Abstract
Despite antiretroviral therapy (ART), HIV infection promotes cognitive dysfunction and neurodegeneration through persistent inflammation and neurotoxin release from infected and/or activated macrophages/microglia. Furthermore, inflammation and immune activation within both the central nervous system (CNS) and periphery correlate with disease progression and morbidity in ART-treated individuals. Accordingly, drugs targeting these pathological processes in the CNS and systemic compartments are needed for effective, adjunctive therapy. Using our in vitro model of HIV-mediated neurotoxicity, in which HIV infected monocyte-derived macrophages (MDM) release excitatory neurotoxins, we show that HIV infection dysregulates the macrophage antioxidant response and reduces levels of heme oxygenase-1 (HO-1). Furthermore, restoration of HO-1 expression in HIV-infected MDM reduces neurotoxin release without altering HIV replication. Given these novel observations, we have identified dimethyl fumarate (DMF), used to treat psoriasis and showing promising results in clinical trials for multiple sclerosis, as a potential neuroprotectant and HIV disease-modifying agent. DMF, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and neurotoxin release. Two distinct mechanisms are proposed; inhibition of NF-κB nuclear translocation and signaling, which could contribute to the suppression of HIV replication, and induction of HO-1, which is associated with decreased neurotoxin release. Finally, we found that DMF attenuates CCL2-induced monocyte chemotaxis, suggesting that DMF could decrease recruitment of activated monocytes to the CNS in response to inflammatory mediators. We propose that dysregulation of the antioxidant response during HIV infection drives macrophage-mediated neurotoxicity and that DMF could serve as an adjunctive neuroprotectant and HIV disease modifier in ART-treated individuals.
Introduction
HIV-1 infection of the central nervous system (CNS) can result in cognitive, motor, and behavioral abnormalities, collectively known as HIV-associated neurocognitive disorders (HAND) (1, 2). Early in the course of infection, HIV traffics into the brain via infected monocytes and lymphocytes (3) and despite antiretroviral therapy (ART) persists in parenchymal microglia and perivascular macrophages (4-6). HIV infection of the CNS results in the immune activation of resident glia, and because HIV cannot infect neurons, neuronal damage is mediated by neurotoxins released by these infected and/or activated macrophages, microglia and astrocytes. Although the severity of HAND has been significantly reduced through the widespread use of ART, the prevalence and associated morbidity remain high (~50%) (7, 8). The persistence of HAND in individuals effectively controlled for systemic viral load is incompletely explained, although recent evidence suggests that prolonged inflammation in both the CNS and periphery may be responsible (9-11).
Chronic systemic inflammation is tightly linked to morbidity and mortality in ART-treated patients, which suggests that adjunctive anti-inflammatories or immune modulators may improve clinical outcomes. Despite undetectable serum viral loads, measures of systemic inflammation correlate to cerebral spinal fluid (CSF) immune activation, CNS inflammation and HAND (9-11). It has been proposed that elevated peripheral inflammation mediates neurocognitive decline by increasing the transendothelial migration of infected and/or activated monocytes into the brain (10, 12). An increased number of microglia and macrophages in the CNS correlates with the severity of pre-mortem HAND, demonstrating the importance of these cell types in mediating neurological impairment (4, 13, 14). Some of the most striking evidence linking peripheral inflammation to HAND derives from the strong association between early and persistent damage caused to gut-associated lymphoid tissue (GALT) by HIV infection (or SIV infection in macaques), increased microbial translocation, systemic immune/monocyte activation and HAND progression (9, 10, 15, 16). Therefore, reducing inflammation in the periphery as well as within the CNS is expected to improve neurocognitive impairment in HIV-infected patients.
Fumaric acid esters (FAEs), including dimethyl fumarate (DMF) and its primary in vivo metabolite monomethyl fumarate (MMF), are a class of compounds that have anti-inflammatory and immune modulating effects in vitro and in vivo. Fumaderm, a formulation of DMF and other FAEs, has been used in Europe since 1995 as an effective treatment for psoriasis; its mechanism of action is attributed to modulation of T cell activation and infiltration into plaques (17). DMF is currently under investigation for use in multiple sclerosis (MS) and a recently completed Phase III study demonstrated a significant benefit in suppressing relapses, disease progression and brain lesion inflammation (18). Using the rodent model of MS, experimental allergic encephalomyelitis (EAE), it was shown that DMF reduces the recruitment of monocytes into areas of active demyelination in the brain (19). In in vitro model systems, DMF has been shown to inhibit pro-inflammatory cytokine production and NF-κB signaling via inhibition of nuclear translocation (19-22). Furthermore, DMF induces the expression of Nrf2-driven antioxidant response genes, including heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase 1 (NQO1) (23, 24). Notably, induction of HO-1 expression in human monocytes by hemin has been associated with suppression of HIV-1 replication (25).
Because HIV replication can be strongly driven by NF-κB activation and nuclear translocation, we hypothesized that DMF treatment of HIV-infected monocyte-derived macrophages (HIV/MDM) would result in attenuation of HIV replication, immune activation and neurotoxin production. Our in vitro system models macrophage-mediated neurotoxicity during HIV infection by utilizing human MDM and rat cerebrocortical neuronal cultures. In this system, HIV infection of MDM results in the release of low molecular weight excitotoxins that injure neurons through excessive activation of N-methyl-D-aspartate (NMDA) receptors (26-28). In this study we demonstrate that DMF attenuates HIV replication, nuclear translocation of NF-κB subunits and TNFα production in human MDM. Furthermore, supernatants from DMF and MMF-treated HIV/MDM cultures are markedly less neurotoxic to primary neurons than those from non-treated HIV/MDM cultures. Suppression of neurotoxin production is mediated by induction of HO-1 in HIV/MDM, and this suppression of neurotoxin production can occur even without suppression of HIV replication. Finally, DMF and MMF also reduce CCL2-induced chemotaxis in human monocytes. This study demonstrates that DMF inhibits key steps in HAND pathogenesis through distinct effects on HIV replication and macrophage-mediated neurotoxin production and DMF should be considered as an adjunctive therapeutic for ameliorating the neurological complications of HIV infection.
Materials and Methods
Reagents
Stock solutions of dimethyl fumarate and monomethyl fumarate (Sigma, St. Louis, MO) were prepared in DMSO and stored at −20°C until use. Tin (IV) mesoporphyrin IX dichloride (SnMP) and cobalt (III) protoporphyrin IX chloride (CoPP; Frontier Scientific, Logan, UT) were prepared in 1N NaOH and stored at −20°C until use. Stock solutions of Ara-C (Sigma), phytohemagglutinin (PHA; Sigma), TNFα (R&D Systems, Minneapolis, MN) and CCL2 (Peprotech, Rocky Hill, NJ) were prepared in filter-sterilized distilled water and stored at −20°C. Stock solutions of efavirenz (NIH AIDS Research and Reference Reagent Program, Germantown, MD) were prepared in DMSO and frozen at −80°C until use.
Isolation and culture of human monocyte-derived macrophages (MDM)
All human studies were reviewed and approved by the Institutional Review Board at the University of Pennsylvania. Human monocytes were prepared from PBMCs of healthy donors and isolated by Ficoll density gradient centrifugation as previously described (26, 29). Monocytes were plated at 1×106 cells per well to Cell-Bind 6-well plates (Corning, Lowell, MA) and cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 10% horse serum, 1% non-essential amino acids with 50 U/mL penicillin/streptomycin at 37°C, 6% CO2. Cells were cultured for 7-8 days and visually inspected for MDM differentiation before use in HIV-infection experiments. MDM were cultured for 7-10 days before use in non-infectious experiments.
