Abstract
Perinatally HIV-infected (PHIV+) youth are surviving into adolescence and young adulthood. Understanding the sexual development of PHIV+ youth is vital to providing them with developmentally appropriate HIV prevention programs. Using pooled data (N = 417) from two longitudinal studies focused on HIV among youth (51% female; 39% HIV+) and their caregivers (92% female; 46% HIV+), we compared the rate of sexual onset during adolescence across four youth-caregiver combinations: PHIV+ youth with HIV+ caregivers (12%); PHIV+ youth with HIV− caregivers (27%); HIV− youth with HIV+ caregivers (34%); and HIV− youth with HIV-caregivers (27%). Youth with HIV− caregivers were more likely than other youth-caregiver groups to have had their sexual onset. Youth with HIV+ caregivers reported a slower rate of onset of penetrative sex across the adolescent years. We discuss our findings by highlighting the role that both youth and caregiver HIV status play in the onset of sexual behavior across adolescence.
Keywords: HIV, perinatal infection, adolescence, caregiver, sex
INTRODUCTION
With the advent of effective and widespread use of antiretroviral treatment (ART) in the United States (US), children born with HIV are now surviving into adolescence and young adulthood, living prolonged and healthier lives (Abrams, 2004; New York City Department of Health and Mental Hygiene, 2007). As perinatally HIV-infected (PHIV+) youth are aging into young adulthood in the US, they face urgent developmental decisions related to HIV-illness management, vocation, peer relationships, and dating. Complex decisions about sexual behavior debut, warrant particular attention given public health concerns about primary/secondary HIV prevention and implications for the overall sexual and reproductive health of PHIV+ youth. Moreover, given the common challenges of HIV treatment adherence, the risk of maternal to infant transmission among PHIV+ females cannot be understated.
During adolescence, youth become curious about sexual relationships, begin to initiate romantic and intimate partnerships, and typically have their first sex (Brooks-Gunn & Paikoff, 1997). National data suggest that close to half of high school aged youth (46%) in the US have ever had sexual intercourse (Centers for Disease Control and Prevention, 2008). For youth born with HIV, their sexual development and behavior is complicated by early and lifelong exposure to HIV infection and treatment, both of which not only affect health but also penetrate the central nervous system (CNS). Related challenges may include pubertal delays Buchac, Rogol, Lindsey et al., 2003); significant neurodevelopmental and cognitive problems (Brouwers, Belman & Epstein, 1991; Smith, Malee, Charurat et al., 2000; Nozyce, Lee, Wiznia et al., 2006); and potentially greater social and emotional immaturity compared to same aged HIV− peers (Donenberg & Pao, 2005; Havens & Mellins, 2008). These challenges may influence the onset of sexual behavior among PHIV+ youth, possibly resulting in early onset of risk behavior due to impulsivity or judgment problems, or conversely delayed onset due to developmental delays. Given the potential for transmission of not only HIV, but multidrug resistant strains of the virus due to non-adherence, understanding the sexual development of PHIV+ youth is vital to provide developmentally, age-appropriate HIV prevention programs to this population.
The literature on the first sex and development of sexual (risk) behavior in PHIV+ youth is limited, with mixed findings. Some researchers examining PHIV+ youths’ sexual behaviors have found rates of sexual activity to be the same or slightly lower than in other populations (Brogly, Watts, Ylitalo et al., 2007; Jennings, Ellen, Deeds et al., 2009; Mellins, Elkington, Bauermeister et al., 2009). In contrast, other studies have found that PHIV+ youth are starting to engage in risky sexual behavior (i.e. unprotected sex), in addition to other risky behavior such as substance use (Elkington, Bauermeister, Brackis-Cott et al., 2009), and are at greater risk of becoming pregnant (Ezeanolue, Wodi, Patel, Dieudonne, & Oleske, 2006).
Our limited understanding of PHIV+ youth’s sexual behavior and the role of PHIV infection on sexual risk may be attributable to several methodological considerations. First, some studies examining PHIV+ youth’ sexual behaviors have focused on samples of pre- to early adolescents whose rates of behavior may be relatively low (Mellins et al., 2009). Second, the majority of studies examining PHIV+ youth’s’ sexual behaviors has often relied on cross-sectional analyses that preclude examination of HIV infection on the development of sexual behavior across time and stages of adolescence (Bauermeister, Elkington, Brackis-Cott, et al., 2009; Ezeanolue et al., 2006; Frederick, Thomas, Mascola et al., 2000). Finally, researchers with longitudinal data have either a) reported on the sexual behavior of HIV+ youth without distinguishing mode of infection (perinatal vs. behavioral vs. transfusion) (Wiener, Battles & Wood, 2007), b) excluded PHIV+ youth from samples of HIV-infected youth (Brown, Schultz, Parsons et al., 2000; Murphy, Steers, & Dello Stritto, 2001), or c) not included a comparable reference group of HIV− youth (Wiener et al., 2007). Comparative studies using appropriate control groups are necessary to determine if any differences in the sexual development in PHIV+ youth are associated with HIV infection or to other sociodemographic or contextual factors.
