Abstract
Background
Problems inhibiting non-adaptive behaviors have been linked to an increased risk for substance use and other risk taking behaviors in adolescence. This study examines the hypothesis that abnormalities in neural activation during inhibition in early adolescence may predict subsequent substance involvement.
Methods
Thirty eight adolescents from local area middle schools, ages 12–14, with very limited histories of substance use, underwent functional magnetic resonance imaging (fMRI) as they performed a go/no-go task of response inhibition and response selection. Adolescents and their parents were then followed annually with interviews covering substance use and other behaviors. Based on follow-up data, youth were classified as transitioning to heavy use of alcohol (TU; n=21), or as healthy controls (CON; n=17).
Results
At baseline, prior to the onset of use, youth who later transitioned into heavy use of alcohol showed significantly less activation than those who went on to remain non to minimal users throughout adolescence. Activation reductions in TU at baseline were seen on no-go trials in 12 brain regions, including right inferior frontal gyrus, left dorsal and medial frontal areas, bilateral motor cortex, cingulate gyrus, left putamen, bilateral middle temporal gyri, and bilateral inferior parietal lobules (corrected p < .01, each cluster ≥ 32 contiguous voxels).
Conclusions
These results support the hypothesis that less neural activity during response inhibition demands predicts future involvement with problem behaviors such as alcohol and other substance use.
Keywords: alcohol, adolescence, fMRI, inhibition, go/no-go
1. Introduction
For many, substance (e.g., alcohol and cannabis) use initiation begins in middle school, with rates of use increasing dramatically through adolescence. For instance, according to recent community surveys, alcohol is the most widely used intoxicant among 8th graders, with 15% reporting drinking in the past 30 days, expanding to 44% by 12th grade. More alarmingly, 8% of 8th graders and 25% of 12th graders report past 2-week heavy drinking (≥5 drinks per occasion) (Johnston et al., 2009). Similarly, 12% of 8th graders report cannabis use in the past year, and rates increase to 30% by 12th grade; other drug use increases from 7% to 17% from 8th to 12th grade for annual or more frequent use (Johnston et al., 2009). These data indicate that a sizable proportion of adolescents use substances.
A range of risk factors influences the probability of initiation and intensity of substance use in adolescence. Youth with a family history of alcohol use disorder (AUD) are more likely to initiate drinking and develop AUD than youth without such familial background (Cloninger et al., 1986; Schuckit, 1985). Initiating alcohol use at an earlier age (i.e., < age 14) is also linked to an increased risk for future AUD (DeWit et al., 2000; Grant and Dawson, 1997). Additional factors influencing adolescent substance use risk are childhood behavioral problems (Stice et al., 1998; Zucker et al., 2008) and disinhibitory features (Hill et al., 1999; Kirisci et al., 2006) such as impulsivity (Ernst et al., 2006; McGue et al., 2001), aggression (Brook et al., 1992; Clapper et al., 1995), and conduct disorder (Boyle and Offord, 1991; King et al., 2004; Kuperman et al., 2005). Early pubertal onset (Downing and Bellis, 2009), positive alcohol or other drug use expectancies (Killen et al., 1996; Simons-Morton, 2004), and depressive symptoms (Henry et al., 1993) also potentiate the likelihood of substance involvement. Understanding the mechanisms by which these factors contribute to adolescent substance use may refine the foci of secondary prevention efforts.
Inhibition, the ability to suppress inappropriate or impulsive behaviors, is a risk factor of particular interest as it may relate to future adolescent substance use either directly or indirectly, through externalizing behaviors (Iacono et al., 2008). Inhibition matures across childhood with the capacity to inhibit a response apparent as early as infancy (Diamond and Goldman-Rakic, 1989) yet the ability to consistently inhibit a behavior or action developing well into adolescence (Luna et al., 2004; Luna et al., 2001). Multiple inhibitory tasks show increasing cognitive control with age, including Stroop (Tipper et al., 1989), go/no-go (Luciana and Nelson, 1998), stop signal (Greenberg and Waldman, 1993; Ridderinkhof et al., 1999; Williams et al., 1999), and antisaccade tasks (Fischer and Weber, 1998; Fukushima et al., 2000; Luna et al., 2004; Munoz et al., 1998). For example, go/no-go studies have shown decreasing false alarm rates across development with young adults recording significantly less false alarms than children (Casey et al., 1997; Jonkman, 2006). Similarly, antisaccade studies have shown that children commit more errors than adults, with rates of correct inhibitions improving through adolescence into adulthood (Fischer and Weber, 1998; Fukushima et al., 2000; Luna et al., 2004; Munoz et al., 1998).
Underlying the development of more efficient inhibitory control are changes in brain structure and function subserving these higher order processes. Synaptic pruning, cortical thinning, and myelination of white matter tracts occur throughout adolescence (Gogtay et al., 2004; Sowell et al., 2002). Prefrontal cortex activity appears to change prominently as inhibitory networks develop. Increased BOLD response in prefrontal regions has been reported among children (<age 12) compared to adolescents and adults during go/no-go (Booth et al., 2003; Casey et al., 1997) and antisaccade tasks (Velanova et al., 2008). However, less prefrontal response in children and adolescents compared to adults has been reported during flanker (Bunge et al., 2002), stop (Rubia et al., 2000), and Stroop tasks (Adleman et al., 2002), and one investigation found adolescents and adults to activate prefrontal cortex similarly during an antisaccade task (Velanova et al., 2008). These inconsistent results could be due to small sample sizes, chance, or differences in developmental stages across studies.
