Abstract
The canonical Wnt-β catenin signaling pathway plays an important role in thymocyte development and T cell migration but little is known about its role in naïve-to-effector differentiation in human peripheral T cells. We show that activation of Wnt-β catenin signaling arrests human peripheral blood and cord blood T lymphocytes in the naïve stage and blocks their transition into functional T effector cells. Wnt signaling was induced in polyclonally activated human T cells by treatment either with the GSK3β inhibitor TWS119 or the physiological Wnt agonist Wnt-3a and these T cells preserved a naïve CD45RA+ CD62L+ phenotype compared to control activated T cells that progressed to a CD45RO+ CD62L- effector phenotype and this occurred in a TWS119 dose-dependent manner. TWS119-induced Wnt signaling reduced T cell expansion, as a result of block in cell division, and impaired acquisition of T cell effector function, measured by degranulation and IFN-γ production in response to T cell activation. The block in T cell division may be attributed to the reduced IL-2Rα expression in TWS119-treated T cells that lowers their capacity to utilize autocrine IL-2 for expansion. Altogether, our data suggest that Wnt-β catenin signaling is a negative regulator of naïve-to-effector T cell differentiation in human T lymphocytes. The arrest in T cell differentiation induced by Wnt signaling might have relevant clinical applications such as to preserve the naïve T cell compartment in antigen-specific T cells generated ex vivo for adoptive T cell immunotherapy.
Introduction
The canonical Wnt-β catenin signaling pathway regulates the progression of thymocyte development at different stages (1–3). However, the role of this signaling pathway in post-thymic peripheral T lymphocytes is less understood. Resting and effector peripheral T cells express components of the Wnt signaling pathway. Wnt proteins induce T cell production of matrix metalloproteinases which are required for peripheral T cell transmigration (4). TCR activation also leads to changes in expression patterns of Wnt-targeted transcription factors in peripheral T cells (5). These results indicate that the Wnt signaling pathway is active in peripheral T cells and it may play a role during T cell activation and differentiation. We undertook this study to elucidate the role of the canonical Wnt signaling pathway in naïve-to-effector differentiation of human peripheral blood and cord blood T lymphocytes. This question is relevant both to the fundamental understanding of human T cells and our practical ability to expand therapeutic T cells ex vivo for treating patients with cancer. In particular, human T cells expanded ex vivo almost invariably acquire an effector phenotype which may limit their ability to persist in vivo after adoptive transfer into the patient. Manipulation of the Wnt pathway might help preserve the naïve phenotype of T cells during these ex vivo cultures.
In the canonical Wnt pathway, a destruction complex comprised of scaffold proteins (adenomatous polyposis coli (APC), axis inhibition protein 1 (Axin)) and two kinases (glycogen synthase kinase 3β (GSK3β) and casein kinase 1 (CK1)) phosphorylates and promotes βTRCP-mediated ubiquitination and degradation of the β catenin molecule in the absence of Wnt ligand (6). Nineteen different Wnt ligands identified in humans are all lipid-modified glycoproteins. Engagement of cell surface Frizzled/LRP 5/6 receptor complexes by Wnt ligands including Wnt1 and Wnt3a recruits the protein Disheveled to the receptor complex inducing phosphorylation of LRP 5/6 by CK1 and GSK3β. This recruits Axin to the plasma membrane and disassembles the destruction complex, allowing accumulation of cytoplasmic β catenin which can then translocate to the nucleus. β catenin associates with transcription factors from the T cell factor (Tcf) or lymphoid enhancing binding factor (Lef) family including Tcf-1, Tcf-3, Tcf-4 and Lef-1 and activates expression of target genes including Tcf7 (7), Lef1 (8), Nlk (9) and Jun (10).
Willinger et al. reported that mature human CD8+ T lymphocytes downregulate the expression of Wnt transcription factors Tcf-1 and Lef-1 upon activation but shifts the pattern of splicing to favor the stimulatory isoforms of these transcription factors rather than the inhibitory isoforms, suggesting that Wnt signaling may play a complex role in peripheral T cell differentiation (5). It has been reported that induction of canonical Wnt signaling in pmel-1 transgenic TCR mouse CD8+ T cells in vitro arrests effector T cell differentiation and function (11). This observation agrees with another study in which activation of Wnt-β signaling in mouse T cells obtained by genetic modification to express a nondegradable β catenin inhibited T cell activation at the proximal stages of TCR signaling and also arrested effector T cell proliferation and function (12). Importantly, Gattinoni et al. (11) found that in addition to arresting effector cell differentiation, induction of Wnt signaling in pmel-1 mouse T cells generated a distinct population they called ‘T memory stem’ (Tscm) cells. These Tscm cells expressed high levels of Sca-1, Bcl-2 and CD122, preserved a CD44lowCD62Lhigh naïve phenotype even after undergoing several cycles of cell division, rapidly released cytokines upon antigen encounter, and had superior proliferative and anti-tumor activity in vivo compared to central or effector memory T cells (11).
Successful translation of these findings in human T cells could lead to an important clinical application to maintain naïve T cells in ex vivo cultures, using Wnt signaling, for adoptive transfer. Infusion of naïve T cells that have a greater potential to persist and expand in vivo may improve the objective clinical responses in cancer patients as previously observed in mouse models (13, 14). We investigated the role of canonical Wnt signaling in naïve-to-effector T cell differentiation in human T lymphocytes. We found that induction of Wnt signaling, using graded doses of synthetic GSK3β inhibitor TWS119 or the native agonist Wnt-3a, preserves a naïve phenotype (CD45RO– CD45RA+ CD62L+) in activated CD4+ and CD8+ peripheral T cells. These Wnt-induced phenotypically naïve cells also showed reduced effector T cell function in response to polyclonal stimulation and in antigen-specific redirected T cells. In addition, Wnt signaling impaired T cell activation by inhibition of proximal TCR signaling and significantly blocked T cell division. Similar effects were observed in T cells derived from cord blood, which can be considered a better source of immature T cells compared to those circulating in peripheral blood (15). However, Wnt-induced T cells could be rescued from arrest in proliferation by exogenous γ chain cytokines allowing them to become effector T cells. We also found that Wnt signaling induced by TWS119 does not imprint on the T cell phenotype and functions as these cells acquire proliferative and effector function when restimulated in the absence of TWS119. Therefore, our results using human T lymphocytes support a model in which Wnt signaling inhibits effector T cell differentiation and this observation may have relevant clinical implications in T cell based immunotherapy.
Materials and methods
Cell isolation and selection
Mononuclear cells were isolated using Ficoll gradient from peripheral blood collected from healthy donors at Gulf Coast Regional Blood Center (Houston, Texas) or from research cord blood units obtained from MD Anderson Cord Blood Bank. Samples were collected according to local IRB-approved protocols. T cells were positively selected from peripheral blood mononuclear cells (PBMCs) or cord blood derived mononuclear cells (CBMCs) by magnetic-activated cell sorting using CD3, CD4 or CD8 microbeads (Miltenyi Biotec). CD45RO- and CD45RA-expressing T cells were sorted by negative selection using appropriate microbeads (Miltenyi Biotec).
T cell activation and culture
T cells were activated with plate-coated OKT3 (Ortho Biotech; 1 μg/ml) and anti-CD28 (clone CD28.2, Becton Dickinson; 1 μg/ml) antibodies and cultured for 7 days. TWS119 (GSK3β inhibitor) (EMD Biosciences) and recombinant human Wnt-3a (R&D systems) were used at the indicated concentrations in culture. TWS119 was resuspended in dimethyl sulfoxide (DMSO, Sigma). Human recombinant interleukins IL-15 (Peprotech), IL-7 (Peprotech) and IL-2 (Proleukin) were added to the cells at 5 ng/mL, 10 ng/mL and 50 U/mL respectively for indicated experiments. Phytohemagglutinin (PHA, Sigma) was used at 5 μg/mL to activate T cells polyclonally for selected experiments. Antigen-redirected T cells were generated by transduction of primary human T cells with a chimeric antigen receptor (CAR) directed against CD19 as previously described (16). These cells were retrovirally transduced (~25% CAR+) and cultured in the presence of DMSO or 3 μM TWS119 and cytokines IL-15 (5ng/ml) and IL-7 (10 ng/ml).
