Abstract
In congenital heart block (CHB), binding of maternal anti-SSA/Ro antibodies to fetal apoptotic cardiocytes impairs their removal by healthy cardiocytes and increases uPA/uPAR-dependent plasmin activation. Since the uPA/uPAR system plays a role in TGF beta activation, we evaluated whether anti-Ro binding to apoptotic cardiocytes enhances plasmin-mediated activation of TGF beta thereby promoting a profibrosing phenotype. Supernatants from co-cultures of healthy cardiocytes and apoptotic cardiocytes bound by IgG from a mother whose child had CHB (apo-CHB-IgG) exhibited significantly increased levels of active TGF beta compared to supernatants from co-cultures of healthy cardiocytes and apoptotic cardiocytes preincubated with IgG (apo-nl-IgG) from a healthy donor. Treatment of the culture medium with anti-TGF beta antibody or TGF beta inhibitor (SB431542) abrogated the luciferase response thereby confirming TGF beta dependency. Increased uPA levels and activity were present in supernatants generated from cocultures of healthy cardiocytes and apo-CHB-IgG cardiocytes compared to healthy cardiocytes and and apo nl-IgG cardiocytes, respectively. Treatment of apo-CHB-IgG cardiocytes with anti-uPAR or anti-uPA antibodies or plasmin inhibitor aprotinin prior to coculturing with healthy cardiocytes attenuated TGF beta activation. Supernatants derived from cocultures of healthy cardiocytes and apo-CHB-IgG cardiocytes promoted Smad2 phosphorylation and fibroblast transdifferentiation as evidenced by increased SMAc and collagen expression, which decreased when fibroblasts were treated with supernatants from cocultures pretreated with uPAR antibodies. These data suggest that binding of anti-Ro antibodies to apoptotic cardiocytes triggers TGF beta activation, by virtue of increasing uPAR-dependent uPA activity, thus initiating and amplifying a cascade of events that promote myofibroblast transdifferentiation and scar.
Introduction
Organ injury induced by antibodies characteristic of Sjogren’s Syndrome and Systemic Lupus Erythematosus may share in common a link between apoptosis and ultimate fibrosis 1. The signature histologic lesion of autoimmune-associated congenital heart block (CHB) is fibrosis of the atrioventricular node and more rarely the surrounding myocardium and endocardium 2,3. The mechanism by which maternal anti-SSA/Ro-SSB/La antibodies initiate and finally eventuate in cardiac scarring has been challenging to define, in part because the target cardiac antigens are normally sequestered intracellularly 1,4. In vitro and in vivo studies suggest that apoptosis may be a key step in facilitating the accessibility of intracellular antigen to extracellular maternal autoantibodies. Previous studies utilizing fetal cardiac myocytes demonstrated that binding of anti-SSA/Ro-SSB/La antibodies to apoptotic cardiocytes impairs their removal by healthy cardiocytes and increases urokinase plasminogen activator (uPA)/urokinase plasminogen activator receptor (uPAR)–dependent plasmin activation 5,6. Immunohistochemical evaluation of the atrioventricular nodal region from fetuses dying with CHB has revealed exaggerated cardiocyte apoptosis accompanied by both intense transforming growth factor-β (TGF beta) immunoreactivity in the extracellular fibrous matrix and infiltrating macrophages in close proximity to myofibroblasts (transdifferentiated fibroblasts with scarring potential) 7–9.
TGF beta is a pleiotropic cytokine that is ubiquitously expressed by all cells and tissues 10. TGF beta is secreted as a small or large noncovalent complex in which mature TGF beta is complexed to latency associated peptide (LAP), or LAP and latent TGF beta binding protein (LTBP), respectively. TGF beta, when complexed, is prevented from binding the TGF beta receptor. For TGF beta to signal through its receptor, complex TGF beta must be converted to to active TGF beta, a process defined as latent TGF beta activation. Activation can occur chemically or biologically, the latter through proteolytic or non-proteolytic mechanisms. The activation of TGF beta is a complex and tightly regulated process, both temporally and spatially. Although most latent TGF beta is sequestered within the extracellular matrix, TGF beta may also be tethered on the cell surface. Regardless of the state of storage, all TGF beta requires activation for biologic effect 10,11. Proteases play a central role in injury and are involved in TGF beta activation, affecting bioavailability through processing the pro-TGF beta, indirect activation of TGF beta and direct TGF beta activation. Plasmin, generated following proteolysis of plasminogen by the serine protease uPA, was the first protease to have documented TGF beta activating capacity. Plasmin-dependent activation of TGF beta is promoted by the surface localization of uPA to its receptor. Plasmin can release active TGF beta from the latent complex due to proteolytic cleavage of the latency-associated peptide 10.
Given the significance of both plasmin and TGF beta signaling in inflammation and organ injury, we tested the hypothesis that binding of anti-SSA/Ro antibodies to the surface of apoptotic cardiocytes leads to uPA/uPAR activation and subsequent plasmin-dependent TGF beta activation and fibrosis. This was experimentally approached using co-cultures of healthy and apoptotic human fetal cardiac myocytes and autoantibodies isolated from mothers of children with CHB. Evidence of a biologic effect of TGF beta activation was sought by evaluation of Smad2 phosphorylation in separately cultured cardiac fibroblasts. Fibrosing phenotypes were assessed by SMAc and collagen expression.
Materials and Methods
Reagents, commercial antibodies
Pan-anti-TGF beta antibody, active and latent TGF beta were purchased from R&D Systems (Minneapolis, MN). Anti-α-SMAc and anti-tubulin antibody were from Sigma (St. Louis, MI). Secondary Alexa-568 was from Molecular Probes, Inc. (Eugene, OR). Aprotinin was from Sigma. Anti-uPA and anti-uPAR antibodies were from American Diagnostica (Stamford, CT). Anti-Phospho-Smad2 antibody was from Cell Signaling (Boston, MA). Anti-Col1A antibody was from Santa Cruz Biotechnologies (Santa Cruz, CA). Plasminogen free serum was from Enzyme research (South Bend, IN). Millcell cell culture plates with inserts (Millicell-PCF 3 μm filter) were from Millipore (Bedford, MA). Collagen Type-1 culture slides were from BD Biosciences (Bedford, MA). The small molecule inhibitor of TGF beta (SB431542) was purchased from Sigma.
Human IgG preparations
Human IgG was routinely isolated using a Protein A-IgG isolation kit (Pierce, Rockford, IL). Samples were processed by application to Detoxi-Gel Endotoxin Removing Gel (Pierce) to remove any contaminating LPS (<1 pg/ml). Protein concentrations of each IgG fraction and affinity purified antibody (Ab) were assessed by a protein quantification kit (Pierce). The Ab preparations included: IgG fractions (0.3 mg/ml) isolated from: 1) four anti–SSA/Ro-SSB/La-positive mothers whose children have CHB (CHB-IgG, CHB1-IgG, CHB2-IgG, CHB3-IgG 2) healthy control absent any autoantibodies (nl-IgG). The specific clinical manifestations of the mothers are as follows CHB: Systemic lupus erythematosus (SLE), CHB1: undifferentiated autoimmune disease, CHB2: Sjogren’s Syndrome, CHB3: Sjogren’s Syndrome. In addition, affinity purified Abs to the 60 kDa Ro component were generated from the sera of another SSA/Ro-positive mother of a child with CHB (Sjogren’s Syndrome) by affinity column chromatography using the Ro60 recombinant protein coupled to cyanogen bromide-activated Sepharose 4B as previously described 7.
