Abstract
Primary progressive aphasia (PPA) is an insidiously progressive clinical syndrome that includes at its core an impairment in language. From a clinical perspective, there are a variety of diagnostic challenges; international consensus has only recently been reached on the nomenclature for specific clinical subtypes. There are at present no established treatments, and efforts to develop treatments have been hampered by the lack of standardized methods to monitor progression of the illness. This is further complicated by the multiplicity of underlying neuropathologies. Although measures developed from work with stroke aphasia and from work with disorders such as Alzheimer’s disease and frontotemporal dementia have provided a valuable foundation for monitoring progression, PPA presents unique challenges to clinicians aiming to quantify impairments for the purposes of full characterization and monitoring, and ultimately with the goal of designing clinical trials of interventions to make a meaningful difference in patients’ lives. In this review, I will summarize the main points made in my presentation at the 2010 International Conference on Frontotemporal Dementia, expand from there to summarize our current approach to monitoring progression of PPA, and finally will outline some ideas about goals for the development of better tools for this purpose.
Keywords: Magnetic resonance imaging, Cerebral cortex, Primary progressive aphasia, Frontotemporal dementia, Clinical trials, Biomarkers
Introduction
Primary progressive aphasia (PPA) is a syndrome that involves the insidiously progressive loss of language abilities largely in the absence of other cognitive, behavioral, or neurologic impairments at least for the first few years of its course (Mesulam 1982; Kertesz et al. 1994; Hodges 2001; Mesulam and Weintraub 2008; Grossman 2010). It has been considered to be a form of Frontotemporal Lobar Degeneration (FTLD) (Neary et al. 1998; McKhann et al. 2001), although some forms of PPA may have Alzheimer or other types of pathology. Scientifically, these disorders are of great interest in part because they strike the language networks of the brain with surprising specificity, which may shed light on the pathobiology of neurodegenerative disorders and on the neurobiology of language function. Clinically, these disorders are devastating to the patients and families who experience them.
Unfortunately, at present, despite substantial effort in several clinical trials of pharmacologic agents (Reed et al. 2004; Kertesz et al. 2008; Johnson et al. 2010) and preliminary efforts with rehabilitative techniques (Henry et al. 2008), we lack proof of the efficacy of any form of treatment for PPA. Although there have been a number of important advances in clinical research related to PPA in the past few years, a critical barrier to the development of new treatments is the lack of standard assessment instruments that could be used in clinical trials. There are a variety of efforts currently underway to develop new methods to improve the sensitivity and specificity of diagnosis, monitor progression over time, and predict molecular pathology.
Why should we care about improved ability to quantify the types of impairments in PPA and their severity and to monitor progressive change in these impairments over time? First, in our clinical care, it is important to be able to provide clear information to patients and families regarding relative strengths and weaknesses that the patient has in communication abilities; this may serve as a foundation for the development of compensatory strategies. And at each return visit, it is valuable for the clinician to be able to clearly indicate whether there appears to have been change since the previous visit and if so the types and magnitude of change. It is also critical for the clinician to communicate this information to professional peers in clinical care and in clinical research. Finally, as alluded to above, these measures form the bedrock of rationally designed clinical trials.
The Clinical Phenotypes of PPA
The core clinical phenotype of PPA is an insidious-onset, gradually progressive impairment of language production, object naming, syntax, or word comprehension that is apparent during conversation or through speech and language assessments. There are several excellent descriptions of the general recommended diagnostic approach (Mesulam and Weintraub 2008; Gorno-Tempini et al. 2011).
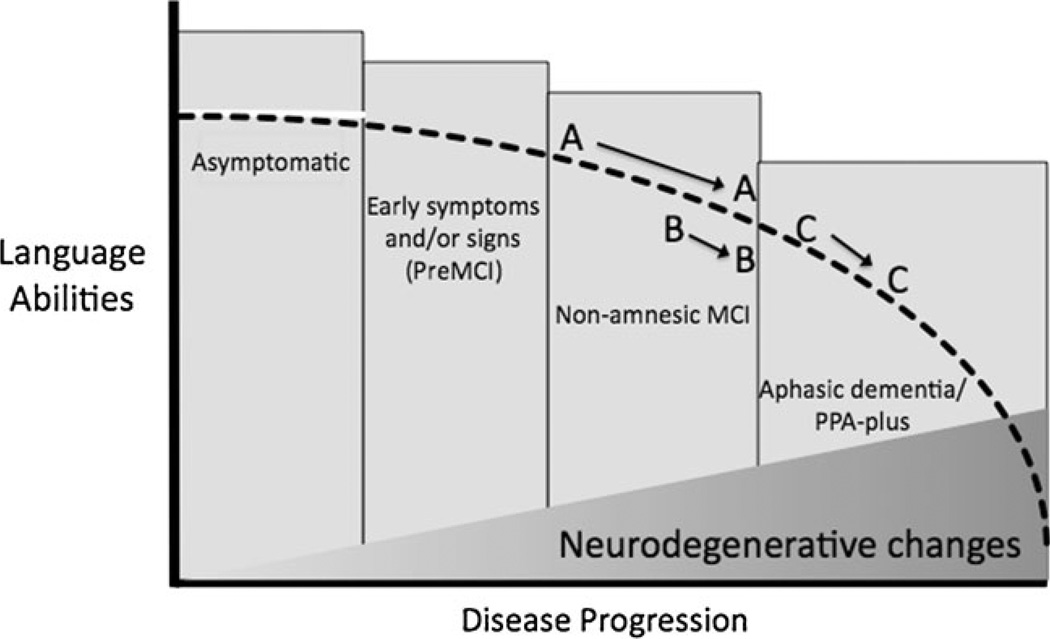
In our practice and clinical research, we find it helpful to conceptualize PPA within the same general framework as Alzheimer’s disease (AD) and related neurodegenerative disorders, which are thought of as progressing in three clinical phases with respect to global function: asymptomatic/preclinical, mildly symptomatic/mild cognitive impairment (MCI), and dementia (see Fig. 1). Because the pathophysiologic process of most neurodegenerative diseases likely takes place over years prior to the development of symptoms, all of these conditions are thought to involve a phase in which neurodegenerative changes are developing in the brain but are not yet producing symptoms. In the earliest clinical phase, patients may describe increasingly effortful speech but may be able to perform well on many tests of language function. For some patients, this phase may last for a number of years. As the condition progresses, impairments become evident on psycholinguistic tests, such as measures of naming, verbal fluency, or picture description. At this clinical stage, many patients are still functioning relatively normally in complex activities of daily living and are capable of performing well on neuropsychological tests of cognitive domains other than language, such as memory, executive function, or visuospatial ability. Nevertheless, by this time, neuronal loss and neuropathologic change have damaged core brain regions subserving language, and a diagnosis can often be supported by demonstrating the expected abnormalities of brain structure or function with neuroimaging tests. Finally, patients enter a dementia phase in which other cognitive, behavioral, and/or motor domains outside of language are affected, and the person develops the need for assistance by others in daily activities (this phase is sometimes called “aphasic dementia” or “PPA-plus” (Rogalski and Mesulam 2009)). This phase may occur after many years, or within a relatively short time frame after diagnosis. From a strict standpoint, Mesulam and colleagues have argued for the relative sparing of other non-language abilities for at least the first 2 years of the clinical syndrome. Although it is often said that the course of the illness progresses over approximately 7–10 years from diagnosis to death, recent studies suggest that some forms of PPA may be slowly progressive for 12 or more years (Hodges et al. 2010), with reports of up to 20 years depending on how early a diagnosis is made. Although very little research has been performed on prognosis in PPA, one study suggests that patients may maintain independence in activities of daily living for 6–7 years after the onset of symptoms and that better functional outcomes may be associated with several baseline characteristics including fluent aphasia (Le Rhun et al. 2005).