Complete T cell medium contained 45% RPMI (Thermo Scientific) and 45% Click’s medium (Irvine Scientific) supplemented with 10% heat inactivated fetal calf serum (FCS) (Hyclone), 100 U/ml penicillin, 100 mg/ml streptomycin and 2 mM GlutaMax™ (Invitrogen). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Flow cytometry
Cells were stained with antibodies (BD Biosciences) coupled to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP) or allophycocyanin (APC) against the indicated molecules. Routinely, 1 × 106 cells were stained with the indicated antibody or appropriate isotype controls for 20 minutes at 4°C, washed in phosphate-buffered saline (PBS) containing 1% FCS and resuspended for FACS analysis.
To examine intracellular IFNγ production and surface CD107 expression, T cells were collected after 7 days culture with control DMSO or TWS119 and restimulated overnight with 10 ng/ml phorbol myristate acetate (PMA, Sigma Aldrich) and 1 uM ionomycin (EMD Biosciences). For intracellular staining, cells were treated with protein transport inhibitor (Brefeldin A), fixed, permeabilized and stained in saponin-containing buffer (17).
The effector function of the CAR redirected T cells was examined by coculturing them with Raji cells at a 5:1 effector:target ratio in the presence or absence of DMSO or 3 μM TWS119 as indicated. After 5 days of culture, the residual Raji and T cells in coculture were identified by their CD20 and CD3 expression respectively, using flow cytometry. Non-transduced (NT) DMSO or TWS119 treated T cells served as negative controls.
Cells were analyzed by CellQuest software on a BD FACS Calibur cytometer. For each sample, a minimum of 10,000 events was analyzed.
Enzyme-linked immunosorbent assay (ELISA)
T cells were collected after 7 days culture with control DMSO or TWS119 and restimulated with 10 ng/ml phorbol myristate acetate (PMA, Sigma Aldrich) and 1 uM ionomycin (EMD Biosciences). For antigen-specific cells, the DMSO or TWS119-treated CAR redirected T cells were cocultured with Raji cells at a 5:1 ratio. After 24 hours, the supernatant of the cultures was collected and analyzed for IFNγ, IL-2 or IL-17 using 96-well plates coated with the specific antibodies by ELISA, according to the manufacturer’s instructions (R&D Systems). Other cytokines such as TNFα, IL-4 and IL-10 were analyzed using cytometric bead array (CBA) for human Th1 and Th2 cytokines (BD Biosciences).
Cell proliferation and apoptosis
T cell proliferation was evaluated in a carboxyfluorescein succinimidyl ester (CFSE) dilution assay. Briefly, freshly isolated T cells were labeled with 1.5 μM CFSE (Invitrogen) according to the manufacturer’s instructions. After 7 days of culture, the progressive dilution of CFSE corresponding to each cycle of cell division was evaluated by flow cytometry. T cell apoptosis was measured at the end of a week’s culture by staining the cells for Annexin-V and 7-aminoactinomycin D (7AAD) (Becton Dickinson).
Immunoblot analysis
For Western blot analysis, complete cell lysates were prepared from T cells cultured with control DMSO or TWS119 for 6 or 24 hours as indicated. Cell lysates were resolved by sodium dodecyl sulfate-polyacrylamidegel electrophoresis. Expression of β catenin, PLCγ, LAT, pY783 PLCγ and pY132 LAT were detected using antibodies purchased from Cell Signaling Technology and Abcam. Immunoblots were developed using enhanced chemiluminescence detection reagents (Amersham Biosciences). As a loading control, the blots were probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific monoclonal antibody (Santa Cruz Biotechnology).
Quantitative RT-PCR
T cells were activated and cultured with control DMSO or TWS119 for indicated times, washed and total RNA was isolated using the RNeasy Mini column purification kit (Qiagen). cDNA was synthesized using High Capacity RNA to cDNA kit (Applied Biosystems) according to the manufacturer’s instructions. Expression of genes Nlk, Fzd7, Tcf7, Jun and Lef1 was evaluated by quantitative RT-PCR (qPCR) using specific primers/probes purchased from Applied Biosystems. The ΔCT of the gene was normalized to the ΔCT of GAPDH and fold-change in expression was expressed relative to untreated cells.
Statistics
Results are presented as mean ± SD. Student’s t test was used to determine the statistical significance of differences between samples (p values calculated as 0 were depicted as p<0.001).
Results
TWS119 activates the canonical Wnt-β catenin signaling pathway
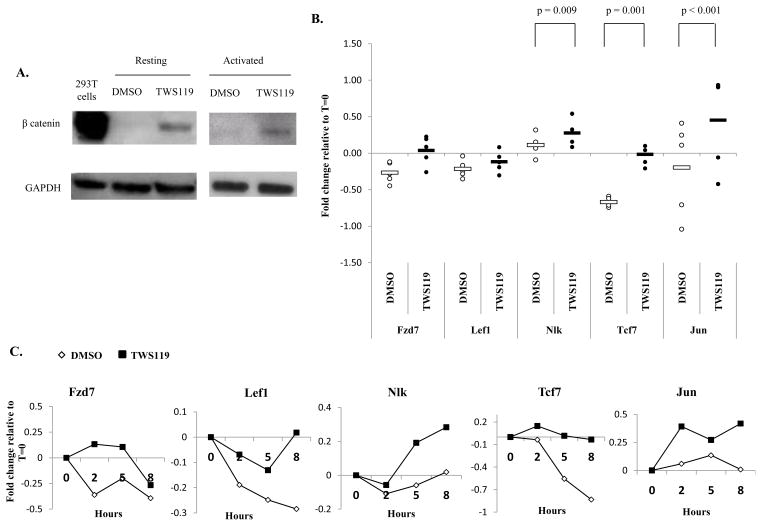
First we wanted to determine whether treatment with the GSK3β inhibitor, TWS119, induces the canonical Wnt signaling pathway in human T cells as has been observed in mouse T cells (11). As illustrated in Fig 1a, resting and activated human CD3+ T lymphocytes treated with TWS119 for 6 hours showed a large increase in β catenin accumulation relative to cells treated with DMSO, indicating that canonical Wnt signaling is activated upon TWS119 treatment in human T cells.
Figure 1. Treatment with TWS119 causes activation of canonical β-catenin-Wnt signaling.
(A) Western blot analysis of β catenin expression in CD3+ T cells cultured with DMSO or 7 μM TWS119 with or without activation for 6 hours. 293T cells were used as a positive control for β catenin expression and GAPDH served as loading control. Immunoblot data from one of 2 independent experiments. (B) qRT-PCR analysis of Wnt target genes in activated CD3+ T cells cultured with DMSO or 7 μM TWS119 for 8 hours. Fold change in expression of genes was calculated with respect to 0 hours and the data are shown in a log scale. Data summarize mean ± SD of 5 independent experiments. (C) Time course of Wnt target gene expression in activated T cells treated with DMSO or 7 μM TWS119 for 0, 2, 5 and 8 hours analyzed by qRT-PCR. Data from one of 2 independent experiments.