One important contextual factor to examine in the sexual development of PHIV+ youth is the role of caregiver HIV infection. By definition, all PHIV+ youth are born to an HIV+ mother; however, some PHIV+ youth may have been raised by another caregiver, particularly if their mothers’ are too ill or have died. To date, no prior studies of PHIV+ youth have had appropriate comparison groups that would permit examination of both caregiver and youth HIV status variables simultaneously. Studies examining the relationship between caregiver HIV status and HIV-negative youth’s’ sexual risk behaviors have found caregiver’s positive status was associated with earlier first sex and greater likelihood of sexual risk behavior in youth (Lee, Lester & Rotheram-Borus, 2002; May, Lester, Ilardi, & Rotheram-Borus, 2006; Rotheram-Borus, Draimin, Reid & Murphy, 1997). Conversely, other studies have found no differences or lower rates of sexual (risk) behavior among uninfected youth with HIV+ mothers compared to youth with HIV-mothers (Mellins, Brackis-Cott, Dolezal, & Meyer-Bahlburg, 2005; Mellins, Dolezal, Brackis-Cott, Nicholson, Warne, & Meyer-Bahlburg, 2007; Leonard, Gwadz, Cleland, Vekaria & Ferns, 2008; Murphy, Herbeck, Marelich, & Schuster, 2010). To our knowledge, however, no study has examined prospectively how youth’ sexual development is contextualized by their own and their caregivers’ HIV infection. Contextualizing PHIV+ first sex can inform the development of targeted HIV prevention interventions, an important public health priority for the numerous urban minority youth both infected and affected by HIV.
We had the unique opportunity to examine the role of youth and caregiver HIV status on the onset of oral sex, penetrative sex (i.e. anal and/or vaginal sex), in addition to unprotected penetrative sex by combining baseline and follow-up data from two relatively large, longitudinal behavioral studies: 1) a study of perinatally HIV-exposed youth (both infected and uninfected); and 2) a study of HIV− youth with and without HIV+ caregivers. The resulting sample comprised both PHIV+ and HIV− youth with either HIV+ or HIV− caregivers, all of whom were recruited from similar neighborhoods in New York City (NYC), one of the epicenters of the US HIV epidemic. We compared youth’ rates of the onset of oral and penetrative (vaginal and/or anal) sexual behavior and unprotected penetrative sex across ages of adolescence based on the four youth-caregiver combinations (i.e., PHIV+ youth with HIV+ caregivers; PHIV+ youth with HIV− caregivers; HIV− youth with HIV+ caregivers; and HIV− youth with HIV+ caregivers).
METHODS
Participants and Procedures
Data were combined from the baseline and follow-up assessments of two longitudinal studies, Risk and Resilience in Youth with HIV+ mothers (R&R; Mellins, Brackis-Cott, Dolezal, Leu, Valentin, & Meyer-Bahlburg, 2008) and Child and Adolescent Self-Awareness and Health Project (CASAH; Mellins et al., 2009). Both studies were designed to examine differences in mental health and behavioral health outcomes, as well as sexual and drug use risk behaviors among youth and caregiver dyads.
Both study samples were drawn from general pediatric and HIV primary care clinics at medical centers, and a network of HIV care providers based in the same inner-city environments in New York City with high HIV seroprevalence. In both studies, caregiver-youth dyads were excluded if one of the dyad had severe cognitive impairment (e.g. severe mental deficiency, autism and other pervasive developmental disorders) that precluded understanding study questions. For both R&R and CASAH, trained, bilingual interviewers administered all measures. The mean time between interviews was 35 months for R&R and 20 months for CASAH. For both studies, Institutional Review Board approval was obtained from all study sites. All caregivers provided written informed consent for themselves and youth; youth provided assent. Monetary reimbursement for time and travel was provided. Caregivers and children were interviewed separately, but simultaneously when possible. Further details regarding the data pooling procedures of these datasets are discussed elsewhere (Elkington, Robbins, Bauermeister, Abrams, McKay, & Mellins, 2011), and briefly outlined below.
