Abstract
The histone deacetylase inhibitor, largazole 1 was synthesized by a convergent approach which involved several efficient and high yielding single pot multistep protocols. Initial attempts using t-butyl as thiol protecting group proved problematic and synthesis was accomplished by switching to trityl protecting group. This synthetic protocol provides a convenient approach to many new largazole analogues. Three side chain analogues with multiple heteroatoms for chelation with Zn2+ were synthesized and their biological activities were evaluated. They were less potent than largazole 1 in growth inhibition of HCT116 colon carcinoma cell line and in inducing increases in global H3 acetylation. Largazole 1 and the three side chain analogues had no effect on HDAC6 as indicated by the lack of increased acetylation of α-tubulin.
Introduction
A major challenge in cancer chemotherapy is the development of drugs that selectively kill cancer cells without affecting normal cells for increased potency and reduced toxic side effects. Changes in gene expression due to mutation, loss, or rearrangement are associated with cancer initiation and progression. With few exceptions, these changes have been difficult to target therapeutically. A new paradigm that is being extensively investigated as an anticancer drug development strategy is the targeting of epigenetic regulation of gene expression. Epigenetics refer to heritable alterations in gene expression that are not a result of changes in DNA sequences. In cancer, aberrant epigenetic silencing of several genes including tumor suppressor genes is a common occurrence.1 Aberrant epigenetic silencing occurs as a result of aberrant promoter region CpG island DNA methylation in concert with changes in covalent modification of histone proteins.1g Enzymatic modifications such as acetylation, methylation and phosphorylation of the lysine and arginine residues of the N-terminus of histones regulate the access to DNA by transcriptional factors thereby regulating gene expression.2 Histone acetylation modulated by two protein families, histone acetyltransferases (HATs) and histone deacetylases (HDACs) is the most extensively studied of these processes.3 Most importantly, epigenetic changes, unlike DNA sequence changes are reversible and thus the interest in targeting epigenetic gene regulation as an anticancer strategy.
HDACs are a class of metalloenzymes responsible for removal of acetyl group from lysine residues of histone and other nonhistone substrate proteins. The deacetylated histone, positively charged at physiological pH, interacts with the negatively charged DNA phosphate backbone and can form transcriptionally inactive condensed forms of chromatin. These epigenetic changes can result in silencing of tumor suppressor genes promoting cancer initiation and/or progression. In some cases, inhibition of HDACs can be used to restore hyperacetylation to derepress or activate these undesirably silenced genes.4 HDAC proteins are a family of at least 18 enzymes and are classified into four groups based on their size, cellular localization, active catalytic site numbers and homology to yeast HDAC proteins:5 Class I: HDAC1, -2, -3, and -8; Class IIa: HDAC4, -5, -7, and -9; Class IIb: HDAC6 and -10; Class III: Sirtuin proteins and Class IV: HDAC11. The HDAC proteins are associated with basic cellular functions and disease states such as cancer, but the importance of individual isoforms in cell function and cancer biology is not well understood. However, class I isoforms are regarded as promising cancer targets.6 Two HDAC inhibitors suberoylanilide hydroxamic acid (SAHA, Vorinostat) and FK228 (romidepsin) have recently been approved by the USFDA for the treatment of cutaneous T-cell lymphoma (CTCL). SAHA with a hydroxamic acid moiety as the metal binding domain is a pan-inhibitor of HDACs. FK228 has a thiol masked as a cyclic disulfide bond with a cysteine residue of the depsipeptide core. Many other HDACis are at different stages of clinical investigation7 with a preference for isoform-selective or class-selective inhibitors for higher therapeutic potential.5 X-ray crystallography and SAR studies of HDAC inhibitors have shown that three key structural elements are involved in the binding of the inhibitor to the active site of the HDAC protein:8 (a) a metal binding domain, which chelates with the Zn2+ cation present at the active site; (b) a surface recognition cap group which interacts with hydrophobic residues on the rim of the active site; and (c) a linker, which mimics the N-acetyl lysine side chain of histone and occupies a hydrophobic channel positioning the zinc binding moiety and the cap group in binding orientation.
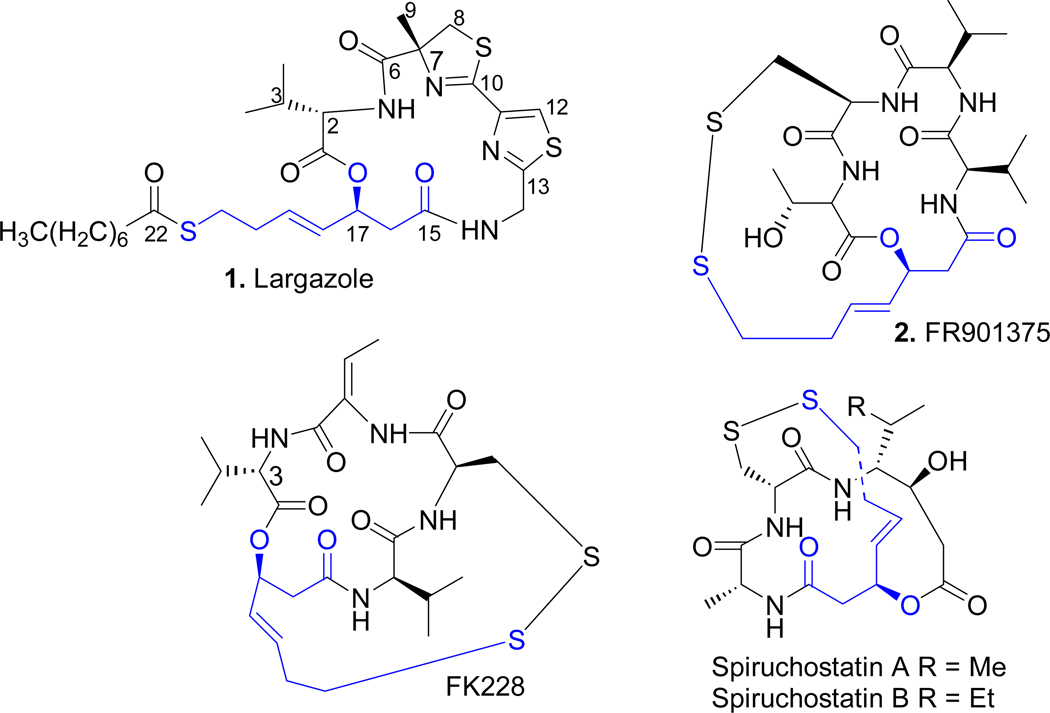
Recently, a new natural product largazole (1, Figure 1), isolated from a marine cyanobacterium of the genus Symploca was reported to possess remarkable selective activity against highly invasive transformed human mammary epithelial cells (MDA-MB-231) and transformed fibroblastic osteosarcoma (USOS) cells over the normal cell lines (NMuMG and NIH3T3, respectively).9
Figure 1.
Natural HDAC inhibitors with anticancer properties
Mechanistic studies showed that largazole 1 is an inhibitor of HDACs.10 It is a prodrug that undergoes hydrolysis of the thioester group by cellular esterases and/or lipases to release a free thiol function which constitutes the domain that chelates Zn2+. The depsipeptide ring system represents the surface recognition cap group. The two domains are connected by a four-carbon olefinic linker. A striking similarity of largazole 1 to other natural HDAC inhibitors such as FK228,10b FR901375 210b and spiruchostatins (Fig. 1) is that they all contain a masked 3-hydroxy-7-mercapto-4-heptenoic acid side chain. Upon activation, they release the free thiol as the active species.
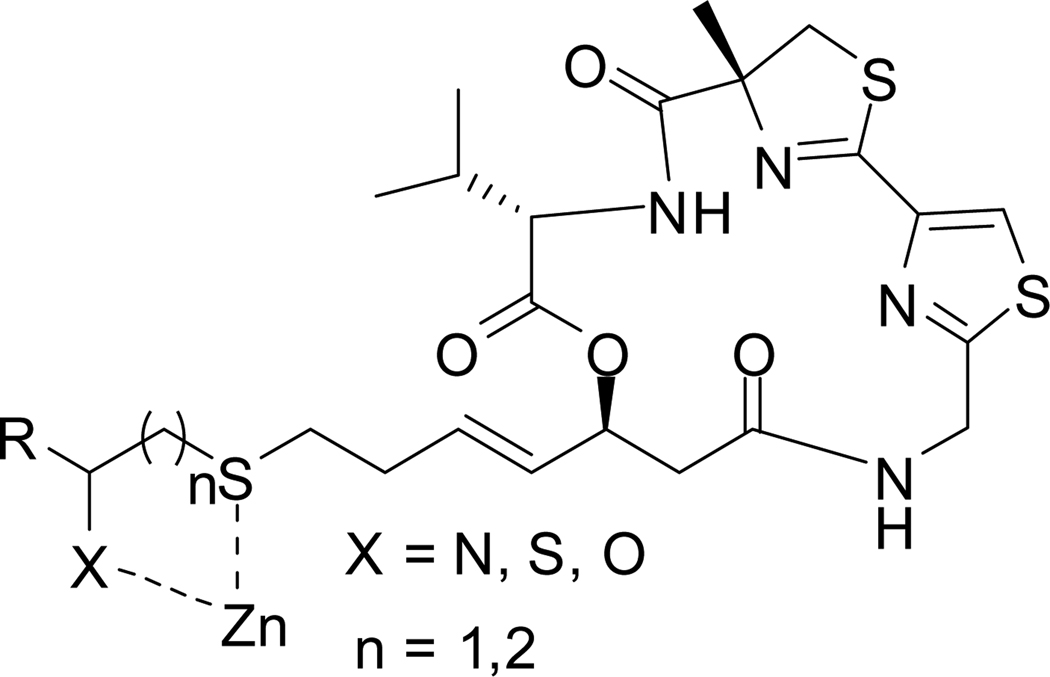
Due to its highly selective activity on specific cancer cell lines and a strong and exceptional picomolar inhibitory bias for Class I HDACs (HDAC1, -2 and -3) over Class II HDAC6, largazole 1 has been subjected to extensive investigation since its discovery in 2008.10b A number of synthetic approaches to largazole 1 have appeared since then.10a, b, 11 Its high potency and selectivity, along with the presence of a metal-binding domain and a surface recognition group interacting with differently conserved regions of the receptor, make largazole 1 a fascinating lead molecule for further structural optimization in pursuit of molecules of higher potency and/or selectivity. An array of largazole analogues has been synthesized and their biological properties evaluated to uncover some of the SAR requirements of the molecule.10–11, 11c, 11g–i These studies have focused mostly on alterations in the cap group and to a lesser extent on the side chain. The four-atom linker between the hydrophobic cap group and the Zn2+ coordinating group in largazole 1 and other HDACis with a masked thiol as the Zn2+ binding domain such as FK228, spiruchostatins, and FR901375 2 appears to be the optimal distance for highest potency in comparison to side chains with 5–7 atoms which are common among most HDACis.10d, 11c Replacement of the zinc-binding thiol of largazole 1 with a number of benzamide and thioamide head groups failed to produce more potent analogues.10c The active sites of HDAC isoforms are highly conserved. However, the presence of an internal cavity within the active site has been exploited in designing isoform-selective HDACis with substituted benzamide derivatives as the zinc-binding group.12 Therefore, it is conceivable that structural alterations in the zinc-binding domain of largazole 1 to modulate metal binding affinity may take advantage of such minor structural differences within the active site to develop isoform or class-selective inhibitors. Isoform-selective inhibitors may help understand the role of different isoforms in cellular processes and disease states. Our approach to modifying the metal-binding domain consisted of introducing a second heteroatom in the side chain 2–3 atoms apart from sulfur and is thus capable of interacting with Zn2+ via 5/6-membered cyclic transition state (Figure 2).