The expression of genes downstream of canonical Wnt signaling (Fzd7, Lef1, Nlk, Tcf7 and Jun) was examined by qRT-PCR. Control DMSO-treated activated T cells downregulated expression of Fzd7, Lef1 and Tcf7 relative to untreated cells and this is consistent with previously published observations (5) (Fig 1b, c). However in the presence of TWS119, activated T cells showed upregulation of Fzd7, Nlk and Jun genes or reduced downregulation of Lef1 and Tcf7 compared to DMSO-treated cells. This trend of higher expression of Wnt target genes in TWS119-treated cells compared to DMSO-treated cells was observed for all genes with statistically significant differences in the case of Nlk, Jun and Tcf7 (Fig 1b). This further supports the claim that TWS119 activates the Wnt pathway in human T cells.
Induction of Wnt signaling by TWS119 preserves the subset of CD45RA+ CD62L+ cells in polyclonally activated T cells
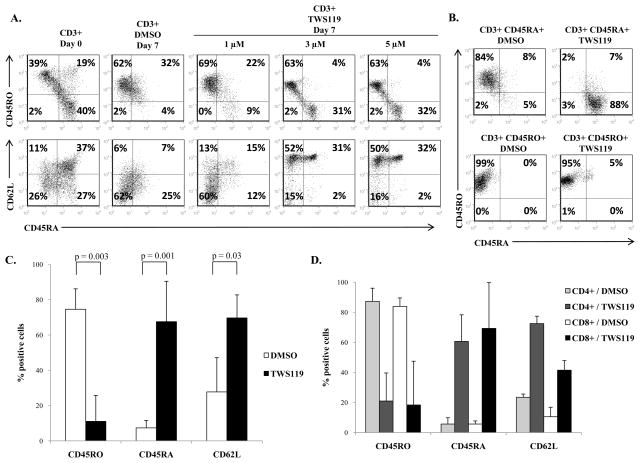
Previous reports have shown that Wnt signaling inhibits splenic T cell differentiation in mouse T cells (11). The difference in expression patterns of transcription factors and receptors of the Wnt signaling pathway between naïve and effector human T cells indicates that this pathway plays a significant, if complex, role in naïve-to-effector human T cell differentiation as well (4, 5). To test if Wnt signaling inhibits differentiation of naïve human T cells to effector cells, naïve cells were polyclonally activated and cultured with graded doses of TWS119 for a week. Freshly isolated T cells consisted of both naïve (CD45RA+ CD62L+) and effector cells (CD45RO+ CD62L-) (Fig 2a). As expected, upon activation with OKT3 and anti-CD28 antibodies, naïve T cells progressed to antigen-experienced cells, showing decline in the expression of CD45RA and acquisition of the alternatively spliced variant, CD45RO (18). By day 7, the control cells possessed predominantly effector T cell characteristics such as elevated expression of CD45RO and low expression of CD62L (Fig 2a). In contrast, activated T cells treated with TWS119 retained the subset of naïve CD45RA+ CD62L+ cells in a dose-dependent manner. We selected 3 μM of TWS119 for our subsequent in vitro experiments since it was the lowest dose that produced an effect on T cell phenotype with minimal toxicity, while higher doses (> 5 μM) resulted in substantial reduced viability of T cells by day 7 of culture (data not shown).
Figure 2. TWS119 treatment blocks transition of CD45RA+ cells into CD45RO+ cells and preserves a subset of phenotypically naïve T cells.
(A) Flow cytometry analysis of naïve and effector T cell markers - CD62L, CD45RA and CD45RO - on freshly isolated CD3+ T cells at baseline (left panels) and after activation and 7 days culture with DMSO or different doses of TWS119 (right panels). Plot from one of 3 independent experiments. (B) Analysis of CD45RO and CD45RA expression on CD3+ T cells, further selected for CD45RA or CD45RO, activated and cultured with DMSO or 3 μM TWS119 for 7 days. Plot from one of at least 3 independent experiments (C) Comparison of CD45RA, CD45RO or CD62L expression on CD3+ CD45RA+ selected cells after activation and culture with DMSO or 3 μM TWS119 for 7 days. Data summarize mean ± SD of 4 independent experiments. (D) Comparison of CD45RA, CD45RO or CD62L expression on CD45RA+ selected cells, further selected for CD4 or CD8, after activation and culture with DMSO or 3 μM TWS119 for 7 days. Data summarize mean ± SD of 3 independent experiments.
The increase in the CD45RA+ subset after TWS119 treatment could be the result of a block in the transition of T cells from the naïve CD45RA+ to the effector CD45RO+ phenotype or due to reversion of CD45RO+ cells to CD45RA+ cells (19). To determine whether TWS119 treatment blocks CD45RA-CD45RO transition, CD3-selected cells were purified based on the expression of CD45RA prior to treatment with TWS119. As expected, the great majority of CD45RA-selected cells lost CD45RA and expressed CD45RO after activation in the presence of DMSO (75% ± 12%). In contrast, most of the CD45RA+ cells retained expression of CD45RA when activated in the presence of TWS119 (68% ± 23%) (Fig 2b, upper panels). This indicates that induction of Wnt signaling blocked the transition of CD45RA-selected cells into effector CD45RO+ cells. We wanted to test if TWS119 treatment also causes reversion of CD45RO+ cells to CD45RA+ cells. CD45RO-selected T cells maintained CD45RO expression by day 7 after activation irrespectively of treatment with DMSO (85% ± 21% CD45RO+) or TWS119 (86% ± 10% CD45RO+) (Fig 2b, lower panels), indicating that TWS119 does not cause reversion of CD45RO-selected cells to CD45RA+ cells. Fig 2c summarizes the significant differences in CD62L, CD45RA and CD45RO expression in naïve CD45RA-selected T cells in response to TWS119 compared to DMSO-treated control cells. TWS119-treated cells showed higher expression of CD62L (70% ± 13% vs. 28% ± 19%, p = 0.03) and CD45RA (68% ± 23% vs. 7% ± 4%, p = 0.001) and low expression of CD45RO (11% ± 15% vs. 75% ± 12%, p = 0.003) compared to control cells. We also examined the effect of Wnt signaling on the expression of other naïve/memory markers such as CD127, activation markers such as 41BB and CD69 and costimulatory molecules such as CD27 and CD28 (Suppl Fig 1a). There was a significant decrease in the expression of CD28, 41BB and CD69 on TWS119-treated T cells compared to control cells. TWS119 treatment also maintained high expression of CD127 and CD27.
These results were observed in CD3-selected T cells which include both CD4+ and CD8+ T cells. To test if Wnt signaling induced similar effects in both CD4+ and CD8+ T cells, CD4 and CD8-selected T cells were further purified based on CD45RA expression, then activated and treated with or without TWS119 for 7 days. As illustrated in Fig 2d, induction of Wnt signaling in CD4 and CD8-selected cells produced similar naïve phenotypes. We also examined the expression of transcription factors, chemokine receptors and cytokines associated with the Th1, Th2, Th17 and Treg subsets (Suppl Fig 1b, c). TWS119-treated cells showed reduced expression of transcription factors including T-bet, RORγ, Gata-3 and FoxP3, chemokine receptors such as CXCR3 and CCR4 and the cytokines TNFα, IL-4 and IL-17. Expression of CCR6 which was downregulated by activation in DMSO control cells was maintained by TWS119. These data suggest that TWS119 does not skew CD4+ T cells to any specific subset (Th1, Th2, Th17 or Treg), but rather has a similar inhibitory effect on all the subsets. Our results demonstrate that TWS119 affected both CD4+ and CD8+ T cells equally, preserving a naïve subset by blocking the transition of CD45RA+ cells into CD45RO+ cells.