Fig. 1.
Model of the clinical trajectory of primary progressive aphasia as a function of biological disease progression within the asymptomatic/mild cognitive impairment (MCI)/dementia framework. Letters indicate hypothetical patients: patient A and patient B both fit within an overall clinical classification of MCI at baseline and longitudinal follow-up, although patient starts at a milder level of impairment at baseline but progresses at a faster rate than patient B. Patient C is initially more impaired (mild aphasic dementia) than patient B but progresses at a similar rate. Vertical boxes depict clinical classification of overall functional status. Dashed black line illustrates gradual decline of language abilities; filled gray section at bottom indicates gradual progression of neurodegenerative pathologic changes in language systems of the brain
Once neuroprotective or disease-modifying medications aiming to slow disease progression are developed to the point that they can be tested in clinical trials, it would be ideal to initiate them as early as possible (DeKosky and Marek 2003). For example, if the illness could be confidently identified at its earliest MCI stage when patients are still relatively intact in complex activities of daily living, a rational clinical trial outcome would be to delay the onset of aphasic dementia for at least a year or more. To approach this goal, we need to improve our capability to confidently identify individuals in the earliest stages of PPA. We also need to develop better instruments to grade the severity of the condition at baseline and to monitor decline over time in order to demonstrate a slowing in the rate of progression. The development of such instruments is complicated by the clinicopathologic heterogeneity of PPA.
Clinicopathologic Subtypes of PPA
The clinical syndrome of PPA has multiple subtypes, each of which appears to be probabilistically associated with a distinct type of neuropathology. In the last 5–10 years, three major subtypes of PPA have been defined—non-fluent/agrammatic, semantic, and logopenic—each characterized by distinct profiles of language impairment and regional atrophy within the language networks (Grossman; Neary et al. 1998; Gorno-Tempini et al. 2004; Mesulam et al. 2009; Gorno-Tempini et al. 2011). The core features of the non-fluent/agrammatic variant of PPA are agrammatism in language production and effortful speech, often with inconsistent speech sound errors and/or a motor speech impairment. The semantic variant of PPA is characterized primarily by prominent anomia and single word comprehension deficits. The core features of the logopenic variant of PPA are deficits in word retrieval (in spontaneous speech and confrontation naming) and sentence repetition. Spontaneous speech is slow with frequent pauses due to significant word finding problems without frank agrammatism. Speech production deficits are therefore distinct from those of patients with the non-fluent/agrammatic variant, who also speak in a slow and halting manner, but with output that is dysprosodic with motor speech errors and/or agrammatism. Despite substantial progress, there is still a need to better define these subtypes, including better methods to identify motor speech abnormalities since these appear to be strong indicators of underlying tauopathies.
Although these subtypes are relatively easily recognizable in their typical forms, there is still a subset of patients who do not cleanly fit into one of these clinical subtype categories. Nevertheless, emerging data are beginning to suggest that the non-fluent/agrammatic, semantic, and logopenic subtypes are largely, though not exclusively, associated with underlying tau, TDP-43, and Alzheimer pathologies, respectively (Josephs 2008). Increasing effort is being devoted to trying to understand these clinicopathologic relationships because if medications are developed to target specific molecular abnormalities, it will be critical to be able to identify PPA patients whose illness is associated with the particular abnormality the medication is targeting.
Monitoring Progression of Overall Clinical Status
Once a diagnosis of PPA is made, an important goal for the clinician is to determine the overall level of impairment, which will serve as a baseline against which longitudinal monitoring can be performed. In our practice and in the National Alzheimer’s Coordinating Center Uniform Data Set methodology (although there is still no consensus on this issue in the broader PPA community), an initial objective is to determine whether the patient would fit the designation of MCI or dementia, optimally via a structured clinical interview with the patient and someone who knows the patient well, typically a spouse or adult child. Questionnaire-based instruments can be helpful for this purpose, particularly those that aim to ascertain the type and degree of impairments in instrumental and basic activities of daily living (Johnson et al. 2004; Mioshi et al. 2007; Wicklund et al. 2007; Mioshi and Hodges 2009). Overall level of impairment can be graded using a variety of clinician-rated scales, such as the Clinical Dementia Rating scale (Morris 1993) with the supplemental Language and Behavior ratings, the Clinician Global Impression of Change (Knopman et al. 2008), and related measures of global dementia severity.
It is more challenging to utilize performance-based tests, such as the Mini-Mental State Exam, Addenbrooke’s Cognitive Exam (ACE), Alzheimer’s Disease Assessment Scale, or neuropsychological test batteries in part because of the effects of language deficits on non-language domains of performance (Knopman et al. 2008). While we believe these evaluations are useful for a variety of purposes, we always encourage the patient to use his/her most functional response modality, which may be writing. Even then, a language deficit can be as problematic in writing as in speech and may impede the patient’s ability to display his knowledge. Therefore, we have added multiple-choice response options to tests for measuring orientation, memory, and other abilities. The National Alzheimer’s Coordinating Center Uniform Data Set workgroup is developing a Frontotemporal Dementia Module that should provide additional insights with respect to performance-based instruments. With these caveats in mind, however, a recent study demonstrated that the ACE is useful for monitoring longitudinal change over time in patients with PPA (Leyton et al. 2010).