Isolation and culture of cardiac myocytes and fibroblasts from human fetal tissue
Human fetal cardiocytes and human fetal fibroblasts were cultured as described 8. Briefly, human fetal hearts of gestational ages 16–24 weeks were aseptically obtained after elective termination of normal pregnancy by dilatation and evacuation. This was done in accordance with the guidelines of the Institutional Review Board and after obtaining consent from the mothers. The aorta was cannulated for continuous perfusion of the coronary arteries using a Langendorff preparation 9. The heart was treated with collagenase A (type III), which was recirculated for <20 min. The heart dissociated spontaneously, allowing cells to slowly drip and fall on a Petri dish containing 0.25% trypsin, 1 mM EDTA in HBSS. Clumps of cells were dissociated and the resulting suspension was poured over a cell strainer. Cells were centrifuged and the pellet was resuspended in 20 ml of culture medium (DMEM supplemented with 10% FBS, 50 U/ml penicillin, 50 U/ml streptomycin, 100 mg/ml gentamicin, 1 mM nonessential amino acid (Life Technologies, Rockville, MD), 0.1 mM essential medium vitamins (Life Technologies), 2 mM glutamine, 0.1 mM Na pyruvate).
The cell isolate contained both cardiac myocytes and fibroblasts. Separate enriched cultures of each cell type were generated by an initial adhesion step in which 1.2 × 107 cells were plated per 75-cm2 culture flask in DMEM plus 20% FCS (20 min, 37°C). The nonadherent cells (cardiac myocytes) were centrifuged and plated at <1.2 × 107 cells per 75-cm2 culture flask and grown in 5% CO2 at 37°C. After 4 days in culture, spontaneous contraction (30–40 beats per min) was observed under phase-contrast microscopy. Greater than 75% of the cells were stained by a murine monoclonal anti-α actinin (sarcomeric) Ab, which is specific for α-skeletal muscle actinin and α-cardiac muscle actinin. The antibody stains Z lines and dots in stress fibers of skeletal and cardiac muscle, but not in nonsarcomeric muscle elements such as connective tissue, epithelium, nerves, or smooth muscle 3.
To obtain cardiac fibroblasts, the primary isolate was plated in flasks (20 min at 37°C). Fibroblasts at passages 3–5 were routinely used in these studies. Fibroblast enrichment in the cell culture was (fibroblasts are rapidly proliferating vs myocytes) greater than 90%, as assessed using mAb clone IB10 (F-4771; Sigma-Aldrich, St. Louis, MO), which recognizes fibroblasts.
Activation of Apoptotic Pathway in Human Fetal Cardiocytes
For induction of apoptosis, cardiocytes were transferred to serum-free media containing 0.5% BSA (Sigma-Aldrich, Sydney, Australia) and 0.5 μmol/L staurosporine for 5 hours at 37°C, followed by washing and further incubation in medium for 12 hours. Apoptosis was confirmed by microscopic observation of cell size, morphology, and flow cytometric analysis of phosphadylserine exposure by binding of Annexin V–FITC (catalog no. 556419, BD Pharmingen; 1:50) according to the recommendations of the manufacturer.
Engulfment Assay
For engulfment assays, healthy human fetal cardiocytes were plated in a 24-well culture plate(50,000/well) (Becton Dickinson) and the next day apoptotic cardiocytes (rendered apoptotic as described above) were added to cultured healthy cardiocytes to achieve a ratio of 2:1 apoptotic cells: healthy cardiocytes. Before addition, the apoptotic cardiocytes in a total volume of 1 mL of DMEM plus 10% FCS were preincubated with various antibody preparations, which included the following: CHB-IgG(0.3 mg/mL) (“opsonized”), nl-IgG (0.3 mg/mL) (“nonopsonized”), Affinity purified anti-Ro60-IgG (0.015 mg/ml) (“opsonized”), rabbit anti-uPAR and mouse anti-uPAR (1 μg/mL) (American Diagnostica); and anti-HLA (1 μg/mL) (Sigma-Aldrich). To determine the contribution of plasmin to TGF beta activity, 10 μg/ml aprotinin was added to the apoptotic cells prior to incubation with the healthy cardiocytes. To determine the specificity of TGF beta activation of the PAI-1 promoter in the TMLC cells, 10 nm SB431542 or 50 μg/ml of pan-TGF beta antibody were added during the co-culture assays. In the engulfment assays using cell culture plates with inserts to inhibit cell contact, healthy cells were plated in the lower chamber of the culture plate and apoptotic cardiac myocytes were added in a ratio of 2:1 in the upper chamber followed by overnight incubation.
TGF beta Activity Assay
TGF beta was assayed as described. Briefly, TMLC cells (mink lung epithelial reporter cells that stably express a portion of the plasminogen activator inhibitor 1 promoter) were suspended at 15 × 105 cells/ml in DMEM containing 10% FCS. TMLC were plated first at 100 μl per microtiter in a 96-well culture plate (Microtest III plates, Falcon, Franklin Lakes, NJ) and allowed to attach overnight. Cells were washed and sample supernatants were added at 100 μl/well and cultured for 24 hr. Luciferase activity was measured in triplicate using the Bright-Glo detection system (Promega, Madison, WI), and luminescence was determined using a Synergy 2 Biotek microplate reader (Winooski, VT) and reported as relative light units (RLU). Inter variability in RLU measurements observed between assays was attributed to the cardiocytes derived from different donors.
ELISA
Total TGF beta in supernatants was measured using a TGF beta enzyme-linked immunosorbent assay system (R&D Systems, Minneapolis, MN). This system is designed to measure active TGF beta. Total TGF beta was assayed after acid activation by addition of 1N HCl to samples. Total uPA was measured using a uPA enzyme-linked immunosorbent assay system (American Diagnostica).
uPA Activity Assay
uPA activation was determined using a chromogenic assay. Apoptotic cardiocytes were treated with the following antibodies: CHB-IgG or nl-IgG for 30 minutes at RT. In separate experiments, either anti-uPA or anti-uPAR, following preincubation with nl-IgG or CHB-IgG, or aprotinin were added during the coculture experiments. 100μl of sample supernatant was added in triplicate to each well of a 96-well plate. Ten microliters of human plasminogen (0.2 mg/mL in PBS) was added, and after a 2 hr incubation at 37°C, 15 μl of chromogenic substrate for plasmin (Spectrozyme PL) was added to each well. The chromogenic substrate was prepared by diluting 50 μmol into 27 ml of lysis buffer and then neutralized to pH 7.0 with 0.6 mL of 1 N HCl. The reaction product was read on a Bio-Tek spectrophotometer (405 nm; Bio-Tek Instruments, Winooski, Vt)at 1 hr, and the results were compared with a standard curve generated by serial dilutions of high-molecular-weight uPA.
Immunofluorescence
Fibroblasts were seeded on collagen coated chamber slides and serum starved overnight before the addition of the sample supernatants. Immunodetection was performed on cells after fixation with 3% paraformaldehyde (Fisher Scientific, Fair Lawn, NJ) in PBS (pH7.4) for 15 min at RT. After blocking non-specific binding with 3% normal goat serum (Jackson ImmunoResearch, West Grove, Pa), cells were incubated with the appropriate primary antibodies and then with secondary antibody, anti-mouse Alexa Fluor dye 568(Molecular Probes) at RT. Cells were stained with 2 μg/mL Hoechst 33258 for 30 min at 37°C and embedded in Vectashield mounting medium (Vector Laboratories). For each sample at least 6 images from 3 independent experiments were analyzed. Cells were viewed with Axioplan, Carl Zeiss Meditec, Thornwood, NY microscope as indicated. For the Axioplan, images were captured with a charge-coupled device camera (SPOT-2; Diagnostic Instruments (Sterling Heights, Michigan); processed by PhotoShop (Adobe Systems, Mountain View, California). The images were subsequently combined and processed with Image J software (NIH, Bethesda, Md; http://rsb.info.nih.gov/ij).
