Abstract
Background
A prevailing hypothesis is that the set of genes that underlie the endophenotypes of alcoholism overlap with those responsible for the addicted state. Functional ethanol tolerance, an endophenotype of alcoholism, is defined as a reduced response to ethanol caused by prior ethanol exposure. The neuronal origins of functional rapid tolerance are thought to be a homeostatic response of the nervous system that counters the effects of the drug. Synaptic proteins that regulate neuronal activity are an important evolutionarily conserved target of ethanol.
Methods
We used mutant analysis in Drosophila to identify synaptic proteins that are important for the acquisition of rapid tolerance to sedation with ethanol. Tolerance was assayed by sedating flies with ethanol vapor and comparing the recovery time of flies after their first sedation and their second sedation. Temperature-sensitive paralytic mutants that alter key facets of synaptic neurotransmission, such as the propagation of action potentials, synaptic vesicle fusion, exocytosis, and endocytosis were tested for the ability to acquire functional tolerance at both the permissive and restrictive temperatures.
Results
The shibire gene encodes Drosophila Dynamin. We tested two temperature-sensitive alleles of the gene. The shits1 allele blocked tolerance at both the permissive and restrictive temperatures while shits2 blocked only at the restrictive temperature. Using the temperature-sensitive property of shits2 we showed that Dynamin function is required concomitant with exposure to ethanol. A temperature-sensitive allele of the Syntaxin 1A gene, Syx1A3-69, also blocked the acquisition of ethanol tolerance.
Conclusion
We have shown that shibire and Syntaxin 1A are required for the acquisition of rapid functional tolerance to ethanol. Furthermore, the shibire gene product, Dynamin, appears to be required for an immediate early response to ethanol that triggers a cellular response leading to rapid functional tolerance.
Introduction
Alcoholism is a chronic disease in which individuals are driven to drink excessively despite the negative health and social consequences. It is a disease of changed behavior and it is clear that an individual's propensity for alcoholism results from both genetic and environmental components. The interplay of these components, however, produces various ethanol-induced endophenotypes, making it difficult to model the full behavioral tapestry of alcoholism. While alcoholism does not lend itself to study in a model organism, its endophenotypes do. In the context of alcoholism, an endophenotype is a trait frequently observed in alcoholics (e.g. decreased sensitivity or alcohol resistance, impulsivity) or a response to alcohol exposure (e.g. a drop in body temperature, pharmacodynamic tolerance). It is anticipated that the mechanistic origins of the endophenotype overlap with those of alcoholism (Lesch, 2005). While we cannot yet replicate addiction in the fly, we can probe the origins of an alcoholism endophenotype: functional tolerance, defined as a reduced response to ethanol caused by prior ethanol exposure (Cowmeadow et al., 2005). This type of tolerance is not metabolic in origin (due to a change in the rate of uptake or clearance of the drug), but rather arises from the changed responsiveness of cells. With regard to the nervous system, functional tolerance represents a form of drug-induced neural plasticity (Atkinson, 2009). The acquisition of tolerance has been implicated in the transition to an addicted state due to neuroadaptive changes occurring in the brain (Koob, 1996). Tolerance is ideal for study in model organisms because it can be reliably induced and tested using physiological and behavioral assays.
Although the neural organization of insects is substantially different from higher vertebrates, there is conservation of function at the molecular level (Jeibmann and Paulus, 2009). Candidate gene approaches using mutation analysis have proven successful in the invertebrate model Drosophila melanogaster to identify genes essential for the capacity to develop functional alcohol tolerance. The adult fly appears to be ideal for studying functional tolerance because prior ethanol exposure does not enhance the rate of ethanol clearance (Cowmeadow et al., 2005; Scholz et al., 2000). A number of genes that have been identified as important for the acquisition of functional tolerance in Drosophila, such as homer, hppy, and slo, also participate in the production of mammalian alcohol tolerance (Urizar et al., 2007; Corl et al., 2009; Cowmeadow et al., 2006; Atkinson, 2009). This demonstrates the predictive value of Drosophila studies in understanding the molecular processes that generate tolerance in mammals.
The synapse is an important target of ethanol in invertebrate and vertebrate model systems. We have shown that the voltage-gated Ca2+-activated K+ channel (BK channel) regulates synaptic activity in response to volatile solvents and is needed for the acquisition of rapid tolerance to ethanol in flies (Ghezzi et al., 2010; Cowmeadow et al., 2005). The BK channel has been further implicated in ethanol phenotypes in higher vertebrates (Liu et al., 2006; Brodie et al., 2007). In the rat neurohypophysial system, ethanol potentiates BK channel activity, triggers the internalization of ethanol sensitive channels, and alters gene expression so that replacement channels are refractory to ethanol (Pietrzykowski et al. 2004, 2008). Another evolutionarily conserved synaptic target of ethanol is the Homer protein, an integral part of the protein scaffold at the post-synaptic density that regulates synaptic function (Szumlinski et al., 2005; Urizar et al., 2007; Hayashi et al., 2009). Based on these observations we wanted to further explore the possibility that other synaptic proteins are involved in rapid tolerance to ethanol.
We used mutants that dynamically affect synaptic transmission in the adult nervous system and measured the capacity of these flies to acquire tolerance. The alleles we tested were temperature-sensitive paralytic mutants in genes that produce key facets of synaptic neurotransmission, such as propagation of neuronal action potentials (paralytic) and synaptic vesicular events including fusion (comatose), exocytosis (Syntaxin 1A), and endocytosis (shibire). By reversibly inhibiting synaptic function, in vivo, we have shown that the shibire and Syntaxin 1A genes are required for the acquisition of rapid functional tolerance to ethanol and have further provided exciting evidence that the shibire gene product, Dynamin, might be an early trigger that initiates the pathways responsible for rapid functional tolerance in the adult nervous system.
Methods
Drosophila stocks & fly husbandry
We used the wild-type stock Canton S (CS) and the temperature-sensitive paralytic stocks shits1, shits2, parats1, comtst17, and Syx1A3-69, which are mutant alleles of the genes shibire, paralytic, comatose, and Syntaxin 1A, respectively. The fly stocks were obtained from the Bloomington Drosophila Stock Center and raised on standard cornmeal agar medium at 20–22 °C under a 12/12 hour light/dark cycle. Flies were manipulated under CO2 anesthesia. To avoid any effects of the CO2 on ethanol sedation or tolerance, the flies were stored in food vials for 24 hours after CO2 sedation prior to all behavioral assays.
Administration of ethanol
Ethanol admixed with humidified air was administered to age-matched (8–10 days) female flies using an inebriator (Cowmeadow et al., 2005). Solvent-resistant tubing was used to deliver air from a wall supply to the inebriator. The air supply was split and passed through two flow meters set to 15 ml/minute; one air stream was used for control treatments and the other for ethanol treatments. Each stream then entered a water bubbler consisting of a 250 ml Erlenmeyer flask with a #10 rubber stopper to humidify the air. The flask contained about 100 ml of distilled deionized water with a hole in the rubber stopper just large enough to place a plastic 10 ml pipette through it. The air stream passed through the pipette, bubbled through the water and exited the sidearm of the flask through tubing. In the case of the control, this tubing led directly to a control treatment chamber. In the case of the ethanol vapor stream, this tubing transited through a three-way valve to an ethanol bubbler (Kimble Chase, Vineland, NJ) containing 25 ml of 100% ethanol and then to the treatment chamber. The three-way valve performed the role of a switch delivering either ethanol or the humidified air stream directly. The bubblers were placed in a 65 °C water bath to help ethanol evaporation. The ethanol vapor stream exiting the three-way valve entered a trap to collect any condensing ethanol before entering the treatment chamber.
The treatment chamber consisted of six standard plastic vials. Two microfuge tube racks, one along the top of the six vials and the other along the bottom, clamped the vials and held them in place. The top rack had holes drilled to allow access to the tops of the vials. Above the top rack was a manifold dividing the incoming stream of humidified air or ethanol vapor into six individual streams, each guided through the top rack into one of the vials. An airtight seal was created between the vials and the top microfuge rack using a sheet of Viton®. A fine mesh was placed over the end of the tubing projecting inside each vial to prevent flies from entering the tubing. Using a heated 25-gauge needle, four holes were poked in the bottom of each vial to allow air to exit the system. Nine to twelve age-matched female flies were placed into each vial and either ethanol vapor or humidified air was pumped through the vials.