Monitoring Progression of Speech and Language Impairment
Patients with PPA seek medical attention at different levels of severity—some patients seek neurologic/language evaluation at a very mild stage while others are surprisingly impaired by the first evaluation—and the rate at which symptoms progress varies considerably across patients (see Fig. 1). Therefore, the first step in monitoring speech/language is a comprehensive baseline assessment that goes beyond the language components of general cognitive measures used in AD. The baseline speech/language assessment provides key information to determine clinical subtype, which will be increasingly important as clinical trials targeting specific pathologies become available. The baseline assessment, if sensitive enough, might reveal the earliest speech/language signs and symptoms and help us to identify those patients who meet inclusion criteria for a trial. Furthermore, knowing the baseline level of impairment is necessary to determine the efficacy of interventions including drug treatments or speech/language therapy.
Speech/language assessment has been done using a variety of performance-based tests from stroke aphasiology, such as the Western Aphasia Battery (WAB) (Kertesz 1982) and Boston Diagnostic Aphasia Examination (BDAE) (Goodglass et al. 2001), pediatric speech-language pathology, such as the Curtiss–Yamada Comprehensive Language Evaluation–Receptive (CYCLE) (Gorno-Tempini et al. 2004), and tools recently developed for the PPA population, including the Northwestern Anagram Test (NAT) (Weintraub et al. 2009). A motor speech exam is also typically done to determine the presence of apraxia of speech, which can be the presenting symptom in the non-fluent/agrammatic subtype or may be a distinct subtype (Ogar et al. 2005; Josephs et al. 2006).
We have found that performance-based testing is only one component of the evaluation. There are often discrepancies between test performance and ability to communicate in daily life, which we observe directly or learn about through interviewing the partner. In addition, psycholinguistic measures typically do not award credit for use of alternative communication strategies and compensatory efforts, which can significantly improve a patient’s functional communication ability. We therefore consider the partner interview an important part of the assessment and also obtain questionnaires about daily communication functioning from the partner (and patient when possible). To integrate these sources of information, we recently developed a clinical rating scale that expanded upon the CDR Supplemental Language rating scale (Knopman et al. 2008), the Progressive Aphasia Severity Scale (PASS) (Sapolsky et al. 2010). The scale is used to rate presence and severity of impairment (0=normal to 3=severe impairment) in ten domains of language (see Table 1). Ratings are made using the clinician’s best judgment of the overall level of impairment in each domain based on information from a structured interview with the patient and partner and from the language assessment. The ratings represent a snapshot of the patient’s overall functioning in daily life in discrete domains of speech/language and can be used at baseline and as a monitoring tool. There is also a functional communication domain, which takes into account the patient’s ability to communicate despite the impairment (i.e., ability to use compensatory strategies). The scores in each domain can be summed to give a PASS Sum-of-Boxes measure, analogous to the CDR Sum-of-Boxes measure, representing a finer grading of overall level of impairment.
Table 1.
Domains of the progressive aphasia severy scale
| PASS domain | Description |
|---|---|
| Articulation | Ability to say sounds and syllables accurately and effortlessly |
| Fluency | Degree to which speech flows easily or is interrupted by hesitations, fillers, pauses; reduced fluency is associated with decreased phrase length and words per minute |
| Syntax and grammar | Use of word forms (run, ran), functor words (the, an), and word order when forming phrases and sentences in most used modality (speech or writing) |
| Word retrieval and expression | Ability to express the intended word through primary modality (speech or writing) |
| Repetition | Ability to repeat words, phrases, sentences; difficulty should not be attributable to working memory problem; do not penalize for sound distortions resulting from apraxia of speech or dysarthria |
| Auditory comprehension | Ability to understand spoken phrases and sentences (e.g., conversation, commands) |
| Single word comprehension | Ability to understand spoken or written single words |
| Reading | Ability to decode and understand written material; difficulty should not be attributable to an elementary visual problem |
| Writing | Ability to write and spell; difficulty should not be attributable to an elementary motor problem |
| Functional communication | Ability to communicate despite the speech/language impairment; ability to compensate for impairment |
Each language domain is rated on a scale of 0 (normal), 0.5 (questionable or very mild impairment), 1 (mild impairment), 2 (moderate), or 3 (severe)
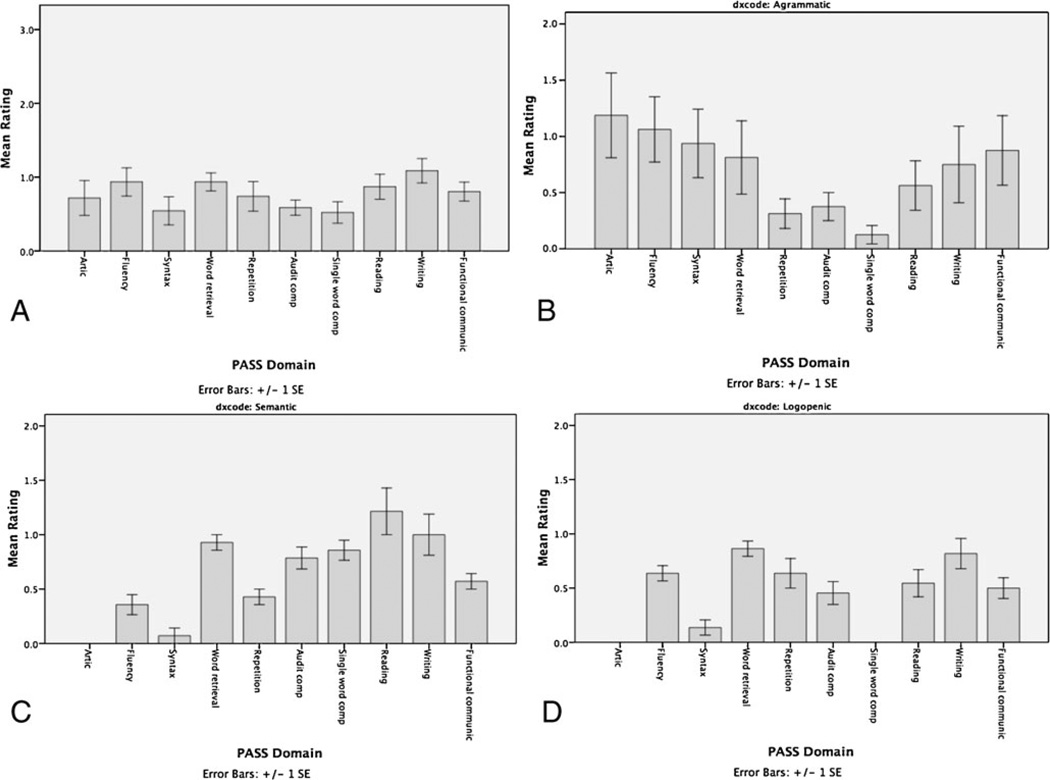
Because the PASS allows us to rate individual domains of speech/language, it captures the pattern of relative strengths and weaknesses at baseline and at each successive evaluation. This can be depicted graphically as a summary profile of impairments (Fig. 2). The profiles of the clinical subtypes differ as expected.