Immunoblotting
For immunoblot analysis, cells were collected from culture dishes, washed 3 times in PBS, and lysed in solubilization buffer (50 mmol/L Hepes, pH 7.4, buffer containing 0.1% Triton X-100, 10% glycerol, 0.5% deoxycholic acid, 150 mmol/L NaCl, 50 mmol/L NaF, 1 mmol/L NaVO4, 100 μg/mL phenylmethylsulfonyl fluoride, 2 μg/mL leupeptin, 2 μg/mL pepstatin, and 10 μg/mL aprotinin) for 30 minutes at 4°C. The lysates were centrifuged at 15,000 rpm for 15 minutes at 4°C and protein content was measured using a Bio-Rad protein assay kit, with serum albumin as a standard. Lysate (20 μg) was subjected to SDS-PAGE under reducing conditions and transferred onto nitrocellulose membranes. The membranes were blocked with 1% BSA in wash buffer for 1 hr at room temperature (RT), followed by an overnight incubation with the appropriate antibodies in the same buffer at 4°C. The membranes were subsequently washed and secondary horseradish peroxidase–conjugated antibodies were added. Proteins were detected using the ECL chemiluminescent detection reagents (Invitrogen) according to the instructions of the manufacturer. Where appropriate, membranes were stripped with β-mercaptoethanol and subjected to Western blotting for α-tubulin.
Statistics
For statistical analysis, the Mann–Whitney test or the Paired t test where appropriate, were used to compare medians or means between groups in the luciferase, plasminogen and TGF beta ELISA assays. P<0.05 was considered statistically significant. Analyses were performed with GraphPad Prism (GraphPad Software, San Diego, CA).
Results
Latent TGF beta is activated by CHB-IgG, anti-Ro60 but not anti-HLA treated apoptotic cardiocytes
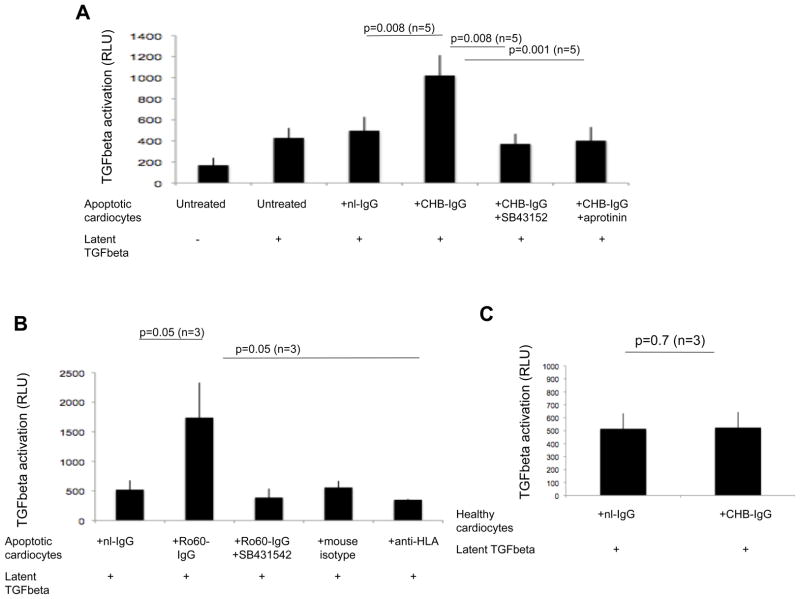
We initially addressed whether the increased plasmin generated by uPA/uPAR induced by anti-SSA/Ro 60 binding to apoptotic cardiocytes resulted in increased activation of exogenously provided latent TGF beta. Cultured cardiac myocytes were rendered apoptotic by exposure to staurosporine, and apoptosis was confirmed by microscopic observation of cell size, morphology, and flow cytometric analysis of phosphadylserine exposure by binding of Annexin V–FITC. The addition of latent TGF beta (1 ng/ml) to apoptotic cardiocytes treated with CHB-IgG, but not nl-IgG, increased luciferase activation of a mink epithelial TGF beta reporter cell line (TMLC) (1021±192 CHB-IgG+ltTGFbeta RLU vs 496±131 nl-IgG+ ltTGFbeta RLU; p=0.008; n=5) (Figure 1A). This activity was diminished to baseline levels following the addition of the TGF beta receptor kinase inhibitor (SB431542) (1021±192 CHB-IgG+ltTGFbeta RLU vs 371±96 nl-IgG RLU; p=0.008; n=5), indicating that the luciferase activation was specific to TGF beta. In the presence of aprotinin, which inhibits a range of proteases including plasmin, the CHB-IgG dependent luciferase activation was significantly attenuated (1021±192 CHB-IgG+ltTGFbeta RLU vs 402±129 nl-IgG RLU; p=0.001; n=5) (Figure 1A). Opsonization of the apoptotic cardiocytes with affinity-purified anti-Ro60 also resulted in activation of latent TGF beta, which was diminished by the small molecule inhibitor (SB431542) (1740±350 Ro60-IgG RLU vs 521±159 nl-IgG RLU; p=0.05; n=3) (Figure 1B). An additional control included incubation of apoptotic cardiocytes with anti-HLA antibodies, which also bind the surface of apoptotic cardiocytes. This condition did not activate latent TGF beta (1740±350 Ro60-IgG RLU vs 350±11 anti-HLA RLU; p=0.05; n=3) (Figure 1B). In contrast to apoptotic cells, healthy cardiocytes treated with either CHB-IgG or nl-IgG did not activate latent TGF beta (514±108 CHB-IgG RLU vs 524±119 nl-IgG RLU; p=0.6; n=3) (Figure 1C) indicating a requirement for surface exposed Ro60 on the apoptotic cells, since CHB-IgG does not bind healthy cells6.
Figure 1. CHB-IgG treated apoptotic cardiocytes activate latent TGF beta.
(A) TMLC-based assessment of the luciferase activity generated by incubation of apoptotic cardiocytes in the presence or absence of latent TGF beta (1 ng/ml). Y axis represents the relative luciferase units (RLU) and x axis each experimental condition of apoptotic cardiocytes (either untreated or treated with nl-IgG, CHB-IgG, CHB-IgG+ SB431542 or CHB-IgG+aprotinin respectively) in the presence of 1 ng/ml of latent TGF beta. (B) TMLC-based assessment of the luciferase activity generated by incubation of nl-IgG, Ro60-IgG, mouse isotype or anti-HLA-treated apoptotic cardiocytes in the presence or absence of latent TGF beta (1 ng/ml). (C) TMLC-based assessment of the luciferase activity generated by incubation of nl-IgG or CHB-IgG treated healthy cardiocytes in the presence of latent TGF beta (1 ng/ml). Sample number of independent experiments is indicated and error bars represent ±SEM of the mean. p values are given above the experimental conditions and considered significant when less that 0.05.