Figure 2.
Largazole analogues targeted to Zn2+ binding motif
Chemistry
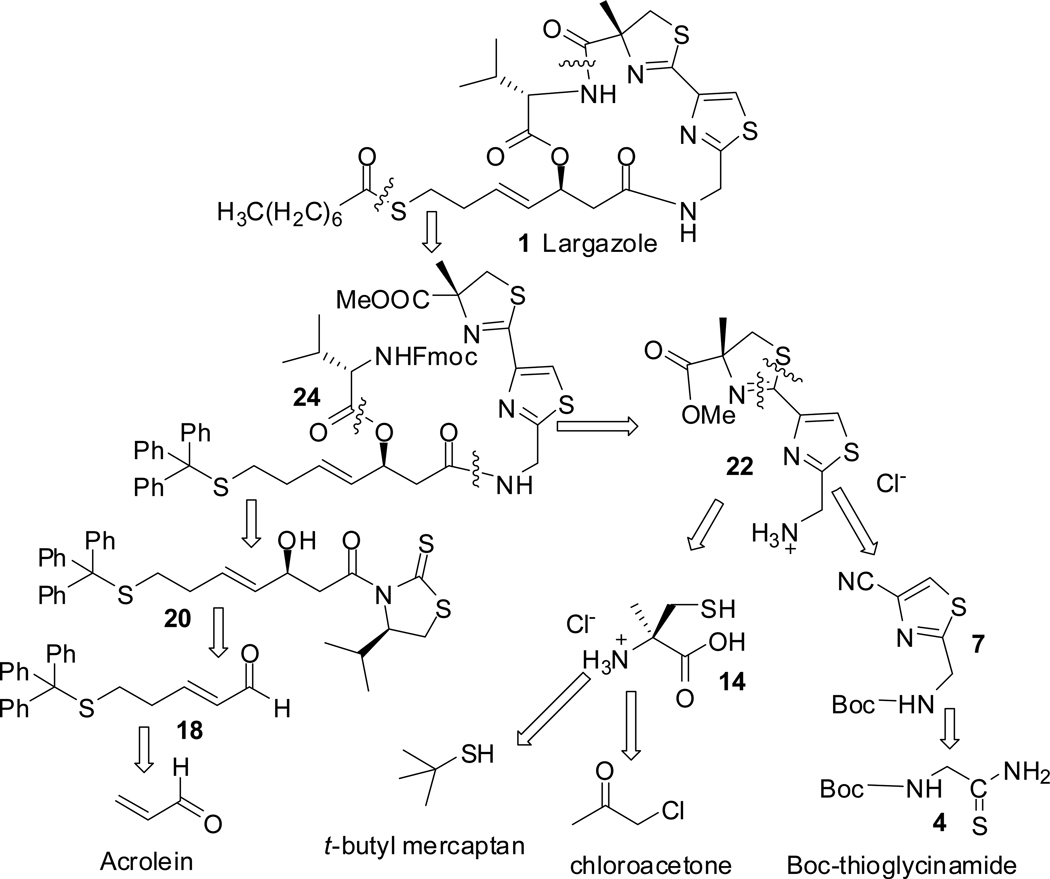
The convergent synthetic approach adopted as shown in retrosynthetic analysis (scheme 1) involved macrolactamization and side chain thioesterification of the intermediate 24 which could be derived by acyl transfer from fragment 20 to 22 followed by coupling with Fmoc-valine. Intermediate 22 can be synthesized from the thiazole nitrile 7 and (R)-α-methylcysteine hydrochloride 14. Synthesis of 14 would also generate its enantiomer useful for making the C-7 epimers of largazole 1 and analogues. Fragment 20 is the product of acetate aldol reaction of the aldehyde 18 using acetyl Nagao as the chiral auxiliary.13 In our initial approach when t-butyl was used as the thiol protecting group, deprotection and thioesterification to produce the final product became problematic (vide infra).
Scheme 1.
Retrosynthetic analysis of largazole 1
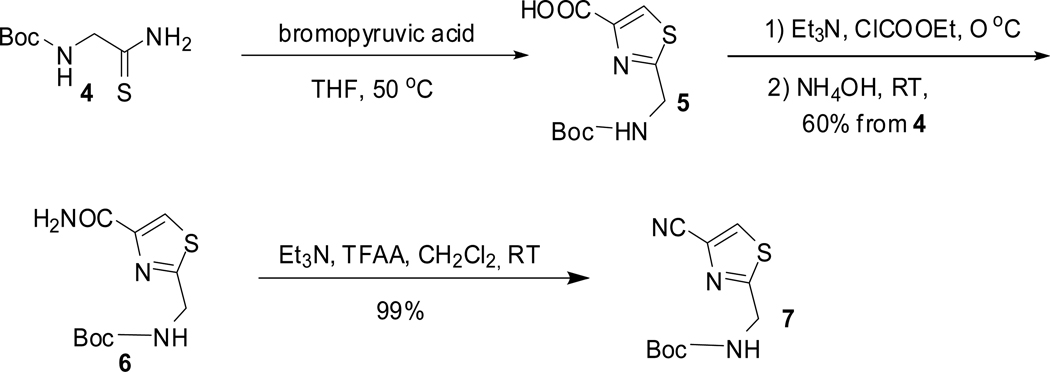
The synthesis of thiazole derivative 7 is shown in scheme 2. Thiazole amide 6 was made in 60% overall yield from boc-thioglycinamide 4 and bromopyruvic acid in a one-pot reaction in which the initially formed carboxylic acid, after removal of water, was activated and treated with ammonia. This one-pot protocol was found to be more efficient and gave the product in higher yields and in a much shorter period of time than the previously reported methods.11a Amide 6 was converted to the nitrile 7 using standard conditions in 99% yield.11a
Scheme 2.
Synthesis of fragment 7
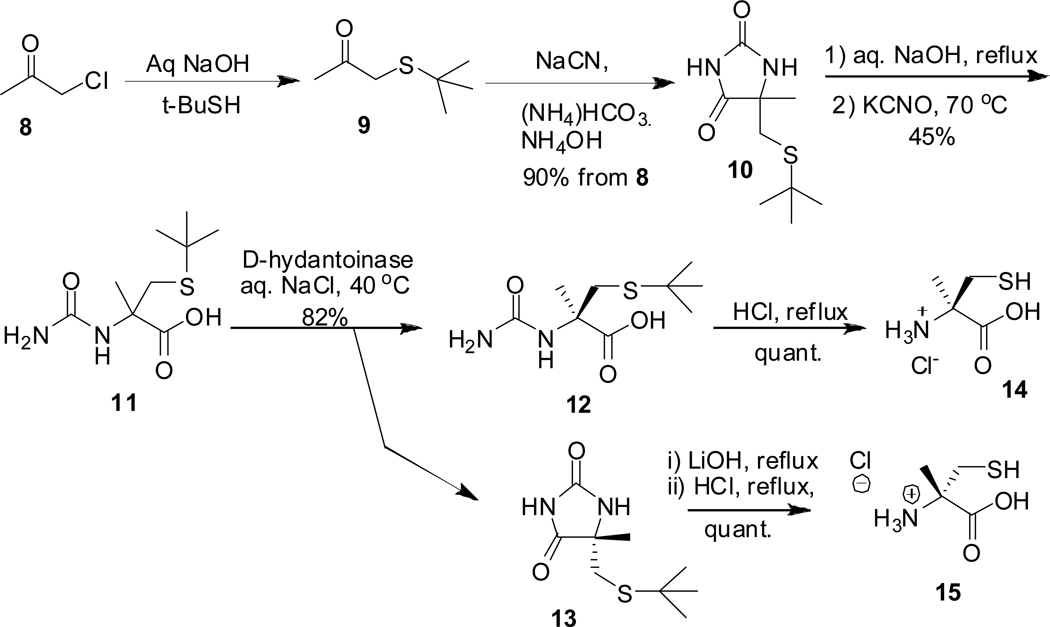
The synthesis of (R)-α-methylcysteine HCl 14 and its (S)-enantiomer 15 was achieved via the enzymatic resolution of racemic precursor 11 (scheme 3).14 Reaction of chloroacetone 8 and t-butyl mercaptan under basic conditions gave 9, which was subjected to Bucherer-Berg conditions to produce hydantoin 10. This one-pot protocol gave 90% yield of 10 after two steps. Hydantoin 10 was converted to 11 in one pot by hydrolysis with aqueous NaOH followed by KCNO treatment. Resolution of 11 with D-hydantoinase afforded 12 and 13 which were converted to 14 and 15 respectively, in quantitative yields.14
Scheme 3.
Synthesis of (R)- and (S)-α-methylcysteine
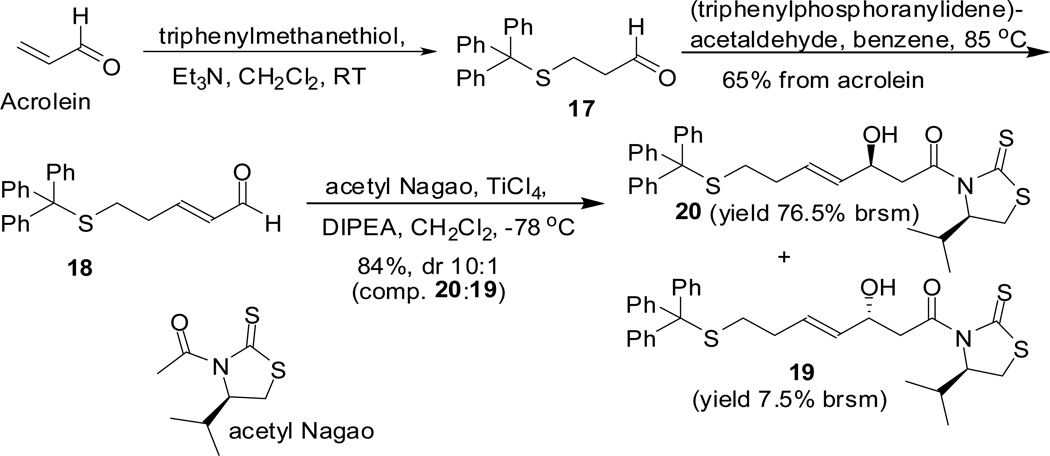
Turning our attention to the synthesis of 20 (Scheme 4), conjugate addition of triphenylmethanethiol to acrolein 16 gave 17, which was used in a Wittig reaction to yield 18.15 This one-pot protocol gave 65% overall yield for the two steps. Acetate aldol condensation of the aldehyde 18 with acetyl Nagao auxiliary was used to synthesize 20 in 84% diastereomeric excess.13 The S-configuration of the newly created chiral center of molecule 20 was established by modified Mosher ester analysis.16
Scheme 4.