Fig. 2.
a Profile of impairments at first evaluation in all domains in our initial sample of 23 PPA patients. Profiles of each PPA subtype are depicted, including b non-fluent/agrammatic, c semantic, and d logopenic. For each graph, on the x-axis, the individual domains described in Table 1 are depicted; Global is the overall rating on the CDR Supplemental Language rating. Articulation (Artic), Auditory Comprehension (Aud Comp), Functional Communication (Funct)
In addition to a refined characterization of baseline types and level of impairment, we also use the PASS to monitor the emergence of problems in initially unaffected areas and the rate at which impaired domains worsen. Besides providing a quantitative basis for communication with families and peers, monitoring symptom severity and the emergence of new impairments in this way is also critical for planning speech/language therapy and use of alternative communication strategies and devices. We are also currently investigating whether baseline PASS assessment data are useful for predicting the domains of most prominent impairment (and relative preservation) over the subsequent 2–4 years, which would be very valuable for discussions about prognosis.
More information about the PASS, including questionnaires and training materials, can be found on our MGH FTD Unit website at http://www.ftd-boston.org/. We are currently working with collaborators to further assess the multi-center reliability of this instrument.
Imaging Biomarkers for Diagnosis and Monitoring of Progression
Imaging biomarkers of brain anatomy, function, and molecular composition are already essential tools for diagnosis of most neurodegenerative diseases and will almost certainly be critical for monitoring progression. For the purposes of clinical trials, these tools can be thought of as useful for identifying a relatively homogenous sample of participants (i.e., for the identification of disease-specific brain abnormalities and the exclusion of those lacking typical disease-associated abnormalities, much as is done in stroke or multiple sclerosis). They can also be thought of as potential outcome measures, although the clinical validation requirements called for by the Food and Drug Administration make this a taller hurdle. Readers interested in a detailed discussion of some of these issues in the field of Alzheimer’s disease are referred to recent publications (Dickerson and Sperling 2005; Hampel et al. 2010).
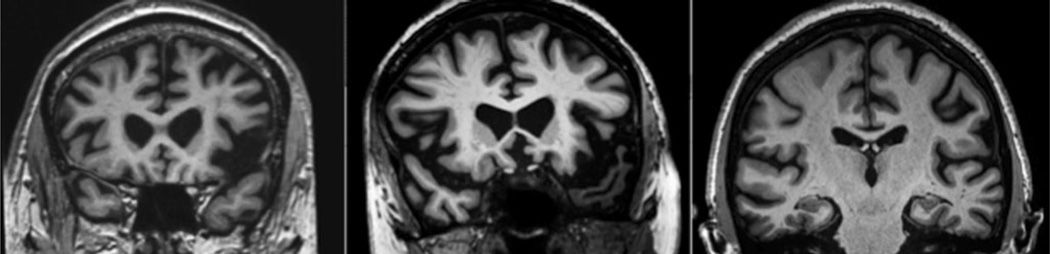
Although routine clinical MRI scans in patients with mild PPA are often interpreted as within normal limits, careful visual inspection of specific cortical regions can often provide the clinician with evidence of subtle atrophy in critical nodes of the language network. The ability to see these regions clearly depends heavily on the type of MRI scan obtained, and we typically employ a high-resolution (1 mm isotropic) coronal T1-weighted scan, which is a sequence that is becoming much more widely available on common MRI platforms; this type of sequence should be requested in the MRI protocol, and clinicians should review the results directly themselves in consultation with a radiologist or other imaging expert (see Fig. 3 for case examples).