Tolerance assay
The tolerance assay was done as described in Cowmeadow et al. (2005) with slight modifications. In brief, age-matched female flies were pooled from a single set of culture bottles and divided into two equal groups of vials (the control group and the experimental group). The experimental group was sedated using an ethanol-saturated airstreams while the control group was mock sedated. After sedation, the animals were allowed to recover in a fresh-air environment and then returned to food vials for 24 hours.
On the second day, the test for tolerance was performed by comparing the recovery from sedation between the two groups. Both groups were sedated in tandem using an ethanol-saturated air stream. The control and experimental vials were interdigitated in the chambers to minimize any position effect within the testing apparatus. This was the first ethanol exposure for the control animals and the second ethanol exposure for the experimental animals. Ethanol was administered until all the flies in the ethanol chamber were sedated. Sedated flies were scored as those that were lying on their backs or sides or those lying prostrate with their legs splayed in a non-standard posture. After sedation, the ethanol vapor was replaced with fresh air, and their recovery period was monitored. Flies are said to have recovered from sedation once they regain postural control. Tolerance was quantified by counting the number of flies recovered from sedation in each vial approximately once every minute starting from the time ethanol was first applied to the time the flies recovered. The results for both groups were graphed as the percentage of flies recovered from sedation over time. The mean and SEM were calculated, and the SEM error bars for each data point were plotted. To determine significance, we used a log-rank time-to-event statistical test that evaluates the entire recovery curve (Hosmer et al., 2008).
Heat-shock protocols
All heat-shock treatments were performed in glass or plastic vials heated in an aluminum heat block (VWR, Radnor,PA). For longer heat-shocks the vials were kept in a Digitherm® incubator (Tritech Research, Los Angeles, CA) maintained at 30 °C. To avoid desiccation, cellulose acetate plugs (Flugs™, Genesee Scientific, San Diego, CA) were inserted to the bottom of the vials and moistened with 1 ml of water. The heat-shock protocol varied based on the temperature-sensitive properties of each mutant; for the shi and para alleles, shits2 and parats1, the heat shock was administered at 30 °C for 5 hours and the experiment was also repeated with a shorter duration of heat shock for 30 minutes for shits2; for the comatose allele, comtst17, we used 35 °C for 30 minutes.
Ethanol plus heat shock
To determine the effect of the temperature-sensitive mutations on the capacity to acquire tolerance, ethanol was administered and as soon as the flies were sedated they were shifted to an incubator maintained at the restrictive temperatures for the times listed above. The flies were then moved to food vials overnight on a circadian 12/12 day-night cycle. These animals were then sedated with ethanol vapor and their rate of recovery determined 24 hours after their first sedation as described above.
Quantification of mRNA
Flies were sedated with ethanol vapor one time as described above. Three groups received ethanol vapor and three groups received humidified air. Six hours after sedation, the flies were frozen in liquid nitrogen, and the heads were isolated by vortex decapitation and sieving. RNA was isolated from fly heads using a single-step RNA isolation protocol (Ghezzi et al., 2004). RNA was then quantified spectrophotometrically (NanoDrop Technologies, Wilmington, DE). A 40 µl reverse-transcription reaction using 200 ng of RNA was performed following the manufacturer's protocol using oligo(dT)20 primers and Superscript® II (Life Technologies Corporation, Carlsbad, CA). A standard curve reverse transcription reaction was also performed (800, 400, 200, 100 and 50 ng). The cDNA product was diluted 1 to 5 by adding 160 µl of ddH2O and 5 µl was used in a 25 µl PCR reaction using gene-specific primers (Integrated DNA Technologies, Coralville, IA). The SYBR® Green® based detection protocol was used for real-time amplification and analysis using the ABI Prism 7700 Sequence Detection system (Life Technologies Corporation, Carlsbad, CA). The mRNA abundance was quantified using the Standard Curve method (Applied Biosystems manual, Life Technologies Corporation, Carlsbad, CA) and normalized to an internal control, Cyclophilin 1 (Cyp1). The mean mRNA abundance was plotted with SEM error bars. Significance was calculated using the Student's t-test. The primer sets used were as follows: shiF- CGTCAACTGGGCAACCAAGTCATT, shiR- ATACGGACGCGATCCACCTTTCAT, Syx1AF- CGCAGCACTCGACGTTGT, Syx1AR- CGGTCTGCGTGCGATTG, Cyp1F- ACCAACCACAACGGCACTG, Cyp1R- TGCTTCAGCTCGAAGTTCTCATC.
Measurement of Ethanol Concentrations using Gas Chromatography
Ethanol concentration was measured five hours after sedation using liquid-phase gas chromatography with slight modifications. Immediately following sedation by the saturated ethanol airstream four groups of ten flies each were transferred into 1.5 mL centrifuge tubes containing 700 uL of toluene. An additional four groups of ten flies each were allowed to recover in the fresh air stream for five hours and then transferred into 700 ul of toluene. Flies were immediately crushed with a pestle. The tubes were then centrifuged and the supernatant was collected and transferred to glass vials for gas chromatography. A dilution series of ethanol in toluene was prepared for the generation of a standard curve. Gas chromatography was performed with a 310G gas chromatograph equipped with a 60 meter Restek MXT®-1 column (SRI Instruments, Torrance, CA). Data were collected and analyzed using SRI Instruments Peak Simple software according to the manufacturer's instructions. Final ethanol concentration was determined by interpolating the ethanol peak area values from each sample to the standard curve with known ethanol concentrations.
Results
We assayed for behavioral tolerance using the loss-of-righting-reflex assay. A population of age-matched female flies was split into two groups with one group receiving a sedating dose of ethanol and the second group receiving a mock sedation. Twenty-four hours after the first sedation, both groups received a sedating dose and the recovery was monitored (protocol shown in Fig. 1A.) If the pre-treated experimental animals recovered earlier than the naïve control group, then the flies acquired tolerance. In this paper we report tolerance as a binary event: for a given fly stock, if the recovery curve of the pre-treated animals is significantly left-shifted with respect to the recovery curve of the naïve animals then the stock is said to be capable of acquiring tolerance. In our assay, we have not observed a reproducible correlation between initial sensitivity for ethanol sedation and the capacity to acquire ethanol tolerance. Furthermore, although we observe day-to-day differences in the absolute sensitivity of the flies to ethanol sedation, we observe that the capacity to acquire tolerance is a reliable metric. Figure 1B shows a tolerance test performed on a wild-type Canton S stock.