TWS119-induced Wnt signaling impairs acquisition of effector function of T cells
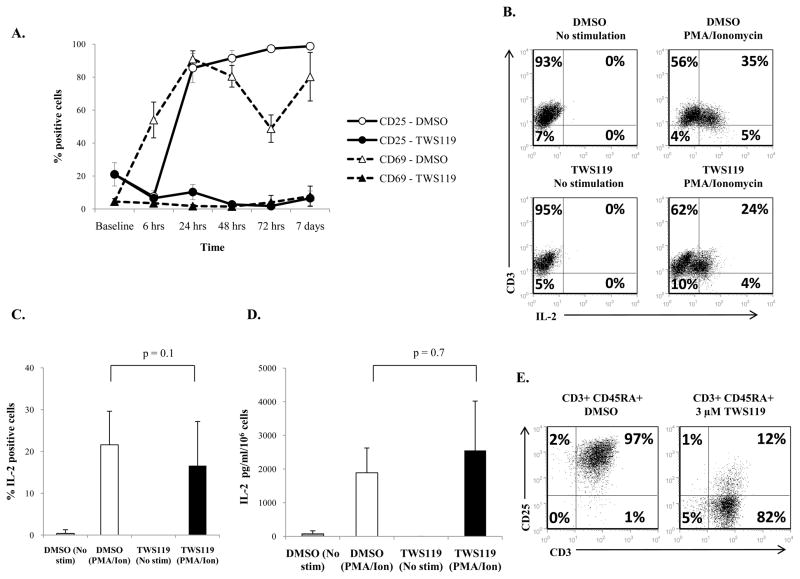
Since TWS119 inhibited naïve-to-effector T cell differentiation in terms of phenotype, we wanted to determine whether TWS119 treatment also inhibited the acquisition of effector function of T cells. CD3-selected T cells, activated and expanded for a week with or without TWS119, were stimulated overnight with PMA and ionomycin to elicit a polyclonal effector T cell response measured as IFNγ and CD107 expression (a marker for degranulation). As shown in Fig 3a and 3b, control cells that were stimulated with PMA/ionomycin expressed IFNγ (41% ± 16%) and CD107 (53% ± 10%) compared to unstimulated cells. In contrast, fewer TWS119-treated cells expressed IFNγ (17% ± 11%, p = 0.004) or CD107 (11% ± 10%, p = 0.001) in response to PMA/ionomycin. There was a significant decrease in the effector response of TWS119-treated cells in response to PMA/Ionomycin compared to control cells (Fig 3b). These results were further confirmed by ELISA detecting secreted IFNγ from PMA/ionomycin stimulated cells. TWS119-treated cells secreted significantly less IFNγ (22706 ± 5815 pg/ml) than did control cells (41306 ± 3081 pg/ml, p = 0.02) (Fig 3c).
Figure 3. TWS119 treatment impairs acquisition of T cell effector function.
(A and B) Evaluation of IFNγ production and degranulation by CD3+ cells cultured for a week in DMSO or 3 μM TWS119 by flow cytometry. (A) Representative plot and (B) percentages of DMSO or TWS119 treated CD3+ cells that express CD107 and intracellular IFNγ in response to PMA/ionomycin stimulation. Data summarize mean ± SD of at least 4 independent experiments. (C) ELISA assessment of IFNγ release in supernatant of PMA/ionomycin stimulated T cells that had been treated for a week with DMSO or 3 μM TWS119. Data summarize mean ± SD of 3 independent experiments. (D and E) Evaluation of cytotoxic function and cytokine production of antigen-specific T cells cultured with DMSO or 3 μM TWS119 cells with CD19+ Raji cells at 5:1 ratio for 5 days. Non-transduced (NT) DMSO or TWS119 treated T cells served as negative controls. (D) Representative plot of CD20+ residual tumor cells in coculture of CAR CD19 redirected T cells with Raji cells. Data from one of 2 independent experiments. (E) Cytokines in the 24 hours supernatant of coculture of CAR T cells with Raji cells assessed by CBA. Data summarize mean ± SD of 3 independent experiments.
To study the effect of Wnt signaling on effector function in an antigen-specific setting, CAR CD19 redirected T cells were cocultured with CD19-expressing Raji cells in the presence of DMSO or TWS119 for 5 days. While the DMSO control CAR CD19 cells were able to eliminate the tumor cells from the culture, the TWS119 treated CAR CD19 cells were unable to eliminate the tumor cells completely (Fig 3d). The production of IFNγ and TNFα in the coculture of TWS119 CAR CD19 T cells and Raji cells was also significantly lower than the control DMSO CAR CD19 T cells (Fig 3e). Therefore, Wnt signaling induced by TWS119 inhibits full acquisition of effector function of T cells.
TWS119 induced Wnt signaling arrests the expansion of polyclonally activated T cells
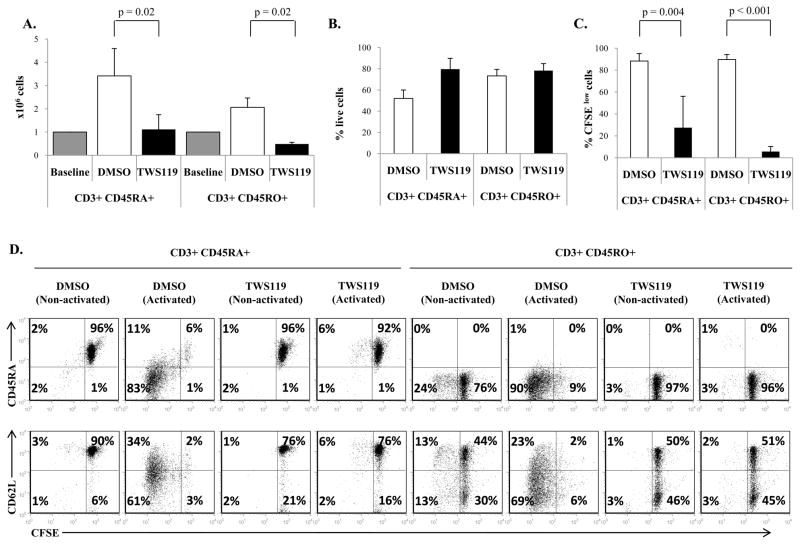
The transition of naïve into effector T cells induced by T cell activation is accompanied by cell proliferation. Since Wnt signaling inhibits naïve-to-effector T cell differentiation, we wanted to determine if Wnt signaling also arrests concomitant T cell expansion of naïve or effector T cells. As shown in Fig 4a, both CD3+ CD45RA+ and CD3+ CD45RO+ cells numerically expanded 2- to 3-fold in response to polyclonal activation by day 7 of culture. In contrast, TWS119 treatment severely limited expansion of both T cell subsets. To discover if this TWS119-mediated effect was the result of increased cell death induced by TWS119, impaired cell proliferation or both, we analyzed apoptosis and cell division by Annexin V-7AAD staining and CFSE dilution respectively. As shown in Fig 4b, TWS119 treatment did not cause a significant increase in cell death compared to control DMSO-treated cells in either CD3+ CD45RO+ (78% ± 6% and 73% ± 6% Annexin-Vnegative 7AADnegative for TWS119 and DMSO-treated cells respectively, p = 0.4) or CD3+ CD45RA+ cells (80% ± 10% and 52% ± 8% Annexin-Vnegative 7AADnegative for TWS119 and DMSO-treated cells respectively, p = 0.08). In contrast, in terms of proliferation, while control activated CD3+ CD45RA+ and CD3+ CD45RO+ cells divided robustly (88% ± 7% and 90% ± 5% diluted CFSE), activated cells treated with TWS119 did not proliferate significantly (27% ± 29% and 6% ± 5% diluted CFSE, p = 0.004 and <0.001 respectively) similar to non-activated cells (Fig 4c and 4d). As expected, proliferating DMSO-treated CD3+ CD45RA+ cells lost expression of CD45RA and CD62L but TWS119-treated cells maintained their CD62L and CD45RA expression (Fig 4d). Therefore Wnt signaling reduced the expansion of T cells by causing an arrest in cell division.
Figure 4. Treatment of T cells with TWS119 reduces cell expansion by blocking proliferation.