HIV infection of MDM
Prior to infection, MDM were treated with efavirenz (5nM or 20nM), MMF and/or DMF (1-100μM), as indicated, for 1 hour. All wells were normalized for the vehicles appropriate for drug treatments (DMSO and/or NaOH). Differentiated MDM were exposed to 50ng (p24 ELISA, equivalent to 1.82 ± 0.22 kcpm/μL by reverse transcriptase (RT) activity assay) of HIV-1 Jago (R5 strain) or 89.6 (R5/X4 strain) for 24 hours. HIV-Jago is a macrophage tropic, CSF isolate from a patient with confirmed HIV-associated dementia (29). Virus stocks were prepared by the University of Pennsylvania Center for AIDS Research Virology Core. Supernatants from HIV-infected or non-infected (Mock) MDM were collected every 2-4 days and stored at −80°C. Supernatants were monitored for HIV replication by quantifying viral RT activity, as analyzed by the amount of radiolabeled deoxythymidine incorporation.
Subcellular fractionations and Western blot analysis
For whole cell lysate collection, cells were rinsed twice with ice-cold PBS and lysed in 75mM Tris-HCl (pH 6.8), 15% glycerol, 3.75mM EDTA, 3% SDS and supplemented with Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN) and PhosSTOP phosphatase inhibitor cocktail (Roche Applied Science).
To assess for nuclear translocation of NF-κB proteins, differentiated MDM were treated with DMF for 24 hours, exposed to TNFα (1 ng/mL) for 10 minutes and fractionated. To prepare nuclear extracts, cells were rinsed twice in ice-cold PBS and lysed on ice for 10 minutes in 10mM HEPES (pH 7.9), 10mM KCl, 10mM EDTA, 1mM DTT, 0.4% Nonidet P-40, supplemented with protease and phosphatase inhibitors. Nuclei were pelleted for 3 minutes at 16,000 × g and the supernatant (cytoplasmic fraction) was collected and stored at −20°C. The nuclear pellet was resuspended in 20mM HEPES (pH 7.9), 400mM NaCl, 1mM EDTA, 10% glycerol, 1mM DTT, protease and phosphatase inhibitors and incubated at 4°C on a rocking platform at 200rpm for 2 hours. After centrifugation at 16,000 × g for 5 minutes, supernatants (nuclear fractions) were collected and stored at −20°C. All protein concentrations were determined by the Detergent Compatible (DC) protein assay (Bio-Rad Laboratories, Hercules, CA).
Cell lysates were subjected to SDS-PAGE as previously described (26) using the following antibodies: rabbit anti-HO-1 (Stressgen/Enzo Life Sciences, Farmingdale, NY), mouse anti-NQO1 (Abcam, Cambridge, MA), mouse anti-Nrf2 (R&D Systems), rabbit anti-RelB (Cell Signaling Technologies, Danvers, MA), rabbit anti-NF-κB p65 (Cell Signaling), rabbit anti-NF-κB p50 (Cell Signaling), rabbit anti-poly (ADP-ribose) polymerase (PARP) (Cell Signaling), mouse anti-GAPDH (Advanced Immunochemical, Long Beach, CA), and species-specific HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA or Cell Signaling). For densitometry analysis, films were scanned and a fixed cursor area centered over each band was assessed for pixel density using ImageJ (NIH, Bethesda, MD).
MDM-mediated neurotoxicity
Rat cerebrocortical neuronal cultures were prepared from embryos of Sprague-Dawley rats at day 17 of gestation, as previously described (26). All procedures were within the ARRIVE guidelines for animal research, and in accordance with protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Cells were plated in tissue culture dishes pre-coated with poly-L-lysine (Peptides International, Louisville, KY) and maintained in neurobasal media plus B27 supplement (Invitrogen, Carlsbad, CA) at 37°C and 5% CO2. Forty-eight hours after plating, cells were treated with 10μM Ara-C. After 7 days in vitro (DIV), approximately one-half volume of fresh media was added to the cells in order to counteract effects of evaporation. All cultures were used between 14 and 16 DIV.
Cell-based microtubule-associated protein 2 (MAP2) ELISAs were performed on primary rat cerebrocortical cells plated at a density of 6×104 cells per well in 96-well plates. Following a 24 hour exposure to HIV/MDM supernatant, cultures were fixed and fluorescently labeled as described (30, 31) using the following reagents: mouse anti-MAP2 (Covance, Princeton, NJ), goat anti-mouse β-lactamase TEM-1 conjugate (Invitrogen), and Fluorocillin Green substrate (Invitrogen). Fluorescence intensity was measured using a fluorometric plate reader with the 480/520 nm filter set. Macrophage supernatant was applied at a 1:10-1:50 dilution; the dilution that gave values within the linear range of the assay is presented.
Immunofluorescence
Primary rat cerebrocortical cells were plated at a density of 2×105 cells per 35mm dish with glass coverslips. Following exposure to HIV/MDM supernatant for 24 hours, cultures were fixed and fluorescently labeled as described (26) using the following reagents: mouse anti-MAP2 (Sigma) and species-specific Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories), and Hoescht 33342 (Invitrogen).
LDH assay
Soluble lactate dehydrogenase (LDH) in HIV/MDM culture supernatant was measured using the Cytotoxicity Detection KitPLUS (Roche Applied Science) according to manufacturer’s instructions.
Electrophoretic Mobility Shift Assay (EMSA)
Following 24 hours of pretreatment with DMF, human MDM were exposed to 1 ng/mL TNFα for 10 minutes and nuclear protein extracts were isolated as described. 8μg of nuclear protein was assessed for NF-κB-DNA binding with an EMSA kit (Panomics, Santa Clara, CA), used according to manufacturer’s directions. The labeled oligonucleotide for NF-κB p50 binding, 5′-AGTTGAGGGGACTTTCCCAGGC-3′, was used.
Cytokine detection in culture supernatants
The concentration of TNFα in culture supernatants was detected using an ELISA kit (Invitrogen) and used according to manufacturer’s instructions. Uninfected MDM were treated with 0.067% DMSO (vehicle) or DMF for 24 hours prior to exposure to 10 μg/mL PHA for 6 hours. Supernatants were collected and frozen at −80°C until assayed.
Chemotaxis assay
Monocyte chemotaxis was assayed using the Chemicon QCM 96-well (5μM pore size) Migration kit (Millipore, Temecula, CA) according to manufacturer’s directions. Freshly isolated human monocytes were plated at a density of 2×105 cells/well in serum- and growth factor-free culture media to the upper chamber in the presence of DMF, MMF or vehicle (0.02% DMSO). CCL2 (300 ng/mL) was added to the lower chamber and cells were incubated at 37°C and 6% CO2 for 6 hours (32-34). Exposure of monocytes to 300 ng/mL CCL2 for 6 hours most consistently induced chemotaxis, with an average of 35.3 ± 20.2% above baseline. All cells that had migrated through the insert, including those adhered to bottom of the membrane, were collected. For quantification, cells were lysed and labeled with CyQuant GR dye. Fluorescence was read with the 480/520 nm filter set on a fluorometric plate reader.
Flow cytometry
Human PBMCs were cultured in RPMI supplemented with 10% FBS and 50 U/mL penicillin/streptomycin at 37°C and 5% CO2. Following 6 hours of treatment with the indicated concentrations of DMF or DMSO vehicle, cells were washed with ice-cold FACS buffer (PBS, 1% BSA, 0.1% NaN3) and stained with CD11b-PE (clone ICRF44, eBioscience, San Diego, CA), CD14-PE/Cy7 (M5E2, BioLegend, San Diego, CA), and CCR2-PerCP/Cy5.5 (TG5, BioLegend) antibodies. Mouse IgG2a-PerCP/Cy5.5 (MOPC-173, BioLegend) was used as the isotype control for CCR2 staining. Antibody-stained cell suspensions were pretreated with 4′6′-diamidino-2-phenylindole (DAPI) to identify dead cells. Flow cytometry was performed on a LSR-II (BD Biosciences, Franklin Lakes, NJ). Doublets were excluded using forward side scatter-height versus forward side scatter-width and side scatter-height versus side scatter-width parameters. Data were analyzed using FlowJo (Tree Star, Ashland, OR). Monocytes were identified as CD11b+CD14+ cells.