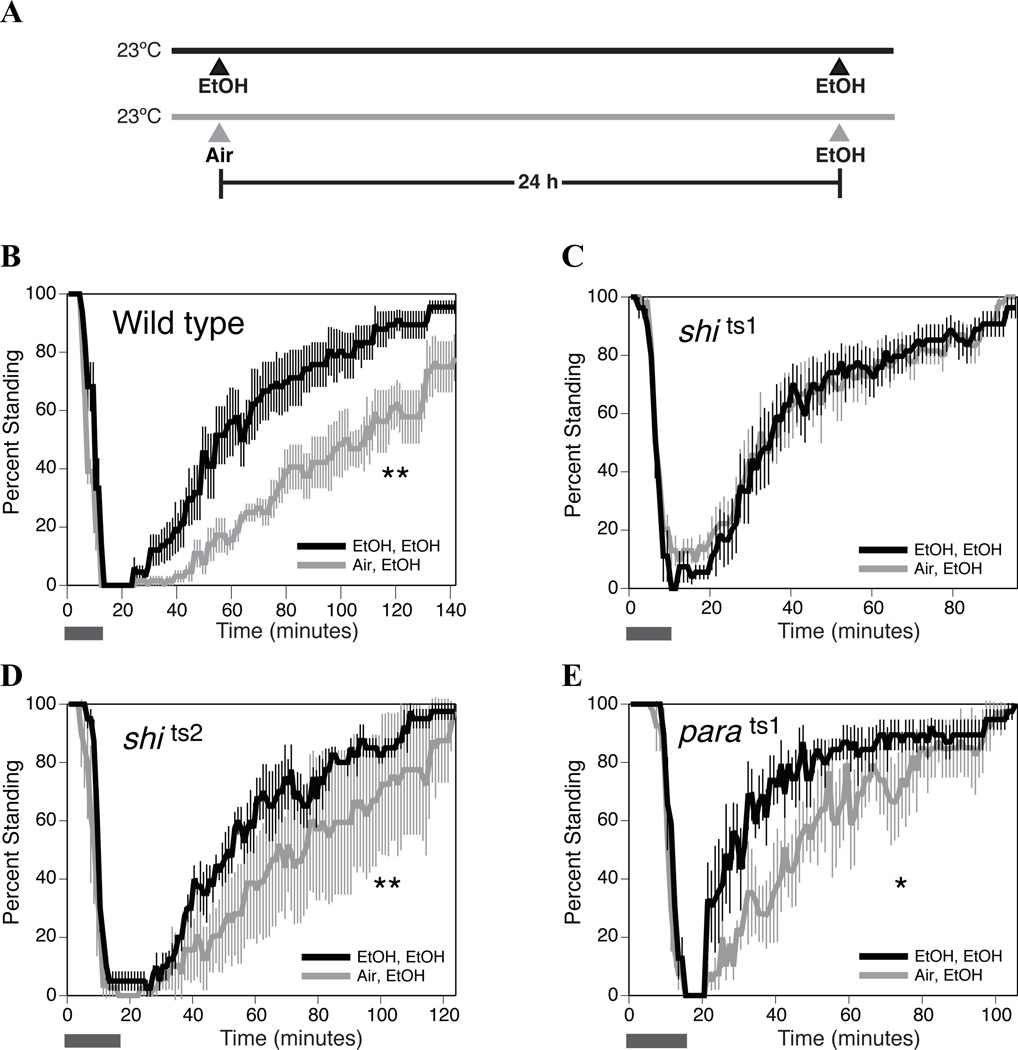
Figure 1. The shits1 allele is a mutant allele of the shibire gene that interferes with the capacity to acquire ethanol tolerance.
A) Schematic of the two-day protocol used to determine if a stock can acquire tolerance to ethanol sedation. A stock is said to be capable of acquiring tolerance if it recovers more rapidly from its second sedation than from its first sedation. The black timeline represents the group that receives two consecutive ethanol sedations (black triangles labeled EtOH). The time between sedations is 24 hours. The rate of recovery is determined after the second ethanol sedation. The gray timeline represents the group that will receive a single ethanol sedation. In this group, age-matched adult females are mock sedated (gray triangle labeled Air), and 24 hours later the flies experience their first ethanol sedation (gray triangle labeled EtOH). The rate of recovery from that first sedation is measured. B–E) Shown are plots of flies as they respond to a sedating dose of ethanol vapor. Plotted is the percentage of flies that exhibit normal postural control. The gray horizontal bars under each plot identify the period during which a saturated ethanol vapor stream is pumped into the vials. Once all of the flies are sedated, the flies are allowed to recover in a fresh-air environment. At this point, recovery from ethanol sedation was monitored. B) Canton S wild-type flies are able to acquire tolerance to ethanol (n=6 repeats). C) Flies that carry the shits1 mutation in the shibire Dynamin gene are unable to acquire tolerance (n=6 repeats). D) However, flies that carry the shits2 mutation in the shibire Dynamin gene are able to acquire tolerance at the permissive temperature (n=4 repeats). E) Flies that carry the parats1 mutation in the paralytic voltage-gated Na+ channel gene are able to acquire tolerance (n=4 repeats). Statistical significance was determined by the log rank test (*p<0.05; **p<0.01). The error bars represent the SEM for each data point.
We used this behavioral assay to screen important synaptic mutants that perturb different stages of the synaptic vesicle cycle. Using temperature-sensitive paralytic mutants, we were able to reversibly inhibit synaptic signaling in vivo and test the role played by these synaptic genes in the acquisition of ethanol tolerance.
Temperature-sensitive shibire mutants disrupt the capacity to acquire tolerance
In our investigation of the relationship between synaptic proteins and the acquisition of tolerance, we examined two temperature-sensitive paralytic alleles of the shibire gene. The shibire gene encodes for the Drosophila homolog of the synaptic vesicle recycling protein, Dynamin (van der Bliek and Meyerowitz, 1991; Chen et al., 1991). The shits1 and shits2 alleles are point mutations in the crucial GTPase domain of the gene and exhibit reversible paralysis at the restrictive temperature (30 °C) (van der Bliek and Meyerowitz, 1991). The flies are not paralyzed at the permissive temperature (23°C). Paralysis is caused by a conditional blockade in synaptic transmission that is caused by defective vesicle recycling (Grigliatti et al., 1973; Koenig et al., 1983). This phenotype shows remarkable conservation across phylogeny and a homologous mutation in mammalian Dynamin-1 causes temperature-sensitive impairment of vesicle endocytosis (Damke et al., 1995).
We performed tolerance assays at the permissive temperature (23 °C) for both alleles and observed that the shits1 allele (Fig. 1C) is incapable of acquiring 24 hour tolerance after a prior sedation with ethanol whereas the shits2 allele (Fig. 1D) is able to acquire tolerance.
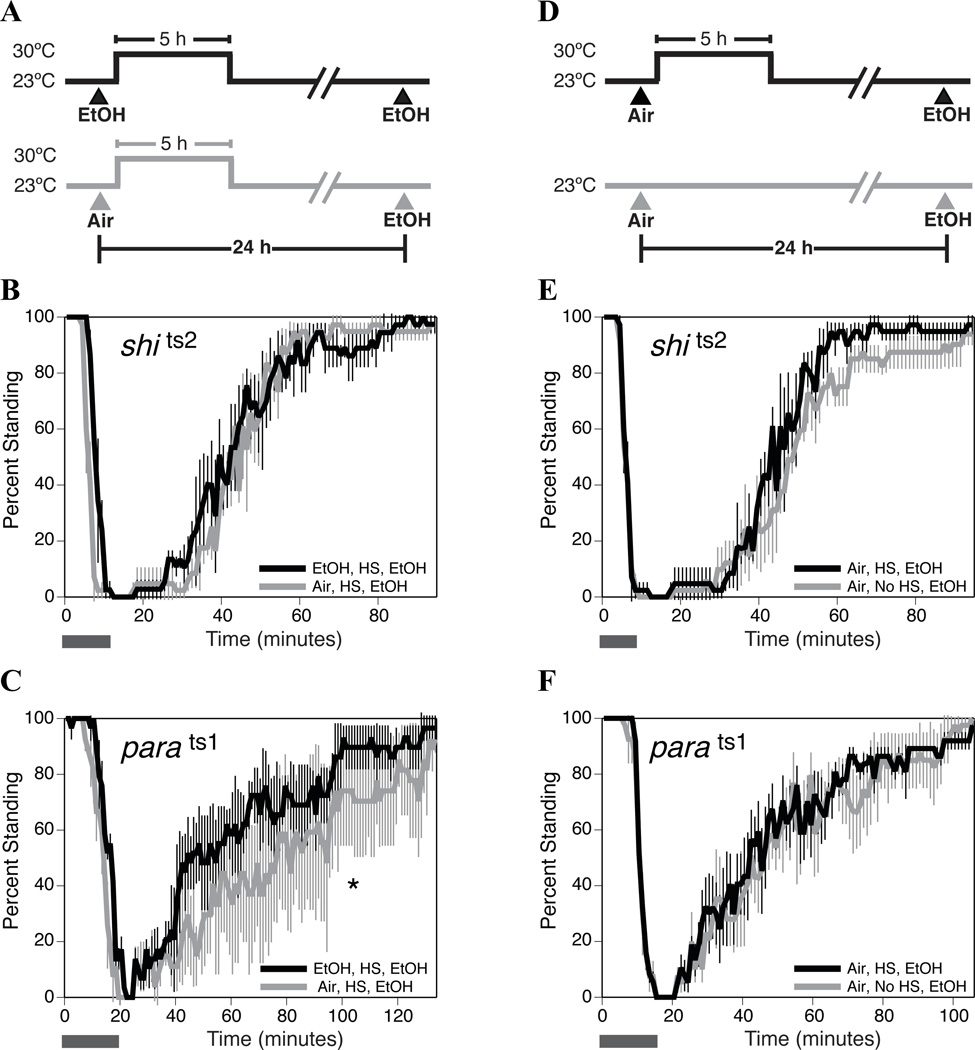
To determine whether shits2 is capable of acquiring tolerance at the restrictive temperature, we incubated the flies at the restrictive temperature of 30 °C immediately following the first ethanol sedation. We showed that a 5 hour heat shock, initiated as soon as the flies were ethanol sedated, could block the acquisition of tolerance (Fig. 2A & B). We chose a 5 hour heat shock because it was the longest period of time we could keep shits2 flies paralyzed without any loss of viability and furthermore, because gas chromatography showed that the ethanol has been completely cleared by 5 hours post sedation (immediately following sedation fly hemolymph concentatraion was approximately 170 mM +/− 20mM ethanol, after 5 h incubation in fresh air ethanol in the flies was not detectable by GC). This heat treatment protocol does not block the acquisition of tolerance by wild type flies (data not shown).
