS and then maintained in the experimental medium for an additional 24 hours. The transfected cells with KIS siRNA maintained in the absence of FGF-2 showed that there was neither cell proliferation activity nor cytotoxic effect by transfection (Fig. 4B). When the transfected cells with KIS siRNA were maintained in FGF-2 containing medium, there was a marked decrease of cell proliferation (36% reduction) compared with that of nontransfected cells maintained with FGF-2. Control siRNA had no effect on the FGF-2-stimulated cell proliferation. Cdc25A inhibitor also blocked cell proliferation activity of FGF-2 (49% reduction) compared with that of cells maintained with FGF-2 (Fig. 4C). These results suggested that both KIS and Cdc25A are involved in the FGF-2-induced cell proliferation in hCECs, similar to that seen in rCECs. To determine whether KIS was involved in phosphorylation of p27 at the Ser10 site, we transfected cells with KIS siRNA and performed immunoblotting. In the KIS siRNA transfected cells, not only was the KIS protein level greatly reduced, but the phosphorylation of p27 at Ser10 site was blocked. In contrast, there was no effect on the phosphorylation of p27 at the Thr187 site or on the protein level of Cdc25A (Fig. 5). Cdc25A inhibitor blocked the phosphorylation of p27 at the Thr187 site, but not at the Ser10 site, and had no effect on the KIS protein level (Fig. 6). These results indicated that there was no cross talk between the specific enzymes involved in phosphorylation of p27, KIS, and Cdc25A. Of great importance, these pathways of hCECs for phosphorylation at both the Ser10 and Thr187 sites induced by FGF-2 stimulation are identical to those of rCECs.
Figure 4.
Induction of KIS and Cdc25A through PI 3-kinase and ERK1/2 and their involvement in cell proliferation induced by FGF-2 stimulation. (A) The serum-starved hCECs were pretreated with PI 3-kinase inhibitor or MEK1/2 inhibitor for 2 hours and then maintained in DMEM with FGF-2 for 4 hours to detect KIS and 12 hours to detect Cdc25A. At the end of treatment, cells were lysed and then immunoblotted with the designated antibody. Actin was used to control protein concentration on immunoblot analysis. The results represent data obtained in three independent experiments. (B) After transfection with KIS siRNA, the CECs were maintained with DMEM or DMEM with FGF-2 for 24 hours. Cell proliferation was determined using MTT assay as previously described in Figure 1. Data were normalized to untransfected cells. The graphs represent the mean ± SEM from three independent experiments (*P = 0.0114, paired t-test). (C) To determine the effect of Cdc25A inhibition on cell proliferation, the serum-starved cells were pretreated with BN82002 for Cdc25A inhibition for 2 hours and then maintained in DMEM with or without FGF-2 for 24 hours. Cell proliferation was determined and data were normalized to unstimulated cells. The graphs represent the mean ± SEM from three independent experiments (**P = 0.0087, paired t-test). BN, BN82002.
Figure 5.
Inhibitory effect of siRNA to KIS on phosphorylation of p27 at the Ser10 site, but not at the Thr187 site. Human CECs were transfected with KIS siRNA. After transfection, cells were maintained with DMEM or DMEM with FGF-2 for an additional 24 hours. Total protein extracts were prepared and immunoblotted with designated antibodies. Actin was used to control protein concentration on immunoblot analysis. The results represent data obtained in three independent experiments. C, negative control cells which were transfected with negative control siRNA (Silencer Select Negative Control; Ambion).
Figure 6.
Inhibitory effect of Cdc25A inhibitor on phosphorylation of p27 at the Thr187 site, but not at the Ser10 site. The serum-starved hCECs were pretreated with or without Cdc25A inhibitor for 2 hours and then maintained in DMEM with FGF-2 for the designated time. At the end of treatment, the phosphorylation of p27 at the Ser10 site and the Thr187 site was detected with immunoblotting. Actin was used to control protein concentration on immunoblot analysis. The results represent data obtained in three independent experiments.