Risk and resilience participants and procedures
Full study procedures are described in depth in several previous publications (Mellins et al., 2007; 2009). In brief, research participants included caregiver-youth dyads of either HIV− early adolescents and their HIV+ birth mothers or uninfected or untested birth mothers. Caregiver-youth dyads were eligible if the youth was between 10 and 14 years of age, the mother was the birth parent of the youth, and the mother and youth had lived together for at least the past six months. All caregiver-youth dyads were recruited between 1998 and 2000. Among the 294 eligible families approached for the study, 14% refused to participate primarily due to time constraints, and 11% frequently cancelled or failed to show up for interviews. The remaining 220 (75%) caregiver-youth dyads completed the baseline interview. R&R became a follow-up study after additional funds were obtained two years into the project; thus, the mean time between baseline and follow-up was 35 months, with youth ranging in age from 13–19 year (Mellins et al., 2007). Considering R&R was not initially designed as a longitudinal study, we were able to retain 65% of the baseline sample. Baseline and follow-up data from 143 dyads are included here (67 HIV+ mothers with HIV− youth and 76 HIV− mothers with HIV− youth).
CASAH participants and procedures
Full study procedures in CASAH are also described in multiple previous publications (Mellins et al., 2009, Mellins, Brackis-Cott, Leu et al., 2009). To summarize, research participants were youth aged 9 to 16 years perinatally exposed to HIV (as confirmed by medical providers) who had a caregiver with legal capacity to sign consent for the child participation (foster care parents cannot provide consent for child participation in behavioral research in NYC). Participants were recruited, between 2003 and 2005. Of the 443 eligible participants, 11% refused contact with the research team and 6% could not be contacted by the site study coordinators. A total of 367 (83%) caregiver-youth dyads were approached, of whom N = 340 were enrolled (77% of eligible families). An additional 15 caregiver-youth dyads were removed as they did not complete both baseline interview sessions; the baseline final sample was N = 325. CASAH is an ongoing longitudinal study and the first follow-up was conducted at approximately 18 months post baseline (M = 20 months; youth age ranging from 11–19 years). We were able to retain 84.3% of CASAH participants between baseline and follow-up. In this analysis, we include the N = 274 dyads with follow-up data, representing PHIV+ youth with HIV+ caregivers (N = 49) or with HIV− caregivers (N = 113); and HIV− youth with HIV+ caregivers (N = 74) or with HIV− caregivers (N = 38).
Merged Sample (Table 1)
Table 1.
Descriptive Statistics of Youth and their Caregivers (N = 417)
| Mean | SD | N | % | |
|---|---|---|---|---|
| HIV+ Caregivers | 192 | 46 | ||
| Caregiver is Bioparent | 273 | 65 | ||
| Caregiver is Female | 382 | 92 | ||
| Education of Caregiver (years) | 11.73 | 3.02 | ||
| Household Income | 4.86a | 2.57 | ||
| Poverty (less than $25k) | 289 | 69 | ||
| Youth’s age (years) | 12 | 1.99 | ||
| HIV+ Youth | 163 | 39 | ||
| Female Youth | 211 | 51 | ||
| Race | ||||
| Hispanic/Latino | 164 | 40 | ||
| Black/African American | 222 | 53 | ||
| Other Race/Ethnicity | 31 | 7 | ||
| Caregiver-Youth Category | ||||
| NegY-NegCg | 114 | 27 | ||
| PozY-NegCg | 113 | 27 | ||
| NegY-PozCg | 141 | 34 | ||
| PozY-PozCg | 49 | 12 |
Mean Household Income approximates the $20,000–25,000 range.
Across the pooled samples (N = 545), approximately half were male, and the majority were African American or Hispanic. At baseline, participants had a mean age of 12.1 years (SD = 1.9), and the majority were African American or Hispanic (Table 1). The majority of caregivers were females and all caregivers were birth mothers in R&R compared to 48.6% in CASAH. At baseline among the HIV+ youth in CASAH, the majority had been told their diagnosis (70.4%) and were currently receiving ART (N = 194; 84%). Their median HIV RNA viral load was 3200 copies/ml (SD = 26,383 copies/ml); 35% had undetectable viral loads (≤ 400 copies/ml) and 5% had viral load values ≥ 100,000 copies/ml.
Attrition Analyses
In order to determine if excluded and non-excluded participants had comparable means across study variables of interest and whether we could generalize our findings to the entire sample, we conducted preliminary attrition analyses between participants included in our analyses (N = 417) to those who were excluded from this analysis due to missing data at baseline or follow-up (N = 128).