Enhanced activation of TGF beta in cocultures of healthy cardiac myocytes with CHB-IgG bound apoptotic cardiac myocytes
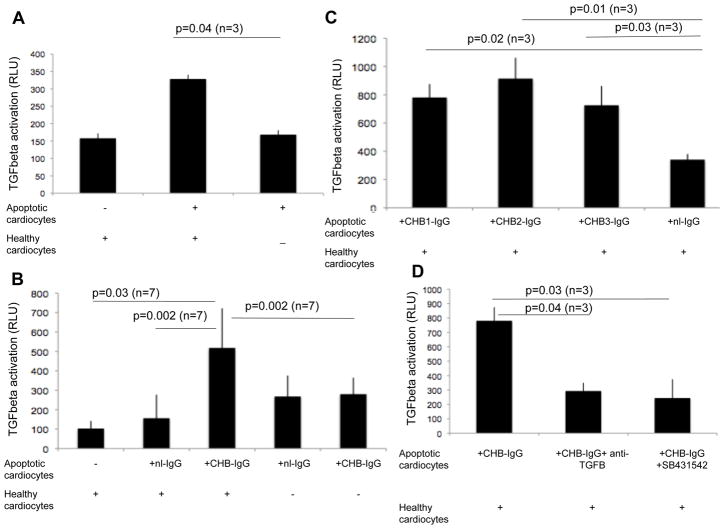
Having established that apo-CHB-IgG cardiocytes activate exogenously added latent TGF beta, we next addressed whether healthy cardiocytes undergoing efferocytosis of apoptotic cardiocytes were the source of latent TGF beta. Initial experiments demonstrated that efferocytosis of apoptotic cardiocytes by healthy cardiocytes resulted in enhanced activation of TGF beta, albeit the levels were modest (158± 14 healthy cards RLU vs 328±12 healthy+apo cards RLU; p=0.04; n=3) (Figure 2A). Since the binding of CHB-IgG to apoptotic cardiocytes results in the generation of plasmin 5, we investigated whether the addition of apo-CHB-IgG cardiocytes to healthy cardiocytes resulted in TGF beta activation similar to that observed when latent TGF beta was exogenously added (Figure 1A). Supernatants generated following an overnight incubation of healthy cardiocytes and apo-CHB-IgG cardiocytes exhibited a significant increase in luciferase activation compared to supernatants generated following incubation of healthy cardiocytes with apo-nl-IgG cardiocytes (nl-IgG-treated) (511± 204 CHB-IgG RLU vs 156±121 nl-IgG RLU; p=0.002; n=7) or compared to supernatants from healthy cardiocytes alone (Figure 2B)(511± 204 CHB-IgG RLU vs 103±39 healthy cardiocytes RLU; p=0.03; n=7). Supernatants from apo-nl-IgG or apo-CHB-IgG cardiocytes showed no increase over background in luciferase activation (511± 204 apo-CHB-IgG from cocultures with healthy cardiocytes RLU vs 280±85 apo-CHB-IgG alone RLU; p=0.002; n=7). (Figure 2B).
Figure 2. Cocultures of healthy cardiocytes with CHB-IgG bound apoptotic cardiocytes activates TGF beta.
(A) TMLC-based assessment of the luciferase activity generated by cocultures of healthy cardiocytes with apoptotic cardiocytes or apoptotic cardiocytes alone. Y axis represent relative luciferase units (RLU). (B) TMLC-based assessment of the luciferase activity generated by cocultures of healthy cardiocytes with apo-nl-IgG or apo-CHB-IgG cardiocytes or antibody treated cardiocytes alone. (C) TMLC-based assessments of the luciferase activity generated by cocultures of healthy cardiocytes with apoptotic cardiocytes treated with either nl-IgG or different IgG fractions CHB-IgG sera (CHB1, CHB2, CHB3). (D) Inhibition of TGF beta luciferase activation of TMLC cells exposed to supernatants of cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes in the presence of either pan anti-TGF beta antibody or small molecule TGF beta inhibitor (SB431542). Sample number of independent experiments is indicated and error bars represent ±SEM of the mean. p values are given above the experimental conditions and considered significant when less that 0.05.
Similar results were obtained from cocultures of apoptotic cardiocytes opsonized with three other CHB-IgG sera (CHB1, CHB2, CHB3), (780± 94 CHB1-IgG RLU vs 340±40 nl-IgG RLU; p=0.02; n=3), (914± 147 CHB2-IgG RLU vs 340±40 nl-IgG RLU; p=0.01; n=3) and (725± 136 CHB3-IgG RLU vs 340±40 nl-IgG RLU; p=0.03; n=3) respectively (Figure 2C). Because other growth factors, in addition to TGF beta, can contribute to PAI-promoter activation, supernatants generated as described above were pretreated with either a pan-anti-TGF beta antibody or the specific small molecule inhibitor of TGF beta (SB431542) prior to incubation with the TMLC cells. These additions attenuated TGF beta activation (736± 151 CHB-IgG RLU vs 229±89 CHB-IgG + anti-TGFbeta RLU; p=0.04; n=3) and (736± 151 CHB-IgG RLU vs 185±37 CHB-IgG + (SB431542) RLU; p=0.03; n=3) (Figure 2D).
CHB-IgG-induced uPA/uPAR-dependent plasminogen activation is responsible for TGF beta activation in cocultures of CHB-IgG bound apoptotic cardiocytes and healthy cardiocytes
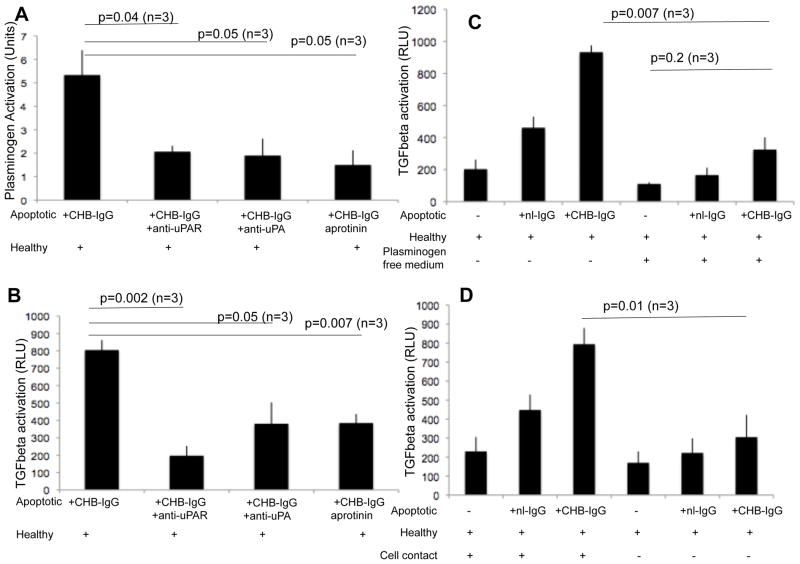
Since aprotinin, a protease inhibitor, decreases the activation of latent TGF beta generated by apo- CHB-IgG cardiocytes (Figure 1A), we evaluated whether plasmin-induced TGF beta activation was dependent on the uPA/uPAR-dependent pathway. Apo-CHB-IgG or apo-nl-IgG cardiocytes were subsequently treated with either anti-uPA or uPAR antibodies or the plasmin-inhibitor aprotinin. Treated apoptotic cardiocytes were co-cultured with healthy cardiocytes and supernatants collected after 24 hr. Supernatants were assessed with a chromogenic assay to monitor the plasmin activity. As previously shown, 5 enzymatic activity was decreased when apo-CHB-IgG cardiocytes were subsequently treated with antibodies against either uPAR (5.3±1.1 CHB-IgG U/ml vs 2±0.2 CHB-IgG+anti-uPAR U/ml; p=0.04; n=3), or uPA (5.3± 1.1 CHB-IgG U/ml vs 1.9±0.7 CHB-IgG+anti-uPA U/ml; p=0.05; n=3) or when aprotinin was present (5.3± 1.1 CHB-IgG U/ml vs 1.5±0.6 CHB-IgG+aprotinin U/ml; p=0.05; n=3) (Figure 3A). The effect of these various inhibitory antibodies on TGF beta activity was assessed by incubating the TMLC cells with the supernatants from the efferocytosis assays. TGF beta activation was attenuated when plasminogen activation was suppressed by either anti-uPA or anti-uPAR antibodies or by aprotinin (805± 57 CHB-IgG RLU vs 197±56 CHB-IgG+anti-uPAR RLU; p=0.002; n=3), (805± 57 CHB-IgG RLU vs 381±123 CHB-IgG+anti-uPA RLU; p=0.05; n=3) and (805± 57 CHB-IgG RLU vs 385±52 CHB-IgG+aprotinin RLU; p=0.007; n=3) respectively (Figure 3B).