Statistics
All quantifications are expressed as mean ± standard error of mean. Statistical comparisons were made by Student’s t-test, one-way ANOVA plus Newman-Keuls post hoc test or post hoc test for linear trend, as indicated. All graphs were generated and statistical analyses were performed using GraphPad Prism software (San Diego, CA), and values of p<0.05 were considered significant.
Results
DMF and MMF inhibit HIV replication in human MDM
Dimethyl fumarate (DMF), and its in vivo primary metabolite monomethyl fumarate (MMF), inhibit NF-κB signaling, suppress the production of inflammatory mediators and induce an antioxidant response in a variety of cell types (19-22, 24, 35, 36). NF-κB signaling has been established as a major pathway of HIV transcriptional regulation, and recent studies have implicated the antioxidant response enzyme, HO-1, as a negative regulator of HIV replication in monocytes (25, 37). Therefore, we hypothesized that DMF could modulate HIV replication in human macrophages through one or both of these mechanisms. Human MDM were treated with DMF or MMF and then examined for virus replication. As shown in Figure 1, exposure of MDM to DMF (A) or MMF (B) attenuated HIV replication in a dose-dependent manner, as determined by culture supernatant reverse transcriptase levels. Suppression of replication in MDM was seen with the R5 CSF HIV strain, Jago (Figure 1) and the prototypic R5/X4 strain, 89.6 (Supplemental Figure 1). As shown in Supplemental Table I, HIV replication was inhibited by an average of approximately 30% at MMF concentrations achieved in vivo after single dose administration (4.4μM in CSF and 6.5μM in plasma) (23, 38). No drug toxicity was detected at concentrations up to 100μM in HIV-infected MDM (HIV/MDM) (Figure 1C, 1D) and non-infected MDM (data not shown). DMF demonstrated additive effects in attenuating HIV replication when used in combination with efavirenz, a non-nucleoside reverse transcriptase inhibitor (Supplemental Figure 2A). There was no observed cellular toxicity when DMF was used in combination with efavirenz (Supplemental Figure 2B).
FIGURE 1.
Dimethyl fumarate and monomethyl fumarate attenuate HIV replication in human MDM. Human MDM infected with 50ng HIV (p24 ELISA, equivalent to 1.82 ± 0.22 kcpm/μL by reverse transcriptase (RT) activity assay) were treated with DMF (A) or MMF (B) over the course of infection at the indicated concentrations (1-30μM) or with 20nM of the non-nucleoside reverse transcriptase inhibitor, efavirenz (EFZ). Culture supernatants were collected every 2-3 days, as indicated, and HIV replication was quantified by RT activity. C, DMF and D, MMF cause no cytotoxicity in HIV/MDM as assessed by LDH assay of supernatants harvested at day 14 post infection. Maximum (Max) LDH release represents the soluble LDH release following cell lysis. RT curves are representative of 3-4 independent experiments, with each replicate performed on cell preparations from different donors. LDH assays represent data averaged from 3-5 individual donors. All statistical comparisons were made by one-way ANOVA plus Newman-Keuls post hoc testing, ***p<0.001 vs. EFZ.
DMF and MMF reduce HIV/MDM-mediated neurotoxicity
We and others have shown that HIV-infected MDM release potent neurotoxins that injure neurons through over-activation of N-methyl-D-aspartate receptors (NMDAR) and that this excitotoxicity is mediated by glutamate and other low molecular weight NMDAR agonists (26, 29, 39). Although the mechanisms underlying neurotoxin production in HIV/MDM are not fully understood, suppression of HIV replication in MDM generally suppresses such neurotoxicity, as demonstrated by treatment with efavirenz (Figure 2). Similarly, in addition to suppressing HIV replication (Figure 1), DMF (Figure 2A) and MMF (Figure 2B) also reduce HIV/MDM neurotoxin production in a dose-dependent manner, as assessed by neuronal survival in our in vitro HIV neurotoxicity model. Representative images of HIV/MDM-mediated neurotoxicity and the protective effects of DMF and MMF are shown (Figure 2C), where surviving neurons are labeled for MAP2 (microtubule-associated protein 2). DMF and EFZ used in combination resulted in additive effects on the suppression of macrophage-mediated neurotoxicity (Supplemental Figure 2C), demonstrating that DMF may successfully reduce HIV replication and macrophage-mediated neurotoxicity that is not fully suppressed by ART. This neuroprotection is due to drug effects on the macrophages, as DMF and MMF do not prevent HIV/MDM mediated neurotoxicity when applied directly to the neurons prior to addition of HIV/MDM supernatants (data not shown).
FIGURE 2.
DMF and MMF reduce HIV/MDM mediated neurotoxicity. Rat cerebrocortical cultures were exposed to supernatant from HIV-infected macrophages that were treated with DMF (A) or MMF (B) at the indicated concentrations (1-30μM) during the course of infection. Neuronal survival was assessed by MAP2 ELISA and expressed as a percentage of untreated (UT) cultures (n = 6; ***p<0.001 vs. Vehicle). C, Representative images of rat cerebrocortical cultures immunofluorescently stained for MAP2 (red) and Hoescht 33324 (blue) following 24 hours treatment with the indicated HIV/MDM supernatant. Scale bar represents 50μm. All statistical comparisons were made by one-way ANOVA plus Newman-Keuls post hoc testing.
DMF inhibits NF-κB nuclear entry, DNA binding and TNFα production in human MDM
NF-κB and TNFα are part of a positive feedback loop that regulates the transcriptional activity of the HIV long terminal repeat (LTR). In unstimulated cells, NF-κB is unable to bind DNA due to its association with inhibitory κB (IκB) proteins, which sequester NF-κB in the cytoplasmic compartment (40-42). Following exposure to an activating stimulus such as TNFα, NF-κB is rapidly freed from the inhibitory complex and translocates into the nucleus to induce transcriptional activation of viral and host genes. NF-κB proteins are major modulators of the HIV LTR and are among the most potent activators of proinflammatory and inflammatory genes. Five members of the mammalian NF-κB/Rel family have been described, including c-Rel, NF-κB1 (p50/p105), NF-κB2 (p52/p100), RelA (p65), and RelB. Functional NF-κB complexes are composed of heterodimer complexes containing p65, c-Rel, or RelB bound to p50 or p52 (40, 43, 44). Exposure to activating stimuli, such as TNFα, induces the nuclear accumulation of NF-κB proteins, DNA binding by NF-κB p50 and transcription from the HIV LTR (45).
To determine if DMF and MMF inhibit the nuclear translocation of NF-κB proteins in MDM, DMF and MMF-treated MDM were stimulated with TNFα and subjected to subcellular fractionations before detection of NF-κB subunits by Western blotting. DMF and MMF each inhibited TNFα-induced nuclear accumulation of RelB, p65 and p50 in a dose-dependent manner (Figure 3A, 3B). We also demonstrate that DMF inhibited the formation of the NF-κB p50-DNA complex, as assessed by EMSA (Figure 3C). Because NF-κB signaling also induces expression of inflammatory mediators, we assessed the effects of DMF treatment on TNFα release from MDM. In agreement with previous reports of DMF decreasing the release of inflammatory mediators from multiple cell types, including TNFα, IL-1β and IL-6, (24, 46) we found that DMF suppresses release of TNFα from PHA-activated MDM (Figure 3D). Furthermore, DMF also markedly suppressed HIV-induced TNFα release from MDM (Figure 3E). Thus, DMF and its primary metabolite, MMF, inhibit NF-κB translocation and signaling events that contribute to the positive feedback loop that modulates HIV transcription in infected and activated MDM.