Some investigations offer explanations for the mixed results in the development of inhibition and the prefrontal cortex. Tamm and colleagues (2002) suggest a differential effect for regions within the frontal lobe, with increasing activation in inferior frontal, orbital frontal, and insular gyri, and decreasing activation in middle and superior frontal areas across adolescence. A meta-analysis of functional magnetic resonance imaging (fMRI) studies using various go/no-go tasks in adults suggest that differences among studies are task dependent; tasks with greater cognitive demands evoke additional brain regions that underlie cognitive functions beyond response selection (e.g., working memory) (Simmonds et al., 2008). The same theory could be applied to all developmental studies of inhibition where differences in neural recruitment during inhibition could be accounted for by the type (e.g., go/no-go stop, Stroop, antisaccade) and complexity of the inhibitory tasks.
In addition to the prefrontal cortex, other neural regions are recruited during tasks of inhibition. Activation within the anterior cingulate, parietal and occipital lobes have been observed during tasks requiring response inhibition, and have been more pronounced among adults relative to children (Adleman et al., 2002; Velanova et al., 2008). Subcortically, activation has also been shown in the caudate, though more so in adolescents compared to adults (Rubia et al., 2000). These investigations highlight the complexity of the neural mechanisms responsible for the maturation of inhibition.
Understanding the development of inhibition at the behavioral and neural level is of great importance when considering inhibition within the context of substance use. The few studies examining brain function and response inhibition in youth at risk for developing substance use disorders suggest that less BOLD response in frontal regions is related to risk for substance use; however, the relationship between this frontal activation and future substance use among adolescents is yet to be determined. Among adults with AUD, deficits in executive functioning, specifically in inhibition, persist into abstinence periods, suggesting that functions underlying planning, decision making and response inhibition may be uniquely affected by alcohol consumption (Davies et al., 2005; Fein et al., 2004; Noel et al., 2007). The question remains whether aberrant inhibitory networks in childhood and adolescence are themselves risk factors for substance use or rather substance use interrupts inhibition and the maturation of neuronal substrates of inhibition. The aim of the present study was to investigate the hypothesis that altered neural activation during inhibition in adolescents with limited substance use experience predicts subsequent substance involvement.
To that end, adolescents who had not yet had any notable substance use involvement (i.e., at project baseline) completed a go/no-go task during fMRI, and were then followed quarterly to assess for emergence and persistence of substance use (i.e., follow up). Based on follow-up (M= 4.2 years) alcohol use information, adolescents were classified into one of two alcohol use groups: Transitioned to Heavy Use (TU) and Healthy Controls (CON). Some TU youth also endorsed other substance use at follow-up, predominantly cannabis. We examined the relationship between baseline BOLD response during inhibitory trials of a go/no-go task, and follow-up substance use outcome. Previous studies using this go/no-go task in high-risk adolescents have observed less activation in frontal and posterior parietal regions than low-risk youth (Anderson et al., 2005; Schweinsburg et al., 2004b). Based on previous fMRI studies concerning development, familial risk for substance use, and prediction of relapse (Paulus et al., 2005), we hypothesized that at baseline, when no youths had more than 3 lifetime substance use episodes, those who would transition into heavy alcohol use by mid-adolescence (TU youth) would show less BOLD response in frontal regions than youth who would remain relatively substance-free throughout this developmental period (CON youth), and this would be specific to inhibition trials rather than response selection trials.
2. Methods
2.1 Participants
Participants were ages 12–14 at the start of this longitudinal study (Anderson et al., 2005; Bava et al., 2011; Hanson et al., 2010; Medina et al., 2008; Pulido et al., 2009; Schweinsburg et al., 2004b; Spadoni et al., 2008; Squeglia et al., 2009). Recruitment was through mailings sent to homes of students attending local standard public middle schools. Parents were preliminarily screened by phone, then potentially eligible youths and parents were each administered separate diagnostic interviews to confirm eligibility. Assent and consent, as approved by the University of California, San Diego Human Research Protections Program, were obtained from participants and their parents, respectively, each year of participation.
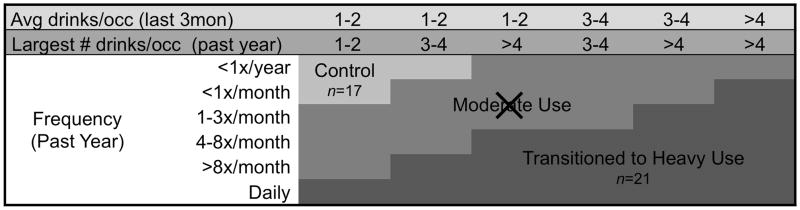
A large number of exclusionary criteria helped rule out potential confounds. At baseline, participants were excluded for: prenatal exposure to substances (i.e., >2 drinks during a given week or any illicit substance use); premature birth (i.e., born prior to 35th gestational week); left handedness; lifetime history of any traumatic brain injury with loss of consciousness >2 minutes, medical problem, neurological abnormality, learning disability, psychiatric disorder, or use of medications affecting the brain or cerebral blood flow (e.g., psychotropic medications); uncorrectable sensory impairment; MRI contradictions; parental history of bipolar I, psychotic disorder, or antisocial personality disorder; and more than 3 lifetime substance use occasions. Eligible adolescents completed interviews, urine toxicology, breathalyzer screens, neuropsychological testing, and neuroimaging at baseline (i.e., prior to the onset of heavy substance use and when age 12–14 years), and thereafter completed quarterly substance use interviews. Self-report data were verified with biannual parent interviews, breathalyzer screening, and urine toxicology. After a mean follow-up of 4.2 years, adolescents were classified as TU (n=21) if they reported heavy alcohol use or CON (n=17) if drinking was infrequent and there were no heavy drinking episodes (see Figure 1 for classification).
Figure 1.
Follow-up alcohol use and classification, based on the distribution of drinking characteristics of adolescent boys and girls observed in the first 2 years of this project (Schweinsburg et al., 2005; Tapert et al., 2004). The current study includes participants from two of the three classifications shown, transitioned into heavy alcohol use (TU; n=21) and controls (CON; n=17).