Figure 2. The shits2 allele blocks the capacity to acquire tolerance in a temperature-sensitive manner.
A) Schematic of the experimental paradigm that produced the results of panels B and C, showing the timing of the ethanol sedations, the mock sedations, and the heat pulse. The heat pulse began less than 30 seconds after the end of the first ethanol sedation. B & C) Ethanol sedation and recovery curves as described in figure 1. The horizontal bar represents the period during which ethanol vapor is delivered. B) A five-hour paralysis-inducing heat pulse completely disrupted the acquisition of tolerance by shits2 flies (n=3 repeats). C) A five-hour paralysis-inducing heat pulse did not prevent parats1 flies from acquiring tolerance to ethanol sedation (n=3 repeats). D) Schematic of the experimental paradigm that produced the results of panels E and F. The purpose of this protocol was to determine whether a 5 hour heat pulse alters the sensitivity of flies to ethanol. E & F) Ethanol sedation and recovery curves as described in figure 1. The horizontal bar represents the period during which ethanol vapor is delivered. E) A five-hour paralysis-inducing heat pulse did not alter the ethanol sensitivity of shits2 flies (n=3 repeats). F) A five-hour paralysis-inducing heat pulse did not alter the ethanol sensitivity of parats1 flies (n=4 repeats). Statistical significance was determined by the log rank test (*p<0.05; **p<0.01). The error bars represent the SEM for each data point.
While the shits1 allele disrupted tolerance at the permissive temperature (Fig. 1C), shits2 only interfered with tolerance when the flies were incubated at the restrictive temperature following sedation (cf. Fig. 2B and Fig. 1D). There are documented reports about differences in the potencies of these two mutant alleles with regard to temperature-kinetics and recovery from paralysis (Chen et al., 2002). The differences in the ethanol phenotypes could arise due to these properties. The inability of shits2 mutants to acquire tolerance at the restrictive temperature could be produced by the blockade in neural signaling or might be more specifically linked to the shibire gene product, Dynamin. To determine if the impairment in the capacity to acquire tolerance is produced by a generalized reduction in neuronal activity, we used the parats1 allele to inhibit neural signaling. The parats1 mutation is a temperature-sensitive mutation in the predominant voltage-gated neuronal sodium channel gene (paralytic) that also blocks neural activity at the restrictive temperature (Suzuki et al., 1971; Nelson and Wyman, 1990). These flies were able to acquire tolerance at both the permissive (Fig. 1E) and restrictive temperatures (Fig. 2C). Heat treatment by itself did not alter the innate sensitivity of either shits2 or parats1 flies to ethanol sedation (Fig. 2D, E & F).
Dynamin activity is required during ethanol intoxication for the acquisition of tolerance
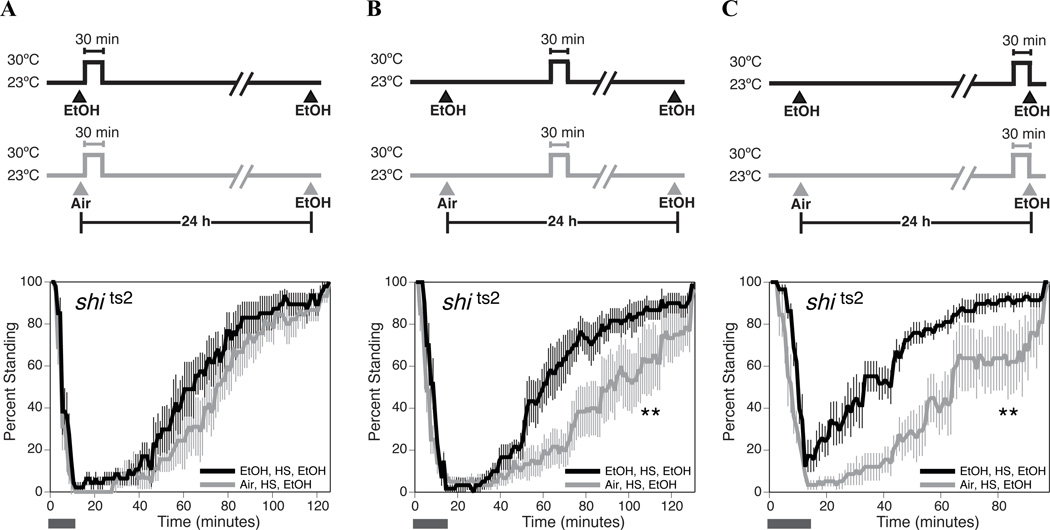
Because shits2 blocks ethanol tolerance at the restrictive but not at the permissive temperature, we were able to use temperature shifts to determine when normal Dynamin activity was required for the production of tolerance. For these experiments, we used a 30 minute heat shock and not the 5 hour heat shock because manipulating flies for the longer time period had the potential of disrupting the fly circadian rhythm. Flies were shifted from room temperature to 30 °C for 30 minutes at three different time points between the first and second ethanol sedation; immediately after ethanol sedation, after complete recovery (3 h) and before the tolerance test on day 2 (23.5 h; Fig. 3). We observed that incubating shits2 flies at the restrictive temperature interfered with tolerance only if the heat treatment occurred immediately after ethanol sedation (Fig. 3A). We also tested a ~1 minute heat shock, however, it did not block the acquisition of 24 hour tolerance even though the flies exhibited the paralytic phenotype caused by the shits2 mutation (data not shown). When the thirty-minute temperature shift occurred 3 hours or 23.5 hours after recovery from ethanol sedation it had no effect on the acquisition of tolerance (Fig. 3B & 3C). This result implicates Dynamin in an early step in the process of generating tolerance, probably in sensing of the drug itself or in sensing the sedated state.
Figure 3. A functional Dynamin protein is required for the initiation of tolerance but not for the maintenance of tolerance.
Age-matched shits2 females were used in all assays, and the time between the first treatment (EtOH or Air) and the second treatment (EtOH) was 24 hours. For each panel, the figure at the top represents the treatment protocol that produced the recovery curves shown at the bottom. Recovery curves are as described in figure 1. The horizontal bar represents the period during which ethanol vapor is delivered. A) A 30 minute heat pulse immediately after ethanol sedation blocks the acquisition of tolerance in shits2 flies. B) A 30 minute heat pulse delivered three hours after ethanol sedation does not interfere with the acquisition of tolerance in shits2 flies. C) A 30 minute heat pulse delivered 23.5 hours after ethanol sedation does not interfere with the acquisition of tolerance in shits2 flies. Statistical significance was determined by the log rank test (*p<0.05; **p<0.01). Error bars are SEM for each data point (n=6 repeats).