Fig. 3.
Representative ultrahigh-resolution T1-weighted MRI scans (0.38 mm in-plane), illustrating asymmetric inferior frontal opercular atrophy in non-fluent/agrammatic PPA (left), temporopolar atrophy in semantic PPA (middle), and inferior parietal atrophy in logopenic PPA (right). Careful visual inspection of high resolution MRI scans, best done for this purpose in the coronal plane, will often reveal atrophy in the predicted localization if the subtype of PPA is clinically typical
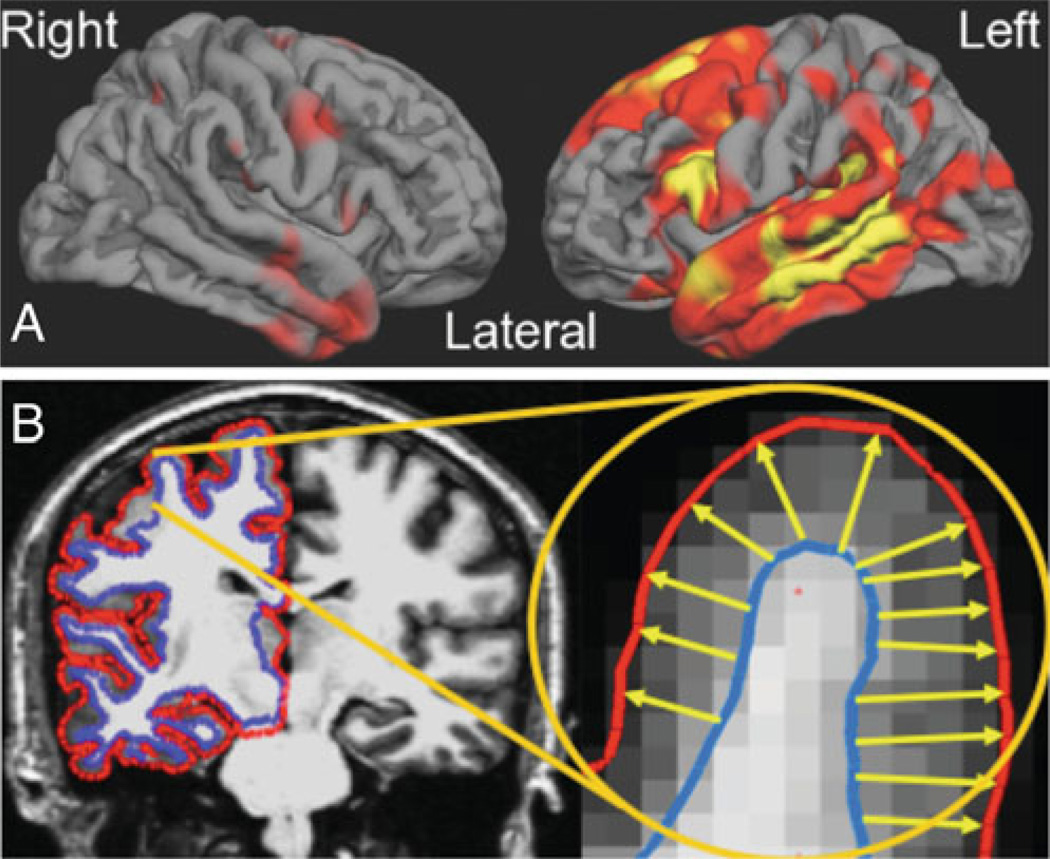
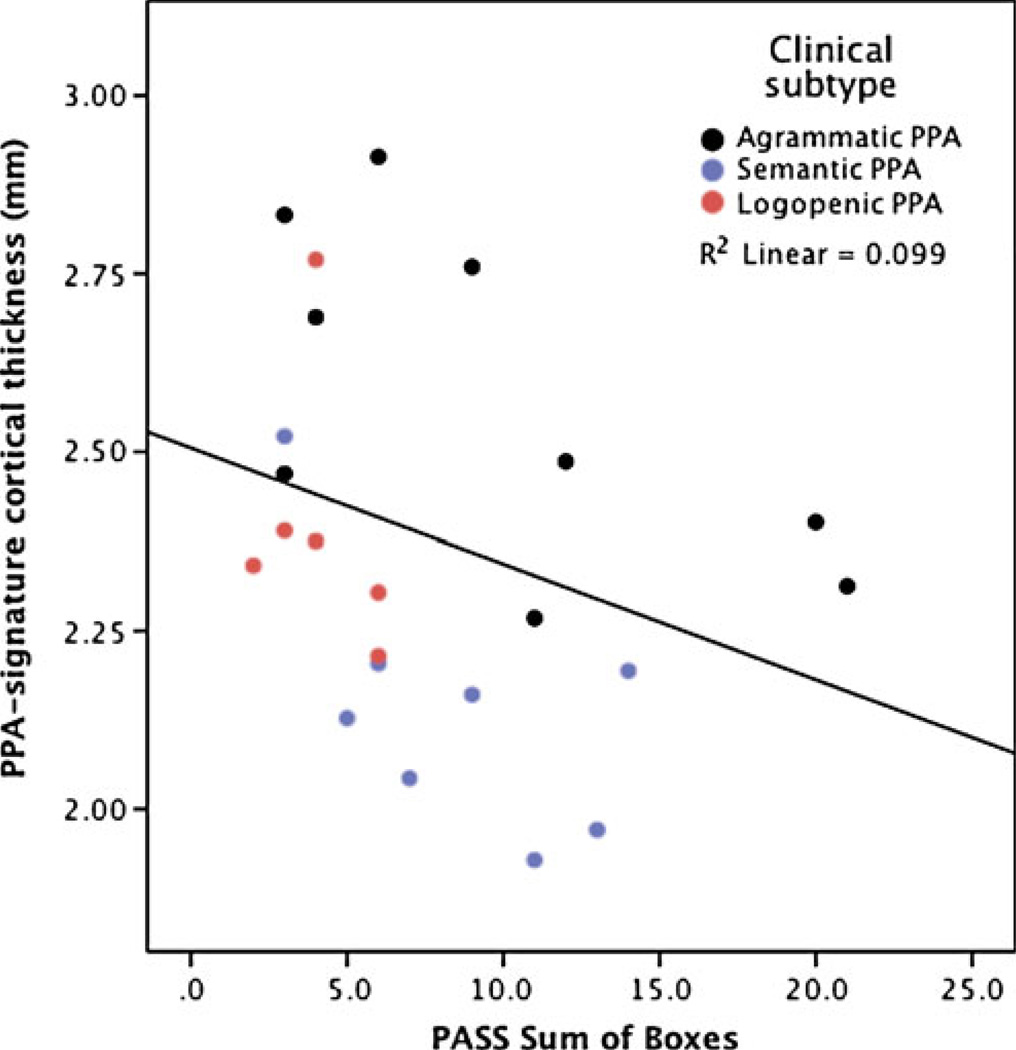
There has been great progress aimed at mapping focal brain abnormalities in PPA and relating these abnormalities to types of language deficits by comparing groups of clinically characterized patients to controls or to other disease groups (Rohrer et al. 2009; Gorno-Tempini et al. 2004; Peelle et al. 2008). Most of the techniques used in such studies that aim to map atrophy and rates of progression in PPA are at present still basic imaging neuroscience research methodology, not yet tools that are available for clinical use. One such technique under development in our group consists of cortical thickness mapping, which has the ability to measure the thickness of the cerebral cortex with submillimeter precision. This method can be used to localize regions of cortical atrophy in patient groups compared to controls. In contrast to AD, in which the “signature” of regional cortical atrophy includes medial and lateral temporal, medial and lateral parietal, and often frontal atrophy with a relatively symmetric pattern (Dickerson et al. 2009), PPA typically has a distinct asymmetric perisylvian atrophy pattern (Fig. 4), which correlates with the overall degree of impairment on the PASS clinical instrument (see Fig. 5).
Fig. 4.
Quantitative analysis of MRI data is still a research tool but it is becoming more apparent that these methods need to be translated into clinically efficient tools since measures of neuroanatomic abnormalities can be very useful as biomarkers for diagnosis and probably longitudinal monitoring. a The regional pattern of cortical thinning in PPA, shown here for a group of 23 PPA patients (including all three subtypes) vs. controls is highly asymmetric and localized in perisylvian regions of the language networks. b The map is generated from semi-automated measurement of the thickness of the cerebral cortex; comparison of the cortical thickness of a group of patients vs. a group of controls may reveal regionally specific thinning even though the magnitude of such thinning may be 0.1–0.2 mm
Fig. 5.