2.2 Measures
2.2.1 Substance use
The Customary Drinking and Drug Use Record (CDDR) (Brown et al., 1998) assessed lifetime use of alcohol, nicotine, and other drugs at baseline. Two measures assessed substance use at follow-up. The CDDR assessed the quantity and frequency of past year and past 3-month alcohol, tobacco, and other drug use, withdrawal/hangover symptoms, and DSM-IV abuse and dependence criteria. Additionally, the Timeline Followback (TLFB) (Sobell and Sobell, 1992), administered to the youth and corroborated by an informant (e.g., parent), assessed participant’s alcohol and drug use for the past 30 days using temporal cues to facilitate recall. Collectively, CDDR and TLFB substance use information were used to determine group membership at follow-up.
2.2.2 Family background
At baseline, socioeconomic status was calculated using the Hollingshead scale, incorporating level of education and occupation for each parent (Hollingshead, 1965). The Family History Assessment Module (FHAM) (Rice et al., 1995) was administered at baseline to both biological parents and the participant to determine participant’s biological family history of AUD. A composite of youth and parent reports was used to create an index of family history density, calculated per subject; a weight of 0.50 was assigned to each parent with a history of alcohol dependence and 0.25 per grandparent with such history (range 0–2) (Zucker et al., 1994).
2.2.3 Mood
Mood was assessed 30 minutes prior to imaging using the Beck Depression Inventory (Beck, 1978) and the Spielberger State Trait Anxiety Inventory (Spielberger et al., 1970).
2.2.4 Development
On the Pubertal Development Scale, adolescents confidentially indicated current levels of pubertal development at baseline by selecting statements corresponding to their particular stage (e.g., has not begun yet, definitely underway, seems complete) for five sex-specific items (Petersen et al., 1988).
2.2.5 Neurocognition
At baseline, estimated verbal IQ was determined using the Vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), and right-handedness was confirmed with the Edinburgh Handedness Inventory (Oldfield, 1971).
2.2.6 Psychopathology
Adolescents’ externalizing behaviors were assessed using the Achenbach system at baseline via parent report on the Child Behavior Checklist (CBCL) (Achenbach and Rescorla, 2001), and at follow-up via CBCL or, for participants 18 years of age and older, the Adult Self-Report (ASR) (Achenbach and Rescorla, 2003). Conduct disorder problems were assessed by both youth and parent report with the Conduct Disorder Questionnaire, which assesses the number and frequency of DSM-IV criteria for conduct disorder (Brown et al., 1996; Myers et al., 1995).
2.2.7 Go/no-go task
Adolescents performed a go/no-go task (Schweinsburg et al., 2004b; Tapert et al., 2007) during fMRI acquisition. The task consisted of trials presenting either a fixation cross or a blue shape. Participants were asked to press a button when they saw a small circle, large circle, or large square (go stimuli), but to refrain from pressing the button (i.e., inhibit their response) when they saw a small square (the no-go stimulus). Fixation (rest) trials were pseudo randomly interspersed between go and no-go trials (total task time: 6 minutes 24 seconds). The task was administered to all participants prior to scanning to ensure comprehension of task instructions.
2.3 Procedures
At baseline, adolescents completed a 1-hour neuroimaging session in a 1.5 Tesla General Electric Signa LX scanner. Participants viewed task stimuli through a mirror attached to the head coil. Whole brain echo planar imaging was collected for each subject. Specifically, a structural image was collected in the sagittal plane using an inversion recovery prepared T1-weighted three-dimensional spiral fast-spin echo sequence (repetition time = 2,000 ms; echo time = 16 ms; field of view = 240 mm; resolution 0.9375 mm × 0.9375 mm × 1.328 mm; 128 continuous slices; acquisition time 8:36) (Wong et al., 2000). The go/no-go task was administered approximately 20 minutes after starting scanning. Images were collected in the axial plane using T2-weighted spiral gradient recall echo imaging (repetition time = 3,000 ms; echo time = 40 ms; flip angle = 90°; field of view = 240 mm; 20 continuous slices; slice thickness = 7 mm; in-plane resolution = 1.875 mm × 1.875 nm; 128 repetitions; acquisition time 6:24).
2.4 Behavioral Analysis
Between groups analysis of demographic variables were done using independent sample t-tests (e.g., age, SES, pubertal stage) and chi-square (gender). Between groups differences in the go/no-go task were assessed with five independent sample t-tests, using the following dependent measures: go reaction time, percent correct inhibition, percent correct hits, D′ (a measure of accuracy in discriminating between no-go and go stimuli), and β (a measure of response bias) (Green and Swets, 1966).
2.5 Data Processing and Analysis
Imaging data were processed using Analysis of Functional NeuroImages (afni.nimh.nih.gov) (Cox, 1996). First, motion in the time series was corrected by registering each acquisition to a repetition that was found to be minimally deviant (Paulus et al., 2004). Motion correction applied for 3 displacement and 3 rotational parameters were retained for each repetition per participant. Two raters then examined the data for any remaining visible motion within the series, and censored such repetitions from further analysis. Participants with more than 20% of repetitions censored were excluded from analysis (n=1, not described in this paper). On average, 6% of repetitions were removed. Groups did not differ on number of repetitions removed or on any of the 6 motion parameters. For the TU group, the average rotational (roll, pitch, and yaw) and displacement movements (superior, left, posterior) were 0.07°, 0.26°, 0.11°, 0.18 mm, 0.06 mm, and 0.09 mm, respectively. For CON, these were 0.06°, 0.11°, 0.08°, 0.09 mm, 0.04 mm, and 0.05 mm, respectively. No significant differences were found between groups for task-correlated motion, as determined by correlating the task reference function with each of the six motion parameters.