The SNARE protein Syntaxin 1A is required for ethanol tolerance
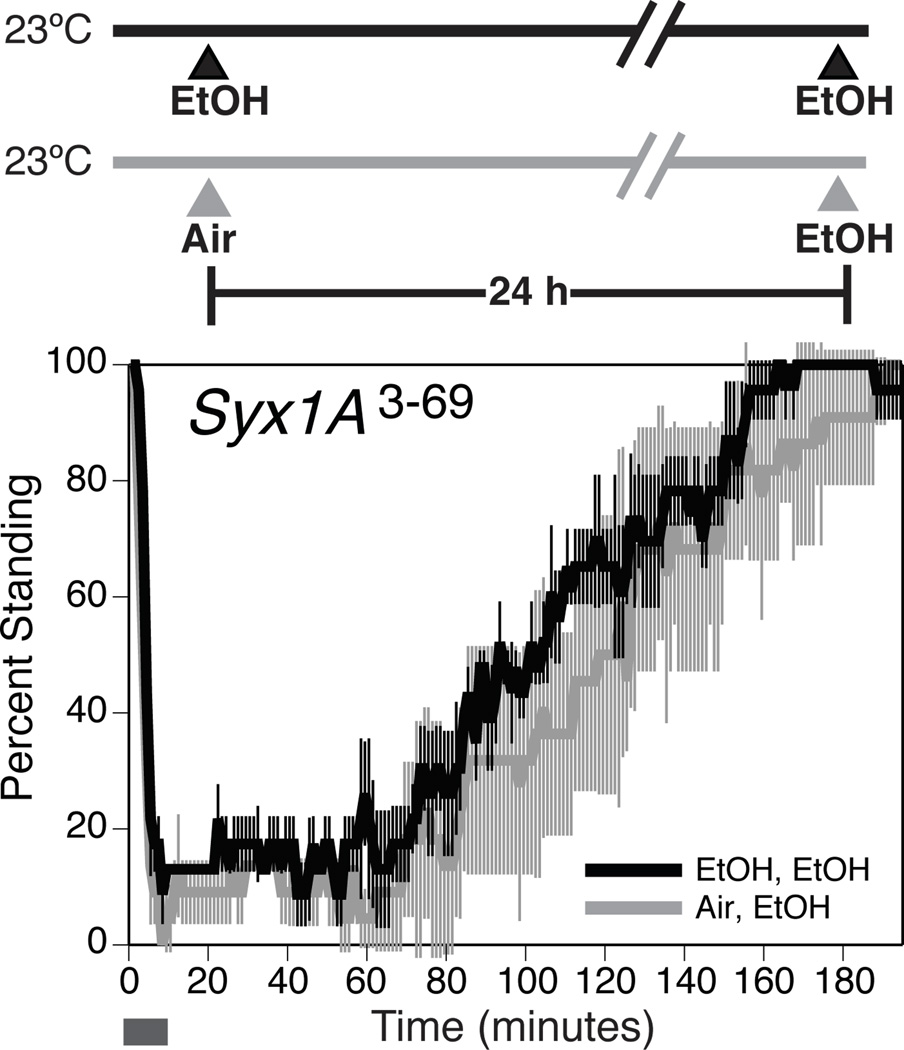
Syntaxin 1A is a part of the SNARE protein complex and functions as a t-SNARE (Soluble NSF attachment receptor) protein that is required in the target membrane for vesicular fusion (Bennett et al., 1992; Littleton et al., 1998). The Syntaxin 1A protein has been shown to interact with voltage-gated K+ channels and regulate neurotransmitter release (Singer-Lahat et al., 2007; Feinshreiber et al., 2009; Regev et al., 2009). The mutant, Syx1A3-69, is a temperature-sensitive mutant allele of Syntaxin 1A (Littleton et al., 1998). The flies carrying this allele failed to acquire tolerance at the permissive temperature (Fig. 4).
Figure 4. The mutant allele of Syntaxin 1A, Syx1A3-69, interferes with the capacity to acquire ethanol tolerance.
At the top of the figure is shown the treatment protocol that produced recovery curves shown at the bottom of the figure. The recovery curve is as described in figure 1. The horizontal bar represents the period during which ethanol vapor is delivered. Statistical significance was determined by the log rank test (*p<0.05; **p<0.01). Error bars are SEM for each data point (n=3 repeats).
Only some synaptic protein mutants block ethanol tolerance
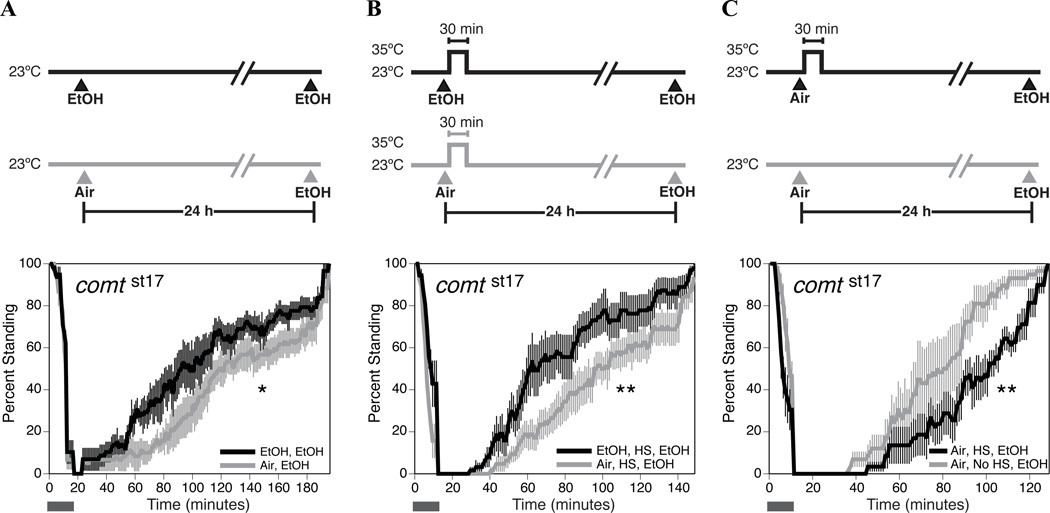
We also tested a conditional temperature-sensitive paralytic mutant allele of the comatose gene (Sudhof, 1995). The comatose gene encodes the Drosophila NSF protein (N-ethylmaleimide-sensitive factor, dNSF1) that is critical for vesicular transport and regulates vesicle fusion and exocytosis at the synapse (Ordway et al., 1994). The ATPase activity of NSF is thought to prime vesicles and promote docking and fusion of the vesicles at the pre-synaptic membrane. The allele, comtst17 is a temperature-sensitive allele of this gene. The mutant phenotype of this allele is manifested at the restrictive temperature of 35 °C (Siddiqi and Benzer, 1976). At the restrictive temperature, comtst17 mutants have an activity-dependent reduction in neurotransmitter release and a buildup of docked synaptic vesicles and 7S synaptic SNARE complexes leading to paralysis (Littleton et al., 1998; Kawasaki et al., 1998). We used the comtst17 mutant to test whether the comatose gene product is required for ethanol tolerance.
The comatose allele, comtst17, did not interfere with the ability to acquire tolerance at the permissive temperature (Fig. 5A). We also incubated the comtst17 flies at the restrictive temperature (35 °C/30 min) immediately following the first ethanol sedation and tested for tolerance the following day. The comtst17 stock was able to acquire tolerance even after incubation at 35 °C (Fig. 5B). Controls for heat alone showed that comtst17 animals exhibited increased sensitivity to the drug after a prior heat treatment (Fig. 5C). Comparable results were obtained for another temperature-sensitive allele of the comatose gene, comttp7 (data not shown).
Figure 5. A temperature-sensitive mutation in the comatose gene does not prevent the acquisition of tolerance to ethanol sedation.
Age-matched females were used in all assays, and the time between the first treatment (EtOH or Air) and the second treatment (EtOH) was 24 hours. For each panel, the figure at the top represents the treatment protocol that produced the recovery curves shown at the bottom. Recovery curves are as described in figure 1. The horizontal bar represents the period during which ethanol vapor is delivered. A) The comtst17 mutation does not interfere with the capacity to acquire ethanol tolerance at the permissive temperature. B) The comtst17 mutation does not prevent the acquisition of tolerance when flies are heat shocked for 30 minutes immediately after their first ethanol sedation. C) Heat shock alone sensitizes comtst17 flies to the effects of ethanol. Statistical significance was determined by the log rank test (*p<0.05; **p<0.01). Error bars are SEM for each data point (n=6 repeats).