Discussion
Coordinate regulation of intracellular signaling pathways is important to promote cell proliferation induced by mitogen stimulation; cell cycle progression is tightly controlled and influenced by the expression, activity, and subcellular localization of key components of both positive and negative cell cycle regulators.28 Positive regulators, including the cyclins and their catalytic partner Cdks, are essential for cells to progress through each phase of the cell cycle. Likewise, negative regulators, including the cyclin kinase inhibitors (CKIs), which inhibit the cell cycle at multiple check points through inactivation of cyclin-Cdk complex, are equally essential for the regulatory role in cell cycle progression. Cyclins and CKIs are regulated by transcriptional and posttranscriptional mechanisms that are linked to a variety of signaling pathways, including PI 3-kinase/Akt and ERK1/2.29–31 Among the CKI regulation for G1/S progression in CECs, control of the p27 level is important to mediate cell proliferation in vitro and in vivo: degradation of p27 was induced by mitogen stimulation in vitro,12,19,20 and p27 was also involved in regulating proliferation in corneal endothelium of the developing mouse cornea.32 Our previous publications on the phosphorylation and degradation kinetics of p27 in rCECs12,20,25 indicated that the pp27Ser10 and pp27Thr187 represent two distinct populations of p27 in the G1 phase of the cell cycle. We also showed that phosphorylation of p27 at Ser10 followed by nuclear export of pp27Ser10 and its subsequent degradation in the cytoplasm occurred much earlier than phosphorylation of p27 at Thr187 followed by degradation of pp27Thr187 in the nuclei.12 Our attempt to elucidate the molecular mechanism of p27-dependent cell proliferation led us to the finding that the ERK1/2 pathway is also involved in G1/S transition in parallel to and independent of the PI 3-kinase pathway in rCECs20: both PI 3-kinase and ERK1/2 pathways employ KIS to phosphorylate p27 at Ser10 and activation of Cdk2 through Cdc25A for phosphorylation of p27 at Thr187.
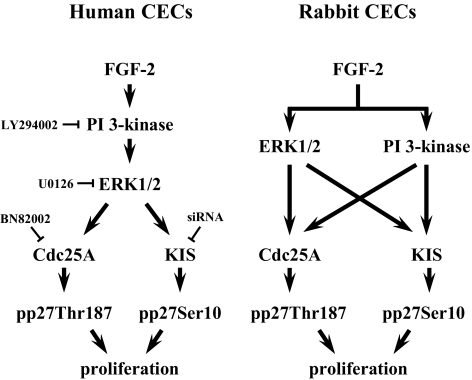
In the present study, we showed the following important observations in hCECs: FGF-2 stimulates cell proliferation through PI 3-kinase and ERK1/2; ERK1/2 works as the PI 3-kinase downstream effecter protein; FGF-2 signaling upregulates the KIS expression necessary for phosphorylation of p27 at Ser10; and FGF-2 signaling upregulates expression of Cdc25A for phosphorylation of p27 at Thr187 (Fig. 7). These findings, taken together, suggest that hCECs employ identical mitogenic signaling pathways triggered by FGF-2 when compared with those in rCECs. The only difference observed between the human and rabbit CEC cell proliferation signaling pathway lies in the cross talk between PI 3-kinase and ERK1/2: ERK1/2 is downstream to the PI 3-kinase/Akt pathways triggered by FGF-2 in hCECs, whereas the PI 3-kinase pathway and ERK1/2 pathway are parallel and independent in rCECs (Fig. 7).20
Figure 7.
Schematic comparison of human and rabbit CECs cell proliferation pathway stimulated by FGF-2. ERK1/2 pathway activated by PI 3-kinase increase cell proliferation through phosphorylation of p27 at both Ser10 and Thr187 residues in response to FGF-2 stimulation in hCECs. In contrast, parallel ERK1/2 and PI 3-kinase pathways induced by FGF-2 increase cell proliferation in rCECs.
Involvement of both PI 3-kinase and ERK1/2 in cell proliferation in the various cell types has been well recognized. Furthermore, the diversity of the cross talk between these two signaling molecules in the cell proliferation pathway induced by various factors is well defined; either a linear coincidence pathway33–35 or a parallel independent pathway36–38 is observed. Furthermore, it has been reported that the two pathways are separately activated in response to the same factor, depending on cell types: a linear pathway was observed in human breast epithelial cells,33 while a parallel pathway was observed in human Schwann cells38 in response to epidermal growth factor stimulation. Although we clearly defined the signal transduction pathway triggered by FGF-2 in human and rabbit CECs, we have not yet deciphered why PI 3-kinase and ERK1/2 pathways are differentially employed in hCECs and rCECs. A plausible explanation might be derived from the cell proliferation ratio between human and rabbit CECs: when compared with the induction ratio of cell proliferation by FGF-2 stimulation measured with MTT assay, induction of cell proliferation of rCECs was 1.4 times higher than that of hCECs.20 We, therefore, assume that cell proliferation is more tightly controlled in hCECs than in rCECs, because the linear signal transduction should more easily control the cellular activity than did the parallel redundant signals.