To determine brain regions involved in inhibition and response selection, fMRI data were deconvolved with a reference function specifying the alternating presentation of task conditions (go, no-go, fixation) (Ward, 2002) and convolved with a gamma variate function to model hemodynamic response (Boynton et al., 1996; Cohen, 1997), while controlling for applied motion corrections and linear trends (Bandettini et al., 1993). This produced two fit coefficients per participant, representing BOLD response contrast for inhibition (no-go) trials relative to baseline, and for response selection (go) trials relative to baseline, for each voxel within the brain. Functional data were transformed into standard space (Talairach and Tournoux, 1988), and resampled into 3.0 mm3 voxels along with the application of a spatially smoothing Gaussian filter (full-width half maximum = 5.0 mm). To examine group differences in BOLD response during inhibition and response selection, two independent samples t-tests (AFNI; 3dttest) compared TU and CON groups on BOLD response contrast for inhibition (no-go) trials relative to baseline, and for response selection (go) trials relative to baseline. A combination of t statistic magnitude and cluster volume thresholding was used to control for Type I error (Forman et al., 1995; Ward, 1997) by only interpreting clusters comprised of at least 32 contiguous voxels (≥864 μl) activated at α<.01, for a brain-wise probability of false positive activations of α<.01. For each resulting cluster, BOLD response data was exported to SPSS 16.0 (Chicago, Il) to screen for outliers, examine for normality of distribution, and conduct analyses.
To confirm group comparison results and to examine the effect of group differences in BOLD response, analyses of covariances (ANCOVAs), controlling for baseline number of conduct problems endorsed and baseline alcohol use, were run for each cluster found to differ between the groups (above). For each analysis, the mean cluster activation was entered as the independent variable and follow-up group membership (i.e., TU vs. CON) as the dependent variable. Following analyses within the TU group (n=21) used multiple regression to examine the extent to which inhibition BOLD response contrast in each cluster that differed between groups might predict intensity of follow-up substance use (follow-up peak drinks on an occasion, average drinks per month, abuse and dependence symptoms, cannabis use, and other substance use) as well as other behavioral characteristics (i.e., attention problems, externalizing behaviors) at follow-up.
3. Results
3.1 Demographic and Substance Use History
At baseline, groups were statistically equivalent on age, gender, socioeconomic status, verbal intellect, mood, and pubertal development. All adolescents (N=38) had minimal to no history of any alcohol or drug use, with a range of 0 to 3 lifetime substance use occasions, with groups being equivalent on cannabis use. The maximum substance use prior to the baseline assessment was a 14 year old male who reported a total of 3 lifetime drinking episodes, one of which involved consuming 4 standard alcoholic beverages, which occurred >3 months prior to his scan date. All subjects denied any tobacco, alcohol, cannabis, or other substance use in the 7 days prior to their scan, which was verified by breathalyzer, urine toxicology, and parent report. Although no participant met diagnostic criteria for conduct disorder at baseline, TU and CON groups differed on the number of lifetime conduct problem behaviors endorsed (M = 3.0 v. 1.2, range = 0–7, p<.01) and lifetime alcohol use episodes (M = 0.6 v 0.0, range = 0–3, p<.05) (see Table 1) at project entry.
Table 1.
Baseline and follow-up characteristics of adolescents who transitioned into substance use and those who did not.
| Healthy Controls | Transitioned to Use | Effect Size Cohen’s d | ||
|---|---|---|---|---|
|
M (SD) n=17 |
M (SD) n=21 |
|||
| BASELINE | Age at baseline | 13.4 (0.7) | 13.9 (0.9) | 0.62 |
| % Female | 52% | 48% | ||
| Hollingshead SES | 18.1 (7.4) | 21.6 (12.7) | 0.34 | |
| Vocabulary T-score | 59.1 (5.8) | 54.9 (7.4) | 0.63 | |
| Familial alcoholism density | 0.2 (0.3) | 0.4 (0.4) | 0.57 | |
| Conduct disorder problem count ** | 1.2 (1.2) | 3.0 (2.6) | 0.89 | |
| Achenbach attention T-score | 51.7 (2.7) | 51.2 (1.9) | 0.21 | |
| Achenbach externalizing T-score | 45.1 (9.0) | 45.3 (7.9) | 0.02 | |
| Beck Depression Inventory total | 2.2 (2.6) | 2.9 (3.1) | 0.25 | |
| Females’ pubertal stage | 3.9 (0.8) | 3.4 (0.5) | 0.75 | |
| Males’ pubertal stage | 3.0 (0.8) | 2.6 (0.7) | 0.53 | |
| Lifetime alcohol use episodes * | 0.0 (0.0) | 0.6 (1.1) | 0.77 | |
| Lifetime cannabis use episodes | 0.0 (0.0) | 0.2 (0.5) | 0.57 | |
| % Correct inhibition (no-go) | 78.7 (0.1) | 79.8 (0.1)a | ||
| % Correct hits (go) | 97.3 (0.3) | 98.4 (0.3)a | ||
| % False alarms | 6.0 (1.5) | 5.9 (1.3)a | ||
| Go reaction time (ms) | 484.6 (57.3) | 475.6 (55.7)a | 0.16 | |
| D-prime (D′) | 2.9 (0.7) | 3.1 (0.7)a | 0.29 | |
| Response bias (β) | 0.2 (0.1) | 0.2 (0.1)a | 0.00 | |
|
| ||||
| FOLLOW-UP | Age at follow-up | 17.7 (1.3) | 18.0 (1.3) | 0.23 |
| Years between baseline and follow-up | 4.4 (0.9) | 3.9 (1.1) | 0.50 | |
| Achenbach Attention T-score | 51.5 (2.8) | 53.4 (3.8) | 0.57 | |
| Achenbach Externalizing T-score** | 40.0 (8.0) | 48.8 (9.4) | 1.01 | |
| Past year drinking days ** | 0.8 (1.6) | 53.3 (43.2) | 1.72 | |
| Lifetime peak drinks/episode ** | 0.5 (0.9) | 10.0 (4.3) | 3.06 | |
| Average drinks per month ** | 0.6 (0.2) | 10.9 (12.3) | 1.18 | |
| Lifetime cannabis use episodes * | 0.2 (0.8) | 114.0 (208.9) | 0.77 | |
| % Any past year cannabis use** | 0% | 81% | ||
| Lifetime other drug use episodes * | 0.0 (0.0) | 3.0 (6.2) | 0.68 | |
| % Any past year other drug use* | 0% | 29% | ||
| Past month substance use days ** | 0.0 (0.0) | 4.6 (5.9) | 1.10 | |
| % Any abuse or dependence criteria met | 0% | 71% | ||
| % Any past year tobacco use ** | 6% | 48% | ||
p<.05
p<.01
TU (n=20)
At follow-up, most TU adolescents reported other drug use as well (lifetime substance use episodes M = 64.6 ± 167.1, range = 0–820). In the TU group, 71% endorsed one or more DSM-IV substance abuse or dependence criteria, with 4 subjects meeting criteria for alcohol dependence, 3 for cannabis abuse, and 1 for cannabis dependence. In contrast, the healthy controls at follow-up reported minimal to no alcohol use, no other substance use, and no abuse or dependence symptomatology (see Table 1).