The PPA-signature of cortical atrophy as measured from MRI scans is a valid reflection of the level of clinical impairment. Correlation between PPA-signature MRI biomarker and PASS Sum-of-Boxes measure of clinical impairment, with higher ratings on this measure indicating more substantial clinical language impairment
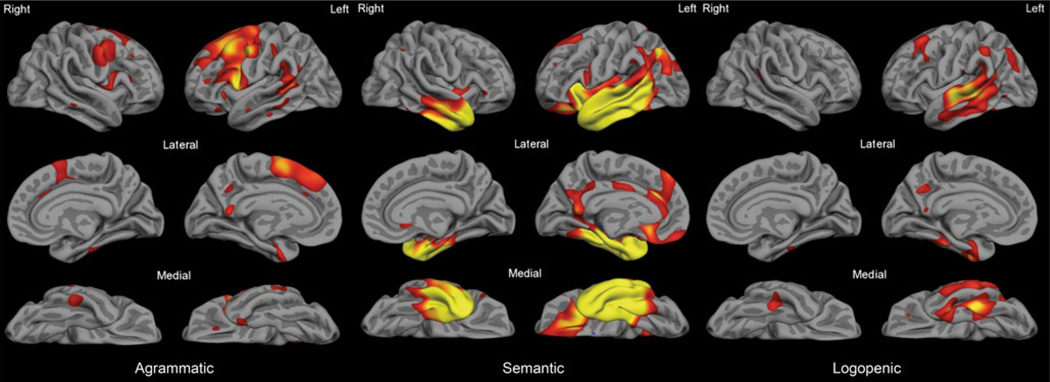
Beyond this global pattern of perisylvian atrophy, the three major subtypes of PPA have distinct atrophy patterns in specific nodes of the language network which relate to impairment in the specific domains of language function that are most compromised in types subtypes (see Fig. 6). Although these patterns appear to be quite consistent across centers (Mesulam et al. 2009; Rohrer et al. 2009; Sapolsky et al. 2010), there has been little effort aimed at localizing and quantifying abnormalities in single individuals. If we aim to use quantitative imaging measures as diagnostic markers, the translation of these imaging methods into tools that can be applied to individual patients will be crucial. Some of these research imaging methods could potentially be translated into tools that can be used clinically in individual patients, but the appropriate reliability and validity studies need to be performed.
Fig. 6.
The three PPA subtypes are associated with distinct patterns of cortical atrophy. PPA-non-fluent/agrammatic patients exhibit primarily left prefrontal atrophy with relative sparing of temporal lobe cortex (left); PPA-semantic patients demonstrate anterior temporal lobe atrophy extending throughout much of the temporal lobe even early in the course (middle); PPA-logopenic patients show mainly caudal superior and middle temporal and inferior parietal atrophy early in the course of their illness
Although both structural MRI and FDG-PET metabolic measures have been used in studies of PPA, there has never been a systematic head-to-head comparison of the relative sensitivity and specificity of these imaging measures in PPA. Do they provide complementary or redundant information? In the field of AD, it appears that MRI and FDG-PET provide complementary information that, together, improve the sensitivity and specificity of diagnosis beyond that provided by either modality alone (Mosconi et al. 2007). Since FDG-PET is becoming more widely available and is approved by Medicare for reimbursement for the differential diagnosis of AD vs. frontotemporal dementia (of which PPA is considered a subtype), it is important to determine the potential benefits of this type of scanning in PPA, particularly since it is still costly.
In addition to FDG-PET, there are several emerging MRI-based measures of brain function, including task-related functional MRI (fMRI), functional connectivity MRI (fcMRI), and perfusion MRI (arterial spin-labeled or ASL MRI). For the most part, these are all currently innovative basic imaging science techniques being used to investigate aspects of brain function, and have not yet been subjected to reliability, validity, or longitudinal studies to determine their potential for translation into useful tools for clinical trials.
As mentioned above, recent post-mortem studies of the pathologic substrates of PPA and related disorders have demonstrated that a substantial portion of patients have AD pathology (Alladi et al. 2007; Mesulam et al. 2008), which has been shown in vivo using PiB PET amyloid imaging (Rabinovici et al. 2008), a novel imaging marker of fibrillar amyloid pathology being used in a variety of research settings but not yet in clinical use. Although a small percentage of patients with the semantic variant of PPA have evidence of AD pathology, it appears that most patients with the logopenic subtype of PPA harbor AD pathology, suggesting that this form of PPA may be an atypical variant of AD. While there are a large number of questions that deserve investigation as follow-up to these initial studies, it is clear that investigations attempting to identify in vivo clinical and imaging markers for use in clinical trials need to include measures that aim to determine whether there are specific characteristics unique to the PPA patients with Alzheimer pathology. Ultimately, it will be important to decide whether these patients should be directed toward disease-modifying clinical trials for AD even though their clinical phenotype is in most cases quite different from that of typical AD. Thus, this imaging method needs to be included in the translational efforts aimed at identifying an optimal set of imaging markers for use in PPA clinical trials.
Efforts are ongoing to develop PET ligands that label fibrillar tau protein, with initial efforts beginning to show promise (Okamura et al. 2005; Small et al. 2006). Ultimately, it may be possible to use molecular markers to “triangulate” the three major subtypes of PPA with the goal of determining whether (1) non-fluent/agrammatic patients exhibit tau-positive PET signals but absent PiB signal consistent with underlying tau pathology, (2) logopenic patients exhibit elevated PiB and tau signal consistent with AD pathology, while (3) semantic patients exhibit no uptake of PiB or tau ligands suggestive of underlying TDP-43 pathology. These PET findings could then be investigated in relation to the quantitative MRI measures described above to determine how well the MRI measures predict molecular abnormalities. The impact of this type of analysis would be to determine whether these additional but expensive molecular markers are needed to increase the specificity of diagnosis, or whether specificity is nearly is good using MRI (and possibly FDG-PET) measures alone. This will be essential information for the planning of treatment trials of compounds that are targeted toward specific molecular abnormalities, which is the case for a growing number of compounds.