Figure 3. Anti-Ro dependent uPA/uPAR plasminogen activation is responsible for the generation of active TGFbeta.
(A) A chromogenic enzymatic activity assay was used to evaluate plasminogen activation of supernatants generated from cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes or apo-CHB-IgG cardiocytes subsequently treated with antibodies against uPA, uPAR or incubated in the presence of aprotinin (10 μg/ml). (B) Inhibition of TGF beta luciferase activation of TMLC cells exposed to supernatants of cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes or apo-CHB-IgG cardiocytes subsequently treated with antibodies against uPA, uPAR or incubated in the presence of aprotinin (10 μg/ml g/ml). (C) Contribution of plasminogen to the CHB-IgG mediated TGF beta luciferase activation. Supernatants of cocultures of healthy cardiocytes with apo-nl-IgG or apo-CHB-IgG cardiocytes conducted in the presence or absence of plasminogen containing media were added to the TMLC cells and after 24 hr incubation luciferase activity was determined. (D) Contribution of cell contact in CHB-IgG mediated TGF beta luciferase activation. Healthy cardiocytes were cocultured with apo-nl-IgG or apo-CHB-IgG cardiocytes either together or separated with cell culture plate inserts to inhibit direct cell contact. After overnight incubation supernatants were collected and added to TMLC cells. Y axis represents relative luciferase units (RLU). Sample number of independent experiments is indicated and error bars represent ±SEM of the mean. p values are given above the experimental conditions and considered significant when less that 0.05.
The source of plasmin mediating of latent TGF beta activation was determined. Coculture assays of healthy and apoptotic cardiocytes were conducted in culture media containing serum devoid of plasminogen. The absence of plasminogen resulted in concomitant loss of CHB-IgG dependent TGF beta luciferase activation (933± 42 CHB-IgG plus plasminogen RLU vs 325±76 CHB-IgG minus plasminogen RLU; p=0.007; n=3) and (170± 72 healthy cardiocytes minus plasminogen RLU vs 325±76 CHB-IgG minus plasminogen RLU; p=0.2; n=3) (Figure 3C).
The necessity for direct cell contact between the healthy cardiocytes and the apoptotic cardiocytes to trigger TGF beta activation was next examined. Coculture assays were performed in which a cell culture plate insert was introduced to separate the healthy cells from the apoptotic cells, while allowing soluble molecules to pass through. Interference with cell contact between the healthy and apoptotic cardiocytes resulted in decreased CHB-IgG dependent TGF beta activation (794± 85 CHB-IgG RLU vs 305±117 CHB-IgG no contact RLU; p=0.01; n=3) (Figure 3D).
Total levels of TGF beta are decreased but uPA levels are elevated in cocultures of CHB-IgG bound apoptotic cardiac myocytes and healthy cardiocytes
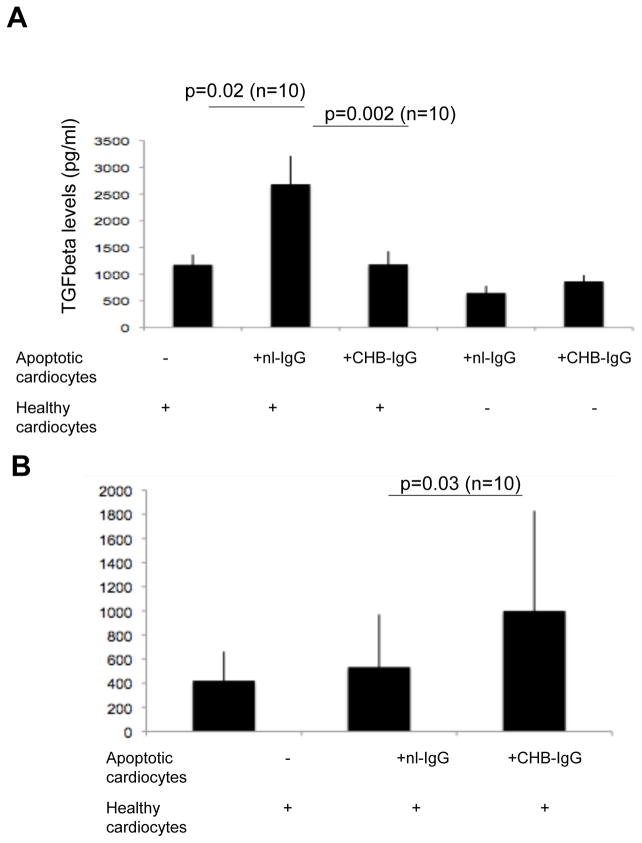
The total (active plus latent) level of TGF beta was evaluated to determine whether the observed increased TGF beta activation in cocultures of apo-CHB-IgG cardiocytes with healthy cardiocytes was due to increased TGF beta protein secretion. Total TGF beta levels were significantly decreased in the supernatants from cocultures of healthy cardiocytes and apo-CHB-IgG cardiocytes compared to apo-nl-IgG (2684±530 pg/ml nl-IgG vs 1183 ± 243 CHB-IgG pg/ml; p=0.002; n=10) as assessed by ELISA (Figure 4A). These data are consistent with the previous observation that the binding of CHB-IgG to apoptotic cardiocytes decreased their efferocytosis by healthy cardiocytes. These same supernatants contained statistically significant levels of uPA protein, (999±830 pg/ml CHB-IgG vs 536±436 nl-IgG; p=0.03; n=10) suggesting that despite the decreased levels of total TGF beta, increased uPA-dependent enzymatic activity results in increased TGF beta activation (Figure 4B).
Figure 4. Total TGF beta levels are decreased but uPA levels are increased in cocultures of apoptotic of CHB-IgG bound cardiocytes with healthy.
(A) 100 μl of supernatants (healthy cardiocytes, cocultures of healthy cardiocytes with apo-nl-IgG or apo-CHB-IgG cardiocytes, apo-CHB-IgG alone and apo-nl-IgG cardiocytes alone) were acid treated with the addition of 1 N HCL in order to measure total levels (active plus latent) of TGF beta. Total TGF beta was then determined by using an enzyme-linked immunosorbent assay system ELISA (R&D Systems). (B) Total uPA levels were measured in supernatants (100 μl) of either healthy cardiocytes, cocultures of healthy cardiocytes with apo-nl-IgG cardiocytes or cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes by enzyme-linked immunosorbent assay (American Diagnostica). Error bars represent ±SEM and p values and experimental sample numbers are indicated.
CHB-IgG-dependent TGF beta activation promotes a scarring phenotype in the cardiac fibroblasts
TGF beta elicits its biologic effects by interacting with TGFbetaRI/II receptors which results in Smad2 phosporylation and its subsequent nuclear translocation and transactivation of gene expression 12. Evidence substantiating an effect of active TGF beta on the cardiac fibroblast was sought by evaluating Smad2 phosphorylation. Elevated phospho-Smad2 was detected when fibroblasts were treated with supernatants of cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes, similar to observed when fibroblasts were directly treated with recombinant TGFbeta (Figure 5).