Ethanol does not alter the expression level of the shibire or the Syntaxin 1A gene
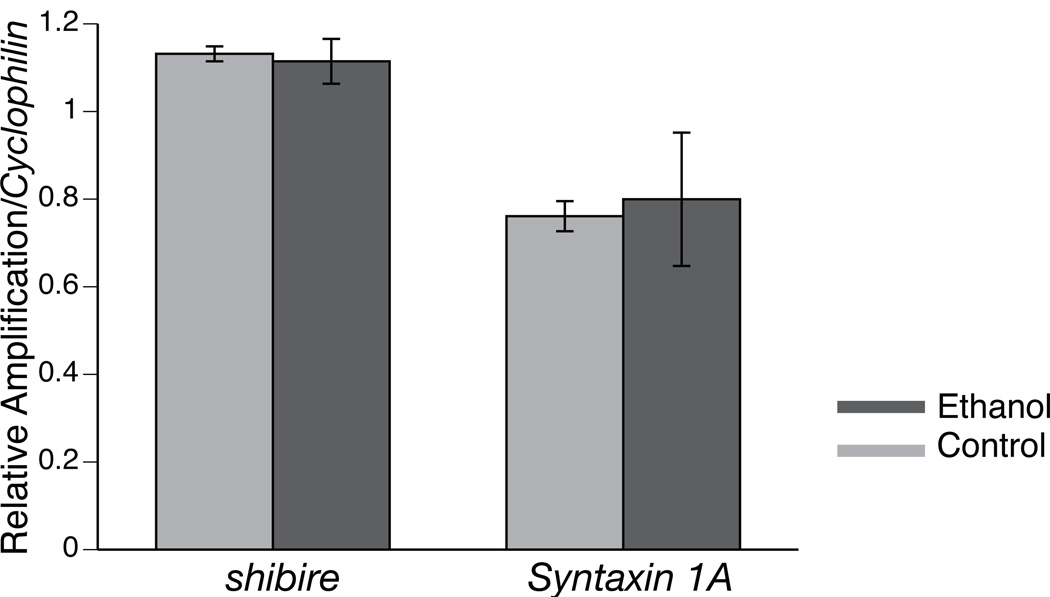
We compared the expression levels of the shibire and Syntaxin 1A genes before and after ethanol sedation. Transcript levels were measured six hours after ethanol sedation by real-time RT PCR using gene-specific primers. This time point was chosen because we had previously shown that ethanol tolerance can be detected at 6 hours post-sedation (Cowmeadow et al., 2005). Furthermore, the slowpoke gene (which is also required for ethanol tolerance) shows induction at this time point (Cowmeadow et al., 2006). As an internal control we used the cell cycle gene, Cyclophilin 1, which we have previously shown to not be induced after ethanol administration. We did not observe an increase in the relative concentration of mRNA from either the shibire gene or the Syntaxin 1A gene (Fig. 6).
Figure 6. Ethanol sedation did not affect the relative abundance of either the shibire or the Syntaxin 1A message.
The mRNA levels of shibire and Syntaxin 1A transcripts in fly heads were measured using real-time reverse transcriptase-PCR six hours after ethanol sedation (Ethanol) or six hours after a mock sedation (Control). Message abundance was normalized relative to mRNA from the internal control gene, Cyp1. Six hours after ethanol sedation, we did not observe an increase in the relative abundance of mRNA from either the shibire or the Syntaxin 1A gene. The error bars are SEM (n=3 repeats.)
Discussion
We used temperature-sensitive mutations that interfere with specific steps in neural signaling to probe the origins of rapid functional tolerance to ethanol sedation. Tolerance can be conceptually partitioned into three categories: chronic, rapid and acute. Chronic ethanol tolerance is the result of repeated ethanol exposure. Rapid tolerance is produced by a single exposure to the drug, measured after drug clearance. Acute tolerance is tolerance that arises while the animal is still being exposed to the drug. It is not yet known whether these states are the products of distinct or common mechanisms. Mechanisms of tolerance can also be classified as metabolic or functional. Metabolic tolerance is produced by a change in the rate of uptake or clearance of the drug while functional tolerance refers to a plastic change in the responsivity of cells to the drug (Atkinson, 2009). Understanding the molecular basis of functional tolerance is relevant to understanding the basis of alcohol addiction. We and others have shown that tolerance observed in adult flies is not due to a change in uptake or clearance of the drug (Cowmeadow et al., 2005; Scholz et al., 2000). In the remainder of this discussion, tolerance will specifically refer to rapid functional tolerance.
Ethanol is a pleiotropic drug that affects a wide variety of cellular processes. This provides a surfeit of drug targets and conflates the identification of the specific molecular processes and gene products that contribute to each ethanol endophenotype. In higher vertebrates ethanol affects proteins that regulate synaptic release leading to cellular and behavioral phenotypes (Beckstead et al., 2000; Crabbe et al., 2006; Boehm et al., 2004; Lovinger and White, 1991; Lovinger et al., 1989; Szumlinski et al., 2005; Liu et al., 2006). We selected temperature-sensitive mutations that target different stages of the synaptic vesicle cycle. The mutant alleles of shibire, comatose and Syntaxin 1A, which are the centerpieces of this work, are well-characterized temperature-sensitive mutations (Grigliatti et al., 1973; Siddiqi and Benzer, 1976; Pallanck et al., 1995; Littleton et al., 1998).
The shibire gene encodes Drosophila Dynamin, a protein that is part of the mechanism for recycling synaptic vesicles. Flies carrying the shits1 allele failed to acquire tolerance at the permissive temperature (Fig. 1C), while a different shibire mutant allele, shits2, eliminated the capacity to acquire tolerance at the restrictive (Fig. 2B) but not the permissive temperature (Fig. 1D). The phenotypic differences between the mutant stocks are not due to genetic background since both the alleles were backcrossed six-fold into a wild-type background. We observed that the inability to acquire tolerance at the permissive temperature segregated with the shits1 allele and the temperature-sensitive blockade of tolerance segregated with the shits2 allele.
How can we account for the fact that shits1, a temperature-sensitive allele, eliminates tolerance at the permissive temperature? Moreover, why do the shits1 and shits2 alleles behave differently in the tolerance assay? Both the shibire alleles that we tested, shits1 and shits2, are point mutations in the critical GTPase domain of the gene (van der Bliek and Meyerowitz, 1991). These alleles produce mutant proteins irrespective of temperature. Their temperature-sensitive character is thought to arise because a higher temperature elicits a change in conformation not experienced by the wild-type protein (Grant et al., 1998). The shits1 allele is known to be phenotypically stronger than the shits2 allele. Animals carrying the shits1 allele show abnormal distribution and stability of Dynamin complexes even at the permissive temperature whereas the shits2 mutation does not have an obvious biochemical phenotype at the permissive temperature (Chen et al., 2002). Earlier studies have shown that even at the larval stage the different potencies of these alleles are apparent. At the restrictive temperature shits1 larvae show an extreme phenotype exhibiting complete paralysis and inability to pupate while the shits2 larvae exhibit some movement and can pupate and eclose (Grigliatti et al., 1973). We believe that the difference in strength of these two alleles likely accounts for the differential effect on the capacity to acquire tolerance: the stronger allele, shits1, blocks tolerance at the permissive temperature while the weaker allele, shits2, only disrupts the acquisition of tolerance at the restrictive temperature.
It is extremely interesting that the blockade of tolerance exhibited by shits2 was effective only if the restrictive temperature shift occurred immediately after ethanol sedation (Fig. 3A). Once the flies recovered from sedation, incubating shits2 mutants at the restrictive temperature did not affect the acquisition of tolerance. We interpret this to mean that the shibire gene product, Dynamin, is required for the initiation of the tolerance response. While rapid tolerance is tolerance measured after drug clearance, acute tolerance is tolerance that occurs during the drug experience. The shibire-sensitive period overlaps with the time period that we would normally assign to acute tolerance. If shits2 blocks acute tolerance at the restrictive temperature then it would mean that acute tolerance and rapid tolerance have a common mechanistic origin. At this time, we do not know whether flies experience acute tolerance. However, it will be important to determine this and test how shits2 affects acute tolerance.