Synthesis of the β-hydroxycarboxamide 20
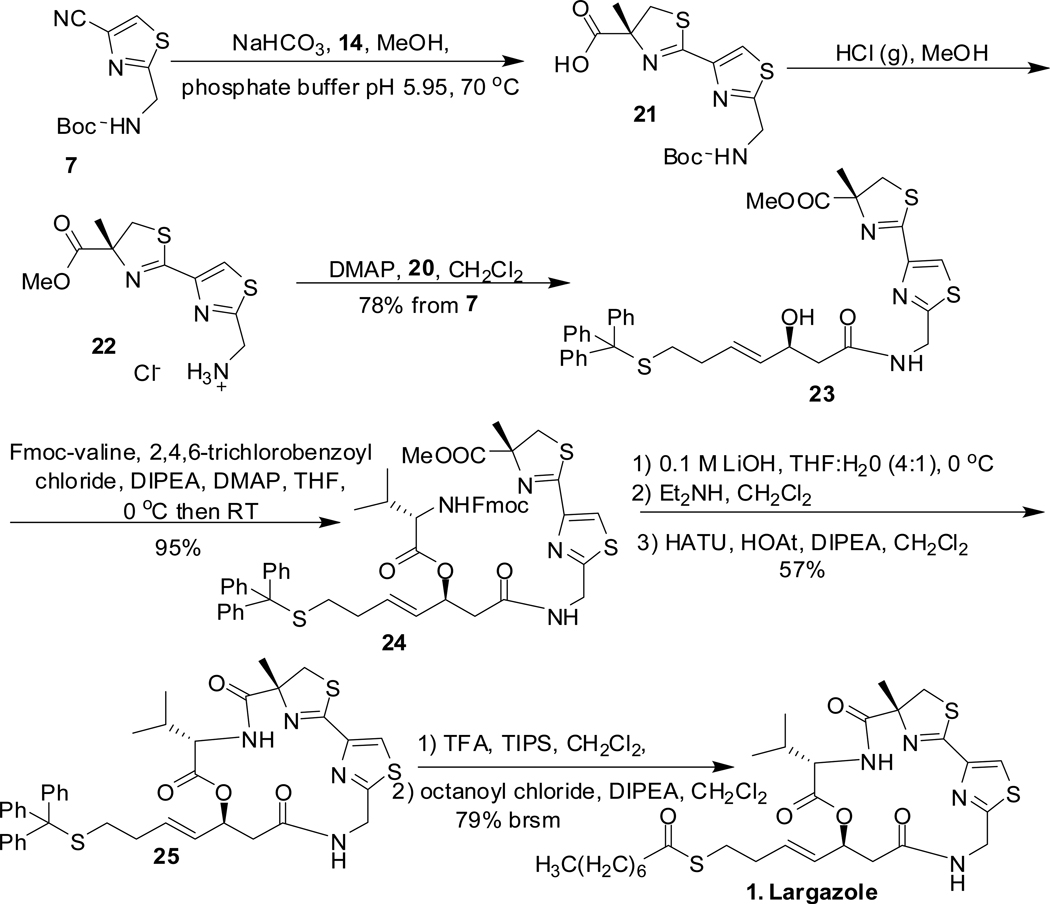
With the necessary building blocks in hand, the assembly of largazole 1 was undertaken as shown in scheme 5. The nitrile 7 was condensed with (R)-α-methylcysteine HCl 14 to obtain the thiazole-thiazoline carboxylic acid 21.11a After simultaneous removal of Boc group and esterification of the free carboxylic acid under acidic conditions in MeOH, acyl group was transferred17 from 20 to 22 to obtain 23. The formation of 21 from nitrile 7 and the one pot conversion of 21 to 23 proved very efficient with 78% yield for 3 steps. Yamaguchi esterification10a, 18 was used to couple Fmoc-valine to 23 to afford the acyclic precursor 24. After saponification and Fmoc group removal, macrocyclization with HOAt, HATU, and Hunig’s base yielded the cyclized product 25 in 56% overall yield over the three steps. Deprotection of trityl group gave largazole thiol which was esterified with octanoyl chloride using Hunig’s base to give largazole 1 in 79% yield (based on recovered largazole thiol in esterification step) over two steps.
Scheme 5.
Synthesis of largazole 1
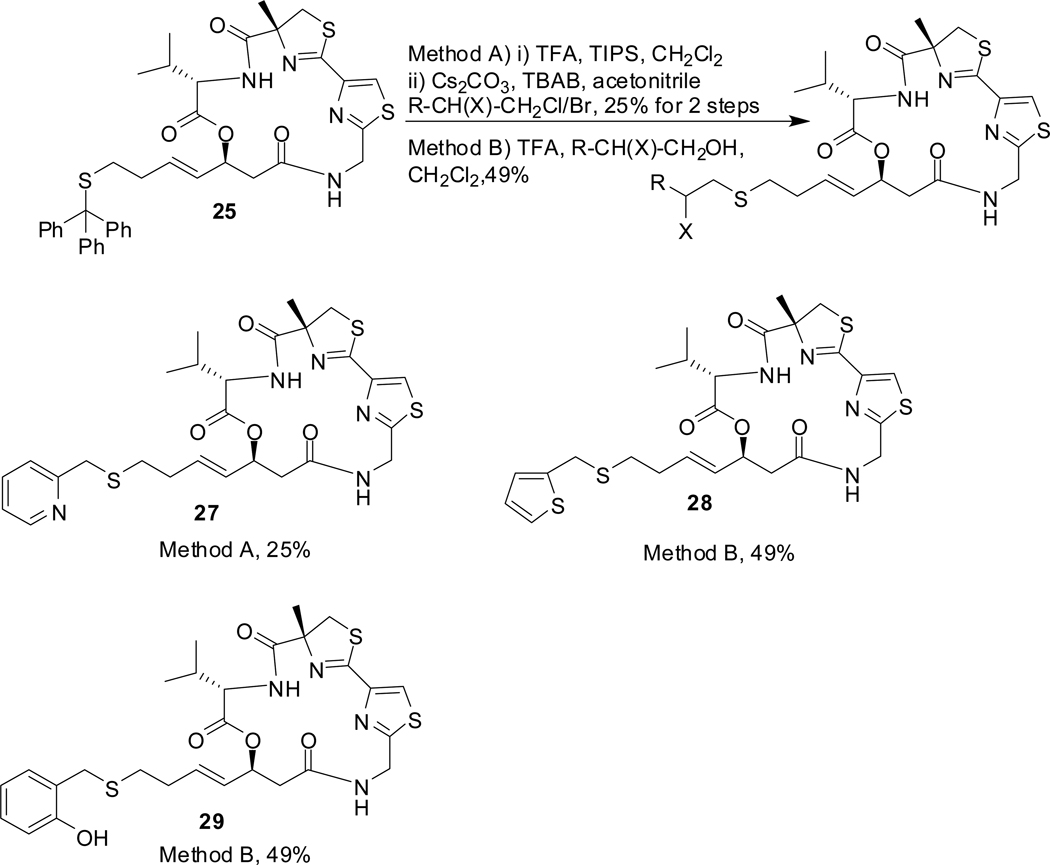
Scheme 6 shows the general method used for the synthesis of largazole analogues with modification in the zinc-binding motif. These analogues are designed such that thiol and a second heteroatom in the side chain are 2–3 atoms apart and are thus capable of interacting with Zn2+ via 5/6 membered cyclic transition states (Figure 2).
Scheme 6.
Synthesis of analogues with modified metal-binding motif
The analogues 27, 28 and 29 were synthesized following the removal of trityl group by nucleophilic substitution of the corresponding precursors by either method A (basic)19 or one-pot method B (acidic)20 as shown in scheme 6.
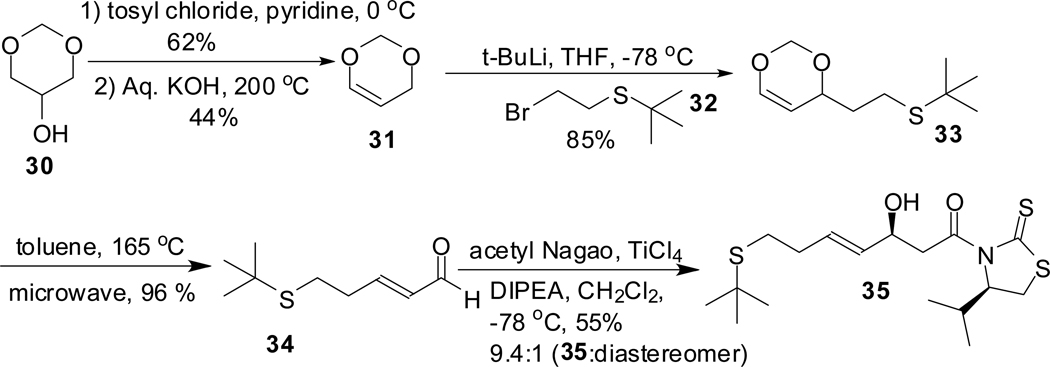
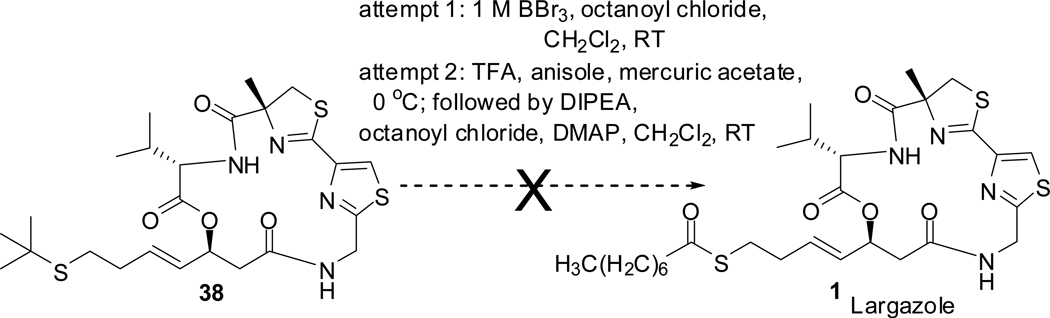
In a previous attempt to synthesize largazole 1 by a similar approach, we used t-butyl group as the protecting group for thiol. Synthetic route to make the t-butyl protected alcohol 35 is depicted in scheme 7. Synthesis of alcohol 35 started from glycerol formal 30 which was converted to dioxene 31 in two steps. Alkylation of 31 with 2-[(2-bromoethyl)sulfanyl]-2-methylpropane 32 via the lithium derivative gave 33. Compound 33 underwent retro Diels-Alder reaction to give the aldehyde 3421 which was used in aldol reaction to furnish the alcohol 35. It was used as described in scheme 5 to make the cyclic core 38. Attempts to remove the t-butyl group as illustrated in scheme 8 (1 M BBr3;22 trifluoroacetic acid, anisole, mercuric acetate23) and esterify with octanoyl chloride to form largazole 1 failed (see supporting information for details). With BBr3, starting material was recovered. Treatment with TFA/anisole/mercuric acetate indicated (TLC) the formation of a polar product which was subjected to thioesterification, but no largazole 1, largazole thiol or starting material was recovered.