3.2 Task Performance
Performance data logging failed for one participant. There were no (p<.05) differences between groups on task performance (percent correct), reaction time, false alarms, D′, or β (see Table 1).
3.3 Imaging Findings
An independent samples t-test revealed 12 clusters where, at baseline, TU adolescents showed significantly less (p<.01) inhibition (no-go trial) BOLD response contrast than CON teens (see Table 2 and Figure 2). There were no clusters in which TU showed significantly more inhibition BOLD response contrast than CON. ANCOVAs controlling for baseline conduct problems and baseline alcohol use replicated these findings (ps<.01). In one of the 12 clusters with significantly different activation between groups, one participant’s BOLD response was deemed an outlier and the subject was excluded from analyses involving signal in that cluster. Multiple regressions examined inhibition BOLD response contrast for each of the 12 clusters as a predictor of follow-up substance use frequency, quantity, and consequences; results were not significant (Type I error controlled with α = .05/12 = .004). However, less baseline inhibition BOLD response contrast in 4 of the 12 clusters was linked to greater follow-up Achenbach Attention Problems above and beyond baseline Attention Problems, follow-up depression, anxiety, age, gender, or ethnicity. This relationship was seen in right inferior frontal gyrus (F1, 30= 5.32, p= .03; β = −0.39, p= .03; R2Δ =15%), left cingulate (F1, 30= 6.29, p= .02; β = −0.42, p= .02; R2Δ =17%), right middle temporal gyrus (F1, 30= 4.27, p= .048; β = −0.35, p= .048; R2Δ =13%), and left inferior parietal lobule (F1, 30= 6.33, p= .02, β = −0.42, p= .02; R2Δ =17%). While a zero-order relationship was also seen between less no-go trial BOLD response in 5 of the 12 regions and greater follow-up Achenbach Externalizing Problems, these relationships were fully accounted for by baseline Achenbach Externalizing Problems.
Table 2.
Inhibition trials (no-go) results: clusters showing significantly different BOLD response at baseline between adolescents who transitioned into substance use (TU; n=21) versus those who remained continuously non-users (CON; n=17) (each cluster was ≥ 32 contiguous voxels, ≥864 μl, with each voxel differing at α < .01).
| Anatomical region | Brodman n Area | Volume (μl) | Talairach Coordinatesa | Effect Size Cohen’s d | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| TU < CONb | ||||||
| L dorsolateral prefrontal cortex | 10,46 | 1755 | 31.5 | −52.5 | 14.5 | 1.47 |
| L superior and middle frontal gyrus | 8,9 | 4995 | 1.5 | −49.5 | 38.5 | 1.33 |
| R inferior frontal gyrus | 45 | 891 | −49.5 | −22.5 | 2.5 | 1.46 |
| B medial frontal gyrus | 10 | 891 | −1.5 | −64.5 | 11.5 | 1.55 |
| B paracentr lobules, cingulate gyrus | 6,31 | 8235 | −1.5 | 43.5 | 59.5 | 1.19 |
| L cingulate | 30,19,29 | 3186 | −1.5 | 58.5 | 5.5 | 2.39 |
| L putamen | - | 1026 | 19.5 | 13.5 | −3.5 | 1.55 |
| L middle temporal gyrus | 21 | 1431 | 55.5 | 49.5 | −3.5 | 1.17 |
| R middle temporal gyrus | 39,19,22 | 1593 | −52.5 | 55.5 | 11.5 | 1.40 |
| L inferior parietal lobule | 40 | 1701 | 46.5 | 37.5 | 53.5 | 1.40 |
| R inferior parietal lobule | 40 | 1377 | −49.5 | 43.5 | 44.5 | 2.66 |
| Pons | - | 1431 | −1.5 | 22.5 | 27.5 | 1.34 |
R right; L left; B bilateral
Coordinates refer to the location of the peak group difference within the cluster
In no region did TU show more no-go response than CON.
Figure 2.