FIGURE 3.
DMF inhibits NF-κB nuclear translocation, DNA binding and TNFα production in human MDM. A, DMF and B, MMF inhibit the nuclear translocation of the NF-κB proteins RelB, p65 and p50 in human MDM in a dose-dependent manner. Cells were treated with DMF or MMF for 24 hours, exposed to TNFα (10 min), separated into cytoplasmic and nuclear fractions and analyzed by Western blotting. Results of densitometry analysis are presented numerically under each panel as the ratio of NF-κB protein to PARP, a nuclear marker and loading control. Blots are representative of 4-6 independent experiments, with each replicate performed on cell preparations from different donors. C, DMF inhibits nuclear NF-κB p50 binding to DNA in TNFα stimulated MDM, as assessed by EMSA. Results of densitometry analysis were normalized to vehicle. D, DMF inhibits the production of TNFα in MDM stimulated with PHA (10μg/mL). Values are expressed as percent TNFα production relative to Vehicle treated cells (227 ± 11.9 pg/mL TNFα in Vehicle). Data are expressed as mean ± SEM and represent data averaged from 4 different donors. E, TNFα production in HIV/MDM is inhibited by DMF treatment. HIV/MDM were treated with DMF (1-30μM) or 20nM efavirenz (EFZ) over the course of infection and culture supernatants from day 14-15 post infection were assayed for TNFα by ELISA. Values represent the mean ± SEM of data averaged from 5 different donors. All statistical comparisons were made by one-way ANOVA plus Newman-Keuls post hoc testing (*p<0.05, **p<0.01, ***p<0.01 vs. Vehicle).
DMF restores the antioxidant response suppressed by HIV infection in MDM
The antioxidant response is one of the cellular adaptive stress responses that can modulate virus replication and host cell survival, as shown in Hepatitis B and Dengue 2 infection models (47, 48). The antioxidant response maintains redox balance and counteracts oxidative damage through induction of proteins that are involved in detoxification of reactive oxygen species (ROS). These proteins are produced by genes with a common promoter element, the antioxidant response element (ARE), and ARE transcription is mediated by Nrf2. Under conditions of low oxidative stress, Nrf2 is kept transcriptionally inactive by Kelch-like ECH-associated protein 1 (Keap1), which sequesters Nrf2 in the cytoplasmic compartment (49). Following exposure to ROS or electrophiles, Keap1 is degraded by the proteasome and Nrf2 translocates to the nucleus to drive expression of numerous genes including, HO-1, NQO1, glutathione peroxidase 1 (GPX1), and genes responsible for glutathione synthesis (glutamate cysteine ligase modifier, glutamate cysteine ligase catalytic subunit and glutathione synthetase). HIV infection is associated with increased ROS production and depressed levels of glutathione, the major intracellular antioxidant (50). We observed a marked reduction in the level of HO-1 expression in HIV/MDM across multiple human donors, with a more modest but nonetheless consistent reduction in GPX1 levels (Figure 4A, 4B). The effects of DMF on Nrf2 and NQO1 levels were more variable among HIV/MDM cultures from different donors, but trended towards increased expression relative to uninfected Mock/MDM (Figure 4A, 4B).
FIGURE 4.
DMF restores the imbalance in the antioxidant response caused by HIV infection. HIV infection of human MDM reduces HO-1 and GPX1 expression, as assessed by Western blotting (A) and quantified by densitometry analysis (B). Values indicate mean ± SEM of 6 different donors. Statistical comparisons were made by two-tailed paired t-test (*p<0.05, **p<0.01 and ***p<0.001 vs. Mock). C, DMF activates the Nrf2-dependent antioxidant response in HIV/MDM and restores HO-1 and GPX1 levels to that found in uninfected Mock cells, as quantified by densitometry analysis (D). E, MMF activates the Nrf2-dependent antioxidant response in HIV/MDM and restores HO-1 and GPX1 levels to that found in uninfected Mock cells, as quantified by densitometry analysis (F). Blots are representative of 3 independent experiments, with each replicate performed on cell preparations from different donors. Densitometry data are expressed as mean ± SEM and represent data averaged from 3 different donors.
Upon exposure of HIV/MDM to DMF, expression of Nrf2, HO-1, GPX1 and NQO1 increased with increasing doses of DMF (Figure 4C), suggesting a restoration of antioxidant responses in HIV-infected MDM. Both HIV infection and DMF increase total levels of Nrf2, suggesting that while HIV infection stabilizes or induces total cellular Nrf2 levels, this is not sufficient for the coordinated transcriptional activation of ARE-regulated genes, such as HO-1 and GPX1. DMF and MMF treatment activates transcription of these ARE-regulated genes in HIV-infected macrophages, possibly by disrupting inhibitory Nrf2-Keap1 interactions (23). DMF restores levels of HO-1 and GPX1 to those observed in uninfected MDM, while NQO1, which is not suppressed during HIV infection, is induced to levels exceeding those in uninfected MDM (Figure 4D). We have also confirmed that MMF can induce the antioxidant response in HIV/MDM (Figure 4E, 4F) and that both DMF and MMF induce the antioxidant response in uninfected MDM (data not shown). DMF induction of antioxidant responses in MDM occurs independently of HIV infection, which is consistent with previous findings describing induction of the antioxidant response by DMF in multiple cell types, including glia and neurons (23, 24).
DMF inhibition of HIV replication and NF-κB signaling is not mediated by HO-1
HIV infection of human MDM results in alterations to the antioxidant response with a striking reduction in HO-1 levels (Figure 4A, 4B). Induction of HO-1 by hemin has been reported to decrease HIV replication in human monocytes, suggesting that DMF’s induction of HO-1 may underlie its antiviral effects (25). We used a pharmacologic inhibitor of HO-1 enzymatic activity, tin mesoporphyrin (SnMP), to determine the potential role for HO-1 in DMF-mediated suppression of HIV replication and NF-κB translocation. As shown in Figure 5A, SnMP had no effect on DMF-mediated HIV suppression, which suggests that DMF does not suppress HIV replication through enhanced HO-1 expression and activity. We found no effect of SnMP on DMF-mediated suppression of HIV replication regardless of donor, level of infection, DMF dose or timing of SnMP addition (data not shown). We also confirmed that SnMP does not inhibit DMF’s suppression of TNFα-induced nuclear accumulation of NF-κB (Figure 5B). In addition, we show that an inducer of HO-1 expression, cobalt protoporphyrin (CoPP) had no effect on TNFα-induced nuclear accumulation of NF-κB (Figure 5B). These results suggest that DMF’s induction of HO-1 does not directly suppress HIV replication or NF-κB signaling.
FIGURE 5.

HO-1 does not mediate the attenuation of HIV replication or NF-κB signaling induced by DMF. A, SnMP, an inhibitor of HO-1 enzymatic activity, does not inhibit DMF-mediated attenuation of HIV replication. DMF and vehicle treated HIV/MDM were exposed to 10μM SnMP from day 6 through day 15 post infection. Culture supernatants were collected every 3 days and assessed for RT activity. RT curves are representative of 3 independent experiments, with each replicate performed on cell preparations from different donors. B, SnMP and CoPP, a specific inducer of HO-1, do not directly affect or alter DMF-mediated inhibition of the nuclear translocation of NF-κB proteins, as assessed by western blotting. Human MDM were treated with 10μM SnMP, 10μM CoPP and/or 100μM DMF for 24 hours before treatment with 1 ng/mL TNFα (10min) and subcellular fractionation. Western blot is representative of 3 independent experiments, with each replicate performed on cell preparations from different donors.