Scheme 7.
Synthesis of t-butyl protected β-hydroxycarboxamide 35
Scheme 8.
Attempted synthesis of largazole 1
Biological Evaluation
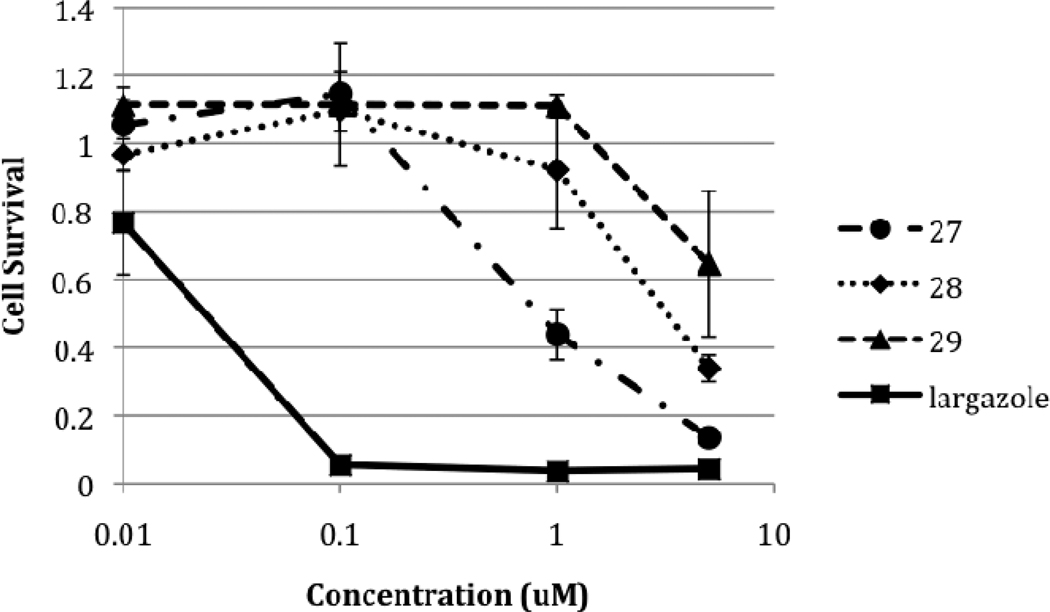
The antiproliferative activity of analogues 27, 28 and 29 were evaluated in the HCT116 colon adenocarcinoma cell line by a standard 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reduction assay (Promega) using largazole 1 as the control as previously reported.24 Figure 3 shows the results of the MTS assay after 96 h of treatment with 10 nM, 100 nM, 1 µM and 5 µM concentrations of the compounds. Largazole 1 inhibited the growth of HCT116 cells with a GI50 of 10 nM whereas the three side chain analogues showed activity only at higher concentrations. The pyridine analogue 27 demonstrated the greatest effect on cell survival of the three with a GI50 of 1.0 µM.
Figure 3.
Growth inhibitory effects of largazole 1 and analogues on HCT116 colon carcinoma cells. Each point represents the mean of two experiments; each with 4 replicates with the error bars indicating the SEM.
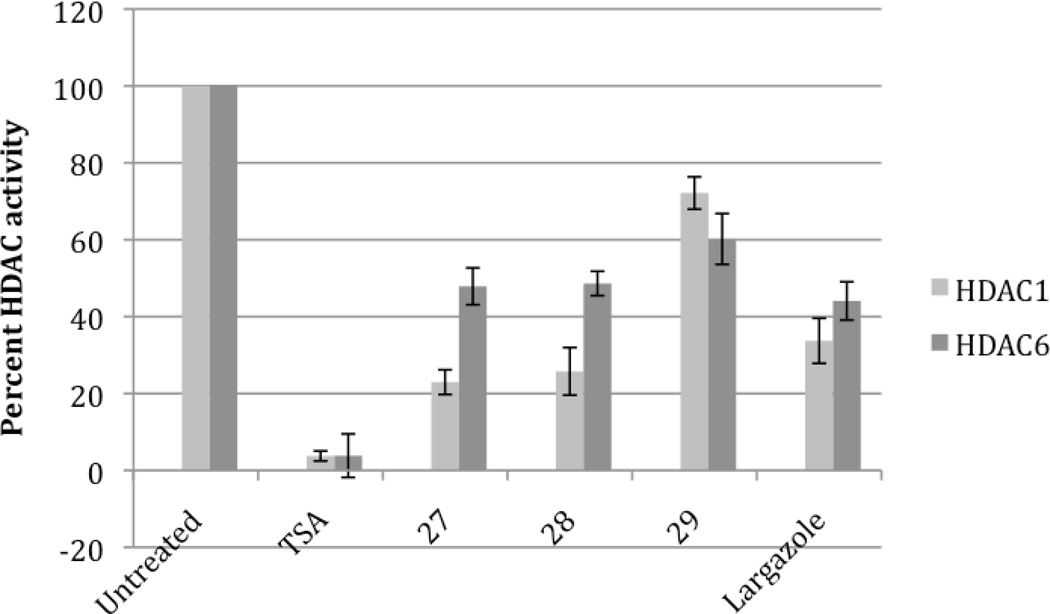
In order to ascertain the inhibitory effects of these analogues, in vitro fluorimetric assays on recombinant HDACs were performed in the presence and absence of the analogues. Analogues 27 and 28 at 1 µM significantly inhibited HDAC1 activity by almost 80 percent (Figure 4). Analogue 29 had a modest inhibition of HDAC1, with only a ~30 percent decrease of activity. Largazole 1 demonstrated a decrease of HDAC1 activity similar to analogues 27 and 28. Assays using HeLa nuclear extracts as a source for class 1 and 2 HDAC activity were nearly identical to what was observed with HDAC1 (data not shown). In vitro evaluation of the inhibition of recombinant HDAC6 enzyme showed ~50% decrease of activity with all of the analogues, including largazole 1, at a concentration of 1 µM (Figure 4).
Figure 4.
In vitro HDAC inhibitory activity – Recombinant HDAC1 and 6 activity was measured with and without the analogues. Reactions were carried out for 1 hour at a concentration of 1 µM for all analogues. HDAC1 showed the greatest inhibition with analogues 27 and 28, while HDAC6 inhibition was more modest and less specific to any of the largazole based analogues.
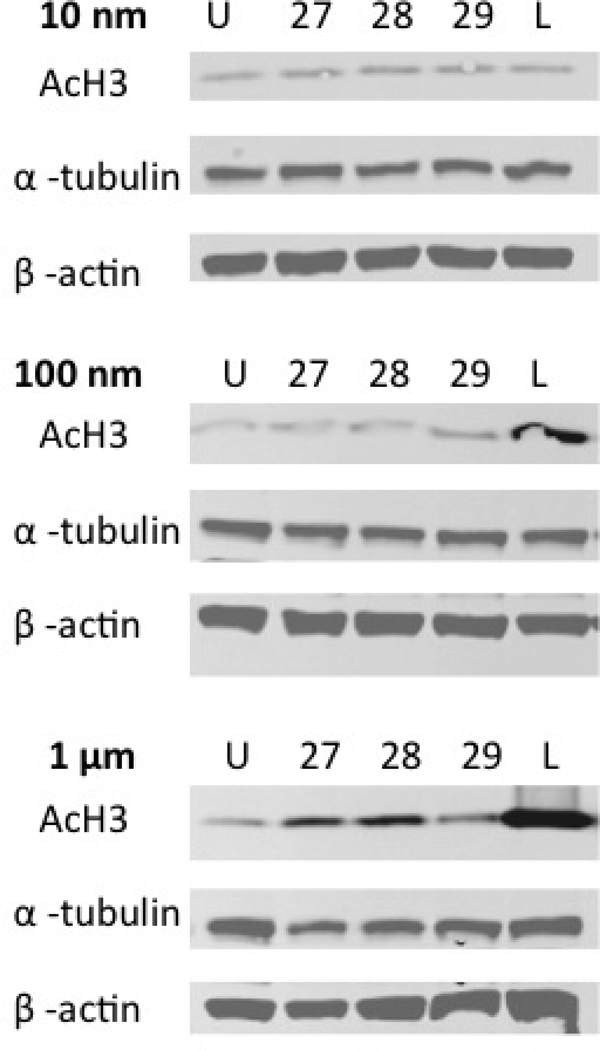
To evaluate the effects of these compounds on HDAC activity in a more physiologically relevant system, we treated a colorectal cancer cell line, HCT116, with these analogues at increasing concentrations for 24 hours. We examined the downstream effects of HDAC inhibition on global histone H3 acetylation by Western blot analysis after 24 hours of cellular exposure to 10 nM, 100 nM, and 1 µM of each compound. No increase in global acetylation was observed in treated cells exposed to any of the compounds at 10 nM (Figure 5). Largazole 1 showed an increase of global acetylation at 100 nM. Analogues 27 and 28 showed a significant increase in global acetylation at 1 µM, though much lower than what was observed with largazole 1.
Figure 5.
Western blot analysis of global acetylated H3 and acetylated α-tubulin proteins following the treatment of HCT116 cells with largazole 1 (L) and analogues at 10 nM, 100 nM and 1 µM concentrations after 24 hours of treatment. Increases of global acetylation were observed with largazole 1 at 100 nM and 1 µM and modest increases with analogues 27 and 28. α-tubulin levels remained unchanged.