Participants excluded from our analyses (N = 128) were more likely to: be older (M = 12.49, SD = 1.71) than our analytic sample (M = 11.96, SD = 1.99; t(242.06) = 2.98; p < .01,. have younger caregivers (M = 41.66, SD = 9.48) than those included in our analyses (M = 45.01, SD =11.65; t(255.39) = −3.31, p < .01), and attrite if their caregiver was a biological parent (χ2(N = 545, df = 1) = 13.91, p < .001). Excluded participants also reported slightly lower mean household income (M = 4.18, SD = 2.36) than our analytic sample (M = 4.83, SD = 2.55; t(543) = −2.06; p < .01). Excluded participants were more likely at baseline to report having engaged in oral sex (χ2(N = 545, df = 1) = 15.36, p < .001), penetrative sex (χ2(N = 540, df = 1) = 6.23, p < .05), and unprotected sex (χ2(N = 539, df = 1) = 5.55, p < .05). Consistent with the loss to follow-up in Risk & Resilience, youth were also more likely to be excluded if they were HIV-negative (χ2(N = 545, df = 1) = 6.42, p < .05). We noted no differential attrition in youth gender, or race/ethnicity. We also found no differences across caregiver HIV status, gender, education, or work status. We found no differential attrition across the four youth-caregiver groups.
Measures
Sexual Behavior
Youth sexual behavior was assessed with an adapted version of the Sexual Risk Behavior Assessment Schedule for Youth (SERBAS-Y; Meyer-Bahlburg, Ehrhardt, Exner, Gruen, & Dugan, 1995) in R&R and the Adolescent Sexual Behavior Assessment (ASBA; Mellins et al., 2007) in CASAH. In brief, both assessments examine a range of sexual behaviors with gateway questions that make the batteries appropriate for the younger children in the study (e.g., if youth deny being touched or having sex, further questions on specific practices and condom use are not asked). The following lifetime sexual behaviors (yes/no) were examined at each follow-up interview: oral sex, penetrative sex (vaginal or anal), and unsafe sex (one or more occasions of penetrative sex without a condom). We aggregated reports of vaginal and anal sex behavior into one variable (“penetrative sex”) given the low frequency of vaginal and anal sex, and the high-risk of transmission associated with both behaviors. We also sought to examine same-sex behaviors, yet we had few cases to carry out these analyses (4 males; 8 females); therefore, we exclude same-sex analyses from the remainder of the manuscript.
Caregiver characteristics
Caregiver HIV status was assessed via several questions about personal HIV-tests and the results. The majority of these reports were confirmed by clinicians as the majority of HIV+ caregivers in both studies were recruited from medical clinics. For data analysis, caregiver’s HIV status was treated as a dichotomous variable (HIV infected vs. uninfected or untested). Caregiver demographics included caregiver age, gender, relationship to the child (birth parent versus non birth parent), educational attainment, and household income. Educational attainment was measured using caregivers’ self-reported years of formal education. Self-reported household income was calculated in ranges (1 = $5,000 or less; 5 = $20,000–25,000; 13 = More than $150,000). We created a dichotomous variable to indicate whether youth lived under the poverty line for New York State (i.e., household income less than $25,000).
Youth characteristics
Youth HIV status was determined via youth enrollment in an HIV primary care clinic, verified by clinicians. Youth demographics included age, gender and race/ethnicity. We collapsed youth’s self-reported race/ethnicity into three dummy variables (African American/Black, Hispanic/Latino, or Other Race/Ethnicity) to facilitate group comparisons. African American/Black youth served as the referent group.
Data Analytic Strategy
After examining the descriptive statistics for our variables of interest, we used HLM (v. 6.08) to design multilevel logistic growth curve models of the cumulative onset of oral and penetrative (vaginal and/or anal) sexual behavior and unprotected penetrative sex across ages of adolescence. While a repeated measures regression performs list-wise deletion for cases with missing values in one or more data points, HLM maximizes all available data because its algorithms do not require information across all waves in order to compute growth estimates for all participants (Raudenbush & Bryk, 2002). Similar to repeated measures regression and survival analyses, however, multilevel modeling allows the total variance to be divided into within-individual variation (Level One Model; i.e., change over time) and between-individual variation (Level Two Model; i.e., person-centered characteristics, e.g., sex).
We used an age-centered approach to model the onset of youth’s sexual behavior over time in our Level One model. The age-centered approach allowed us to estimate the log-odds for youth’s sexual behaviors for any given year in adolescence, with participants contributing their age-specific data to estimate the changes in sexual onset over time. The level-one model analysis comprised two analytic steps. First, we centered our growth curves at age 13; however, given that national data (Eaton, Kann, Kinchen et al., 2008) suggests that six percent of U.S. youth report sexual initiation prior to age 13, we created a coding scheme (see Appendix A) that took into account the full range of participants’ ages at baseline (9 to 16 years old) and adjusted for youth who reported having engaged in sexual activity prior to age 13. A growth parameter was then computed to model sexual onset over time. Each participant contributed to the growth parameter such that the cumulative odds of sexual onset across adolescence were modeled using study participants’ age across both time points (i.e., 9–19 years). To illustrate, among youth who were age 14 at baseline and 16 at follow-up, those who reported their first sex by age 14 (age at baseline) contributed to the cumulative odds (i.e. the growth curve) of having sexual onset by 14 and by 16 (their age at follow-up). When we excluded participants who had been sexually active at both time points, we found the results did not change (data not shown).