Figure 5. Supernatants from cocultures of healthy cardiocytes and CHB-IgG bound apoptotic cardiocytes trigger activation of TGF beta pathway as depicted by increased phospho-Smad2 phosphorylation.
Fibroblasts were prepared as monolayers and seeded on collagen-treated culture slides. Following overnight serum starvation cells were treated with supernatants from healthy cardiocyte cultures, supernatants from cocultures of healthy cardiocytes with isotype control treated apoptotic cardiocytes, cocultures of healthy cardiocytes with anti-uPAR treated apoptotic cardiocytes, cocultures of healthy cardiocytes with apo-nl-IgG cardiocytes, cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes, cocultures of healthy cardiocytes with initially apo-nl-IgG and then anti-uPAR cardiocytes, cocultures of healthy cardiocytes with initially apo-CHB-IgG and then anti-uPAR cardiocytes cocultures of healthy cardiocytes with mouse anti-uPAR treated apoptotic cardiocytes, cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes in the presence of SB431542, or treated with recombinant TGFbeta (100 pg/ml). Cell lysates were collected and subjected to electrophoresis followed by immunoblotting for anti- phospho-Smad2. Equal loading was confirmed with anti-tubulin.
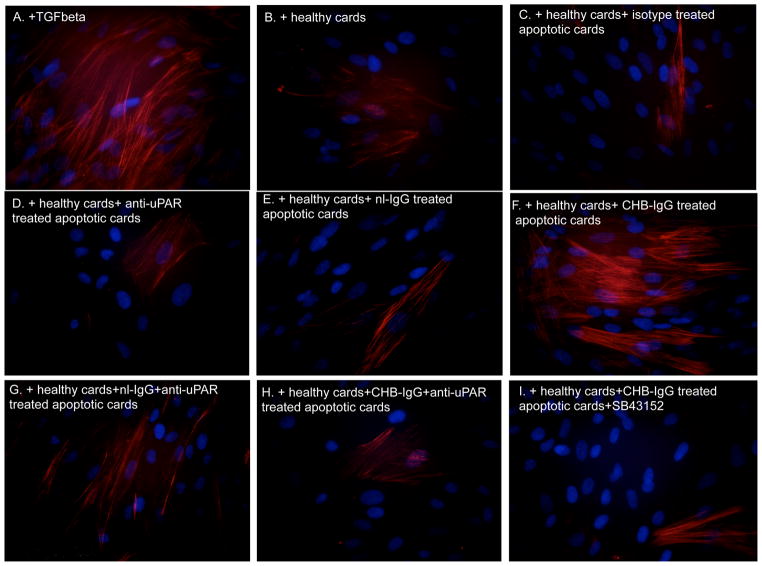
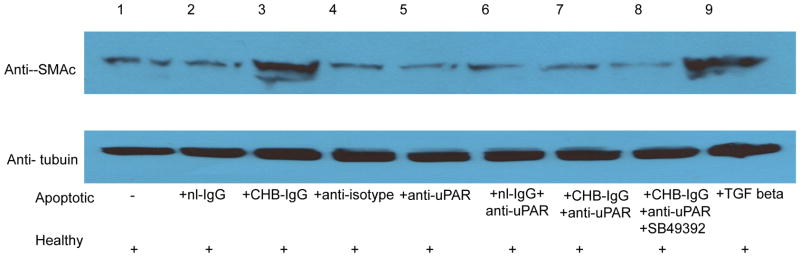
The effect of CHB-IgG mediated TGF beta activation was evaluated for its ability to promote fibrosis on fetal cardiac fibroblasts. We monitored levels of SMAc as a read-out of fibroblast transdifferentiation. Fibroblasts were serum- starved overnight and subsequently treated with supernatants from cocultures of healthy cardiocytes incubated with apo-CHB-IgG or apo-nl-IgG cardiocytes. Increased SMAc expression was observed when fibroblasts were incubated with supernatants derived from cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes (Figure 6F). Addition of recombinant active TGF beta served as a positive control (Figure 6A). Attenuation of SMAc expression was observed when fibroblasts were treated with supernatants generated from cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes subsequently treated with anti-uPAR antibodies (Figure 6H) or SB43152 (Figure 6I). The results obtained by immunofluorescence were paralleled by immunoblot of the fibroblast lysates treated under identical conditions. As illustrated in Figure 7, comparing lane 3 to 9, SMAc protein expression was increased in fibroblasts treated with supernatants from cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes.
Figure 6. Supernatants from cocultures of healthy cardiocytes and CHB-IgG bound apoptotic cardiocytes promote transdifferentiation of human fetal cardiac fibroblasts as depicted by increased smooth muscle actin (SMAc) staining.
Fibroblasts were prepared as monolayers and seeded on collagen-treated culture slides. Following overnight serum starvation cells were treated with recombinant TGF beta (100 pg/ml)(A) or exposed to supernatants from healthy cardiocyte cultures (B), supernatants from cocultures of healthy cardiocytes with isotype control treated apoptotic cardiocytes (C), cocultures of healthy cardiocytes with anti-uPAR treated apoptotic cardiocytes (D), cocultures of healthy cardiocytes with apo-nl-IgG cardiocytes (E), cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes (F), cocultures of healthy cardiocytes with initially apo-nl-IgG and then anti-uPAR cardiocytes (G), cocultures of healthy cardiocytes with initially apo-CHB-IgG and then anti-uPAR cardiocytes cocultures of healthy cardiocytes with mouse anti-uPAR treated apoptotic cardiocytes (H), cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes in the presence of SB431542 (I). Fibroblasts were then fixed, stained and analyzed by fluorescence microscopy (original magnification X40). Results are representative of three experiments.
Figure 7. Supernatants from cocultures of healthy cardiocytes and CHB-IgG bound apoptotic cardiocytes promote fibroblast transdifferentiation as demostrated by increased smooth muscle actin (SMAc) expression.
Fibroblasts were prepared as monolayers and seeded on collagen-treated culture slides. Following overnight serum starvation cells were treated with supernatants from healthy cardiocyte cultures, supernatants from cocultures of healthy cardiocytes with isotype control treated apoptotic cardiocytes, cocultures of healthy cardiocytes with anti-uPAR treated apoptotic cardiocytes, cocultures of healthy cardiocytes with apo-nl-IgG cardiocytes, cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes, cocultures of healthy cardiocytes with initially nl-IgG and then anti-uPAR treated apoptotic cardiocytes, cocultures of healthy cardiocytes with initially apo-CHB-IgG and then anti-uPAR cardiocytes cocultures of healthy cardiocytes with mouse anti-uPAR treated apoptotic cardiocytes, cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes in the presence of SB431542, or treated with recombinant TGF beta (100 pg/ml). Cell lysates were collected and subjected to electrophoresis followed by immunoblotting for anti-SMAc. Equal loading was confirmed with anti-tubulin.