In order to ascertain the effect of the analogues on HDAC6 activity, we studied the levels of α-tubulin acetylation after exposure to 10 nM, 100 nM and 1 µM concentrations. Again, we measured the α-tubulin acetylation using Western blot analysis of whole cell lysates from cells treated for 24 hours. No changes in α-tubulin acetylation were observed by exposure to any of the analogues, including largazole 1 (Figure 5). This concurs with earlier findings that HDAC6 is not a target for largazole 1.10b
Discussion
Histone deacetylase inhibitors that modulate post-transcriptional modification of proteins and target reversible epigenetic repression of gene expression are a promising source of selective anticancer agents. Although the basic cellular functions of individual HDAC isoforms are not fully characterized, the aberrant expression of specific HDAC isoforms in some types of cancer cells suggests that specific isoforms may be targeted to develop selective anticancer agents. With two inhibitors SAHA (vorinostat) and FK228 (romidepsin) already approved by the USFDA for treatment of cutaneous T-cell lymphoma (CTCL) and a number of other candidates in the drug pipelines, HDACis are being extensively investigated as potential selective anticancer agents. SAHA with a promiscuous hydroxamic acid group as the metal-binding domain is a pan-inhibitor of HDACs where as FK228 with a 3-hydroxy-7-mercapto-4-heptenoic acid side chain masked as a disulfide bridge as the metal-binding domain is a class I inhibitor with no effect on HDAC6. Although the thiol group is not as strong a Zn2+ binding functionality as hydroxamic acid,25 FK228 has a higher potency than SAHA due to additional compensatory binding interaction of the cap group with the rim region of the active site. The recently isolated depsipeptide largazole 1 has a high degree of structural and functional similarity to FK228 and like FK228, is a pro-drug of the active thiol. A range of largazole analogues synthesized by a number of groups has unraveled some SAR information related to potency and selectivity. The cyclic cap of the molecule which interacts with the less conserved rim region of the receptor has been the major target of structural modifications. Notable among them are the variable substitution of thiazoline methyl at C-7 or oxidation of thiazoline to thiazole11i and replacement of L-valine with other amino acids10d, 11h without adverse effect on potency, reduction in potency accompanying change of configuration at C210c, 26 and C1710d and conversion of lactone to lactam,10e equipotent replacement of dihydrothiazoline with dihydrooxazoline10c and the substitution of pyridine for thiazole10c to generate the most potent HDACi known to date. Major SAR determinants of the side chain are the preference for a trans-alkene vs a cis-alkene,11h preference for thiol vs esters,11a ketones,11a benzamides and α-thioamides10c as the metal-binding moiety and most importantly the requirement of an optimal four atom linker between the hydrophobic cap group and the Zn2+ coordination thiol group10d, 11c in comparison to 5–7 atom side chains common among most HDACis. We modified the metal-binding domain by introducing a second heteroatom in the side chain 2–3 atoms apart from sulfur to facilitate chelation with Zn2+ via a 5/6-membered cyclic transition state.
We adopted a convergent approach to the synthesis of largazole 1 and used it as a general method to synthesize side chain analogues by nucleophilic substitution of the desired precursors with largazole thiol. In our initial approach to largazole 1 we used t-butyl for thiol protection. The t-butyl protected 5-mercapto-2-pentenal 34 was conveniently obtained by the retro Diels-Alder reaction of the 6- substituted-1,3-dioxene 33. After conversion to the t-butyl protected 3-hydroxy-7-mercapto-4-heptenoic acid derivative 35 it was used in our convergent approach to synthesize t-butyl protected largazole thio ether 38. Attempts to convert 38 to largazole 1 by removal of the t-butyl group by a number of standard protocols and esterification with octanoyl chloride were unsuccessful. This was overcome by switching to trityl protecting group. Largazole 1 and the side chain analogues were synthesized via largazole thiol following removal of trityl under acid conditions.
Largazole 1 inhibited the growth of HCT116 cells with a GI50 of ~10 nM. The three side chain analogues were less active, the pyridine analogue 27 being the most active of the three with a GI50 of ~1.0 µM. The other two analogues were active at higher concentrations. In vitro analysis of HDAC activity demonstrated that all of these analogues have some inhibitory effects on recombinant HDAC1 and HDAC6. The inhibition of HDAC6 observed with analogues 27 and 28 was modest in comparison to their effects on HDAC1 or global class 1 and 2 HDACs. The modest inhibition of HDAC1 by largazole 1 observed is somewhat in contrast to previous reports.10a, b However, the increased global acetylation of H3 in treated cells indicates efficient in situ inhibition of HDAC1. The effects on cellular proliferation are consistent with significant increase of global H3 acetylation observed with largazole 1 at 1 µM concentration and modest induction of global H3 acetylation observed for analogues 27 and 28. There was no change in acetylated α-tubulin levels with any of the compounds indicating they have no effect on HDAC6. The marked difference in the activity of largazole 1 in the cellular assays compared to the in vitro HDAC activity assays can be attributed to the hydrolysis of the pro-drug largazole 1 to largazole thiol by cellular esterases. Thioethers bind weakly to hard or borderline metal ions like Mg2+, Mn2+, Cu2+ and Zn2+.27 These largazole analogues were designed to see whether the introduction of a second hetero atom would lead to stronger binding to the metal ion and also to isoform/class selectivity. However, it is not possible to suggest if the diminished biological activity observed is due to poor affinity of thioether group for Zn2+ ion or incompatibility of the modified metal binding moiety with the HDAC active site. Depsipeptides such as largazole 1 and FK228 constitute a distinct class of HDACis characterized by a masked thiol function as the metal-binding domain and a side chain with a characteristic four-atom spacer between the macrocycle and the metal binding domain. The side chain occupies the hydrophobic channel between the metal binding site and the hydrophobic rim of the active site and positions the two end groups of the inhibitor for binding interaction with the receptor. Despite the high sequence similarity within the active site, presence of discrete binding cavities in the vicinity of the metal ion may be taken advantage of to achieve isoform selectivity as exemplified by HDACis with substituted benzamide metal-binding domains and class I selectivity.12 The exploration of alternative metal-binding domains is the way forward to the development of such isoform and class-selective HDACis.
Conclusion
Largazole 1 and its analogues were synthesized efficiently and in high yields using several one-pot two-reaction protocols. The stereochemistry of 20 was installed by acetate aldol reaction. Macrolactamization with HATU, HOAt and Hunig’s base gave cyclic product 25. An initial attempt to synthesize largazole 1 using t-butyl group to protect thiol proved problematic due to difficulty in removing the protecting group for thioesterification. Using trityl group instead, deprotection followed by thioesterification with octanoyl chloride provided largazole 1. This method provides an efficient synthetic approach to largazole analogues. Three side chain analogues with multiple heteroatoms for metal binding synthesized using this approach were found to be less potent than largazole 1 as measured by growth inhibition in HCT116 colorectal carcinoma cell line and induction of global H3 acetylation. However, the moderate selectivity for HDAC1 over HDAC6 observed with analogues 27 and 28 in the in vitro HDAC inhibitory activity assay may guide the structural modification of the metal-binding motif to design selective HDAC inhibitors. Further studies in this direction are continuing.
Experimental Section
THF was refluxed with Na and benzophenone and freshly distilled prior to use. NMR spectra were recorded on Varian INOVA 600 MHz and Varian VXRS 400 MHz instruments and calibrated using residual undeuterated solvent as internal reference (CDCl3: 1H NMR at δ 7.26, 13C NMR at δ 77.23; D2O: 1H NMR at δ 4.8; DMSO-d6: 1H NMR at δ 2.5, 13C NMR at δ 39.51). Optical rotations were recorded on an AUTOPOL III 589/546 polarimeter. High-resolution mass spectra (HRMS) were recorded on a Micromass LCT Electrospray mass spectrometer at the Central Instrument Facility, the Wayne State University, Detroit, Michigan and on a Micromass Q-Tof II Electrospray mass spectrometer at the Mass Spectrometry and Proteomics Facility, the Ohio State University, Columbus, Ohio. Crude products were purified by flash column chromatography on silica gel (32–63 µ) purchased from Dynamic Adsorbents Inc. and by preparative thin layer chromatography on 1000 µ Uniplates purchased from Analtech Inc. using commercial solvents as specified. HPLC analyses were performed on a Waters 1525 Binary Pump HPLC system with Waters 2487 Dual Wavelength Absorbance Detector on a Symmetry C18 column (reverse phase, 5 µ, 4.6 mm × 150 mm) using a linear gradient of 10–100% H2O:MeOH over 15–20 min; flow rate of 1 mL/min and UV detection at 254 nm. Structural integrity and purity of the test compounds were determined by the composite of 1H and 13C NMR, HRMS and HPLC and were found to be > 95% pure. D-Hydantoinase, recombinant, immobilized from E. Coli was purchased from Fluka Chemie AG (catalog no: 53765; CAS: [9030-74-4]).
tert-Butyl (4-carbamoylthiazol-2-yl)methylcarbamate (6)
A mixture of Boc-thioglycinamide 4 (0.95 g, 5 mmol, 1 equiv) and bromopyruvic acid (0.835 g, 5 mmol, 1 equiv) in dry THF (20 mL) was stirred at 50 °C under nitrogen for 2 h. The reaction mixture was concentrated to dryness and the residue was dried by repeated azeotropic removal of moisture with toluene. The crude carboxylic acid thus obtained was dissolved in dry THF (50 mL), and triethylamine (1.3 g, 12.94 mmol, 2.6 equiv) and ethyl chloroformate (0.681 g, 6.24 mmol, 1.25 equiv) were added at 0 °C and stirred for 30 minutes at the same temperature. Ammonium hydroxide (~ 1.1 g, 31.76 mmol, 5 equiv) was added and the reaction mixture was stirred at room temperature for 2 h. It was concentrated in vacuo and purified by flash column chromatography on silica gel in ethyl acetate/hexanes 33–100% to yield 6 (0.768 g, 60% over two steps from boc-thioglycinamide); mp 153–154 °C (lit.11c mp 153 °C). 1H NMR (400 MHz, CDCl3): δ 8.08 (s, 1H), 7.13 (br s, 1H), 5.94 (brs, 1H), 5.34 (br s, 1H), 4.59 (d, J = 5.6 Hz, 2H), 1.47 (s, 9H) ppm. 13C NMR (100 MHz, CDCl3): δ 169.7, 163.1, 155.8, 149.3, 124.8, 80.8, 42.5, 28.5 ppm.
tert-Butyl (4-cyanothiazol-2-yl)methylcarbamate (7)
To a solution of amide 6 (0.204 g, 0.797 mmol, 1 equiv) in dichoromethane (20 mL) at 0 °C was added triethylamine (0.174 g, 1.725 mmol, 2.16 equiv) followed by dropwise addition of trifluoroacetic anhydride (0.181 g, 0.863 mmol, 1.08 equiv). The reaction mixture was stirred at room temperature for 1 h, concentrated in vacuo, and purified by flash chromatography on silica gel in ethyl acetate/hexanes 20–50% to obtain the nitrile 7 (0.189 g, 99%); mp 84–85 °C (lit.11c mp 84 °C). 1H NMR (600 MHz, CDCl3): δ 7.95 (s, 1H), 5.3 (s, 1H), 4.62 (d, J = 6 Hz, 2H), 1.47 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 171.7, 155.8, 131.1, 126.6, 114, 81, 42.5, 28.5.