Appendix A.
Age-Centered Coding Scheme
| Age | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Coding | −4 | − 3 | − 2 | − 1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
We then tested whether caregiver or youth demographic characteristics were associated with youth’s sexual onset over time. To avoid confounding between caregiver and child HIV status, we created four groups that acknowledge the HIV status of caregivers and youth concurrently: HIV− Youth with HIV− caregivers (NegY-NegCg), HIV− youth with HIV-positive caregivers (NegY-PozCg), HIV-positive youth with HIV− caregivers (PozY-NegCg), and HIV-positive youth with HIV-positive caregivers (PozY-PozCg). These groups allowed us to examine whether different dyad combinations were associated with different rates of sexual onset in our sample. For ease of interpretation, we used the NegY-NegCg (i.e., dyad unaffected by HIV) as the referent group. Finally, because PHIV+ youth were more likely to not be living with a birth parent due to either having lost their biological mother to AIDS-related complications or other factors such as maternal substance abuse during pregnancy (Gadow, Chernoff, Williams et al., 2010; Mellins et al., 2009), we also accounted for the biological relationship between the caregiver and the child (e.g., birth parent vs. caregiver or relative) to avoid confounding (Elkington et al., 2011).
We found no support for random effects in our analyses; consequently, we report our findings as fixed-effect models (i.e., population-average model). We had too few youth aged 18 or 19 in our sample to reliably estimate their contribution to the growth curve. As such, we only model the growth curve up to age 17. For brevity, we only note statistically-significant relationships below.
RESULTS
Sample Characteristics
Descriptive statistics for participants included in this analysis are reported in Table 1. Approximately half of the caregivers were HIV+; two-thirds of caregivers reported being a biological parent. On average, caregivers reported having some high school education and having a household earning of less than $20,000 (69% met criteria for living below the poverty line). Thirty-nine percent of youth included in our analyses were HIV+; 50% were female and most reporting being African American/Black (53%) or Hispanic/Latino (40%).
HIV− youth with HIV+ caregivers (N = 141; 34%) accounted for over a third of the sample, followed by HIV− youth with HIV− caregivers (N = 114; 27%) and HIV+ youth with HIV− caregivers (N = 113; 27%), who accounted for over a quarter of the sample, and HIV+ youth with HIV+ caregivers (N = 49; 12%).
Sexual Behaviors across Adolescence
Prior to our longitudinal analyses, we examined whether onset of youth’s sexual behavior varied by age (see Table 2). By age 13, six participants reported having had oral sex and 12 participants had engaged in penetrative sex, half of whom reported having had unprotected intercourse. When we examined the sexual onset of youth between the ages of 14 and 16, we found 54 participants engaging in oral sex and 83 reporting penetrative sex, of who 24 reported unprotected intercourse. Among youth between the ages of 17 and 19, 30 reported oral sex and 41 reported penetrative sex, 20 of whom reported unprotected sex.
Table 2.
Cumulative prevalence of age of onset of sexual behaviors at follow-up, n (%)
| Age | Total N | Oral Sex | Penetrative Sex | Unsafe Sex |
|---|---|---|---|---|
| 10 | 28 | 1(3.6%) | 2(7.1%) | 2(7.1%) |
| 11 | 39 | 0(0%) | 0(0%) | 0(0%) |
| 12 | 38 | 2(5.26%) | 3(5.3%) | 3(5.3%) |
| 13 | 51 | 3(5.9%) | 7(13.7%) | 1(1.9%) |
| 14 | 79 | 13(16.5%) | 25(31.65%) | 4(5.06%) |
| 15 | 68 | 17(25.0%) | 26(38.24%) | 7(10.3% |
| 16 | 55 | 24(43.6%) | 32(58.2%) | 13(23.6%) |
| 17 | 42 | 20(47.6%) | 28(66.7%) | 13(30.9%) |
| 18 | 15 | 9(60.0%) | 11(73.3%) | 5(33.3%) |
| 19 | 2 | 1(50.%) | 2(100%) | 2(100%) |
Onset of Oral Sex Behavior across Adolescence
When we examined the onset of oral sex across adolescence, we found the cumulative odds of engaging in oral sex doubled with every additional year of age (OR = 2.25, 95% CI: 1.62, 3.12). We found no differences in youth’s onset of oral sex over time across the four caregiver-youth groups, or sociodemographic characteristics.