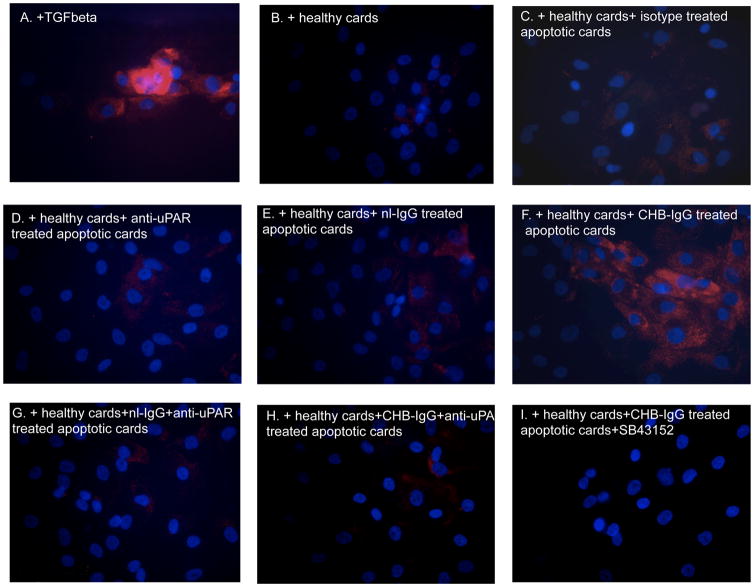
As an additional readout of fibroblast transdifferentiation, collagen expression was assessed in fibroblasts treated with supernatants from cocultures of healthy cells and apoptotic cells treated with the various antibody conditions as above. Increased collagen expression was noted when fibroblasts were incubated overnight with supernatants of healthy cardiocytes with apo-CHB-IgG cardiocytes (Figure 8F).
Figure 8. Supernatants from cocultures of healthy cardiocytes and CHB-IgG bound apoptotic cardiocytes promote transdifferentiation of human fetal cardiac fibroblasts as depicted with increased collagen staining (Col1A) staining.
Fibroblasts were prepared as monolayers and seeded on collagen-treated culture slides. Following overnight serum starvation cells were treated with recombinant TGF beta (100 pg/ml)(A) or exposed to supernatants from healthy cardiocyte cultures (B), supernatants from cocultures of healthy cardiocytes with isotype control treated apoptotic cardiocytes (C), cocultures of healthy cardiocytes with anti-uPAR treated apoptotic cardiocytes (D), cocultures of healthy cardiocytes with apo-nl-IgG cardiocytes (E), cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes (F), cocultures of healthy cardiocytes with initially apo-nl-IgG and then anti-uPAR cardiocytes (G), cocultures of healthy cardiocytes with initially apo-CHB-IgG and then anti-uPAR cardiocytes cocultures of healthy cardiocytes with mouse anti-uPAR treated apoptotic cardiocytes (H), cocultures of healthy cardiocytes with apo-CHB-IgG cardiocytes in the presence of SB431542 (I). Fibroblasts were then fixed, stained and analyzed by fluorescence microscopy (original magnification X40). Results are representative of three experiments.
Discussion
Identification of the molecular components that contribute to the cross talk between inflammation and fibrosis is an important step in linking anti-SSA/Ro antibodies to cardiac injury. A consistent histologic finding in hearts of fetuses dying shortly after the diagnosis of CHB is an abundance of apoptotic cardiocytes 8. By applying this clue to an in vitro culture system of human fetal cardiocytes, we recently demonstrated that the binding of anti-SSA/Ro antibodies results in attenuation of efferocytosis by healthy cardiocytes (supporting the exaggerated apoptosis) and a modification of uPAR expression, uPA activation and ultimately the generation of plasmin 5,6. Our studies presented herein identify a functional and potentially pathologic consequence of the latter enzymatic activity. Apoptotic anti-SSA/Ro bound cardiocytes mediated increased activation of exogenously added latent TGF beta compared to control antibody treated apoptotic cardiocytes. Activation of latent TGF beta secreted during efferocytosis of apoptotic cardiocytes by healthy cardiocytes was significantly increased in supernatants from co-cultures containing anti-SSA/Ro bound apoptotic cardiocytes compared to co-cultures with unopsonized apoptotic cardiocytes. TGF beta activation was dependent on contact between the apoptotic cardiocyte and healthy cardiocyte rather than engulfment per se. The requirement for uPA activity was supported by the decrease in TGF beta activation observed when the opsonized apoptotic cardiocytes were treated with anti-uPAR or anti-uPA antibodies or the plasmin inhibitor aprotinin prior to coculturing with healthy cardiac myocytes. The addition of supernatants derived from cocultures of healthy cardiac myocytes and opsonized apoptotic cardiocytes to cardiac fibroblasts supported the biologic activity of TGF beta as evidenced by Smad2 phosphorylation. The profibrotic consequence of TGF beta activation generated under these conditions was illustrated by myofibroblast transdifferentiation (SMAc staining) of and increased collagen protein expression in the cardiac fibroblasts.
Although TGF beta is important in regulating crucial cellular activities, the full spectrum of molecular mechanisms that promote its activation is still to be delineated. Some of the known activating pathways are cell or tissue specific, whereas others are common to multiple cell types and tissues. Proteases, integrins, pH, and reactive oxygen species are among the currently identified factors that can activate TGF beta 10. Plasmin was the first protease documented to have TGF beta activating capacity, and it has been hypothesized that plasmin-mediated proteolysis of thrombus-associated TGF beta may act as a slow release mechanism for TGF beta following acute injury.
Plasmin has long been considered a protease with fibrinolytic effects, however plasmin is involved in a plethora of cellular processes through it’s cleavage and activation of non-fibrin substrates in the extracellular matrix. Such substrates include MMPs, TGF beta and other growth factors, which can be liberated thereby inducing signaling changes in the resident tissue microenvironment. Supportive evidence for a profibrotic role of plasmin has been provided in several experimental systems. In an experimental model of progressive kidney disease, using Plg−/− and Plg+/+ mice, Vaughan et al 12 demonstrated that the presence of plasmin is necessary to promote fibrosis through epithelial to mesenchylial transition and activation of TGF beta and PAR-1/ERK signaling pathways. Compared with Plg+/+ mice in which fibrosis progresses over time, Plg−/− mice develop fibrosis at a significantly lower pace, which temporally regresses. In murine embryonic PAI-KO fibroblasts, plasmin inhibition attenuates TGF beta activation and subsequent fibrosis 13. Additionally, in a murine model of PAI-1 deficiency, increased plasmin enzymatic activity and TGF beta signaling contributes to cardiac fibrosis 12. Specific to the relationship between organ injury and fibrosis observed in the pathology of CHB, a relative absence of TGF beta has been implicated as a major cause of scarless repair. In another murine model developmentally early stage fetal fibroblasts show decreased plasmin and subsequent TGF beta activity compared to later stage or adult fibroblasts, which contract and repair wounds in a plasmin-TGF beta dependent manner 14. These findings support a dual role for plasmin in the balance between TGF beta mediated fibrosis-fibrinolysis and inflammation.
Our initial experiments showed that apo-CHB-IgG cardiocytes were capable of activating exogenously provided TGF beta compared to control apo-nl-IgG cardiocytes. Binding of the apoptotic surface by Ro60-IgG, but not anti-HLA, surface binding resulted in a TGF beta activation. This observation suggests that the effect of anti-Ro60 is specific and not simply the result of antibody binding to cognate surface exposed antigen. Similar effects were obtained using supernatants derived from cocultures of anti-Ro bound apoptotic cardiocytes and healthy cardiocytes (source of latent TGF beta) in which activation of TGF beta was significantly greater compared to supernatants generated from healthy cardiocytes which efficiently engulfed the unopsonized apoptotic cardiocytes. Minimal activation of TGF beta was observed in the case of apoptotic cells alone, most likely attributed to the TGF beta present in the serum of the culture medium.
Furthermore, blocking plasmin generation by either anti-uPA or anti-uPAR antibodies or protease inhibition led to ablation of the TGF beta activation supporting our hypothesis that the TGF beta activation was attributed to a CHB-dependent uPA/uPAR mediated plasminogen activation. The source of plasminogen was shown to be the serum present in culture media (presumeably in vivo, plasminogen is present in the fetal circulation), since no TGF beta activation was observed during efferocytosis when serum depleted of plasminogen was used.