(2E)-5-[(Triphenylmethyl)thio]-2-pentenal (18)
To a solution of triphenylmethanethiol (6.91g, 25 mmol, 2.09 equiv) in dichloromethane (100 mL) was added acrolein 16 (1.965 g, 35 mmol, 2.9 equiv) and triethylamine (3.56 g, 35 mmol, 2.9 equiv). The resulting mixture was stirred for 1 h at room temperature and was concentrated to give the aldehyde 17 as a white solid, which was used in the next step without purification. A solution of the aldehyde 17 obtained above and (triphenylphosphoranylidene)acetaldehyde (3.64 g, 11.96 mmol, 1 equiv) in dry benzene (150 mL) was refluxed for 8 h. The reaction mixture was concentrated and purified by flash chromatography on silica gel in dichloromethane/hexanes 20–25% to afford aldehyde 18 (5.83 g, 65% over the two steps); mp 140–141 °C. 1H NMR (400 MHz, CDCl3): δ 9.43 (d, J = 8.0 Hz, 1H), 7.42 (dd, J = 2.4, 7.6 Hz, 6H), 7.29 (dt, J = 2.0, 6.8 Hz, 6H), 7.22 (dt, J = 2.4, 7.2 Hz, 3H), 6.60-6.67 (m, 1H), 5.95-6.01 (dd, J = 8.0, 15.6 Hz, 1H), 2.29-2.37 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 194, 156, 144.7, 133.8, 129.7, 128.2, 127, 67.2, 31.9, 30.2.
3S-Hydroxy-1-(4R-isopropyl-2-thioxo-thiazolidin-3-yl)-7-tritylsulfanyl-hept-4E-en-1-one (20)
To a solution of acetyl Nagao chiral auxiliary (1.493 g, 7.355 mmol, 1 equiv) in dichloromethane (60 mL) at 0 °C, was added TiCl4 (1.72 g, 9.05 mmol, 1.23equiv). After stirring the reaction mixture for 5 minutes, cooled to −78 °C and was added of Hunig’s base (1.872 g, 9.2 mmol, 1.25 equiv). It was stirred for 2 h at the same temperature and was added the aldehyde 18 (2.6 g, 7.263 mmol, 0.987 equiv) in dichloromethane (8 mL) dropwise. The reaction mixture was stirred for 1 h at −78 °C. It was removed from cooling bath, treated with water (15 mL), and diluted with dichloromethane (50 mL). The mixture was extracted with dichloromethane; the organic layer was washed with saturated NaCl (40 mL) and dried over anhydrous Na2SO4. It was concentrated in vacuo and the residue was purified by flash chromatography on silica gel in dichloromethane/hexanes 25–90% to obtain the major isomer 20 as a thick yellow oil (1.963 g, 76.5% brsm), and the diastereomer 19 (0.193 g, 7.5%) with recovery of acetyl Nagao chiral auxiliary (0.513 g). Major isomer: [α]D20 - 149 (c 3.7, CHCl3).
1H NMR (400 MHz, CDCl3): δ 7.41 (d, J = 7.2 Hz, 6H), 7.28 (t, J = 7.2 Hz, 6H), 7.21 (t, J = 7.2 Hz, 3H), 5.61-5.55 (m, 1H), 5.46 (dd, J = 6.0, 15.2 Hz, 1H), 5.12 (t, J = 6.8 Hz, 1H), 4.57 (t, J = 5.8 Hz, 1H), 3.56 (dd, J = 2.8, 17.6 Hz, 1H), 3.47 (dd, J = 7.6, 11.6 Hz, 1H), 3.28 (dd, J = 8.8, 17.6 Hz, 1H), 2.99 (d, J = 11.6 Hz, 1H), 2.82 (s, 1H), 2.37-2.32 (m, 1H), 2.21 (t, J = 7.2 Hz, 2H), 2.09 (q, J = 7.2 Hz, 2H), 1.05 (d, J = 6.8 Hz, 3H), 0.97 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 203.1, 172.7, 145, 132, 130.2, 129.7, 128, 126.8, 71.6, 68.6, 66.7, 45.4, 31.6, 31.6, 31, 30.8, 19.3, 18.0.
(R)-2-(2-((tert-Butoxycarbonylamino)methyl)thiazol-4-yl)-4,5-dihydrothiazole-4-carboxylic acid (21)
To a well stirred mixture of the nitrile 7 (0.096 g, 0.4 mmol, 1 equiv) and NaHCO3 (0.232 g, 2.76 mmol, 5.6 equiv) in methanol (5 mL), was added (R)-α-methylcysteine hydrochloride 14 (0.084 g, 0.491, 1.23 equiv) followed by phosphate buffer pH 5.95 (2.5 mL). The reaction mixture was degassed with nitrogen before stirring it under nitrogen at 70 °C for 1 h. It was acidified with 1 M HCl and extracted with ethyl acetate (15 mL) three times. The combined organic extract was washed with saturated NaCl solution, dried over anhydrous sodium sulfate and concentrated to obtain the carboxylic acid 21 (0.137g) which was used in the next step without further purification.
(R)-Methyl 2-(2-((3S-hydroxy-7-(tritylthio)hept-4E-enamido)methyl)thiazol-4-yl)-4-methyl-4,5-dihydrothiazole-4-carboxylate (23)
A solution of carboxylic acid 21 (0.136 g, 0.38 mmol, 1 equiv) in anhydrous methanol (5 mL) was bubbled with HCl gas for 5 minutes. The reaction mixture was stirred overnight and concentrated in vacuo to give compound 22 which was azeotroped using toluene before taking it to the next step. A mixture of above obtained compound 22 and DMAP (0.121 g, 0.992 mmol, 2.61 equiv), in dichloromethane (2 mL) was stirred for 5 minutes, and a solution of aldol product 20 (0.214, 0.38 mmol, 1 equiv) in dichloromethane (1 mL) was added. The reaction mixture was stirred for 1 h, concentrated in vacuo and purified by flash chromatography on silica gel in ethyl acetate/hexanes 20–100% to afford the alcohol 23 (0.191 g, 78% over 3 steps a–c). [α]D20 - 11 (c = 3.55, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.90 (s, 1H), 7.38 (d, J = 8.4 Hz, 6H), 7.26 (t, J = 7.6 Hz, 6H), 7.19 (t, J = 7.6 Hz, 3H), 7.07 (t, J = 6.0 Hz, 1H), 5.50–5.58 (m, 1H), 5.37-5.43 (dd, J = 6.0, 15.2 Hz, 1H), 4.63-4.74 (m, 2H), 4.43 (m, 1H), 3.86 (d, J = 11.6 Hz, 1H), 3.78 (s, 3H), 3.48 (s, 1H), 3.25 (d, J = 11.6 Hz, 1H), 2.34-2.46 (m, 2H), 2.18 (t, J = 7.2 Hz, 2H), 2.05 (q, J = 7.2, Hz, 2H), 1.63 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 173.8, 172.0, 168.1, 162.9, 148.4, 144.9, 132.4, 130.3, 129.7, 128, 126.8, 122.4, 84.6, 69.2, 66.7, 53.1, 42.9, 41.6, 40.9, 31.6, 31.4, 24.1. HRMS-ESI (m/z): [M+H]+ calcd for C36H38N3O4S3, 672.2019; found, 672.2024
(4R)-Methyl 2-(2-((8S)-1-(9H-fluoren-9-yl)-5-isopropyl-3,6,10-trioxo-8-((E)-4-(tritylthio)-but-1-enyl)-2,7-dioxa-4,11-diazadodecan-12-yl)thiazol-4-yl)-4-methyl-4,5-dihydrothiazole-4-carboxylate (24)
To a solution of Fmoc-L-valine (0.09 g, 0.0266 mmol, 1 equiv) in THF (1 mL) at 0 °C, was added Hunig’s base (0.045 g, 0.345 mmol, 1.29 equiv) and 2,4,6-trichlorobenzoyl chloride (0.078 g, 0.32 mmol, 1.2 equiv). The reaction mixture was stirred at 0 °C for 1 h. When TLC indicated formation of the anhydride, alcohol 23 in THF (1 mL) was added to the reaction mixture at 0 °C. It was stirred overnight at room temperature. The reaction mixture was concentrated in vacuo and flash chromatography purification on silica gel in ethyl acetate/hexanes 20–100% yielded the acyclic precursor 24 (0.125 g, 94%). [α]D20 - 12 (c 6.83, CHCl3). 1H NMR (600 MHz, CDCl3): δ 7.89 (s, 1H), 7.76 (d, J = 7.2 Hz, 2H), 7.57 (d, J = 7.2 Hz, 2H), 7.41-7.37 (m, 8 H), 7.30 (t, J = 7.2 Hz, 2H), 7.26-7.29 (m, 6 H), 7.20 (t, J = 7.2 Hz, 3H), 6.74 (t, J = 8.4 Hz, 1H), 5.69-5.65 (m, 1H), 5.61 (dd, J = 6.0, 13.2 Hz, 1H), 5.42 (dd, J = 7.8, 15.0 Hz, 1H), 5.21 (d, J = 7.8 Hz, 1H), 4.7 (d, J = 6.0 Hz, 2H), 4.38 (dd, J = 7.2, 10.8 Hz, 1H), 4.33 (dd, J = 6.6, 10.8 Hz, 1H), 4.19 (t, J = 6.6 Hz, 1H), 4.05 (dd, J = 6.0, 8.4, 1H), 3.85 (d, J = 10.8 Hz, 1H), 3.78 (s, 3H), 3.24 (d, J = 10.4 Hz, 1H), 2.58 (d, J = 6. Hz, 2H), 2.2-2.12 (m, 2H), 2.07-2.01 (m, 2H) 1.62 (s, 3H), 0.9 (d, J = 7.2 Hz, 3H), 0.85 (d, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 173.8, 169.2, 168.6, 163, 156.6, 148.5, 144.9, 143.6, 141.5, 134.2, 129.7, 128.03, 127.9, 127.7, 127.3, 126.8, 125.2, 122.3, 120.15, 84.7, 72.42, 67.2, 66.8, 59.6, 53.1, 47.3, 41.7, 41.6, 41.3, 31.5, 31.3, 31, 29.9, 24.1, 19.2, 18.1. HRMS-ESI (m/z): [M + H]+ calcd for C56H57N4O7S3, 993.3397; found, 993.3389.