Onset of Penetrative Sex across Adolescence
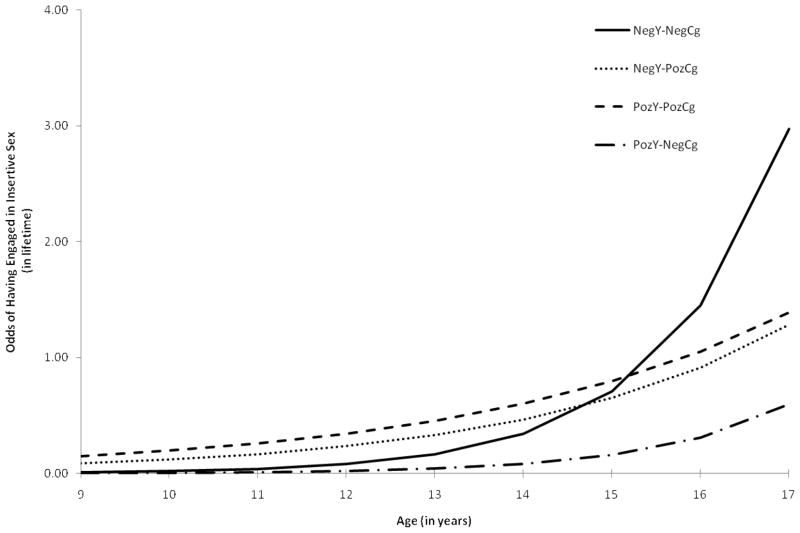
When we examined the cumulative likelihood of onset of penetrative sex across adolescence, we found the odds of engaging in penetrative sex doubled with every additional year (OR = 2.07, 95% CI: 1.55, 2.76), with the likelihood of penetrative sex over time increasing if youth lived with a biological parent (OR = 1.41, 95% CI: 1.05, 1.90). As shown in Figure 1, however, we noted that youth in the NegY-PozCg (OR = .68, 95% CI: .50, .93) and the PozY-PozCg (OR = .64, 95% CI: .45, .92) had a slower rate of onset of sex across adolescence than youth in the NegCg-NegY group, respectively. We found no statistically-significant differences in the rate of penetrative sex across adolescence between youth in the PozY-NegCg and the NegY-NegCg categories. We noted no differences attributable to sociodemographic characteristics in youth’s onset of penetrative sex across adolescence.
Figure 1.
Odds of initiating sexual intercourse across adolescence
Onset of Unprotected Sexual Intercourse across Adolescence
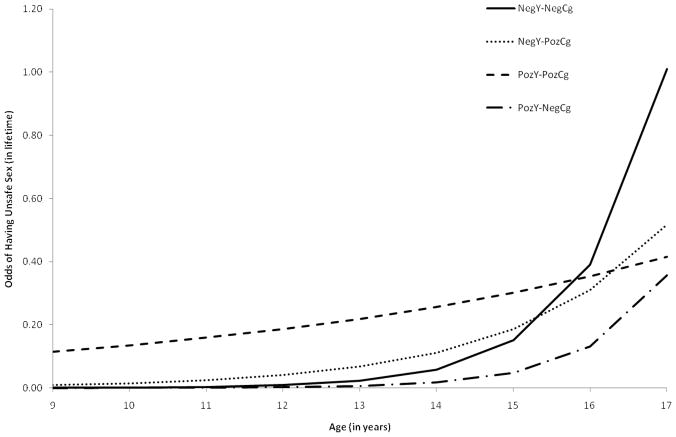
As shown in Figure 2, the cumulative odds of engaging in unprotected sex among the NegY-NegCg doubled with every additional year (OR = 2.58, 95% CI: 1.61, 4.13). As shown in Figure 2, however, we noted that youth in the NegY-PozCg (OR = .64, 95% CI: .43, .96) and the PozY-PozCg (OR = .45, 95% CI: .31, .67) had a slower rate of onset across adolescence than youth in the NegY-NegCg group, respectively. We found no statistically-significant difference in the rate of unsafe sex across adolescence between youth in the PozY-NegCg and the NegY-NegCg categories. The onset of unsafe sex also increased if youth lived with a biological parent (OR = 1.49, 95% CI: 1.02, 2.18). We found no differences in the rate of onset of unprotected sex across any other sociodemographic characteristics.
Figure 2.
Odds of engaging in unprotected sex across adolescence
DISCUSSION
Overall, youth in our sample had a similar prevalence of sexual onset by age 13 (4%) as the national average (6%) (Eaton et al., 2008). The limited number of sexual events reported by age 13 diminishes our ability to make strong claims about the association between onset before age 13 and youth or caregiver HIV status. Consequently, we focus our discussion on the longitudinal trends observed, highlighting the role that both youth and caregiver HIV status play in the onset of sexual behavior across adolescence.