Induction of HO-1 reduces neurotoxin production from HIV/MDM
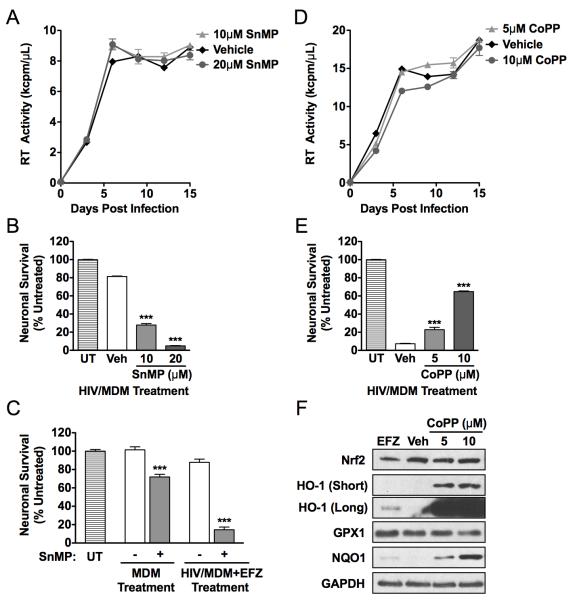
We sought to determine whether the suppression of HIV/MDM neurotoxin production by DMF (Figure 2) was associated with DMF’s suppression of HIV replication and/or induction of HO-1 expression. Inhibiting HIV replication in HIV/MDM can suppress neurotoxin release in vitro, as demonstrated by efavirenz treatment (Figure 2), and similar effects of ART drugs in vivo are thought to account for their ability to limit the severity of HAND in ART-experienced cohorts. While previous studies found that increased HO-1 activity is associated with decreased HIV replication in MDM (25, 37), we found that neither inhibition of HO-1 activity by SnMP treatment of MDM (Figure 6A) nor induction of HO-1 expression by CoPP (Figure 6D, 6F) altered HIV replication. Remarkably, however, SnMP treatment significantly increased the neurotoxicity of MDM supernatant (Figure 6B), even when HIV replication was low or absent (Figure 6C). The increase in MDM-mediated neurotoxicity was a consequence of inhibiting HO-1 activity in the macrophage since SnMP was not toxic when added directly onto neurons (data not shown). And while CoPP does not attenuate HIV replication or inhibit NF-κB signaling, supernatant from CoPP-treated HIV/MDM is significantly less neurotoxic than untreated controls with a similar level of HIV replication (Figure 6E). These studies demonstrate that HO-1 is a critical modulator of neurotoxin production in HIV/MDM and that HO-1 levels can modulate HIV/MDM neurotoxicity without affecting HIV replication.
FIGURE 6.
HO-1 induction reduces neurotoxin production in HIV/MDM without affecting HIV replication. A, SnMP, an inhibitor of HO-1 enzymatic activity, does not directly affect HIV replication and supernatant from these SnMP treated HIV/MDM are significantly more neurotoxic (B), despite equal levels of HIV replication. C, Uninfected Mock/MDM and 20nM efaverinz (EFZ) treated HIV/MDM, which normally produce minimal neurotoxins, are significantly more neurotoxic when treated with 10μM SnMP. D, CoPP, a specific inducer of HO-1 expression, does not directly affect HIV replication and supernatant from these CoPP treated HIV/MDM are significantly less neurotoxic (E), despite high levels of virus replication. F, CoPP treatment exponentially increases HO-1 levels without greatly altering the other components of the antioxidant response, as assessed by Western blotting. For A and C, SnMP or CoPP was added at day 6 post infection onwards and culture supernatants were collected every 3 days and assessed for RT activity. RT curves are representative of 3 independent experiments, with each replicate performed on cell preparations from different donors. For neuronal survival assays, survival was assessed by MAP2 ELISA and expressed as a percentage of untreated (UT) cultures (n = 6; ***p<0.001 vs. vehicle treated paired-condition). Statistical comparisons were made by one-way ANOVA plus Newman-Keuls post hoc testing. Western blot is representative of 3 independent experiments, with each replicate performed on cell preparations from different donors. Two film exposures (short and long) of HO-1 are presented in order to demonstrate the extent of HO-1 induction over basal levels.
DMF and MMF inhibit CCL2 induced chemotaxis in human monocytes
The recruitment of activated and infected monocytes to the CNS in response to CCL2 is a key step in the pathogenesis of HAND (51, 52). In a previous DMF study using the mouse EAE model, DMF reduced macrophage infiltration into the spinal cord in areas of active demyelination (19). We hypothesized that DMF could inhibit chemotaxis of human monocytes in response to chemotactic cytokines, such as CCL2. We found that DMF and MMF inhibited chemotaxis in freshly isolated human monocytes in response to CCL2 in a dose-dependent manner (Figure 7A, 7B). Furthermore, we found that DMF reduced the expression of the CCL2 receptor, CCR2, in freshly isolated human CD11b+CD14+ monocytes within 6 hours of treatment (Figure 7C, 7D), without causing death (Figure 7E). These results indicate that DMF and MMF can decrease monocyte chemotaxis in response to CCL2 and that this effect is associated with downregulation of CCR2 expression.
FIGURE 7.
DMF and MMF reduce CCL2 induced chemotaxis in human monocytes. A, DMF and B, MMF inhibit CCL2-induced chemotaxis in freshly isolated human monocytes in a dose-dependent manner. Values are expressed as percent migration of unstimulated cells (US; 0 ng/mL CCL2) (n = 10-22; **p<0.01 vs. Vehicle). C, DMF decreases CCR2 expression on CD11b+CD14+ PBMCs following 6 hours of treatment, as quantified (D). E, DMF does not cause significant cell death over 6 hours of treatment in freshly isolated human monocytes, as measured by DAPI positivity in CD11b+CD14+ gated PBMCs. For all experiments, values represent data averaged from 3 different donors. All statistical comparisons were made by one-way ANOVA plus Newman-Keuls post hoc testing. Results of post test for linear trend are also presented.
Discussion
Monocytes and macrophages are major reservoirs for HIV in both the periphery and CNS, and they facilitate the spread of virus to target cells, allow for viral persistence and serve as major contributors to inflammation-mediated pathology. Despite current ART, latently infected monocytes and CD4+ T-lymphocytes persist, resulting in inflammation in the periphery and in the CNS in up to 50% of patients on ART (7, 8). While ART will remain the mainstay of HIV therapy, effective adjunctive therapies that suppress inflammation, improve morbidity and improve long-term cognitive outcomes are greatly needed. The immunomodulator DMF, which is effective for the treatment of psoriasis and which shows promising results for multiple sclerosis treatment in recent clinical trails, is an attractive candidate as a safe adjunctive neuroprotectant against HIV. We have demonstrated that physiologically relevant doses of DMF and its primary metabolite, MMF, (23, 38) affect key steps in the pathogenesis of HAND in our in vitro model system by inhibiting HIV replication, neurotoxin production, NF-κB signaling and TNFα production in human MDM and reducing monocyte chemotaxis in response to CCL2. These results suggest that DMF could serve as an effective neuroprotectant in HAND and have beneficial effects on systemic HIV-disease progression as well.