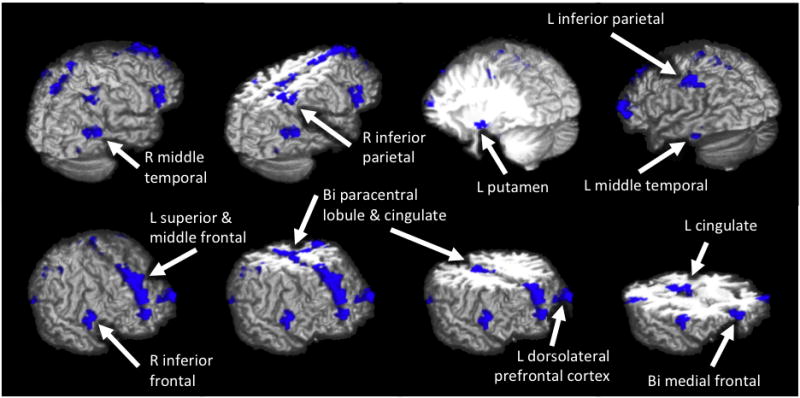
Differences in BOLD response during no-go trials at baseline between adolescents who transitioned into heavy alcohol use (TU; n=21) versus controls (CON; n=17). Areas in blue indicate where future users had significantly less BOLD response during inhibition (no-go) trials relative to baseline than non-users (p<.01, clusters >864 μl). In no region did TU show more no-go response than CON.
For response selection (go) trials, the independent samples t-test at voxelwise α<.01 revealed no differences in BOLD response between groups. In secondary analyses using a more liberal threshold (α<.025), TU showed significantly greater BOLD response than CON during go trials in 4 clusters (see Table 3). There were no clusters during response selection (go) trials where TU showed less BOLD response than CON.
Table 3.
Response selection (go) results: Clusters showing significantly different BOLD response at baseline between adolescents who transitioned into substance use (TU; n=21) versus those who remained continuously non-users (CON; n=17) (each cluster was ≥ 32 contiguous voxels, ≥ 864 μl, with each voxel differing at α < .025)a.
| Anatomical region | Brodman n Area | Volume (μl) | Talairach Coordinates b | Effect Size Cohen’s d | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| CON > TUc | ||||||
| R superior and middle frontal gyrus | 10 | 1890 | −31.5 | −52.5 | 20.5 | 1.46 |
| L cingulate gyrus | 24 | 1134 | 1.5 | −1.5 | 35.5 | 1.49 |
| R precuneus, superior parietal lobule | 7 | 891 | −25.5 | 64.5 | 47.5 | 1.32 |
| R precuneus | 7 | 864 | −34.5 | 70.5 | 35.5 | 1.28 |
R right; L left
At the significance threshold used in Table 2, none of these clusters remained significant.
Coordinates refer to peak group difference.
In no region did TU show more go response than CON.
4. Discussion
This study tested the hypothesis that future alcohol-using adolescents show altered neural activation during inhibitory processing. Consistent with this hypothesis, we found that at baseline, future alcohol-using adolescents showed less activation during inhibitory trials of a go/no-go task, as compared to their matched, healthy control peers. This attenuated activation during inhibition was observed in left dorsolateral prefrontal, left supplementary motor, right inferior frontal and medial frontal, bilateral motor, left cingulate, and left putamen, as well as bilateral middle temporal and inferior parietal regions. Thus, TU adolescents showed less activation than would be anticipated in the areas associated with inhibition and substance use vulnerability (i.e., right inferior frontal, right parietal, and left cingulate) (McNamee et al., 2008). The overall pattern of no-go activation was similar to that reported in other child/adolescent inhibition studies examining the stop signal task (i.e., inferior and medial frontal regions) (Rubia et al., 2000), go/no-go tasks (i.e., frontal, parietal, and cingulate regions) (Casey et al., 1997; Stevens et al., 2007), and the Stroop task (i.e., frontal, parietal, and cingulate regions) (Adleman et al., 2002).
These findings support previous studies of adolescents at risk for substance use disorders (SUD) showing decreased activation patterns despite similar task performance. In response to the same go/no-go paradigm used in this study, youth at familial risk for SUDs showed decreased activation in 4 of the same brain regions: left medial/superior frontal, bilateral medial frontal, right inferior frontal, and bilateral inferior parietal (Schweinsburg et al., 2004a). Similarly, during an anti-saccade task, youth with lower levels of neurobehavioral disinhibition, an indicator of increased SUD risk, also showed less overall frontal activation (McNamee et al., 2008; Schweinsburg et al., 2004b).
While task performance did not differ between TU and CON in the current study, it is possible that in a more challenging task, behavioral differences might accompany the aberrant inferior parietal activation seen in the TU group. Specifically, TU adolescents showed less activation than CON in bilateral inferior parietal regions, an area Bunge and colleagues (2002) found positively correlated with task performance during an inhibition task in both children and adults (Bunge et al., 2002; Schweinsburg et al., 2004b). For our study, it appears that adolescents at risk for substance use escalation are able to perform well behaviorally despite attenuated activation, suggesting efficient prefrontal mediated control.
This pattern of limited neural activity during inhibitory demands was linked to greater, albeit clinically within the normal range, level of externalizing problems, which in turn was linked to greater follow-up externalizing problems that often included substance use. Perhaps more surprisingly, attenuated inhibitory response at baseline significantly predicted follow-up attention problems measured from parent or self-report. This suggests that limited neural engagement during inhibitory demands in early adolescence may indicate a neurodevelopmental trajectory linked to reduced cognitive control in later adolescence. Together, these studies suggest that less responsive frontal cortices during inhibitory demands may underlie a general risk for behaviors that include substance use.
Lending further support to this hypothesis is a study of substance use-related risk in adults where some regions of decreased neural activity found here showed reduced activation during a decision-making task in abstinent amphetamine dependent adults who subsequently went on to relapse. Paulus and colleagues (2005), found that among adults with amphetamine dependence scanned at the end of inpatient treatment hospitalization, those with less activation during decision-making in the right inferior frontal gyrus, right inferior parietal lobule, right middle temporal gyrus, right posterior cingulate, and cingulate gyrus, among other areas, were more likely to relapse in the year after treatment than those who showed more activation in these areas (Paulus et al., 2005). Thus, appropriate recruitment of these areas across task demands may indicate a degree of cognitive effort that is linked to behavioral control and impulse resistance. Less robust neuronal activation may be sufficient to support the cognitive demands of this task, leading to the lack of behavioral response differences reported here. However, this limited degree of activation may be inadequate to sustain more complex real-life inhibitory events. For example, the inhibitory system of future substance using adolescents and relapse prone substance dependent adults may be more vulnerable to environmental perturbations, such as the presence of alcohol or drug cues.