Our data strongly suggests an important role for Dynamin in the ethanol tolerance pathway. In mammals, Dynamin-1 is pre-synaptically concentrated, Dynamin-2 is ubiquitous and Dynamin-3 is preferentially localized to the post-synaptic compartment of the neurons and testicular tissue. The Dynamins are mechanistically involved in membrane vesicle scission including synaptic vesicle recycling, phagocytosis, genesis of podosomes, and cytokinesis (Praefcke and McMahon, 2004). Flies only have a single Dynamin gene, shibire, and its products are thought to fulfill all of the needed roles of mammalian Dynamin-1, -2 and -3 (Praefcke and McMahon, 2004).
In higher vertebrates, Dynamin-3 and Homer proteins interact in the post-synaptic density (PSD) where their scaffolding roles are essential for dendritic spine remodeling and the recycling of AMPA receptors (Gray et al., 2003; Lu et al., 2007). The PSD in mammals has been strongly implicated in ethanol phenotypes affecting neuronal plasticity and leading to addicted states (Chandler, 2003). The PXXF motif of Dynamin-3 binds to an EVH domain within the Homer protein and this causes Dynamin-3 to localize to the PSD (Gray et al., 2003). Sequence analysis of the Drosophila Homer and Dynamin proteins indicate the presence of amino acid motifs required for this protein:protein interaction (unpublished observations). In mammals, mutations in homer have been shown to cause changes in alcohol preference and intake, sensitivity, and to reduce the magnitude of acquired tolerance (Szumlinski et al., 2005; Szumlinski et al., 2007). In flies, Urizar et al. (2007) have shown that a mutant loss-of-function allele of homer enhances ethanol sensitivity and reduces the capacity of flies to acquire chronic tolerance. The observation that mutations in two interacting proteins, Homer and Dynamin, produce related ethanol phenotypes strongly supports the idea that these proteins are key components in the ethanol tolerance pathway.
Another synaptic gene that we demonstrated is required for rapid ethanol tolerance is Syntaxin 1A. Syntaxin 1A is a cytoplasmic membrane protein that together with SNAP-25 and Synaptobrevin forms the SNARE complex which docks synaptic vesicles at the exocytotic active zone (Littleton et al., 1998). The temperature-sensitive allele, Syx1A3-69 did not acquire tolerance at the permissive temperature (Fig. 4). As with shits1, the Syx1A3-69 allele is not temperature-sensitive with regard to the tolerance phenotype. It has been previously demonstrated that, in C. elegans, a mutation in syntaxin can cause strong resistance to isoflurane and halothane and recently, it was shown that over-expression of Syntaxin 1A in PC12 cells blocks the effects of isoflurane on neurotransmitter release (van Swinderen et al., 1999; Herring et al., 2009). Studies have shown that Syntaxin 1A interacts with both Kv2.1 and Kv1.1 and modulates the firing frequency of the pre-synaptic membrane (Singer-Lahat et al., 2007; Feinshreiber et al., 2009). Modulation of the neural refractory period and firing frequency has been shown to be a mechanism through which changes in BK channel gene expression produces tolerance to sedation with the organic solvent benzyl alcohol (Ghezzi et al., 2004; Ghezzi et al., 2010). It is possible that Syntaxin 1A also contributes to the production of tolerance through its influence on the refractory period of neurons.
Mutations in the comatose gene did not interfere with the acquisition of tolerance (Fig. 5A & 5B). The comatose gene encodes the Drosophila neuronal-specific NEM Sensitive Factor, dNSF1 (Pallanck et al. 1995). dNSF1 plays an essential role in the disassembly of the SNARE (Soluble NSF Associated Protein Receptor) complex after fusion or priming of vesicles (Golby et al., 2001). We examined the comtst17 allele, which is a hypomorph that produces paralysis at 37 °C. Biochemically, incubation of the comatose mutants at 37°C results in the stabilization of assembled SNARE complexes (Tolar and Pallanck, 1998). This causes an activity-dependent reduction in neurotransmitter release (Kawasaki et al., 1998). While heat treatment does not block tolerance in comtst17 stocks, we observed that a 30 minute heat pulse 24 hour before ethanol sedation caused the comtst17 animals to be sensitized to their first dose of ethanol (Fig. 5C). We confirmed these findings with another temperature-sensitive allele, comttp7 (data not shown). We cannot account for why a heat treatment delivered such a long time before the ethanol exposure should alter ethanol sensitivity. In spite of this phenotype, the flies are able to acquire tolerance to an ethanol sedation that is delivered immediately before the heat pulse (Fig. 5B). It appears as if the ethanol treatment suppresses the effect of the heat treatment in comtst17 animals. While we do not have a mechanistic explanation for this result, it is consistent with the idea that dNSF1 helps set the sensitivity to ethanol but that wild type function of the protein is not essential for the production of ethanol tolerance.
The proteins encoded by the shibire and Syntaxin 1A genes participate in different steps in synaptic release (vesicle release and vesicle recycling, respectively). However, because of tight coupling between each step in the process of synaptic signaling, it is likely that mutations in either gene produce a generalized disorganization in the synaptic machinery and it is probable that this disorganization prevents the actualization of the tolerance response.
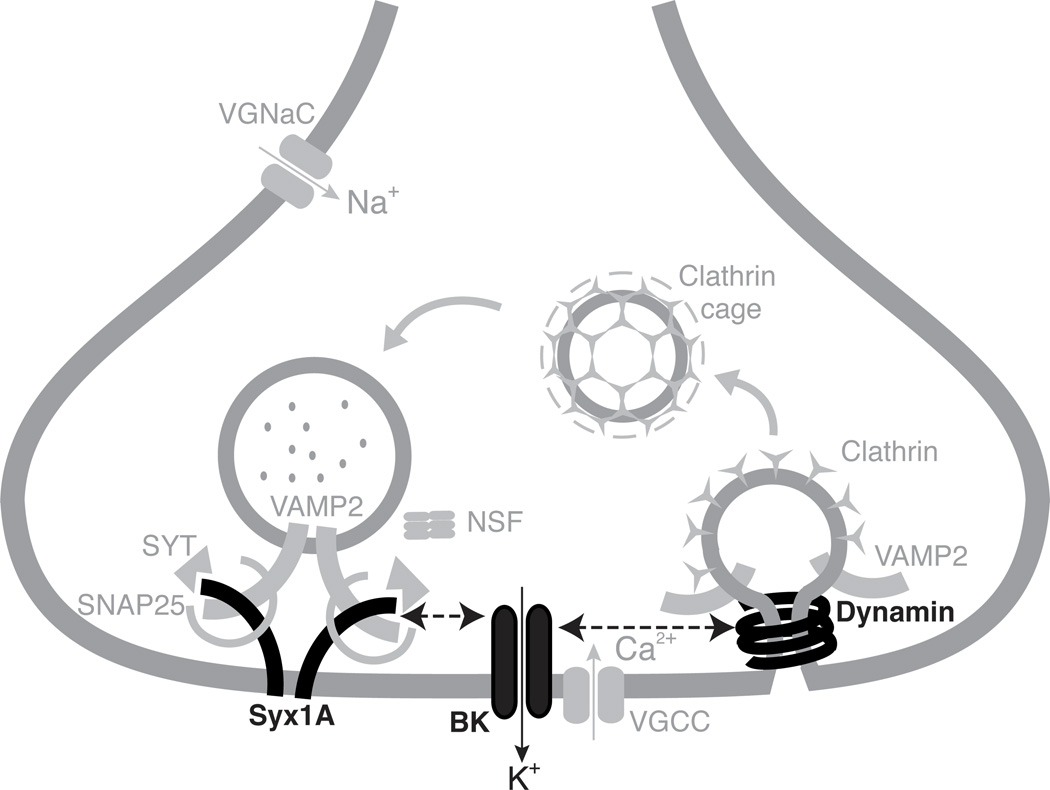
Proteomic analysis of synaptic protein complexes in higher vertebrates have shown that Dynamin-1 physically interacts with BK channels and that BK channels interact with Syntaxin 1A (Figure 7; Gorini et al., 2010; Cibulsky et al., 2005). The interaction between Dynamin and BK channels is particularly interesting since Pietrzykowski et al. (2004) have shown in the rat hypothalamic neurohypophysial explant system that ethanol tolerance occurs concomitantly with the declustering and internalization of BK channels. It is possible that the physical change underlying the removal of BK channels is facilitated by an interaction of the channel with Dynamin-1 and the reason that shibire mutants disrupt the capacity to acquire tolerance is because the BK channel can no longer be properly repositioned. There is no physical evidence yet that Dynamin directly interacts with ethanol. However, with respect to rapid ethanol tolerance, our data indicates that a functional target of ethanol is a protein or process that is located in close proximity to synaptically-localized Dynamin and that probably includes Dynamin, Syntaxin 1A and the BK channel.