We have extensively used rCECs to identify most of the signal transduction involved in EMT.8–12,20,25 However, there has been skepticism concerning the use of nonhuman CECs as a model of hCECs. Nonetheless, obstacles remain to the use hCECs for studying the cell biology: the limited supply of human corneas hampers our effort to obtain the large quantity of cell numbers that are crucial for the long-term engagement of the in-depth study; immortalized hCECs cannot be used to elucidate mechanisms of some cellular activities (i.e., cell cycle progression and cell proliferation); and no alternative cell systems exist. Our current knowledge on the mouse CECs that could have been a good alternative is very poor, while bovine CECs alter their collagen phenotypes when exposed to tissue culture conditions.39 In contrast, our previous studies using rCECs demonstrated that rCECs have a characteristic growth behavior and maintain the basement membrane phenotypes such as type IV collagen, similar to hCECs.40,41 In accordance with these data, the present study also shows that human and rabbit CECs share the removal of p27 through the identical mechanism of p27 phosphorylation, subsequently leading to cell proliferation. Taken together, our study removes the skepticism concerning the use of nonhuman CECs as a model of hCECs and justifies the use of nonhuman CECs. Furthermore, this study clearly demonstrates that rCECs are excellent tools to replace hCECs when studying cell biology of CECs.
Footnotes
Supported by NIH/NEI Grants EY06431 and EY03040, and Research to Prevent Blindness, New York, NY.
Disclosure: J.G. Lee, None; J.-S. Song, None; R.E. Smith, None; E.P. Kay, None
References
- 1. Kreutziger GO. Lateral membrane morphology and gap junction structure in rabbit corneal endothelium. Exp Eye Res. 1976;23:285–293 [DOI] [PubMed] [Google Scholar]
- 2. Geroski DH, Edelhauser HF. Quantitation of Na/K ATPase pump sites in the rabbit corneal endothelium. Invest Ophthalmol Vis Sci. 1984;25:1056–1060 [PubMed] [Google Scholar]
- 3. Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359–389 [DOI] [PubMed] [Google Scholar]
- 4. Joyce NC, Navon SE, Roy S, Zieske JD. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest Ophthalmol Vis Sci. 1996;37:1566–1575 [PubMed] [Google Scholar]
- 5. Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660–667 [PubMed] [Google Scholar]
- 6. Kay ED, Cheung CC, Jester JV, Nimni ME, Smith RE. Type I collagen and fibronectin synthesis by retrocorneal fibrous membrane. Invest Ophthalmol Vis Sci. 1982;22:200–212 [PubMed] [Google Scholar]
- 7. Leung EW, Rife L, Smith RE, Kay EP. Extracellular matrix components in retrocorneal fibrous membrane in comparison to corneal endothelium and Descemet's membrane. Mol Vis. 2000;6:15–23 [PubMed] [Google Scholar]
- 8. Lee HT, Lee JG, Na M, Kay EP. FGF-2 induced by interleukin-1 beta through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J Biol Chem. 2004;279:32325–32332 [DOI] [PubMed] [Google Scholar]
- 9. Lee JG, Kay EP. Common and distinct pathways for cellular activities in FGF-2 signaling induced by IL-1beta in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2009;50:2067–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee HT, Kay EP. Regulatory role of PI 3-kinase on expression of Cdk4 and p27, nuclear localization of Cdk4, and phosphorylation of p27 in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2003;44:1521–1528 [DOI] [PubMed] [Google Scholar]
- 11. Kay EP, Gu X, Smith RE. Corneal endothelial modulation: bFGF as direct mediator and corneal endothelium modulation factor as inducer. Invest Ophthalmol Vis Sci. 1994;35:2427–2435 [PubMed] [Google Scholar]
- 12. Lee JG, Kay EP. Two populations of p27 use different kinetics to phosphorylate Ser10 and Thr187 via PI 3-kinase in response to fibroblast growth factor-2 stimulation. J Biol Chem. 2007;282:6444–6454 [DOI] [PubMed] [Google Scholar]
- 13. Joyce NC, Meklir B, Joyce SJ, Zieske JD. Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci. 1996;37:645–655 [PubMed] [Google Scholar]
- 14. Joyce NC, Harris DL. Decreasing expression of the G1-phase inhibitors, p21Cip1 and p16INK4a, promotes division of corneal endothelial cells from older donors. Mol Vis. 2010;16:897–906 [PMC free article] [PubMed] [Google Scholar]
- 15. Jeffrey PD, Tong L, Pavletich NP. Structural basis of inhibition of CDK-cyclin complexes by INK4 inhibitors. Genes Dev. 2000;14:3115–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reynisdottir I, Polyak K, Iavarone A, Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–1845 [DOI] [PubMed] [Google Scholar]