(A) Cell counts of CD3+ CD45RA+ and CD3+ CD45RO+ cells activated and cultured with DMSO or 3 μM TWS119 for 1 week. Data summarize mean ± SD of at least 3 independent experiments. (B) Assessment of live cell population, denoted by percentage of Annexinnegative 7AADnegative cells, at the end of 1 week of culture with DMSO or 3 μM TWS119. Data summarize mean ± SD of 3 independent experiments. (C and D) Evaluation of T cell proliferation by CFSE dilution of CD3+ CD45RO+ and CD3+ CD45RA+ cells activated or left non-activated and cultured with DMSO or 3 μM TWS119 for 7 days. Percentages of dividing cells after activation represent the CFSElow cells. Data summarize mean ± SD of at least 3 independent experiments.
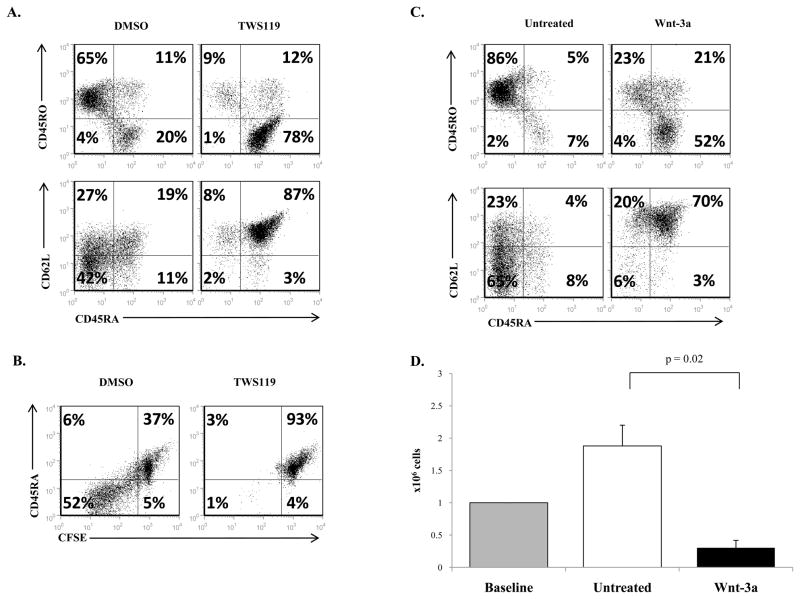
To confirm that TWS119 treatment arrested cell division and blocked transition of CD45RA+ to CD45RO+ cells independently of the mode of T cell activation, PHA was used to activate the T cells instead of OKT3/anti-CD28. As expected, TWS119 treatment preserved the CD45RA+ phenotype in PHA-activated T cells compared to control cells and also blocked cell division (Fig 5a, b). We also confirmed that the observed effects on T cell phenotype were the result of induced Wnt signaling and not off target effects of TWS119. Treatment of PHA-activated cells with physiological canonical Wnt ligand Wnt-3a recapitulated the results seen with TWS119 (Fig 5c, d). In other words, Wnt signaling preserves the naïve CD62L+ CD45RA+ phenotype and limits cell expansion in activated T cells irrespective of the mode of induction of Wnt signaling or mode of T cell activation.
Figure 5. PHA activation of T cells or induction of Wnt signaling by Wnt-3a produces similar effects as OKT3/anti-CD28 activated, TWS119-treated T cells.
(A) Expression of CD62L, CD45RA and CD45RO in T cells activated with 5 μg/ml PHA and cultured with DMSO or 3 μM TWS119 for 7 days. (B) Cells were stained with CFSE and cultured with DMSO or 3 μM TWS119 and the extent of proliferation was measured in terms of CFSE dilution by day 7 of culture. (C) Expression of CD62L, CD45RA and CD45RO in T cells activated and cultured with or without Wnt-3a (5 μg/ml) for 7 days. (D) Cell counts at the 7 days of culture with or without Wnt-3a (5 μg/ml) were determined. Data summarize mean ± SD of 3 independent experiments
Based on the observed effect of Wnt signaling in arresting proliferation and naïve-to-effector differentiation, we predicted that Wnt signaling impairs T cell activation which induces proliferation and differentiation of T cells. The kinetics of expression of early and late T cell activation markers, CD69 and CD25 (20–23), was examined in cells treated with DMSO or TWS119. As expected, activated control cells rapidly upregulated both CD69 and CD25 (Fig 6a). However, TWS119-treated cells continuously maintained low expression of CD69 and CD25 indicating that T cell activation is impaired in these cells (Fig 6a). We also performed immunoblot analysis to examine the effect of TWS119 on the activation status of signaling molecules LAT and PLCγ which are directly downstream of T cell activation. As illustrated in Suppl Fig 1d, the phosphorylation of Y783 in PLCγ and Y132 in LAT was severely impaired in TWS119-treated cells. This is consistent with published observations in mouse T cells in which proximal TCR signaling was inhibited by constitutively active β catenin (12).
Figure 6. TWS119 treatment inhibits expression of activation markers CD25 and CD69 but not production of IL-2.
(A) Evaluation of CD25 and CD69 expression by CD3+ cells treated for a week with DMSO (solid line, open circles and triangles) or 3 μM TWS119 (dotted line, closed circles and triangles) by flow cytometry at the indicated time points. Data summarize mean ± SD of 3 independent experiments. (B and C) Evaluation of IL-2 production by CD3+ cells cultured for a week in DMSO or 3 μM TWS119 by intracellular staining. (B) Representative plot and (C) percentages of DMSO or TWS119 treated CD3+ cells that express intracellular IL-2 in response to PMA/ionomycin stimulation. Data summarize mean ± SD of 5 independent experiments. (D) ELISA assessment of IL-2 release in supernatant of PMA/ionomycin stimulated T cells that had been cultured for a week with DMSO or 3 μM TWS119. Data summarize mean ± SD of 3 independent experiments. (E) Evaluation of CD25 expression on CD3+ CD45RA+ cells treated with DMSO or 3 μM TWS119 for a week. Plot from one of 3 independent experiments.
To begin to investigate the mechanism by which Wnt signaling arrests proliferation of T cells, we tested the ability of these cells to produce IL-2, a cytokine that supports T cell expansion upon activation. CD3-selected T cells activated and expanded for a week with or without TWS119, were stimulated overnight with PMA and ionomycin to induce IL-2 production. Intracellular staining showed comparable numbers of IL-2 producing cells in the TWS119 treatment group (22% ± 8%) and the control group (17% ± 11%, p = 0.1) (Fig 6b, c). These results were confirmed by detection of secreted IL-2 in the supernatant of PMA/ionomycin stimulated T cells by ELISA. There was no significant difference in the amount of IL-2 in the supernatants of DMSO-treated cells (1890 ± 736 pg/ml) and TWS119-treated cells (2554 ± 1466 pg/ml, p = 0.7) (Fig 6d). Therefore, a difference in IL-2 production does not seem to account for the observed Wnt induced arrest in proliferation. However, the ability of these TWS119-treated cells to utilize IL-2 would be affected by the expression of the IL-2R on activated T cells. As illustrated in Fig 6a, the expression of the IL-2Rα chain, CD25, was significantly lower in TWS119-treated cells (7% ± 4%) compared to control cells (99% ± 1%, p < 0.001) at day 7 (Fig 6a, e). This indicates that although TWS119-treated cells are able to produce IL-2, they may have reduced ability to utilize this cytokine for expansion.
The effect of TWS119 on T cell proliferation and differentiation could also be cell extrinsic. As shown in Fig 6e, a small subset of T cells express CD25 after TWS119 treatment (7% ± 4%) and this could contain natural regulatory T cells (nTregs) since Wnt signaling has been reported to enhance nTreg survival (24). Since the presence of Tregs may cause the observed arrest in T cell proliferation and differentiation, CD25-expressing cells were depleted prior to CD3 selection to eliminate nTregs. The cells were then activated and cultured in the presence of DMSO or 3 μM TWS119. At the end of 7 days culture, the subset of CD4+ CD25+ cells was negligible but comparable in both CD3+ and CD3+ CD25depleted cells after TWS119 treatment (Suppl Fig 2a). Furthermore, depletion of CD25+ cells (containing nTregs) did not reverse the impaired expansion of T cells induced by TWS119 treatment (Suppl Fig 2b, c). This indicates that the Wnt-induced effects on T cell phenotype, proliferation and effector differentiation is not mediated by an indirect effect of nTregs.