We have shown that DMF and MMF attenuate macrophage-mediated neurotoxicity following HIV infection by simultaneously attenuating viral replication and inducing HO-1 expression. Furthermore, induction of HO-1 can significantly decrease macrophage-mediated neurotoxicity even without decreasing HIV replication. Consequently, DMF may be an especially relevant therapeutic in patients who have relatively good virologic control but still suffer from neurological complications of HIV. We have shown that HIV infection of MDM results in a dysregulation of the antioxidant response with an especially prominent reduction in HO-1 levels, associated with supernatant neurotoxicity, and that DMF treatment restores HO-1 levels and reduces neurotoxin production in macrophages. In activated microglia, an oxidative burst is required for the release of excitotoxic glutamate (53), demonstrating that alterations to cellular oxidative state can mediate the production and/or release of MDM neurotoxins. DMF’s ability to decrease HIV replication and neurotoxin production by distinct mechanisms makes it an especially attractive therapeutic candidate for HAND. Furthermore, macrophage- and microglia-mediated neurotoxicity contribute to many other neurological disorders including multiple sclerosis, Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease and stroke/reperfusion injury, for which therapeutics for restoring oxidative balance resultant from the disease state have been investigated/prescribed (54).
Numerous proinflammatory factors contribute to HIV disease pathogenesis in both the peripheral and CNS compartments. TNFα, IL-6, IL-1β, IFN-γ and other proinflammatory cytokines are elevated in the blood and CSF of HIV-infected patients (55-58). Among these, TNFα is the most potent mediator of inflammation and is induced early after HIV monocytic infection and its expression continues to increase over the course of infection (59-61). It is well established that TNFα exposure upregulates HIV replication by initiating a signaling cascade that activates the nuclear translocation of NF-κB (62-65). We have shown that DMF and MMF attenuate TNFα-mediated NF-κB signaling in human macrophages and reduce nuclear NF-κB levels, which are expected to decrease transcription from the HIV-LTR. However, the NF-κB and TNFα signaling loop may not entirely mediate DMF’s antiviral activity. Attenuation of HIV replication occurs at low concentrations, as does induction of the ARE, while inhibition of NF-κB signaling may be more relevant at concentrations of 15μM and greater. Future studies are necessary to assess the role of the antioxidant response, including NQO1 and the cellular redox state, in mediating HIV infection and replication. DMF may alter the expression of the HIV co-receptors, CXCR4 and CCR5, similarly to the observed downregulation of cell surface CCR2. It has been reported that antioxidants decrease the stability of mRNA transcripts for CXCR4 and CCR5 in human monocytes, suggesting that DMF treatment may directly reduce HIV entry into human monocytes (66).
However, DMF’s ability to inhibit NF-κB and TNFα signaling following both PHA stimulation and HIV infection has clear implications for the physiologic reduction of neuroinflammation and cytokine induced neuronal injury. Elevated TNFα levels increase monocyte entry into the brain, promote HIV replication, and drive inflammatory cascades, thereby enhancing the production of neurotoxins in the CNS from MDM, microglia and astrocytes (67). Therefore, dampening TNFα-driven processes might also afford neuroprotection against HIV. Indeed, TNFα is linked to glutamine synthetase and glutamate import in macrophages (68), and DMF’s inhibition of TNFα-driven processes may further decrease the release of excitatory neurotoxins, such as glutamate, in HIV/MDM. In human macrophages, we have shown that DMF is a potent suppressor of NF-κB nuclear translocation, subsequent binding to DNA and expression of NF-κB dependent genes. Therefore, DMF is a particularly good therapeutic candidate for pathological states characterized by macrophage driven inflammation and NF-κB signaling.
Although not directly dependent upon HO-1, DMF’s antioxidant properties are likely mediating the inhibition of NF-κB activity. We hypothesize that such effects are due to DMF’s modulation of the macrophage intracellular redox state as activation of the antioxidant response has been shown to block NF-κB activity and HIV transcription (69-71). Furthermore, classical (α and β), novel (δ) and atypical (ζ) PKC isotypes can modulate the nuclear translocation and transcriptional activity of NF-κB and PKC is activated by oxidative stress and inhibited by antioxidants (72-76). In addition to potential effects on PKC, DMF may also affect the phosphorylation of IκB kinases (IKK) and subsequent phosphorylation and degradation of IκB proteins (21, 36). Finally, DMF may affect NF-κB dependent transcription by modulating the preferred composition of NF-κB homo- and heterodimers that form after nuclear translocation has occurred. The intracellular oxidative state can affect levels of NF-κB p50 homodimers, which do not possess transactivation domains and are thought to act as transcriptional repressors of NF-κB heterodimer responsive genes (77-79). We are currently examining the role of DMF and MMF in modulating the activation state of the macrophage, which would affect the cell’s relative sensitivity to pro-inflammatory signals and thereby contribute to decreased NF-κB signaling.
While other antioxidants have been considered as potential therapeutics for HAND, through direct effects on macrophages or neurons, DMF is unique in its ability to inhibit CCL2-induced monocyte chemotaxis. Monocyte transmigration across the blood-brain barrier is dependent upon production of chemokines, such as CCL2, in the CNS and the activation of monocytes in the periphery. Levels of CCL2 in the CSF correlate with CSF viral load and with the clinical severity of HAND (51, 52, 80-83), and CCL2 is produced by brain macrophages, astrocytes and endothelial cells in response to inflammatory mediators and HIV proteins (84-86). Not only does DMF decrease TNFα production and NF-κB signaling in MDM, both of which have been implicated in CCL2 production, but DMF and MMF inhibit CCL2-driven monocyte chemotaxis, possibly by modulation of CCR2 expression. DMF and MMF may modulate the cell surface expression of CCR2 by inducing the antioxidant response and consequently altering the redox state of the cell. It has been demonstrated that direct antioxidants are capable of reducing the transcript stability of CCR2, which has been linked to decreased cell surface expression and CCL2-induced chemotaxis in human monocytes (66). These findings in our in vitro model system predict suppression of transendothelial migration of monocytes into the CNS during HIV infection. Furthermore, it has been reported that DMF modulates adhesion molecule expression in human endothelial cells by inhibiting TNFα-induced expression of ICAM-1, VCAM-1 and E-selectin (35). Expression of each of these adhesion molecules has been linked to monocyte entry into the CNS after HIV infection and down-regulation by DMF is expected to further inhibit monocyte entry into the CNS. Given these findings, DMF should be considered as a potential therapeutic for other neuroinflammatory diseases associated with CCL2-induced recruitment of leukocytes to the CNS.
With this study, we identify dimethyl fumarate as a candidate adjunctive therapy and potential neuroprotectant against HIV. To our knowledge, we are the first to demonstrate that HIV infection dysregulates components of the antioxidant response in human macrophages and that restoration of HO-1 levels, specifically, can reduce macrophage-mediated neurotoxicity. DMF is the first proposed neuroprotectant that reduces CCL2-mediated monocyte chemotaxis as a component of its mechanism of action. Furthermore, we have shown that DMF attenuates HIV replication associated with decreased TNFα and NF-κB signaling. Given these findings, we propose that DMF should be considered a relevant therapeutic candidate for neurological disorders and other complications of HIV-infection mediated by monocyte and macrophage inflammation.
Supplementary Material
Acknowledgements
We would like to thank Dr. Samantha S. Soldan for her technical assistance, intellectual contributions and critical review of the manuscript. We also thank Dr. Stefan Lanker of Biogen Idec, Inc. and Dr. Francisco González-Scarano for their helpful discussions. We gratefully acknowledge Margaret Maronski for expert preparation of primary rodent neuronal cultures. We also sincerely appreciate Dr. Natalia Nedelsky for her thoughtful critique and editing of the manuscript. The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAD, NIH: (efavirenz, cat#4624).
Footnotes
This work was supported by National Institutes of Health R01 Grant (NS-043994; D.L.K.), P50 grant (NS-27405; sub-project PI D.L.K.) and T32 Grant (AG-000255; S.A.C.).