Youth unaffected by HIV (i.e. HIV− youth with HIV− caregivers) reported the steepest rate of onset (i.e. the number of youth who become active by a given age) of penetrative and unsafe sex in the sample. For example, the odds of having had penetrative sex for these youth doubled between ages of 15 and 17 (see Figure 1). There were no significant differences between the behavior trajectories of either HIV− youth or PHIV+ youth with HIV− caregivers. Given that few PHIV+ youth with HIV− caregivers were sexually active across all ages, we may have been unable to detect differences between these two groups. These findings suggest that PHIV+ youth delay their onset of penetrative and sexual risk behavior. Delayed onset of high risk sexual behavior among PHIV+ youth vis-à-vis the increase of sexual behavior by socio-demographically matched sample of HIV− youth suggests that serostatus appears to serve a “protective” function against high risk sexual behavior.
Previous studies have sought to offer plausible explanations for delay in first sex. Immunocompromised youth or those experiencing side effects from ART medications, for example, may be less inclined to engage in sexual activity (Koenig, Pals, Chandwani et al., 2010). Alternatively, it is possible that HIV-infected youth may delay penetrative sex in an effort to avoid potential infecting their partners. In a prior analysis, for example, Bauermeister et al. (2009) found that PHIV+ youth were less likely to engage in penetrative sex than HIV− youth, reporting more touching and oral sex behaviors instead. Moreover, the concern that one must disclose one’s serostatus to sexual and romantic partners prior to relational commitment may discourage PHIV+ youth from engaging in sexual risk behavior. The potential of being rejected or treated differently because of one’s HIV serostatus poses a significant relational risk – one worth delaying or avoiding even at the loss of intimate relationships (Kang, Mellins, Ng, Robinson, & Abrams, 2008). Clinical reports, for example, have suggested that many PHIV+ youth may be less inclined to be sexually active due to internalized stigma resulting from their HIV diagnosis (Marhefka, Valentin, Pinto, Demetriou, Wiznia & Mellins, 2011). Future research examining PHIV+ youth’s motivations to delay sex are warranted; however, rather conceptualizing these explanations as competing alternatives to be tested, we anticipate that these motivations are likely co-occurring.
Findings may also suggest that PHIV+ youth with HIV− caregivers have a delayed but comparable trajectory for both penetrative and unprotected sex over time to youth unaffected by HIV. For example, at age 15 the odds of penetrative sex among PHIV+ youth with HIV-caregivers are 0.16; the same odds of penetrative first sex were reported for HIV-youth with HIV− caregivers two years earlier (Figure 1). Thus, we may expect to see a sharp increase in the odds of engaging in penetrative and unprotected sex in PHIV+ youth with HIV− caregivers as these youth transition into young adulthood if they follow a similar albeit delayed trajectory. Unfortunately, the current analysis only extends to age 17. While these findings are consistent with clinical reports suggesting delays in first sex (Havens & Mellins, 2008), empirical evidence is scarce. These youth are only now surviving into an age where their sexual activity is increasing and can be examined more closely. Future research that follows PHIV+ youth into early adulthood is warranted so that we may better understand their sexual behaviors and desires, and identify similarities and differences to other populations. In addition, prospective data from PHIV+ youth living in other regions where perinatal infection is more prevalent is also warranted. Such information may guide developmentally appropriate HIV risk reduction interventions for these youth, assisting with appropriately timed sexually risk reduction messages (Prendergast, Urada, & Podus, 2001).
Compared to HIV− youth with HIV− caregivers, youth with HIV+ caregivers reported a slower rate of onset of penetrative sex across the adolescent years; this difference emerged irrespective of youth’s HIV status. On average, PHIV+ and HIV− youth with HIV+ caregivers reported engaging in penetrative sex by age 16 and 17 respectively, later than the average age of penetrative first sex for youth unaffected by HIV (age 15) from similar SES backgrounds/communities. Similar trajectories were noted when we examined unprotected sex. Youth with HIV+ caregivers were less likely to report onset of unsafe sex compared to HIV-youth with HIV− caregivers, and instead reported a lagged or delayed trajectory across adolescence (see Figure 2). These findings support past research examining how caregivers’ HIV status may function in a protective role and result in positive youth outcomes, such as better mental health (Elkington et al., in press), and are consistent with some studies that found reduced sexual risk among youth with HIV+ caregivers (Leonard et al., 2008; Murphy et al., 2010).