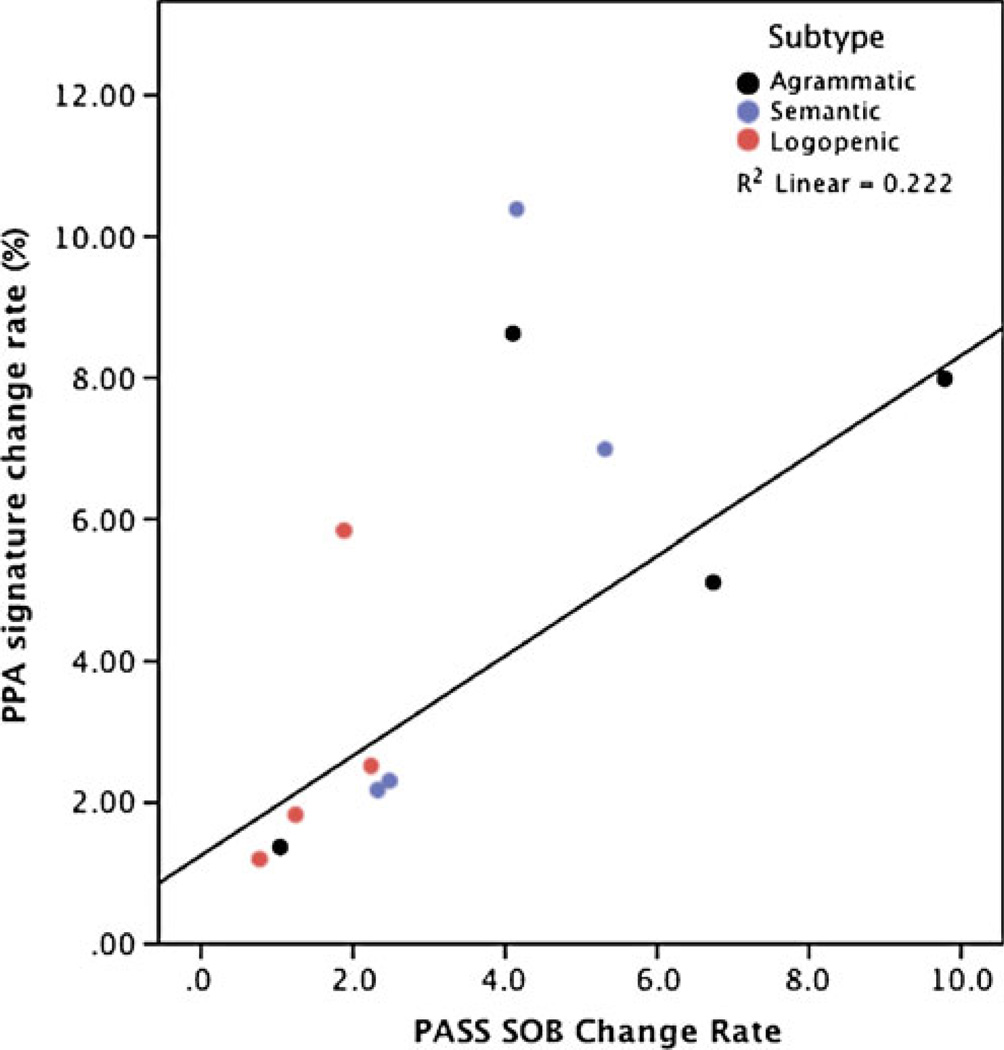
If our long-term goal is to intervene in PPA using forms of disease-modifying therapy, or even to investigate potential plastic responses to forms of speech and language therapy, we need markers of brain structure and function that are sensitive to disease progression. We know that different types of PPA progress at different rates; new data indicate that the non-fluent/agrammatic variant progressing relatively rapidly and the semantic variant more slowly (Hodges et al. 2010). Furthermore, individual patients clearly vary with respect to each other in the level of impairment at initial presentation and in rate of progression. Our preliminary work comparing rate of change on the clinical PASS measure with rate of change in cortical atrophy supports the idea that MRI will provide a clinically valid imaging biomarker of progression (see Fig. 7).
Fig. 7.
Longitudinal change in the PPA-signature MRI biomarker is a valid reflection of progression of clinical impairment. Preliminary evidence indicates that, while there is substantial variability between patients in the rate of decline, there is a reasonable degree of correlation between rate of clinical decline and rate of atrophy within PPA-signature cortical regions in the left hemisphere
Several natural history studies are ongoing in which longitudinal MRI and in some cases PET data are being collected in patients with PPA and related disorders. One goal is to generate data that would enable power calculations to be performed to estimate sample size for disease-modifying clinical trials, much as has been done in AD research; initial data in PPA look promising (Knopman et al. 2009; Gordon et al. 2010). Baseline imaging measures are also being investigated for their potential as predictive markers of clinical or functional-anatomic change, with the goal of determining whether some of these measures could be indicators of patients who may be “rapid progressors”, since this population would be an ideal target for clinical trials.
Other Biomarkers
In addition to imaging biomarkers, efforts are in progress to identify biofluid markers in blood or cerebrospinal fluid (CSF). At present, these are largely targeting CSF amyloid-β and tau abnormalities that are consistent with AD pathology and are primarily directed at early diagnosis and prognostication, as opposed to monitoring of disease progression. A combination of elevated tau and phosphorylated tau accompanied by reduced amyloid-β is strongly suggestive of AD pathology (Shaw et al. 2009). In PPA patients exhibiting such a CSF profile, AD pathology would be strongly suspected, and such patients might then be considered as potential candidates for certain types of AD clinical trials. This CSF molecular biomarker of AD is at present the only such approach that is routinely clinically available for this purpose and reimbursable by many American third-party payors.
With regard to the detection of CSF markers of non-AD tau or TDP-43 pathology, few studies have been conducted to date. Investigations of tau abnormalities are somewhat controversial, with some studies showing elevated tau and others showing reductions of tau. CSF assays for TDP-43 have recently been developed, and their preliminary use so far suggests that further work is needed. Finally, a variety of other proteins are being studied to investigate their potential use in understanding the pathobiology of the underlying diseases and differential diagnosis or monitoring (Grossman 2010).
Conclusions and Future Directions
We hope to see the development of efficacious interventions for PPA in the next decade. These treatments should improve function, slow progression, or both. Some of these treatments, such as specific forms of speech and language therapy, may be developed specifically for PPA and may target all individuals with PPA. Some drug treatments, such as neuroprotective medications, may also focus on this population or the broader population of patients with forms of frontotemporal dementia or kindred disorders. There is also increasing effort being devoted to the development of compounds targeting specific molecular abnormalities that contribute to the pathologic neurodegenerative process of these disorders, such as abnormalities of tau phosphorylation and aggregation.
For these latter compounds, we need to address several critical issues in clinical trial design. First, how do we identify patients with the specific biologic abnormality? This will likely involve a combination of clinical phenotype and one or more types of biomarkers. For example, a clinical trial of a tau agent in frontotemporal dementia could possibly enroll patients with a diagnosis of behavioral-variant or language-variant frontotemporal dementia and biomarker evidence of a tau abnormality. Next, how do we monitor patients to determine whether the treatment is working? We envision the need for a multifaceted approach involving multiple types of clinical instruments as well as imaging or other biomarkers. The clinical instruments need to encompass the spectrum of abnormalities with reasonable sensitivity and specificity. Besides global functional measures, such as the Clinical Global Impression of Change or the Clinical Dementia Rating scale, we also need domain-specific scales of impairment, such as the PASS described here. We also need standardized performance-based measures, probably including both a general cognitive measure such as the Mini Mental State Exam or a similar measure, as well as domain-specific measures such as naming, fluency, or other language tasks. Finally, we will also need to include ratings or measures of other domains, particularly motor and behavioral/affective function since these abilities are typically involved as PPA progresses. Ongoing longitudinal studies will provide critical natural history data that will be useful for planning such trials.