Cytokines overcome the block in T cell proliferation mediated by TWS119 but also cause a loss of the naïve phenotype of T cells
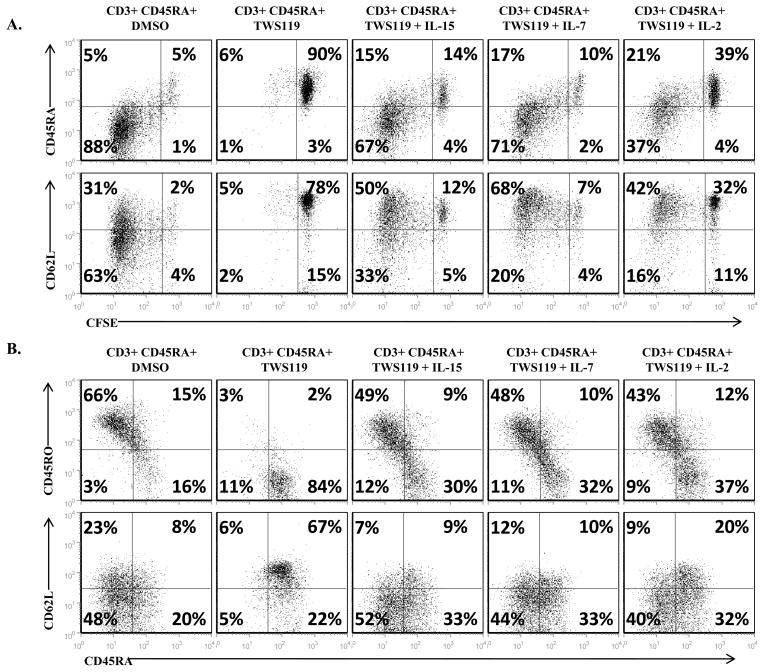
While reduced IL-2Rα expression may explain how TWS119 impairs T cell proliferation in the presence of physiological levels of IL-2 (25), we wanted to test if pharmacological doses of growth factors can rescue the arrested expansion of Wnt-activated cells. Addition of exogenous γ-chain cytokines (IL-15, IL-7 or IL-2) in the presence of TWS119 overcame the block in cell proliferation allowing T cell division as assessed by CFSE dilution assay (Fig 7a) and numeric expansion similar to control cells (data not shown). However, cytokine-induced proliferation also led to a loss of the TWS119-induced naïve phenotype (Fig 7b). By day 7 of culture, the naïve CD45RA+ CD62L+ subset was significantly reduced by the addition of cytokines IL-15, IL-7 or IL-2 to the TWS119-treated cells (from an average of 69% ± 12% to 15% ± 6%, 21% ± 12% and 29% ± 16%, p = 0.015, 0.041 and 0.008 respectively) and was comparable to the DMSO-treated cells (6% ± 3%, p > 0.1). Therefore, addition of cytokines rescued the blocked proliferation of Wnt-arrested T cells and concomitantly led to a loss of the naïve T cell phenotype.
Figure 7. Cytokines can overcome the block in proliferation induced by TWS119 treatment but it leads to a concomitant loss of the naïve T cell phenotype.
(A) Evaluation of proliferation of CD3+ CD45RA+ cells cultured for a week with DMSO or 3 μM TWS119 in the absence or presence of IL-15 (5 ng/ml), IL-7 (10 ng/ml) or IL-2 (50 U/ml) using CFSE dilution assay. Plots show the extent of proliferation in terms of CFSE dilution by day 7 of culture. (B) Analysis by flow cytometry of CD45RA, CD45RO or CD62L expression by these cells at the end of 7 days culture in the absence or presence of the cytokines. Dot plots from one of 3 independent experiments.
Although Wnt signaling induced by TWS119 or Wnt-3a clearly arrests T cell proliferation and naïve-to-effector cell differentiation in our in vitro cultures, we wanted to investigate if these Wnt signaling effects imprint on T cell phenotype and function over a long term. Activated T cells cultured with TWS119 for a week were restimulated with OKT3 and anti-CD28 antibodies and cultured for an additional week in the presence of DMSO or TWS119. As illustrated in Suppl Fig 3a, TWS119-treated cells that were deprived of the drug for the second week did not retain the naïve phenotype and became activated effector cells similar to control DMSO-treated cells. The effector function of these cells as illustrated by production of IFNγ and degranulation (CD107 expression) was slightly reduced or comparable to control DMSO cells (Suppl Fig 3b). In the case of antigen-redirected cells, TWS119-treated CAR CD19 cells that were deprived of this drug upon coculture with the Raji cells were able to recover their cytotoxic function and completely eliminate the tumor cells similar to DMSO-treated CAR CD19 T cells (Suppl Fig 3c). We also conducted experiments where the CD3+ cells were transiently exposed to DMSO or TWS119 for 24 hrs following which the drug was washed out of the culture. As shown in Suppl Fig 3d and e, transient exposure to the TWS119 does not arrest the expansion and activation (CD25, CD69 expression) or preserve the naïve phenotype (CD45RA expression) of these T cells. Therefore, the T cells are arrested in expansion and naïve-to-effector cell differentiation only in the continuous presence of the drug and upon removal of the Wnt-signaling agonist, the T cells overcome this arrest and become effector cells. These results indicate that TWS119 treatment does not imprint on T cell phenotype and function.
TWS119 treatment of cord-blood derived-T cells arrests proliferation and preserves the naïve T cell phenotype
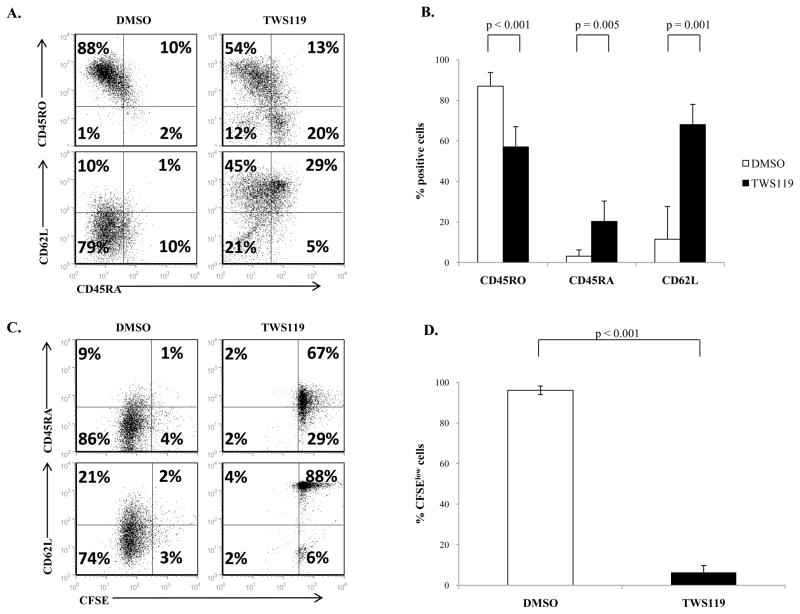
To determine if Wnt signaling impairs proliferation and maintains a naïve phenotype in peripheral T cells irrespective of the source of T cells, we repeated these experiments with umbilical cord-blood derived mononuclear cells, a source of phenotypically and functionally immature circulating T cells (15). As shown in Fig 8a and 8b, activated T cells treated with TWS119 had increased expression of CD45RA (20% ± 11%) and CD62L (68% ± 21%) and reduced CD45RO expression (57% ± 7%) compared to control cells (3% ± 3%, 12% ± 16% and 87% ± 7%, p = 0.005, 0.001 and <0.001 respectively) at the end of a week’s culture. TWS119 also impaired proliferation of cord-blood derived T cells (Fig 8c and 8d). Therefore, TWS119 treatment of T cells derived from umbilical cord blood recapitulated the results seen with T cells isolated from peripheral blood.