Cyclic core 25
To a stirred solution of 24 (133 mg, 0.134 mmol, 1 equiv) in THF/H2O (4:1, 4 mL) at 0 °C, was added 0.1 M LiOH (1.4 mL, 0.14 mmol, 1.045 equiv) dropwise over a period of 15 minutes. After stirring at 0 °C for 1 h, it was acidified with 1 M HCl solution and was extracted with EtOAc three times. The combined organic layer was washed with brine, dried over anhydrous sodium sulfate, and concentrated. The reaction mixture was purified by preparative TLC in ethyl acetate to get the carboxylic acid. It was dissolved in dichloromethane (13 mL) and treated with diethylamine (0.462 g, 6.32 mmol, 47.16 equiv). After stirring at room temperature for 3 h, it was concentrated to dryness to afford the free amino derivative. After drying azeotropically with toluene, it was treated with HATU (0.105 g, 0.276 mmol, 2.06 equiv), HOAt (0.038 g, 0.279 mmol, 2.08 equiv), dichloromethane (130 mL, ~ 1Mmol), and Hunig’s base (0.074 g, 0.575 mmol, 4.29 equiv) and the mixture was stirred for 30 h at room temperature. The reaction mixture was concentrated to dryness and was purified by flash chromatography on silica gel in ethyl acetate/hexanes 10–60% to yield the cyclic core 25 (0.056 g, 57% over three steps from 24). [α]D20 + 2.5 (c 0.95, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.73 (s, 1H), 7.37 (d, J = 8.4 Hz, 6H), 7.26 (t, J = 8 Hz, 6H), 7.20-7.15 (m, 4H), 6.49 (dd, J = 2.8, 9.2 Hz, 1H), 5.68-5.71 (m, 1H), 5.60 (m, 1H), 5.38 (dd, J = 6.8, 15.6 Hz, 1H), 5.19 (dd, J = 8.8, 17.6 Hz, 1H), 4.55 (dd, J = 3.2, 9.6 Hz, 1H), 4.11 (dd, J = 3.2, 17.6 Hz, 1H), 4.01 (d, J = 11.2Hz, 1H), 3.26 (d, J = 11.6 Hz, 1H), 2.77 (dd, J = 9.6, 16.4 Hz, 1H), 2.64 (dd, J = 3.2, 16 Hz, 1H), 2.15-2.19 (m, 2H), 1.98-2.06 (m, 2H), 1.82 (s, 3H), 0.67 (d, J = 6.8 Hz, 3H), 0.50 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 173.7, 169.4, 168.9, 168.1, 164.6, 147.6, 144.9, 133.3, 129.7, 128.1, 126.8, 124.3, 84.6, 72.0, 66.8, 58.0, 43.5, 41.2, 40.8, 34.2, 31.5, 31.4, 29.9, 24.4, 19.1, 16.9.
Largazole (1)
To a solution of 25 (0.033 g, 0.045 mmol, 1 equiv) in dichloromethane (5 mL) at 0 °C was added triisopropylsilane (0.013 g, 0.083 mmol, 1.85 equiv) followed by trifluoroacetic acid (0.307 g, 2.69 mmol, 59.85 equiv). After stirring for 3 h at room temperature, the reaction mixture was concentrated in vacuo. The crude product was purified by flash chromatography on silica gel in 20% ethyl acetate/hexanes to first remove impurity followed by ethyl acetate to obtain the largazole thiol (0.022 g). To a stirred solution of largazole thiol in dichloromethane (7 mL) at 0 °C, was added, Hunig’s base (0.045 g, 0.35 mmol, 7.7 equiv) and octanoyl chloride (0.044 g, 0.27 mmol, 6 equiv). Catalytic DMAP (1 mg) in dichloromethane (0.1 mL) was added to the reaction mixture. After stirring for 4 h at room temperature, reaction was quenched with methanol and the mixture was concentrated in vacuo. Purification of the crude product by preparative thin layer chromatography on silica gel with ethyl acetate as solvent gave largazole 1 (0.010 g, 79%, based on recovered largazole thiol 0.012 g). [α]D20 + 19.5 (c 0.2, CHCl3). 1H NMR (600 MHz, CDCl3): δ 7.77 (s, 1H), 7.15 (d, J = 9.6 Hz, 1H), 6.41 (dd, J = 2.4, 9.0 Hz, 1H), 5.79-5.84 (m, 1H), 5.66 (m, 1H), 5.50 (dd, J = 6.6, 15.6 Hz, 1H), 5.29 (dd, J = 9.6, 18.0 Hz, 1H), 4.61 (dd, J = 3.0, 9.0 Hz, 1H), 4.27 (dd, J = 3.0, 17.4 Hz, 1H), 4.04 (d, J = 11.4 Hz, 1H), 3.28 (d, 11.4 Hz, 1H), 2.90 (t, J = 7.2 Hz, 2H), 2.85 (dd, J = 10.8, 16.2 Hz, 1H), 2.53 (t, J = 7.8 Hz, 2H), 2.31 (q, J = 7.2, Hz, 2H), 2.10-2.12 (m, 1H), 1.87 (s, 3H), 1.62-1.67 (m, 2H), 1.26-1.31 (m, 8H), 0.87 (t, J = 7.2 Hz, 3H), 0.68 (d, J = 7.2 Hz, 3H), 0.5 (d, 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ = 199.6, 174.7, 169.6, 169.1, 168.1, 164.7, 147.7, 133.0, 128.6, 124.4, 84.7, 72.3, 57.9, 44.4, 43.6, 41.3, 40.7, 34.4, 32.5, 31.8, 29.1, 28.1, 25.9, 24.4, 22.8, 19.1, 16.8, 14.3. HRMS-ESI (m/z): [M + Na]+ calcd for C29H42N4O5S3Na, 645.2211; found, 645.2215
Cytoproliferation assay
Cell proliferation was measured using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reduction assay with the CellTiter 96 One solution MTS assay as described by the manufacturer (Promega, Madison, WI). Briefly, cells (3×104 cells/well) were seeded in quadruplicate in 96- well plates and allowed to attach overnight. Media was replaced with 100 µL of fresh medium containing the appropriate concentration of compounds 27, 28, 29, or largazole 1. Following incubation at 37 °C, 5% CO2, 20 µL of CellTiter 96 One Solution was added per well and incubated 1.5 h at 37 °C and absorbance measured at 490 nm.
HDAC activity assays
HDAC activity was assayed using the Enzo Life Sciences (Plymouth Meeting, PA) fluorimetric drug discovery kits with specificity for HDAC1, 6, or using cellular extracts kits. Inhibition of global class 1 and 2 HDAC activity was ascertained using the included HeLa nuclear extracts. Experiments were performed as described by the protocol supplied by the manufacturer. Briefly, the indicated inhibitor, enzyme, and Fluor de Lys substrate were incubated for 1 hour when the developing solution was added and incubated for the designated time prior to reading. Experiments were repeated 3 times and done in triplicate each time. Analogues incubated with the substrate and developer was performed as a control and showed no influence on the fluorescent signal. Data show a representative experiment with error bars indicating standard deviation of the triplicates.
Evaluation of global histone acetylation levels
HCT116 cells were treated for 24 hours with 10 nM, 100 nM, or 1 µM or DMSO. Cell lysates were extracted using RIPA buffer and equal amounts (30 µg/Lane) were loaded onto an SDS-PAGE gel. Standard Western blotting protocols were used using the Invitrogen NuPAGE western blotting system. Primary antibodies used were AcH3 (Millipore), α-tubulin (Sigma), and β-actin (Sigma). Dye-conjugated secondary antibodies from Li-Cor Biosciences were used for detection and scanned using the Odyssey Infrared Detection System (LI-COR Biosciences, Lincoln, NE).
Supplementary Material
Acknowledgments
(R)-α-methylcysteine HCl was initially synthesized in the lab using D-hydantoinase enzyme. We thank Nagase Inc. for providing a sample of (R)-α-methylcysteine HCl at concessionary price when D-hydantoinase became commercially unavailable.
Grant support: NIH CA51085 (to RAC), Samuel Waxman Cancer Research Foundation (to RAC), and Susan G. Komen for the Cure Foundation (to RAC).