Figure 7. The proteins essential for the acquisition of tolerance identified in this study interact in the synaptic terminal.
Dotted lines with double arrowheads indicate that the two proteins interact. Biochemical analysis has shown that the Syntaxin 1A protein physically interacts with BK type Ca2+-activated K+ channels and that BK-type Ca2+-activated K+ channels physically interact with Dynamin. Proteins shown to be required for the acquisition of tolerance are drawn and labeled in black; Syx1A (Syntaxin 1A), BK (BK-type Ca2+-activated voltage-gated K+ channel) and Dynamin. NSF is the product of the comatose gene. Mutations in comatose did not block tolerance. All other items in gray are included to provide context. Figure adapted from Gorini et al. (2010).
Acknowledgements
We thank Xiaolei Li, Rudi Bohm, Roseanna Robles, Ben Troutwine, Brooks Robinson and Jane Kirschman for comments and criticisms. This work was supported by National Institute of Health Grant R01 AA018037 to NSA.
References
- Atkinson NS. Tolerance in Drosophila. J Neurogenet. 2009:1–10. doi: 10.1080/01677060802572937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, et al. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Boehm SLn, et al. gamma-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Brodie MS, et al. Ethanol interactions with calcium-dependent potassium channels. Alcohol Clin Exp Res. 2007;31:1625–1632. doi: 10.1111/j.1530-0277.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Chen ML, et al. Unique biochemical and behavioral alterations in Drosophila shibire(ts1) mutants imply a conformational state affecting dynamin subcellular distribution and synaptic vesicle cycling. J Neurobiol. 2002;53:319–329. doi: 10.1002/neu.10101. [DOI] [PubMed] [Google Scholar]
- Chen MS, et al. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Cibulsky SM, Fei H, Levitan IB. Syntaxin-1A binds to and modulates the Slo calcium-activated potassium channel via an interaction that excludes syntaxin binding to calcium channels. J Neurophysiol. 2005;93:1393–1405. doi: 10.1152/jn.00789.2004. [DOI] [PubMed] [Google Scholar]
- Corl AB, et al. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137:949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, et al. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcohol Clin Exp Res. 2006;30:745–753. doi: 10.1111/j.1530-0277.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, et al. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Damke H, et al. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinshreiber L, et al. Syntaxin modulates Kv1.1 through dual action on channel surface expression and conductance. Biochemistry. 2009;48:4109–4114. doi: 10.1021/bi9002088. [DOI] [PubMed] [Google Scholar]
- Ghezzi A, et al. slo K(+) channel gene regulation mediates rapid drug tolerance. Proc Natl Acad Sci U S A. 2004;101:17276–17281. doi: 10.1073/pnas.0405584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, et al. BK channels play a counter-adaptive role in drug tolerance and dependence. Proc Natl Acad Sci U S A. 2010;107:16360–16365. doi: 10.1073/pnas.1005439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby JA, Tolar LA, Pallanck L. Partitioning of N-ethylmaleimide-sensitive fusion (NSF) protein function in Drosophila melanogaster: dNSF1 is required in the nervous system, and dNSF2 is required in mesoderm. Genetics. 2001;158:265–278. doi: 10.1093/genetics/158.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini G, et al. Dynamin-1 co-associates with native mouse brain BKCa channels: proteomics analysis of synaptic protein complexes. FEBS Lett. 2010;584:845–851. doi: 10.1016/j.febslet.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D, et al. Probable mechanisms underlying interallelic complementation and temperature-sensitivity of mutations at the shibire locus of Drosophila melanogaster. Genetics. 1998;149:1019–1030. doi: 10.1093/genetics/149.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NW, et al. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol. 2003;13:510–515. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Grigliatti TA, et al. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet. 1973;120:107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- Hayashi MK, et al. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, et al. Isoflurane inhibits the neurotransmitter release machinery. J Neurophysiol. 2009;102:1265–1273. doi: 10.1152/jn.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, May S. Applied survival analysis: regression modeling of time to event data. 2008 [Google Scholar]
- Jeibmann A, Paulus W. Drosophila melanogaster as a model organism of brain diseases. Int J Mol Sci. 2009;10:407–440. doi: 10.3390/ijms10020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Mattiuz AM, Ordway RW. Synaptic physiology and ultrastructure in comatose mutants define an in vivo role for NSF in neurotransmitter release. J Neurosci. 1998;18:10241–10249. doi: 10.1523/JNEUROSCI.18-24-10241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Saito K, Ikeda K. Reversible control of synaptic transmission in a single gene mutant of Drosophila melanogaster. J Cell Biol. 1983;96:1517–1522. doi: 10.1083/jcb.96.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Alcohol dependence and gene x environment interaction in emotion regulation: Is serotonin the link? Eur J Pharmacol. 2005;526:113–124. doi: 10.1016/j.ejphar.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Littleton JT, et al. Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron. 1998;21:401–413. doi: 10.1016/s0896-6273(00)80549-8. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. CaM kinase II phosphorylation of slo Thr107 regulates activity and ethanol responses of BK channels. Nat Neurosci. 2006;9:41–49. doi: 10.1038/nn1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JC, Wyman RJ. Examination of paralysis in Drosophila temperature-sensitive paralytic mutations affecting sodium channels; a proposed mechanism of paralysis. J Neurobiol. 1990;21:453–469. doi: 10.1002/neu.480210307. [DOI] [PubMed] [Google Scholar]
- Ordway RW, Pallanck L, Ganetzky B. Neurally expressed Drosophila genes encoding homologs of the NSF and SNAP secretory proteins. Proc Natl Acad Sci U S A. 1994;91:5715–5719. doi: 10.1073/pnas.91.12.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanck L, Ordway RW, Ganetzky B. A Drosophila NSF mutant. Nature. 1995;376(6535):25. doi: 10.1038/376025a0. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, et al. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: decreased ethanol potentiation and decreased channel density. J Neurosci. 2004;24:8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- Regev N, et al. Selective interaction of syntaxin 1A with KCNQ2: possible implications for specific modulation of presynaptic activity. PLoS One. 2009;4:e6586. doi: 10.1371/journal.pone.0006586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, et al. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Siddiqi O, Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1976;73:3253–3257. doi: 10.1073/pnas.73.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Lahat D, et al. K+ channel facilitation of exocytosis by dynamic interaction with syntaxin. J Neurosci. 2007;27:1651–1658. doi: 10.1523/JNEUROSCI.4006-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Suzuki DT, Grigliatti T, Williamson R. Temperature-sensitive mutations in Drosophila melanogaster. VII. A mutation (para-ts) causing reversible adult paralysis. Proc Natl Acad Sci U S A. 1971;68:890–893. doi: 10.1073/pnas.68.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: Implications for addiction. Biochem Pharmacol. 2007 doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, et al. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar LA, Pallanck L. NSF function in neurotransmitter release involves rearrangement of the SNARE complex downstream of synaptic vesicle docking. J Neurosci. 1998;18:10250–10256. doi: 10.1523/JNEUROSCI.18-24-10250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar NL, et al. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27:4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, et al. A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1999;96:2479–2484. doi: 10.1073/pnas.96.5.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]