Figure 8. TWS119 treatment blocks transition of CD45RA+ cells into CD45RO+ cells in cord blood derived T cells.
(A) Representative dot plots and (B) percentage of cells expressing CD45RA, CD62L and CD45RO for CD3+ T cells isolated from CBMCs and cultured with DMSO or 3 μM TWS119 for 7 days. Data summarize mean ± SD of 5 independent experiments. (C and D) Assessment of CBMC-derived CD3+ T cell proliferation using CFSE dilution assay after a week of culture with DMSO or 3 μM TWS119. Data summarize mean ± SD of 3 independent experiments.
Discussion
Although Wnt signaling has been studied extensively in T cell development, its function in peripheral T cells has not been well-defined and to our knowledge, this is the first report evaluating the effects of induction of Wnt signaling on effector T cell differentiation in human T lymphocytes. We found that activation of Wnt signaling blocked the transition of naïve T cells (CD45RA+ CD62L+) to effector T cells (CD45RO+ CD62L-) but did not cause reversion of CD45RO+ cells to CD45RA-expressing cells. Wnt signaling arrested T cell division and impaired the acquisition of effector function in response to polyclonal stimulation. Similar results were also observed in immature peripheral T cells derived from umbilical cord blood. Our phenotypic and functional in vitro data indicate that Wnt signaling inhibits the naïve-to-effector differentiation of human T lymphocytes.
Our findings are consistent with those of Driessens et al. in mice who observed that genetically stabilized β catenin inhibited mouse T cell activation and proliferation and impaired the function of Th1, Th2 and Th17 polarized cells. (12). In particular, those authors demonstrated that β catenin prevented the activation of the LAT/PLCγ1/Ca2+ pathway by diminishing phosphorylation of a specific residue in LAT, leading to defective recruitment of PLCγ1 to LAT. Consistent with these findings, we observed a similar impairment of phosphorylation in these TCR signaling molecules in TWS119-treated human T cells. We found that TWS119-treated cells had significantly reduced expression of IL-2Rα and this may partially explain their impaired proliferation. While the IL-2Rβ and γ chains are sufficient for IL-2R signaling in the presence of high levels of IL-2, the IL-2Rα is required to form the high affinity IL-2R which can utilize low physiological doses of IL-2 for expansion (25, 26). Since IL-2Rα is upregulated in T cells as a result of TCR activation (27, 28) and since inhibition of proximal TCR-induced IL-2Rα expression has been shown to be rescued by treatment with PKC activator PMA and a Ca2+ ionophore (29), we speculate that the Wnt-induced inhibition of IL-2Rα expression illustrated in our experiments may be the consequence of inhibition of the LAT/PLCγ1/PKC/Ca2+ pathway by Wnt signaling. It also has to be noted that TWS119-mediated inhibition of TCR activation would be expected to reduce IL-2 production (30). Although supernatants of TWS119-treated and control cells showed comparable levels of IL-2, a reduction in IL-2 production from TWS119-treated cells may be obscured due to the high consumption of IL-2 by CD25 expressing control activated T cells. Further investigation will be required to fully identify the mechanisms by which Wnt signaling arrests T cell activation and proliferation in human cells.
Our results also agree in part with the findings of Gattinoni et al. who reported that differentiation and production of IFNγ of CD8+ pmel-1 TCR transgenic mouse T cells in response to antigen stimulation were inhibited by TWS119-induced activation of Wnt signaling (11). Our data show that these inhibitory effects of Wnt signaling can be measured not only in CD8+ T cells but also in CD4+ naïve T cells indicating that this pathway is similar in both CD4+ and CD8+ T cells. However, we found that at the lowest dose of TWS119 (3 μM) that was required to obtain significant and consistent preservation of the naïve T cell subset, T cell division was almost completely inhibited. Our experiments examining cell cycle show that the majority of TWS119-treated cells are arrested at the G0/G1 phase in the cell cycle (data not shown). This observation is not in line with Gattinoni’s study in which the CD8+ pmel-1 TCR transgenic mouse T cells retained a significant proliferative capacity while maintaining a naïve phenotype at similar TWS119 doses.
This discrepancy may arise from a number of factors including but not limited to differences in culture conditions of mouse versus human T cells, the intrinsic differences in T cells, namely, transgenic TCR T cells compared to polyclonal or CAR redirected T lymphocytes and also the magnitude of differences in Wnt target gene expression induced by TWS119 in both cases. The large induction of Wnt target genes such as Fzd7 observed by Gattinoni et al. may correlate with higher level of Wnt signaling activity in the pmel-1 TCR transgenic mouse T cells compared to human T lymphocytes and may explain the divergence in the results. The authors also used antigen-presenting cells for activation of mouse T cells compared to our use of antibodies for polyclonal activation of human T cells. However, our overall findings are consistent with conclusions from other studies that Wnt signaling inhibits T cell activation, expansion and naïve-to-effector differentiation.
The Wnt signaling-induced block in expansion and effector function of T cells may play a role in vivo in different situations. For instance, certain tumors such as prostate cancer cells secrete Wnt ligands into the tumor microenvironment (31). This could activate Wnt-β catenin signaling in T cells targeting the tumor and inhibit their expansion and function. Since dysregulation of the Wnt signaling pathway has been implicated in various cancers, several studies are underway to target and inhibit this pathway in tumors (32, 33). Based on our data, inhibition of Wnt signaling at the tumor site may target the tumor in multiple ways – directly by inhibiting the signaling pathway in the tumor cells and indirectly by reversing the inhibitory effects of Wnt signaling on T cells in the tumor microenvironment.
Another possibility is that activation of Wnt signaling in T cells may be induced in a tolerogenic setting. Macrophages and dendritic cells can produce canonical Wnt ligands (34, 35). It has been shown that constitutive expression of β catenin in Tregs extends their survival and improves their suppressive function (24). Stable β catenin expression also induced anergy in CD4+ CD25- cells in terms of reduced proliferation, increased expression of anergy-associated genes (Cblb, GRAIL, Itch) and reduced capacity to induce inflammatory disease in vivo (24). β catenin signaling in intestinal dendritic cells induced tolerance via Treg induction and suppression of effector cells (36). Therefore, our data in conjunction with the literature supports the hypothesis that Wnt signaling may induce a tolerogenic program in immune cells such as dendritic cells and T cells.
In addition to its role in effector T cell differentiation, Gattinoni et al. also reported that Wnt signaling induced a subset of T memory stem cells which possessed a naïve phenotype despite undergoing several cycles of cell division in vitro, and were characterized by self-renewal abilities, long-term persistence and potent anti-tumor function in vivo (11). Recapitulating these results in antigen-specific human T cells using Wnt signaling would have a major impact on clinical applications of adoptive T cell therapy in cancer patients. A current limitation of this approach is indeed the short term persistence of ex vivo expanded antigen-specific T cells upon infusion into the patient which is likely due to the presence of predominantly effector cells with limited life spans (37–39). The infusion of antigen-specific T cells with high renewal capacity would enhance clinical responses in cancer patients, as has been observed upon infusion of memory or naïve T cells (13, 14, 40–42). We tested for the expression of Tscm markers including CD122 and Bcl-2, which were observed in Gattinoni et al., on the TWS119-induced naïve subset of T cells and found no difference in expression of these markers compared to control activated cells (data not shown). However, our results in vitro were obtained using polyclonally activated T cells and this precludes us from evaluating whether Wnt signaling can be used to generate antigen-specific human T memory stem cells with potent anti-tumor effects in vivo.