We are optimistic that the development of all of these kinds of tools is a major goal of the international research community working on PPA, but we continue to need to harmonize methods and invest in infrastructure to encourage data sharing, such as the IMPPACT website and registry for PPA (http://www.ppaconnection.org/). Through such efforts, and in partnership with federal agencies, industry, and private foundations including the Association for Frontotemporal Dementia, we hope to see the burdens of PPA on patients and families lessened over the coming years.
Acknowledgments
This work was supported by grants from the NIA R01-AG29411, R21-AG29840, P50- AG005134, and the Alzheimer’s Association. We express special appreciation to the participants in our program and their families for their valuable contributions. The author thanks Daisy Sapolsky, MS, CCC-SLP for her dedicated collaborative partnership in these efforts.
Footnotes
Financial Disclosure
The author reports no disclosures.
References
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Marek K. Looking backward tomove forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Neuroimaging biomarkers for clinical trials of disease-modifying therapies in Alzheimer’s disease. NeuroRx. 2005;2:348–360. doi: 10.1602/neurorx.2.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston diagnostic aphasia examination, third edition (BDAE-3) Austin: Pro-Ed; 2001. [Google Scholar]
- Gordon E, Rohrer JD, Kim LG, Omar R, Rossor MN, Fox NC, Warren JD. Measuring disease progression in frontotemporal lobar degeneration: a clinical and MRI study. Neurology. 2010;74:666–673. doi: 10.1212/WNL.0b013e3181d1a879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam M, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6:88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, Herholz K, Bokde AL, Jessen F, Hoessler YC, Sanhai WR, Zetterberg H, Woodcock J, Blennow K. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- Henry ML, Beeson PM, Rapcsak SZ. Treatment for lexical retrieval in progressive aphasia. Aphasiology. 2008;22:826–838. doi: 10.1080/02687030701820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR. Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology. 2001;56:S6–S10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Mitchell J, Dawson K, Spillantini MG, Xuereb JH, McMonagle P, Nestor PJ, Patterson K. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133:300–306. doi: 10.1093/brain/awp248. [DOI] [PubMed] [Google Scholar]
- Johnson N, Barion A, Rademaker A, Rehkemper G, Weintraub S. The activities of daily living questionnaire: a validation study in patients with dementia. Alzheimer Dis Assoc Disord. 2004;18:223–230. [PubMed] [Google Scholar]
- Johnson NA, Rademaker A, Weintraub S, Gitelman D, Wienecke C, Mesulam M. Pilot trial of memantine in primary progressive aphasia. Alzheimer Dis Assoc Disord. 2010;24:308. doi: 10.1097/WAD.0b013e3181cf468d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery. New York: Grune and Stratton; 1982. [Google Scholar]
- Kertesz A, Hudson L, Mackenzie IR, Munoz DG. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44:2065–2072. doi: 10.1212/wnl.44.11.2065. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Morlog D, Light M, Blair M, Davidson W, Jesso S, Brashear R. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008;25:178–185. doi: 10.1159/000113034. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, Miller BL, Mercaldo N. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Jr, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, Miller BL, Mercaldo ND. Brain and ventricular volumetric changes in frontotemporal lobar degeneration over 1 year. Neurology. 2009;72:1843–1849. doi: 10.1212/WNL.0b013e3181a71236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rhun E, Richard F, Pasquier F. Natural history of primary progressive aphasia. Neurology. 2005;65:887–891. doi: 10.1212/01.wnl.0000175982.57472.84. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Hornberger M, Mioshi E, Hodges JR. Application of Addenbrooke’s cognitive examination to diagnosis and monitoring of progressive primary aphasia. Dement Geriatr Cogn Disord. 2010;29:504–509. doi: 10.1159/000313980. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the work group on frontotemporal dementia and Pick’s disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. S1owly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Weintraub S. Primary progressive aphasia and kindred disorders. Handb Clin Neurol. 2008;89:573–587. doi: 10.1016/S0072-9752(07)01254-7. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Weintraub S, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66:1545–1551. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioshi E, Hodges JR. Rate of change of functional abilities in frontotemporal dementia. Dement Geriatr Cogn Disord. 2009;28:419–426. doi: 10.1159/000255652. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Kipps CM, Dawson K, Mitchell J, Graham A, Hodges JR. Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology. 2007;68:2077–2084. doi: 10.1212/01.wnl.0000264897.13722.53. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Glodzik-Sobanska L, De Santi S, Rusinek H, de Leon MJ. Early detection of Alzheimer’s disease using neuroimaging. Exp Gerontol. 2007;42:129–138. doi: 10.1016/j.exger.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Ogar J, Slama H, Dronkers N, Amici S, Gorno-Tempini ML. Apraxia of speech: an overview. Neurocase. 2005;11:427–432. doi: 10.1080/13554790500263529. [DOI] [PubMed] [Google Scholar]
- Okamura N, Suemoto T, Furumoto S, Suzuki M, Shimadzu H, Akatsu H, Yamamoto T, Fujiwara H, Nemoto M, Maruyama M, Arai H, Yanai K, Sawada T, Kudo Y. Quinoline and benzimidazole derivatives: candidate probes for in vivo imaging of tau pathology in Alzheimer’s disease. J Neurosci. 2005;25:10857–10862. doi: 10.1523/JNEUROSCI.1738-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Gee J, Moore P, McMillan C, Vesely L, Grossman M. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics. 2008;21:418–432. doi: 10.1016/j.jneuroling.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O’Neil JP, Lal RA, Dronkers NF, Miller BL, Gorno-Tempini ML. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DA, Johnson NA, Thompson C, Weintraub S, Mesulam MM. A clinical trial of bromocriptine for treatment of primary progressive aphasia. Ann Neurol. 2004;56:750. doi: 10.1002/ana.20301. [DOI] [PubMed] [Google Scholar]
- Rogalski EJ, Mesulam MM. Clinical trajectories and biological features of primary progressive aphasia (PPA) Curr Alzheimer Res. 2009;6:331–336. doi: 10.2174/156720509788929264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, Mead S, Beck J, Mummery C, Ourselin S, Warrington EK, Rossor MN, Warren JD. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2009a;49:984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, Ourselin S, Fox NC. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009b;72:1562–1569. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky D, Bakkour A, Negreira A, Nalipinski P, Weintraub S, Mesulam MM, Caplan D, Dickerson BC. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75:358–366. doi: 10.1212/WNL.0b013e3181ea15e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Alzheimer’s disease neuroimaging I. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009;24:408–416. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund AH, Johnson N, Rademaker A, Weitner BB, Weintraub S. Profiles of decline in activities of daily living in non-Alzheimer dementia. Alzheimer Dis Assoc Disord. 2007;21:8–13. doi: 10.1097/WAD.0b013e3180324549. [DOI] [PubMed] [Google Scholar]