Abbreviations used in this paper: MDM, monocyte-derived macrophages; HAND, HIV-associated neurocognitive disorders; DMF, dimethyl fumarate; MMF, monomethyl fumarate; HO-1, heme oxygenase 1; NQO1, NAD(P)H dehydrogenase, quinone 1; Nrf2, nuclear factor E2 related factor 2; GPX1, glutathione peroxidase 1; ART, antiretroviral therapy; EFZ, efavirenz; LTR, long terminal repeat; CNS, central nervous system; CSF, cerebral spinal fluid; MAP2, microtubule-associated protein 2; MS, multiple sclerosis; LDH; lactate dehydrogenase
References
- 1.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurol. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann. Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 3.Dunfee R, Thomas ER, Gorry PR, Wang J, Ancuta P, Gabuzda D. Mechanisms of HIV-1 neurotropism. Curr. HIV Res. 2006;4:267–278. doi: 10.2174/157016206777709500. [DOI] [PubMed] [Google Scholar]
- 4.Petito CK, Cho ES, Lemann W, Navia BA, Price RW. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J. Neuropathol. Exp. Neurol. 1986;45:635–646. doi: 10.1097/00005072-198611000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ho DD, Rota TR, Schooley RT, Kaplan JC, Allan JD, Groopman JE, Resnick L, Felsenstein D, Andrews CA, Hirsch MS. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. New Engl. J. Med. 1985;313:1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- 6.Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 7.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 8.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J. Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 10.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J. Infect. Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 12.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- 13.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann. Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 14.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J. Neuropathol. Exp. Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 15.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 16.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoxtermann S, Nuchel C, Altmeyer P. Fumaric acid esters suppress peripheral CD4- and CD8-positive lymphocytes in psoriasis. Dermatol. 1998;196:223–230. doi: 10.1159/000017903. [DOI] [PubMed] [Google Scholar]
- 18.Trial watch: Phase III success for Biogen’s oral multiple sclerosis therapy. Nat. Rev. Drug Discov. 2011;10:404. doi: 10.1038/nrd3465. [DOI] [PubMed] [Google Scholar]
- 19.Schilling S, Goelz S, Linker R, Luehder F, Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin. Exp. Immunol. 2006;145:101–107. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoof TJ, Flier J, Sampat S, Nieboer C, Tensen CP, Boorsma DM. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br. J. Dermatol. 2001;144:1114–1120. doi: 10.1046/j.1365-2133.2001.04220.x. [DOI] [PubMed] [Google Scholar]
- 21.Seidel P, Merfort I, Hughes JM, Oliver BG, Tamm M, Roth M. Dimethylfumarate inhibits NF-kappaB function at multiple levels to limit airway smooth muscle cell cytokine secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L326–339. doi: 10.1152/ajplung.90624.2008. [DOI] [PubMed] [Google Scholar]
- 22.Loewe R, Holnthoner W, Groger M, Pillinger M, Gruber F, Mechtcheriakova D, Hofer E, Wolff K, Petzelbauer P. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J. Immunol. 2002;168:4781–4787. doi: 10.4049/jimmunol.168.9.4781. [DOI] [PubMed] [Google Scholar]
- 23.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann JC, Listopad JJ, Rentzsch CU, Igney FH, von Bonin A, Hennekes HH, Asadullah K, Docke WD. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J. Invest. Dermatol. 2007;127:835–845. doi: 10.1038/sj.jid.5700686. [DOI] [PubMed] [Google Scholar]
- 25.Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J. Immunol. 2006;176:4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J. Neurosci. 2006;26:981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 28.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Sulcove J, Frank I, Jaffer S, Ozdener H, Kolson DL. Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary isolates and evidence for involvement of the Bcl-2/Bcl-xL-sensitive intrinsic apoptosis pathway. J. Virol. 2002;76:9407–9419. doi: 10.1128/JVI.76.18.9407-9419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White MG, Wang Y, Akay C, Lindl KA, Kolson DL, Jordan-Sciutto KL. Parallel high throughput neuronal toxicity assays demonstrate uncoupling between loss of mitochondrial membrane potential and neuronal damage in a model of HIV-induced neurodegeneration. Neurosci. Res. 2011;70:220–229. doi: 10.1016/j.neures.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, White MG, Akay C, Chodroff RA, Robinson J, Lindl KA, Dichter MA, Qian Y, Mao Z, Kolson DL, Jordan-Sciutto KL. Activation of cyclin-dependent kinase 5 by calpains contributes to human immunodeficiency virus-induced neurotoxicity. J. Neurochem. 2007;103:439–455. doi: 10.1111/j.1471-4159.2007.04746.x. [DOI] [PubMed] [Google Scholar]
- 32.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janic B, Iskander AS, Rad AM, Soltanian-Zadeh H, Arbab AS. Effects of ferumoxides-protamine sulfate labeling on immunomodulatory characteristics of macrophage-like THP-1 cells. PLoS ONE. 2008;3:e2499. doi: 10.1371/journal.pone.0002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiou WF, Ko HC, Wei BL. Evodia rutaecarpa and three major alkaloids abrogate influenza A virus (H1N1)-induced chemokines production and cell migration. Evid. Based Complement. Alternat. Med. 2011;2011:750513. doi: 10.1093/ecam/nep238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandermeeren M, Janssens S, Borgers M, Geysen J. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem. Biophys. Res. Commun. 1997;234:19–23. doi: 10.1006/bbrc.1997.6570. [DOI] [PubMed] [Google Scholar]
- 36.Vandermeeren M, Janssens S, Wouters H, Borghmans I, Borgers M, Beyaert R, Geysen J. Dimethylfumarate is an inhibitor of cytokine-induced nuclear translocation of NF-kappa B1, but not RelA in normal human dermal fibroblast cells. J. Invest. Dermatol. 2001;116:124–130. doi: 10.1046/j.1523-1747.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 37.Devadas K, Hewlett IK, Dhawan S. Lipopolysaccharide suppresses HIV-1 replication in human monocytes by protein kinase C-dependent heme oxygenase-1 induction. J. Leukoc. Biol. 2010;87:915–924. doi: 10.1189/jlb.0307172. [DOI] [PubMed] [Google Scholar]
- 38.Litjens NH, Burggraaf J, van Strijen E, van Gulpen C, Mattie H, Schoemaker RC, van Dissel JT, Thio HB, Nibbering PH. Pharmacokinetics of oral fumarates in healthy subjects. Br. J. Clin. Pharmacol. 2004;58:429–432. doi: 10.1111/j.1365-2125.2004.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J. Neuroimmunol. 2001;117:97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- 40.Baldwin AS. The NF-κB and IκB Proteins: New Discoveries and Insights. Annu. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 41.Ganchi PA, Sun SC, Greene WC, Ballard DW. I kappa B/MAD-3 masks the nuclear localization signal of NF-kappa B p65 and requires the transactivation domain to inhibit NF-kappa B p65 DNA binding. Mol. Biol. Cell. 1992;3:1339–1352. doi: 10.1091/mbc.3.12.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henkel T, Zabel U, van Zee K, Muller JM, Fanning E, Baeuerle PA. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell. 1992;68:1121–1133. doi: 10.1016/0092-8674(92)90083-o. [DOI] [PubMed] [Google Scholar]
- 43.Kilareski E, Shah S, Nonnemacher M, Wigdahl B. Regulation of HIV-1 transcription in cells of the monocyte-macrophage lineage. Retrovirology. 2009;6:118. doi: 10.1186/1742-4690-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann M, Fries H-W, Scheicher C, Keikavoussi P, Kolb-Mäurer A, Bröcker E-B, Serfling E, Kämpgen E. Differential expression of Rel/NF-κB and octamer factors is a hallmark of the generation and maturation of dendritic cells. Blood. 2000;95:277–285. [PubMed] [Google Scholar]