On the other hand, human antigen-specific T cells are expanded ex vivo by repeated stimulations of T cells with antigen-presenting cells (43–45), thus Wnt signaling cannot be induced in this setting since it will inhibit T cell division. However, gene modification of the human T lymphocytes has been implemented to confer them with antigen-specificity by the expression of transgenic TCR or CARs (16, 46–48). Transduction of T cells in the presence of an optimal dose of TWS119 may preserve the naïve status of these cells and could enhance their persistence in vivo after adoptive transfer. Examination of the effect of canonical Wnt signaling on antigen-specific human T cells in in vivo studies may also show more promising results in terms of generation of Tscm cells for adoptive T cell therapy.
In conclusion, we have identified the role of canonical Wnt signaling as a negative regulator in human peripheral T cell effector differentiation. In the presence of Wnt-β catenin signaling, the T cells are arrested in their naïve phenotype, impaired in the acquisition of effector function and show inhibited cell proliferation. Since Wnt signaling does not imprint on the T cell phenotype and effector function, the cells should be able to expand and become effectors upon removal of the Wnt agonist. Therefore, Wnt signaling may be useful for the ex vivo maintenance of naïve T cells for adoptive immunotherapy for cancer.
Supplementary Material
Acknowledgments
We are grateful to Reshma Kulkarni for the phenotypic analysis.
Footnotes
This work was supported in part by R01 CA142636 National Institutes of Health-NCI, W81XWH-10-1-0425 Department of Defense, Technology/Therapeutic Development Award and Cancer Prevention and Research Institute of Texas CPRIT #RP 100484.
Conflict of Interest
The authors have no competing financial interest.
References
- 1.Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002;32:967–971. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 3.Staal FJ, Meeldijk J, Moerer P, Jay P, van de Weerdt BC, Vainio S, Nolan GP, Clevers H. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol. 2001;31:285–293. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 6.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 7.Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Logtenberg T, Clevers H. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 8.Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 9.Zeng YA, Verheyen EM. Nemo is an inducible antagonist of Wingless signaling during Drosophila wing development. Development. 2004;131:2911–2920. doi: 10.1242/dev.01177. [DOI] [PubMed] [Google Scholar]
- 10.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driessens G, Zheng Y, Locke F, Cannon JL, Gounari F, Gajewski TF. Beta-catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase C-gamma1 phosphorylation. J Immunol. 186:784–790. doi: 10.4049/jimmunol.1001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP. Adoptively transferred effector cells derived from naive rather than central memory CD8+ Tcells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris DT, Schumacher MJ, Locascio J, Besencon FJ, Olson GB, DeLuca D, Shenker L, Bard J, Boyse EA. Phenotypic and functional immaturityof human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992;89:10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Micklethwaite KP, Savoldo B, Hanley PJ, Leen AM, Demmler-Harrison GJ, Cooper LJ, Liu H, Gee AP, Shpall EJ, Rooney CM, Heslop HE, Brenner MK, Bollard CM, Dotti G. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010;115:2695–2703. doi: 10.1182/blood-2009-09-242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch KW. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol. 2004;4:931–940. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- 19.Hargreaves M, Bell EB. Identical expression of CD45R isoforms by CD45RC+ ‘revertant’ memory and CD45RC+ naive CD4 T cells. Immunology. 1997;91:323–330. doi: 10.1046/j.1365-2567.1997.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994;12:456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
- 21.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 22.Zola H. Markers of cell lineage, differentiation and activation. J Biol Regul Homeost Agents. 2000;14:218–219. [PubMed] [Google Scholar]
- 23.Schuh K, Twardzik T, Kneitz B, Heyer J, Schimpl A, Serfling E. The interleukin 2 receptor alpha chain/CD25 promoter is a target for nuclear factor of activated T cells. J Exp Med. 1998;188:1369–1373. doi: 10.1084/jem.188.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham JA, Fray M, de Haseth S, Lee KM, Lian MM, Chase CM, Madsen JC, Markmann J, Benichou G, Colvin RB, Cosimi AB, Deng S, Kim J, Alessandrini A. Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3{beta} inhibition. J Biol Chem. 285:32852–32859. doi: 10.1074/jbc.M110.150904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T, Wang R, Russell JH. IL-12 enhances IL-2 function by inducing CD25 expression through a p38 mitogen-activated protein kinase pathway. Eur J Immunol. 2000;30:1445–1452. doi: 10.1002/(SICI)1521-4141(200005)30:5<1445::AID-IMMU1445>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Johnston JA, Bacon CM, Riedy MC, O’Shea JJ. Signaling by IL-2 and related cytokines: JAKs, STATs, and relationship to immunodeficiency. J Leukoc Biol. 1996;60:441–452. doi: 10.1002/jlb.60.4.441. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Shimizu M, Ohira K, Vayuvegula B. T cell activation via the T cell receptor: a comparison between WT31 (defining alpha/beta TcR)-induced and anti-CD3-induced activation of human T lymphocytes. Cell Immunol. 1991;132:26–44. doi: 10.1016/0008-8749(91)90004-u. [DOI] [PubMed] [Google Scholar]
- 28.Leonard WJ, Kronke M, Peffer NJ, Depper JM, Greene WC. Interleukin 2 receptor gene expression in normal human T lymphocytes. Proc Natl Acad Sci U S A. 1985;82:6281–6285. doi: 10.1073/pnas.82.18.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trevillyan JM, Lu YL, Atluru D, Phillips CA, Bjorndahl JM. Differential inhibition of T cell receptor signal transduction and early activation events by a selective inhibitor of protein-tyrosine kinase. J Immunol. 1990;145:3223–3230. [PubMed] [Google Scholar]
- 30.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 31.Hall CL, Kang S, MacDougald OA, Keller ET. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97:661–672. doi: 10.1002/jcb.20735. [DOI] [PubMed] [Google Scholar]
- 32.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, He TC. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 33.Garber K. Drugging the Wnt pathway: problems andprogress. J Natl Cancer Inst. 2009;101:548–550. doi: 10.1093/jnci/djp084. [DOI] [PubMed] [Google Scholar]
- 34.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehtonen A, Ahlfors H, Veckman V, Miettinen M, Lahesmaa R, Julkunen I. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J Leukoc Biol. 2007;82:710–720. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- 36.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, Leitman SF, Rosenberg SA. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cellclones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. JImmunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, Muranski P, Palmer DC, Scott CD, Morgan RA, Robbins PF, Rosenberg SA, Restifo NP. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolle CE, Carrio R, Malek TR. Modeling the CD8+ T effector to memory transition in adoptive T-cell antitumor immunotherapy. Cancer Res. 2008;68:2984–2992. doi: 10.1158/0008-5472.CAN-07-3040. [DOI] [PubMed] [Google Scholar]
- 43.Savoldo B, Cubbage ML, Durett AG, Goss J, Huls MH, Liu Z, Teresita L, Gee AP, Ling PD, Brenner MK, Heslop HE, Rooney CM. Generation of EBV-specific CD4+ cytotoxic T cells from virus naive individuals. J Immunol. 2002;168:909–918. doi: 10.4049/jimmunol.168.2.909. [DOI] [PubMed] [Google Scholar]
- 44.Tsai V, Kawashima I, Keogh E, Daly K, Sette A, Celis E. In vitro immunization and expansion of antigen-specific cytotoxic T lymphocytes for adoptive immunotherapy using peptide-pulsed dendritic cells. Crit Rev Immunol. 1998;18:65–75. doi: 10.1615/critrevimmunol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 45.Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, Brenner MK, Rooney CM. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 46.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, Gopal AK, Pagel JM, Lindgren CG, Greenberg PD, Riddell SR, Press OW. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.