Abbreviations
- HDACs
histone deacetylases
- HAT
histone acetyltransferase
- SAHA
suberoylanilide hydroxamic acid
- MDA-MB-231
invasive transformed human mammary epithelial cells
- U2OS
transformed fibroblastic osteosarcoma cells
- NMuMG
nontransformed mouse mammary gland epithelial cell line
- HCT116
human colorectal carcinoma cell line
- HATU
2-(1H-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- HDACi
histone deacetylase inhibitor
- NMR
nuclear magnetic Resonance
- USFDA
United States food and drug administration
- SAR
structure-activity relationship
- TIPS
triisopropylsilane
- TBAB
tetrabutylammonium bromide
- TFAA
trifluoroacetic anhydride
- HOAt
1-hydroxy-7-aza-benzotriazole
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- TLC
thin layer chromatography
- CTCL
cutaneous T-cell lymphoma
Footnotes
Supporting Information available Experimental procedures, analytical and spectral characterization data and NMR spectra for compounds 10–14, 31–35 and analogues 27–29. NMR spectra for compounds 6–7, 18, 20, 23–25 and largazole 1. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]; (b) Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]; (c) Smith KT, Workman JL. Histone deacetylase inhibitors: anticancer compounds. Int J Biochem Cell Biol. 2009;41(1):21–25. doi: 10.1016/j.biocel.2008.09.008. [DOI] [PubMed] [Google Scholar]; (d) Mai A, Altucci L. Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. Int J Biochem Cell Biol. 2009;41(1):199–213. doi: 10.1016/j.biocel.2008.08.020. [DOI] [PubMed] [Google Scholar]; (e) Bertrand P. Inside HDAC with HDAC inhibitors. Eur J Med Chem. 2010;45(6):2095–2116. doi: 10.1016/j.ejmech.2010.02.030. [DOI] [PubMed] [Google Scholar]; (f) Zhang L, Fang H, Xu W. Strategies in developing promising histone deacetylase inhibitors. Med Res Rev. 2010;30(4):585–602. doi: 10.1002/med.20169. [DOI] [PubMed] [Google Scholar]; (g) Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6(2):107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cloos PAC, Christensen J, Agger K, Helin K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes & Development. 2008;22(9):1115–1140. doi: 10.1101/gad.1652908. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Biel M, Wascholowski V, Giannis A. Epigenetics--an epicenter of gene regulation: histones and histone-modifying enzymes. Angew Chem Int Ed Engl. 2005;44(21):3186–3216. doi: 10.1002/anie.200461346. [DOI] [PubMed] [Google Scholar]
- 3.(a) Mellert HS, McMahon SB. Biochemical pathways that regulate acetyltransferase and deacetylase activity in mammalian cells. Trends Biochem Sci. 2009;34(11):571–578. doi: 10.1016/j.tibs.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hodawadekar SC, Marmorstein R. Chemistry of acetyl transfer by histone modifying enzymes: structure, mechanism and implications for effector design. Oncogene. 2007;26(37):5528–5540. doi: 10.1038/sj.onc.1210619. [DOI] [PubMed] [Google Scholar]
- 4.Conley Barbara A, Wright John J, Kummar S. Targeting epigenetic abnormalities with histone deacetylase inhibitors. Cancer. 2006;107(4):832–840. doi: 10.1002/cncr.22064. [DOI] [PubMed] [Google Scholar]
- 5.Bieliauskas AV, Pflum MKH. Isoform-selective histone deacetylase inhibitors. Chem. Soc. Rev. 2008;37(7):1402–1413. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler KV, Kozikowski AP. Chemical origins of isoform selectivity in histone deacetylase inhibitors. Curr. Pharm. Des. 2008;14(6):505–528. doi: 10.2174/138161208783885353. [DOI] [PubMed] [Google Scholar]
- 7.Garber K. HDAC inhibitors overcome first hurdle. Nat Biotechnol. 2007;25(1):17–19. doi: 10.1038/nbt0107-17. [DOI] [PubMed] [Google Scholar]
- 8.Miller TA, Witter DJ, Belvedere S. Histone deacetylase inhibitors. J. Med. Chem. 2003;46(24):5097–5116. doi: 10.1021/jm0303094. [DOI] [PubMed] [Google Scholar]
- 9.Taori K, Paul VJ, Luesch H. Structure and Activity of Largazole, a Potent Antiproliferative Agent from the Floridian Marine Cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008;130(6):1806–1807. doi: 10.1021/ja7110064. [DOI] [PubMed] [Google Scholar]
- 10.(a) Ying Y, Taori K, Kim H, Hong J, Luesch H. Total Synthesis and Molecular Target of Largazole, a Histone Deacetylase Inhibitor. J. Am. Chem. Soc. 2008;130(26):8455–8459. doi: 10.1021/ja8013727. [DOI] [PubMed] [Google Scholar]; (b) Bowers A, West N, Taunton J, Schreiber SL, Bradner JE, Williams RM. Total synthesis and biological mode of action of largazole: A potent Class I histone deacetylase inhibitor. J. Am. Chem. Soc. 2008;130(33):11219–11222. doi: 10.1021/ja8033763. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bowers AA, West N, Newkirk TL, Troutman-Youngman AE, Schreiber SL, Wiest O, Bradner JE, Williams RM. Synthesis and Histone Deacetylase Inhibitory Activity of Largazole Analogs: Alteration of the Zinc-Binding Domain and Macrocyclic Scaffold. Org. Lett. 2009;11(6):1301–1304. doi: 10.1021/ol900078k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ying Y, Liu Y, Byeon SR, Kim H, Luesch H, Hong J. Synthesis and Activity of Largazole Analogues with Linker and Macrocycle Modification. Org. Lett. 2008;10(18):4021–4024. doi: 10.1021/ol801532s. [DOI] [PubMed] [Google Scholar]; (e) Bowers AA, Greshock TJ, West N, Estiu G, Schreiber SL, Wiest O, Williams RM, Bradner JE. Synthesis and Conformation-Activity Relationships of the Peptide Isosteres of FK228 and Largazole. J. Am. Chem. Soc. 2009;131(8):2900–2905. doi: 10.1021/ja807772w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Nasveschuk CG, Ungermannova D, Liu X, Phillips AJ. A Concise Total Synthesis of Largazole, Solution Structure, and Some Preliminary Structure Activity Relationships. Org. Lett. 2008;10(16):3595–3598. doi: 10.1021/ol8013478. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK, Kulkarni S. Enantioselective Total Synthesis of (+)- Largazole, a Potent Inhibitor of Histone Deacetylase. Org. Lett. 2008;10(17):3907–3909. doi: 10.1021/ol8014623. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Seiser T, Kamena F, Cramer N. Synthesis and biological activity of largazole and derivatives. Angew. Chem., Int. Ed. 2008;47(34):6483–6485. doi: 10.1002/anie.200802043. [DOI] [PubMed] [Google Scholar]; (d) Ren Q, Dai L, Zhang H, Tan W, Xu Z, Ye T. Total synthesis of largazole. Synlett. 2008;15:2379–2383. [Google Scholar]; (e) Numajiri Y, Takahashi T, Takagi M, Shin-ya K, Doi T. Total synthesis of largazole and its biological evaluation. Pept. Sci. 2009;45th:41–44. [Google Scholar]; (f) Wang B, Forsyth CJ. Total synthesis of largazole - devolution of a novel synthetic strategy. Synthesis. 2009;(17):2873–2880. [Google Scholar]; (g) Chen F, Gao A-H, Li J, Nan F-J. Synthesis and Biological Evaluation of C7-Demethyl Largazole Analogues. ChemMedChem. 2009;4(8):1269–1272. doi: 10.1002/cmdc.200900125. [DOI] [PubMed] [Google Scholar]; (h) Zeng X, Yin B, Hu Z, Liao C, Liu J, Li S, Li Z, Nicklaus MC, Zhou G, Jiang S. Total synthesis and biological evaluation of Largazole and derivatives with promising selectivity for cancers cells. Org. Lett. 2010;12(6):1368–1371. doi: 10.1021/ol100308a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Souto JA, Vaz E, Lepore I, Poppler A-C, Franci G, Alvarez R, Altucci L, de Lera AR. Synthesis and biological characterization of the histone deacetylase inhibitor largazole and C7-modified analogues. J. Med. Chem. 2010;53(12):4654–4667. doi: 10.1021/jm100244y. [DOI] [PubMed] [Google Scholar]
- 12.(a) Witter DJ, Harrington P, Wilson KJ, Chenard M, Fleming JC, Haines B, Kral AM, Secrist JP, Miller TA. Optimization of biaryl Selective HDAC1&2 Inhibitors (SHI-1:2) Bioorg Med Chem Lett. 2008;18(2):726–731. doi: 10.1016/j.bmcl.2007.11.047. [DOI] [PubMed] [Google Scholar]; (b) Methot JL, Hamblett CL, Mampreian DM, Jung J, Harsch A, Szewczak AA, Dahlberg WK, Middleton RE, Hughes B, Fleming JC, Wang H, Kral AM, Ozerova N, Cruz JC, Haines B, Chenard M, Kenific CM, Secrist JP, Miller TA. SAR profiles of spirocyclic nicotinamide derived selective HDAC1/HDAC2 inhibitors (SHI-1:2) Bioorg Med Chem Lett. 2008;18(23):6104–6109. doi: 10.1016/j.bmcl.2008.10.052. [DOI] [PubMed] [Google Scholar]; (c) Methot JL, Chakravarty PK, Chenard M, Close J, Cruz JC, Dahlberg WK, Fleming J, Hamblett CL, Hamill JE, Harrington P, Harsch A, Heidebrecht R, Hughes B, Jung J, Kenific CM, Kral AM, Meinke PT, Middleton RE, Ozerova N, Sloman DL, Stanton MG, Szewczak AA, Tyagarajan S, Witter DJ, Secrist JP, Miller TA. Exploration of the internal cavity of histone deacetylase (HDAC) with selective HDAC1/HDAC2 inhibitors (SHI-1:2) Bioorg Med Chem Lett. 2008;18(3):973–978. doi: 10.1016/j.bmcl.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Yurek-George A, Habens F, Brimmell M, Packham G, Ganesan A. Total Synthesis of Spiruchostatin A, a Potent Histone Deacetylase Inhibitor. J. Am. Chem. Soc. 2004;126(4):1030–1031. doi: 10.1021/ja039258q. [DOI] [PubMed] [Google Scholar]
- 14.Ohishi T, N H, Sugawara M, Izumida M, Honda T, Mori K, Nogashima N, Inoue K. Process for producing optically active alpha-methylcysteine derivative. US 2006/0105435 A1. US patent. 2006
- 15.Chen Y, Gambs C, Abe Y, Wentworth P, Jr., Janda KD. Total Synthesis of the Depsipeptide FR-901375. J. Org. Chem. 2003;68(23):8902–8905. doi: 10.1021/jo034765b. [DOI] [PubMed] [Google Scholar]
- 16.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. High-field FT NMR application of Mosher's method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991;113(11):4092–4096. [Google Scholar]
- 17.Su D-W, Wang Y-C, Yan T-H. A novel DMAP-promoted oxazolidinethione deacylation. Application for the direct conversion of the initial chiral thioimide aldols to various ester protecting groups. Tetrahedron Lett. 1999;40(22):4197–4198. [Google Scholar]
- 18.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. A rapid esterification by mixed anhydride and its application to large-ring lactonization. Bull. Chem. Soc. Jpn. 1979;52(7):1989–1993. [Google Scholar]
- 19.Salvatore RN, Smith RA, Nischwitz AK, Gavin T. A mild and highly convenient chemoselective alkylation of thiols using Cs2CO3-TBAI. Tetrahedron Lett. 2005;46(51):8931–8935. [Google Scholar]
- 20.Richter LS, Marsters JC, Jr., Gadek TR. Two new procedures for the introduction of benzyl-type protecting groups for thiols. Tetrahedron Lett. 1994;35(11):1631–1634. [Google Scholar]
- 21.Funk RL, Bolton GL. Metalation and alkylation of 4H-1,3-dioxin: a new beta -acyl vinyl anion equivalent. J. Am. Chem. Soc. 1988;110(4):1290–1292. [Google Scholar]
- 22.Stuhr-Hansen N. The tert-butyl moiety-a base resistant thiol protecting group smoothly replaced by the labile acetyl moiety. Synth. Commun. 2003;33(4):641–646. [Google Scholar]
- 23.Clive DLJ, Hisaindee S, Coltart DM. Derivatized Amino Acids Relevant to Native Peptide Synthesis by Chemical Ligation and Acyl Transfer. J. Org. Chem. 2003;68(24):9247–9254. doi: 10.1021/jo030192r. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SK, Wu Y, Steinbergs N, Crowley ML, Hanson AS, Casero RA, Woster PM. (Bis)urea and (bis)thiourea inhibitors of lysine-specific demethylase 1 as epigenetic modulators. J Med Chem. 2010;53(14):5197–5212. doi: 10.1021/jm100217a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Helquist P, Wiest O. Zinc binding in HDAC inhibitors: a DFT study. J Org Chem. 2007;72(14):5446–5449. doi: 10.1021/jo070739s. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Huang P-H, Chen C-S, Forsyth CJ. Total Syntheses of the Histone Deacetylase Inhibitors Largazole and 2-epi-Largazole: Application of N-Heterocyclic Carbene Mediated Acylations in Complex Molecule Synthesis. J. Org. Chem. 2011;76(4):1140–1150. doi: 10.1021/jo102478x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigel H, Rheinberger VM, Fischer BE. Stability of Metal Ion-Alkyl Thioether Complexes in Solution - Ligating Properties of Isolated Sulfur-Atoms. Inorganic Chemistry. 1979;18(12):3334–3339. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.