Alternatively, reduced activation may indicate a delayed or stunted maturation of inhibitory networks in future substance users. Developmental studies of inhibition have reported increased activation in frontal and parietal regions (Adleman et al., 2002; Bunge et al., 2002; Rubia et al., 2000) and increased connectivity within fronto-parietal networks (Stevens et al., 2007) with increasing age. Less activation may be an indicator that the brain has yet to specify or shift inhibitory function to specialized regions or networks. In further support of this hypothesis are other developmental studies suggesting age-related shifts in regions of activation, finding increased anterior cingulate activation (Adleman et al., 2002), and decreased activation of the caudate (Rubia et al., 2000) from childhood to adulthood.
This study had several limitations. While future users and non-users were equivalent on many demographic variables (i.e., age, gender, socioeconomic status, verbal intellect, mood, stage in pubertal development, and lifetime marijuana use) they differed on the number of conduct disorder problems and lifetime alcohol uses endorsed at baseline, prior to the initiation of regular or heavy substance use. Still, while baseline substance use differed between groups, it was very minimal (M = 0.3 ± 0.9, range = 0–3 lifetime instances), with the majority (N=32 out of 38) never having drunk or used substances at all. Although differences in BOLD response between groups remained after controlling for these factors, the observed activation patterns could be representative of overall greater behavioral problems not specifically related to substance use. Continued longitudinal study of the current sample will help clarify the predictive effect of BOLD response during inhibition on substance use and other life outcomes.
The current findings suggest the potential utility of fMRI in identifying the neural mechanisms underlying the risk factors (e.g., disinhibition) contributing to adolescent substance use. Less neural recruitment of frontal, parietal, temporal, and subcortical areas during inhibition demands in early adolescence may be disadvantageous, and may play a role in adolescents’ abilities to execute more complex decisions and control real-world impulses to engage in risky behavior. Future prospective studies will help clarify whether aberrant activation itself is the risk factor for substance use initiation and disorders, or whether less frontal activation contributes to substance use through other mediating risk factors (e.g., conduct disorder, family history, or maturational pacing). A deeper understanding of the relationship between the networks underlying inhibition and substance use outcomes may eventually lead to improved prevention programs.
Acknowledgments
Role of Funding Source
Research supported by NIAAA: R01 AA13419 (PI: Tapert) & R21 AA019748 (PI: Pulido). The NIAAA had no further role in study design; in the collection; analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Portions of this study were presented at the second International Conference on Applications of Neuroimaging to Alcoholism, New Haven, CT, January 2008. The authors would like to express gratitude to Dr. Sandra A. Brown, Dr. MJ Meloy, Valerie Barlett, Lisa Caldwell, Sonja Eberson, Amanda Gorlick, Alejandra Infante, Jesse Feng, and Sonia Lentz for assistance with subject recruitment and data management, and the participating families.
Footnotes
Contributors
Authors ALN, CP, LMS, ADS, MPP and SFT were responsible for study concept, design, and final protocol. Authors ALN and CP managed the literature searches and summaries of previous work. Authors ALN, CP, LMS, and ADS contributed to the acquisition and analysis of the imaging and behavioral data and undertook the statistical analysis. Author ALN wrote the first draft of the manuscript. All authors, ALN, CP, LMS, ADS, MPP and SFT have contributed to and have approved the final manuscript.
Conflict of Interest
The authors have reported no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont; Burlington: 2001. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms and Profiles. University of Vermont; Burlington: 2003. [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Schweinsburg A, Paulus MP, Brown SA, Tapert S. Examining personality and alcohol expectancies using functional magnetic resonance imaging (fMRI) with adolescents. J Stud Alcohol. 2005;66:323–331. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time- course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bava S, Boucquey V, Goldenberg D, Thayer RE, Ward M, Jacobus J, Tapert SF. Sex differences in adolescent white matter architecture. Brain Res. 2011;1375:41–48. doi: 10.1016/j.brainres.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) Psychological Corp; San Antonio, TX: 1978. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR. Psychiatric disorder and substance use in adolescence. Can J Psychiatry. 1991;36:699–705. [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Whiteman MM, Finch S. Childhood aggression, adolescent delinquency, and drug use: a longitudinal study. J Genet Psychol. 1992;153:369–383. doi: 10.1080/00221325.1992.10753733. [DOI] [PubMed] [Google Scholar]
- Brown SA, Gleghorn A, Schuckit MA, Myers MG, Mott MA. Conduct disorder among adolescent alcohol and drug abusers. J Stud Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Clapper RL, Buka SL, Goldfield EC, Lipsitt LP, Tsuang MT. Adolescent problem behaviors as predictors of adult alcohol diagnoses. Int J Addict. 1995;30:507–523. doi: 10.3109/10826089509048741. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Reich T, Bohman M. Inheritance of risk to develop alcoholism. NIDA Res Monogr. 1986;66:86–96. [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davies SJC, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, Marshall EJ, Boddington S, Lingford-Hughes A. Is there cognitive impairment in clinically ‘healthy’ abstinent alcohol dependence? Alcohol Alcohol. 2005;40:498–503. doi: 10.1093/alcalc/agh203. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget’s AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Downing J, Bellis MA. Early pubertal onset and its relationship with sexual risk taking, substance use and anti-social behaviour: a preliminary cross-sectional study. BMC Public Health. 2009;9:446. doi: 10.1186/1471-2458-9-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Luckenbaugh DA, Moolchan ET, Leff MK, Allen R, Eshel N, London ED, Kimes A. Behavioral predictors of substance-use initiation in adolescents with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Weber H. Effects of pre-cues on voluntary and reflexive saccade generation I. Anti-cues for pro-saccades. Exp Brain Res. 1998;120:403–416. doi: 10.1007/s002210050414. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Hatta T, Fukushima K. Development of voluntary control of saccadic eye movements - I. Age-related changes in normal children. Brain Dev. 2000;22:173–180. doi: 10.1016/s0387-7604(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JM. Signal Detection Theory and Psychophysics. John Wiley and Sons Inc; New York: 1966. [Google Scholar]