Cell contact was necessary for significant TGF beta activation. This was expected since these enzymatic activation reactions are more efficient when focused on the cell surface. For example, receptor anchored plasmin generation on the cell surface is substantially greater than that occurring in solution. Thus cell surface generation of active plasmin on the opsonized apoptotic cardiocyte adjacent to the latent TGF beta secreted by of the resident healthy cell results in enhanced efficient activation. Furthermore, mannose-6-phosphate/insulin like growth factor II-receptor (M6P/IGFII-R), a receptor known to bind latent TGF beta 16, interacts with uPAR on the surface of human monocytes and directly binds plasminogen. Conversion to plasmin within the complex mediates the release of active TGF beta. Thus, a similar mechanism might occur in the coculture system, wherein the increased uPA at the surface of the CHB-IgG opsonized apoptotic cardiocytes interacts with the latent TGF betaeta/M6P/IGFII-R complex on the surface of the healthy cell. Engulfment is not required for this process. Accordingly, impaired clearance of anti-Ro bound apoptotic cardiocytes by healthy cardiocytes and the consequent persistence of CHB-IgG bound apoptotic cardiocytes may promote continued conversion of latent to active TGF beta.
Although the activation of TGF beta is significantly higher when apo-CHB-IgG cardiocytes are cocultured with healthy cells compared to unopsonized apoptotic cardiocytes, the levels of total TGF beta in the conditioned medium are decreased. This observation is consistent with the decreased efferocytosis observed with cocultures of apo-CHB-IgG cardiocytes, assuming the generation of total TFG beta by healthy cardiocytes is dependent on efficient engulfment. Since latent TGF beta is generally in excess and only a small percentage of the total is activated, increased protease activity in the presence of anti-Ro antibodies combined with minimal efferocytosis would be sufficient to lead to significantly more active TGF beta compared to control conditions. Increased uPA levels and activity were found in the presence of anti-Ro antibodies -supporting the protease dependent activation of TGF beta by CHB-IgG generated plasmin.
Functional support for a profibrotic effect of increased TGF beta activation in the pathologic development of CHB was obtained. Increased smooth muscle actin and collagen staining, consistent with transdifferentiation of fibroblasts to myofibroblasts, was observed when fibroblasts were treated with supernatants generated from cocultures of healthy cardiocytes and opsonized apoptotic cardiocytes. This effect was abrogated when uPAR was blocked, exemplifying the tight relationship between plasmin generation and TGF beta signaling pathways. The contribution of macrophages, which are present in the majority of autopsy specimens of affected fetal hearts, may result in an amplification of this profibrotic process. Precedent for this is the observation that supernatants generated by coculturing macrophages with opsonized apoptotic cardiocytes also transdifferentiate cardiac fibroblasts 7. The observed upregulation of SMAc in the presence of healthy cardiocytes and opsonized apoptotic cardiocytes absent macrophages may serve as a minimal threshold to tip the cascade of signaling towards a pro-fibrosing phenotype.
Fibrosis of the atrioventricular node and endocardium may reflect a perilous synergy between two ongoing attempts to clear apoptotic cardiocytes, one “innate” process comprised of resident healthy cardiocytes and the other process “adaptive” represented by recruited professional scavengers, such as macrophages. A key role for uPA-secreting macrophages in the development of cardiac fibrosis has been shown in a mouse model in which recipients of bone marrow transplants from uPA-overexpressing donors, but not nontransgenic donors, developed cardiac macrophage accumulation and fibrosis 17,18.
Collectively, our data further define the pathologic consequences initiated when maternal anti-Ro antibodies bind cognate antigen on the surface of apoptotic fetal cardiocytes. Increased uPAR-dependent uPA activity triggers TGF beta activation thus initiating and amplifying a cascade of events that promote myofibroblast transdifferentiation and scar. These observations provide rationale for therapeutic strategies aimed at attenuating plasmin-mediated TGF beta activation.
Acknowledgments
This work was supported by National Insitutes of Health Grants AR-42455, CA034282 and NO1-AR-4-2271 and 1K01AR060302-01A1
Abbreviations in this papers
- AV
atrioventricular
- CHB
congenital heart block
- nl-IgG
normal IgG
- SMAc
smooth muscle actin
- TGF
transforming growth factor
References
- 1.Chameides L, et al. Association of maternal systemic lupus erythematosus with congenital complete heart block. N Engl J Med. 1977;297:1204–1207. doi: 10.1056/NEJM197712012972203. [DOI] [PubMed] [Google Scholar]
- 2.Frassi M, et al. Neonatal lupus: clinical features and risk of congenital cardiac heart block in newborns from mothers with anti Ro/SSA antibodies. Reumatismo. 2001;53:298–304. doi: 10.4081/reumatismo.2001.298. [DOI] [PubMed] [Google Scholar]
- 3.Brucato A, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44:1832–1835. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briassouli P, Komissarova EV, Clancy RM, Buyon JP. Role of the Urokinase Plasminogen Activator Receptor in Mediating Impaired Efferocytosis of Anti-SSA/Ro-Bound Apoptotic Cardiocytes. Circulation Research. 2010;107:374–387. doi: 10.1161/CIRCRESAHA.109.213629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clancy RM, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy RM, et al. Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. J Immunol. 2002;169:2156–2163. doi: 10.4049/jimmunol.169.4.2156. [DOI] [PubMed] [Google Scholar]
- 8.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–182. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 9.Miranda ME, et al. Accessibility of SSA/Ro and SSB/La antigens to maternal autoantibodies in apoptotic human fetal cardiac myocytes. J Immunol. 1998;161:5061–5069. [PubMed] [Google Scholar]
- 10.Jenkins G. The role of proteases in transforming growth factor-beta activation. International Journal of Biochemistry & Cell Biology. 2008;40:1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 12.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan DE, et al. Genetic Deficiency of Plasminogen Activator Inhibitor-1 Promotes Cardiac Fibrosis in Aged Mice Involvement of Constitutive Transforming Growth Factor-beta Signaling and Endothelial-to-Mesenchymal Transition. Circulation. 2010;122:1200–U1108. doi: 10.1161/Circulationaha.110.955245. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein AM, Pedroja BS, Kang LE, Imas AO, Carmeliet P. Plasminogen Activator Inhibitor-1 Regulates Integrin alpha v beta 3 Expression and Autocrine Transforming Growth Factor beta Signaling. Journal of Biological Chemistry. 2009;284:20708–20717. doi: 10.1074/jbc.M109.018804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman C, Tuan TL, Buckley S, Anderson KD, Warburton D. Contractility, transforming growth factor-beta, and plasmin in fetal skin fibroblasts: Role in scarless wound healing. Pediatric Research. 1998;43:403–409. doi: 10.1203/00006450-199803000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Godar S, et al. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-beta 1. European Journal of Immunology. 1999;29:1004–1013. doi: 10.1002/(SICI)1521-4141(199903)29:03<1004::AID-IMMU1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Stempien-Otero A, et al. Mechanisms of cardiac fibrosis induced by urokinase plasminogen activator. Journal of Biological Chemistry. 2006;281:15345–15351. doi: 10.1074/jbc.M512818200. [DOI] [PubMed] [Google Scholar]
- 18.Moriwaki H, Stempien-Otero A, Kremen M, Cozen AE, Dichek DA. Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circulation Research. 2004;95:637–644. doi: 10.1161/01.RES.0000141427.61023.f4. [DOI] [PubMed] [Google Scholar]