- 45.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflammation. 2010;7:30. doi: 10.1186/1742-2094-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen TH, Tang P, Yang CF, Kao LH, Lo YP, Chuang CK, Shih YT, Chen WJ. Antioxidant defense is one of the mechanisms by which mosquito cells survive dengue 2 viral infection. Virology. 2011;410:410–417. doi: 10.1016/j.virol.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Schaedler S, Krause J, Himmelsbach K, Carvajal-Yepes M, Lieder F, Klingel K, Nassal M, Weiss TS, Werner S, Hildt E. Hepatitis B virus induces expression of antioxidant response element-regulated genes by activation of Nrf2. J. Biol. Chem. 2010;285:41074–41086. doi: 10.1074/jbc.M110.145862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dworkin BM, Rosenthal WS, Wormser GP, Weiss L. Selenium deficiency in the acquired immunodeficiency syndrome. J. Parenter. Enteral Nutr. 1986;10:405–407. doi: 10.1177/0148607186010004405. [DOI] [PubMed] [Google Scholar]
- 51.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J. Infect. Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- 53.Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J. Neurochem. 2007;101:1205–1213. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciardi M, Sharief MK, Thompson EJ, Salotti A, Vullo V, Sorice F, Cirelli A. High cerebrospinal fluid and serum levels of tumor necrosis factor-alpha in asymptomatic HIV-1 seropositive individuals. Correlation with interleukin-2 and soluble IL-2 receptor. J. Neurol. Sci. 1994;125:175–179. doi: 10.1016/0022-510x(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 56.Breen E, Rezai A, Nakajima K, Beall G, Mitsuyasu R, Hirano T, Kishimoto T, Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J. Immunol. 1990;144:480–484. [PubMed] [Google Scholar]
- 57.Kobayashi S, H Y, Kobayashi N, Yamamoto N. Serum level of TNF alpha in HIV-infected individuals. AIDS. 1990;4:169–170. [PubMed] [Google Scholar]
- 58.Ownby R, Kumar A, Benny Fernandez J, Moleon-Borodowsky I, Gonzalez L, Eisdorfer S, Waldrop-Valverde D, Kumar M. Tumor Necrosis Factor-alpha Levels in HIV-1 Seropositive Injecting Drug Users. J. Neuroimmune Pharmacol. 2009;4:350–358. doi: 10.1007/s11481-009-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esser R, Glienke W, von Briesen H, Rubsamen-Waigmann H, Andreesen R. Differential regulation of proinflammatory and hematopoietic cytokines in human macrophages after infection with human immunodeficiency virus. Blood. 1996;88:3474–3481. [PubMed] [Google Scholar]
- 60.Folks T, Kessler S, Orenstein J, Justement J, Jaffe E, Fauci A. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988;242:919–922. doi: 10.1126/science.2460922. [DOI] [PubMed] [Google Scholar]
- 61.Koyanagi Y, O’Brien W, Zhao J, Golde D, Gasson J, Chen I. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988;241:1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 62.Poli G, Kinter A, Justement JS, Kehrl JH, Bressler P, Stanley S, Fauci AS. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc. Natl. Acad. Sci. USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butera S, Roberts B, Folks T. Regulation of HIV-1 expression by cytokine networks in a CD4+ model of chronic infection. J. Immunol. 1993;150:625–634. [PubMed] [Google Scholar]
- 64.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 65.West MJ, Lowe AD, Karn J. Activation of Human Immunodeficiency Virus Transcription in T Cells Revisited: NF-kappaB p65 Stimulates Transcriptional Elongation. J. Virol. 2001;75:8524–8537. doi: 10.1128/JVI.75.18.8524-8537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saccani A, Saccani S, Orlando S, Sironi M, Bernasconi S, Ghezzi P, Mantovani A, Sica A. Redox regulation of chemokine receptor expression. Proc. Natl. Acad. Sci. USA. 2000;97:2761–2766. doi: 10.1073/pnas.97.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, Graves MC, Witte M, Kim KS. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 68.Porcheray F, Leone C, Samah B, Rimaniol AC, Dereuddre-Bosquet N, Gras G. Glutamate metabolism in HIV-infected macrophages: implications for the CNS. Am. J. Physiol. Cell Physiol. 2006;291:C618–626. doi: 10.1152/ajpcell.00021.2006. [DOI] [PubMed] [Google Scholar]
- 69.Schreck R, R P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roederer M, Staal FJ, Raju PA, Ela SW, Herzenberg LA. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-L-cysteine. Proc. Natl. Acad. Sci. USA. 1990;87:4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Staal FJ, R M, Raju PA, Anderson MT, Ela SW, Herzenberg LA, Herzenberg LA. Antioxidants inhibit stimulation of HIV transcription. AIDS Res. Hum. Retroviruses. 1993;9:299–306. doi: 10.1089/aid.1993.9.299. [DOI] [PubMed] [Google Scholar]
- 72.Asehnoune K, Strassheim D, Mitra S, Yeol Kim J, Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell. Signal. 2005;17:385–394. doi: 10.1016/j.cellsig.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-thetas is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 74.Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J. Biol. Chem. 1991;266:6188–6194. [PubMed] [Google Scholar]
- 75.Gopalakrishna R, Chen Z-H, Gundimeda U. Modifications of cysteine-rich regions in protein kinase C induced by oxidant tumor promoters and enzyme-specific inhibitors. Methods Enzymol. 1995;252:132–146. doi: 10.1016/0076-6879(95)52016-3. [DOI] [PubMed] [Google Scholar]
- 76.Gopalakrishna R, Gundimeda U, Chen Z-H. Cancer-Preventive Selenocompounds Induce a Specific Redox Modification of Cysteine-Rich Regions in Ca2+-Dependent Isoenzymes of Protein Kinase C. Arch. Biochem. Biophys. 1997;348:25–36. doi: 10.1006/abbi.1997.0334. [DOI] [PubMed] [Google Scholar]
- 77.Cristofanon S, Morceau F, Scovassi AI, Dicato M, Ghibelli L, Diederich M. Oxidative, multistep activation of the noncanonical NF-kappaB pathway via disulfide Bcl-3/p50 complex. FASEB J. 2009;23:45–57. doi: 10.1096/fj.07-104109. [DOI] [PubMed] [Google Scholar]
- 78.Hoberg JE, Popko AE, Ramsey CS, Mayo MW. IkappaB Kinase alpha-mediated derepression of SMRT potentiates acetylation of RelA/p65 by p300. Mol. Cell. Biol. 2006;26:457–471. doi: 10.1128/MCB.26.2.457-471.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou LZH, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic. Biol. Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]
- 80.Letendre SL, Lanier ER, McCutchan JA. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. J. Infect. Dis. 1999;180:310–319. doi: 10.1086/314866. [DOI] [PubMed] [Google Scholar]
- 81.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc. Natl. Acad. Sci. USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann. Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 83.Sozzani S, Introna M, Bernasconi S, Polentarutti N, Cinque P, Poli G, Sica A, Mantovani A. MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression. J. Leukoc. Biol. 1997;62:30–33. doi: 10.1002/jlb.62.1.30. [DOI] [PubMed] [Google Scholar]
- 84.Gu L, Rutledge B, Fiorillo J, Ernst C, Grewal I, Flavell R, Gladue R, Rollins B. In vivo properties of monocyte chemoattractant protein-1. J. Leukoc. Biol. 1997;62:577–580. doi: 10.1002/jlb.62.5.577. [DOI] [PubMed] [Google Scholar]
- 85.Guillemin GJ, Croitoru-Lamoury J, Dormont D, Armati PJ, Brew BJ. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia. 2003;41:371–381. doi: 10.1002/glia.10175. [DOI] [PubMed] [Google Scholar]
- 86.Lehmann MH, Masanetz S, Kramer S, Erfle V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J. Cell. Sci. 2006;119:4520–4530. doi: 10.1242/jcs.03231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.