- Greenberg LM, Waldman ID. Developmental normative data on the test of variables of attention (T.O.V.A.) J Child Psychol Psychiatry. 1993;34:1019–1030. doi: 10.1111/j.1469-7610.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. Am J Drug Alcohol Abuse. 2010;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B, Feehan M, McGee R, Stanton W, Moffitt TE, Silva P. The importance of conduct problems and depressive symptoms in predicting adolescent substance use. J Abnorm Child Psychol. 1993;21:469–480. doi: 10.1007/BF00916314. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. The Monitoring the Future National Survey Results on Adolescent Drug Use: Overview of Key Findings, 2009. National Institute on Drug Abuse; Bethesda, MD: 2009. [Google Scholar]
- Jonkman LM. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood: a Go/Nogo ERP study. Brain Res. 2006;1097:181–193. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Killen JD, Hayward C, Wilson DM, Haydel KF, Robinson TN, Taylor CB, Hammer LD, Varady A. Predicting onset of drinking in a community sample of adolescents: the role of expectancy and temperament. Addict Behav. 1996;21:473–480. doi: 10.1016/0306-4603(95)00077-1. [DOI] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Reynolds M, Vanyukov M. Individual differences in childhood neurobehavior disinhibition predict decision to desist substance use during adolescence and substance use disorder in young adulthood: a prospective study. Addict Behav. 2006;31:686–696. doi: 10.1016/j.addbeh.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Chan G, Kramer JR, Bierut L, Bucholz KK, Fox L, Hesselbrock V, Numberger JI, Jr, Reich T, Reich W, Schuckit MA. Relationship of age of first drink to child behavioral problems and family psychopathology. Alcohol Clin Exp Res. 2005;29:1869–1876. doi: 10.1097/01.alc.0000183190.32692.c7. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–1173. [PubMed] [Google Scholar]
- McNamee RL, Dunfee KL, Luna B, Clark DB, Eddy WF, Tarter RE. Brain activation, response inhibition, and increased risk for substance use disorder. Alcohol Clin Exp Res. 2008;32:405–413. doi: 10.1111/j.1530-0277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Myers MG, Brown SA, Mott MA. Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcohol Clin Exp Res. 1995;19:1528–1536. doi: 10.1111/j.1530-0277.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Noel X, Bechara A, Dan B, Hanak C, Verbanck P. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21:778–786. doi: 10.1037/0894-4105.21.6.778. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Tapert SF, Liu TT. Trend detection via temporal difference model predicts inferior prefrontal cortex activation during acquisition of advantageous action selection. Neuroimage. 2004;21:733–743. doi: 10.1016/j.neuroimage.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards MH, Boxer AM. A self report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pulido C, Anderson KG, Armstead AG, Brown SA, Tapert SF. Family history of alcohol-use disorders and spatial working memory: effects on adolescent alcohol expectancies. J Stud Alcohol Drugs. 2009;70:87–91. doi: 10.15288/jsad.2009.70.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Band GPH, Logan GD. A study of adaptive behavior: effects of age and irrelevant information on the ability to inhibit one’s actions. Acta Psychologica. 1999;101:315–337. [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Genetics and the risk for alcoholism. JAMA. 1985;254:2614–2617. [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Barlett VC, Killeen LA, Caldwell LC, Pulido CP, Brown SA, Paulus MP, Tapert SF. fMRI of Response Inhibition Across Adolescent Development. Annual Meeting of the International Neuropsychological Society; Baltimore, MD. 2004a. [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann NY Acad Sci. 2004b;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons-Morton B. Prospective association of peer influence, school engagement, drinking expectancies, and parent expectations with drinking initiation among sixth graders. Addict Behav. 2004;29:299–309. doi: 10.1016/j.addbeh.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press, Inc; Totowa, NJ, US: 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Barrera M, Jr, Chassin L. Prospective differential prediction of adolescent alcohol use and problem use: examining the mechanisms of effect. J Abnorm Psychology. 1998;107:616–628. doi: 10.1037//0021-843x.107.4.616. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231 – 1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP, Bourque TA, Anderson SH, Brehaut JC. Mechanisms of attention: a developmental study. J Exp Child Psychol. 1989;48:353–378. doi: 10.1016/0022-0965(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for FMRI Data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 1997. [Google Scholar]
- Ward BD. Deconvolution Analysis of FMRI Time Series Data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 2002. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank LR. Single slab high resolution 3D whole brain imaging using spiral FSE. Proc Intl Soc Magn Reson Med. 2000;8:683. [Google Scholar]
- Zucker RA, Donovan JE, Masten AS, Mattson ME, Moss HB. Early developmental processes and the continuity of risk for underage drinking and problem drinking. Pediatrics. 2008;121(Suppl 4):S252–272. doi: 10.1542/peds.2007-2243B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms: I. Biopyschosocial variation among pathways into symptomatic difficulty. In: Babor TF, Hesselbrock V, Meyer RE, Shoemaker W, editors. Types of Alcoholics: Evidence from Clinical, Experimental and Genetic Research. The New York Academy of Sciences; New York: 1994. pp. 134–146. [DOI] [PubMed] [Google Scholar]