- 17. Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684 [DOI] [PubMed] [Google Scholar]
- 18. Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816 [DOI] [PubMed] [Google Scholar]
- 19. Kikuchi M, Zhu C, Senoo T, Obara Y, Joyce NC. p27kip1 siRNA induces proliferation in corneal endothelial cells from young but not older donors. Invest Ophthalmol Vis Sci. 2006;47:4803–4809 [DOI] [PubMed] [Google Scholar]
- 20. Lee JG, Kay EP. PI 3-kinase/Rac1 and ERK1/2 regulate FGF-2-mediated cell proliferation through phosphorylation of p27 at Ser10 by KIS and Thr187 by Cdc25A/Cdk2. Invest Ophthalmol Vis Sci. 2011;52:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512 [DOI] [PubMed] [Google Scholar]
- 22. Zhu XH, Nguyen H, Halicka HD, Traganos F, Koff A. Noncatalytic requirement for Cyclin A-cdk2 in p27 turnover. Mol Cell Biol. 2004;24:6058–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujita N, Sato S, Katayama K, Tsuruo T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2002;277:28706–28713 [DOI] [PubMed] [Google Scholar]
- 24. Boehm M, Yoshimoto T, Crook MF, et al. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J. 2002;21:3390–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JG, Kay EP. Involvement of two distinct ubiquitin E3 ligase systems for p27 degradation in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2008;49:189–196 [DOI] [PubMed] [Google Scholar]
- 26. Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004;45:1743–1751 [DOI] [PubMed] [Google Scholar]
- 27. Kay EP, Gu X, Ninomiya Y, Smith RE. Corneal endothelial modulation: a factor released by leukocytes induces basic fibroblast growth factor that modulates cell shape and collagen. Invest Ophthalmol Vis Sci. 1993;34:663–672 [PubMed] [Google Scholar]
- 28. Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169 [DOI] [PubMed] [Google Scholar]
- 29. Roberts EC, Shapiro PS, Nahreini TS, Pages G, Pouyssegur J, Ahn NG. Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signaling pathways in somatic cell mitosis. Mol Cell Biol. 2002;22:7226–7241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239 [DOI] [PubMed] [Google Scholar]
- 31. García Z, Kumar A, Marqués M, Cortés I, Carrera AC. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. EMBO J. 2006;25:655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshida K, Kase S, Nakayama K, et al. Involvement of p27KIP1 in the proliferation of the developing corneal endothelium. Invest Ophthalmol Vis Sci. 2004;45:2163–2167 [DOI] [PubMed] [Google Scholar]
- 33. LeVea CM, Reeder JE, Mooney RA. EGF-dependent cell cycle progression is controlled by density-dependent regulation of Akt activation. Exp Cell Res. 2004;297:272–284 [DOI] [PubMed] [Google Scholar]
- 34. Mahimainathan L, Ghosh-Choudhury N, Venkatesan BA, Danda RS, Choudhury GG. EGF stimulates mesangial cell mitogenesis via PI3-kinase-mediated MAPK-dependent and AKT kinase-independent manner: involvement of c-fos and p27Kip1. Am J Physiol Renal Physiol. 2005;289:F72–F82 [DOI] [PubMed] [Google Scholar]
- 35. Pérez J, Torres RA, Rocic P, et al. PYK2 signaling is required for PDGF-dependent vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2011;301:C242–C251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sinha D, Bannergee S, Schwartz JH, Lieberthal W, Levine JS. Inhibition of ligand-independent ERK1/2 activity in kidney proximal tubular cells deprived of soluble survival factors up-regulates Akt and prevents apoptosis. J Biol Chem. 2004;279:10962–10972 [DOI] [PubMed] [Google Scholar]
- 37. Hayashi H, Matsuzaki O, Muramatsu S, et al. Centaurin-alpha1 is a phosphatidylinositol 3-kinase-dependent activator of ERK1/2 mitogen-activated protein kinases. J Biol Chem. 2006;281:1332–1337 [DOI] [PubMed] [Google Scholar]
- 38. Monje PV, Athauda G, Wood PM. Protein kinase A-mediated gating of neuregulin-dependent ErbB2-ErbB3 activation underlies the synergistic action of cAMP on Schwann cell proliferation. J Biol Chem. 2008;283:34087–34100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sage H, Pritzl P, Bornstein P. Secretory phenotypes of endothelial cells in culture: comparison of aortic, venous, capillary, and corneal endothelium. Arteriosclerosis. 1981;1:427–442 [DOI] [PubMed] [Google Scholar]
- 40. Kay EP, Smith RE, Nimni ME. Basement membrane collagen synthesis by rabbit corneal endothelial cells in culture. J Biol Chem. 1982;257:7116–7121 [PubMed] [Google Scholar]
- 41. Kay EP, Nimni ME, Smith RE. Stability of collagen phenotype in morphologically modulated rabbit corneal endothelial cells. Invest Ophthalmol Vis Sci. 1984;25:495–501 [PubMed] [Google Scholar]