The more prominent role of caregiver HIV infection with respect to reduced youth sexual activity and risk warrants continued investigation. Although caregivers living with HIV often remain conflicted about disclosing their HIV serostatus to family members (Murphy, 2008), they are more inclined and uniquely positioned to initiate discussions about HIV-risk behaviors, sexual activity, and HIV transmission with their children compared to HIV− caregivers (O’Sullivan, Dolezal, Brackis-Cott, Traeger, & Mellins, 2005). For many HIV+ caregivers, parenting in the context of their own HIV infection provides an impetus to make decisions that could minimize the likelihood that their children engage in HIV-risk behaviors (Tompkins, Henker, Whalen, Axelrod, & Comer, 1999; Kirshenbaum, Hirky, Correale et al., 2004). As such HIV+ caregivers may prioritize discussing preventive HIV behavior and monitoring their children’s social and/or sexual relationships – parenting factors associated with reduced child sexual risk and delayed sexual onset (Murphy et al., 2010; Kirshenbaum et al., 2004). HIV-affected families may also have greater access to a broad range of preventive services, which may also influence key family processes known to reduce sexual risk behavior in youth. At present, however, we were unable to account for these family processes in our analyses. Alternatively, youth may be involved in their caregiver’s care and treatment and may assist the caregiver in managing the family (Reyland, Higgins-D’Alessandro, & McMahon, 2002). For such youth, assuming a care giving role may provide a sense of purpose and future orientation, which may translate into active steps towards reducing their vulnerability to HIV transmission or (re)infection. In order to build efficacious HIV-prevention interventions and promote sexual health in youth infected and affected by HIV, continued empirical efforts are necessary to understand the role of caregiver HIV infection, in addition to exploring further the family characteristics and strengths of and challenges faced by families affected by caregiver HIV infection.
Not all youth in the current study resided with a biological parent. We found that youth who lived with a biological parent had greater odds of having engaged in penetrative and unprotected sex across adolescence than those who lived with a non-biological caregiver, even after accounting for youth and caregiver HIV status. Youth living with a non-biological caregiver in this sample, by definition had all lost or been removed from the home of an HIV+ parent. These youth may perceive higher threats regarding the potential sequelae of engaging in sexual behavior and may be more willing to delay their first sex given previous losses. Alternatively, non-biological caregivers may also have access to a greater number of services to assist with childrearing. Limited access to services data, however, precludes our ability to examine whether this factor confounds the observed relationship between residing with a biological caregiver and sexual risk.
The lack of associations between youth’s sexual onset over time and other socio-demographic factors such as gender, race/ethnicity, and poverty are inconsistent with prior literature. The absence of differences across some of these sociodemographic factors, however, may be attributable to our selection criteria. Participants were recruited from medical centers based in the same inner-city environments in New York City, resulting in less variability across sociodemographic characteristics within our sample. Consistent with a recent study of perinatally HIV-infected and perinatally HIV-exposed youth from another large US based cohort-study (Mellins, Tassiopolous, Malee et al., in press), we failed to find gender differences in onset of sexual behavior. Future research examining what factors contribute to the absence of a gender disparity in sexual behavior is warranted.
Our findings have several limitations deserving mention. First, these are secondary data analyses involving pooled data from two studies of youth who were recruited at different times with different lengths of time between study follow-ups, and thus differences in outcomes may reflect historical or cohort differences between the two study samples. For example, HIV+ caregivers in CASAH (the later cohort) may have been healthier due to improved ART regimens. The impact of study timing on the association between caregiver HIV status and youth sexual development is unclear. Second, our attrition analyses suggested that we lost older youth who had engaged in higher rates of sexual behavior at baseline. Consequently, our analyses may provide conservative estimates of youth’s sexual onset and the exclusion of these participants from analyses may have influenced our ability to detect other statistically-significant relationships. Furthermore, we did not have data across all years of adolescence for each participant and not all youth had reached age 18 or 19 years, or had their first sex. Finally, the sample is also a convenience sample, largely recruited from either HIV primary care clinics or medical clinics that may not reflect the larger population of urban youth, either infected or affected by HIV, particularly those outside NYC and not followed in HIV care or medical clinics. Thus, study findings may reflect a form of selection bias whereby HIV+ caregivers and their youth who were functioning less well were less likely to be found seeking medical services of any kind, and thus not enrolled in the study. Although we attempted to recruit both study samples from similar communities based on the demographics of pediatric HIV disease, other factors (e.g., differential rates of study refusal) may have altered the group effects. Finally, as noted, among the HIV− youth in R&R, we were unable to distinguish between perinatally HIV exposed and unexposed.
These limitations not with standing, the current study represents an important step in understanding how living with perinatal HIV or caregiver HIV infection influences youth’s decisions regarding onset of sexual behavior. Youth infected or affected by HIV (HIV+ caregiver) in our sample appeared to be sexually delayed. While reasons for this delay require further study, providers are well positioned to address issues related to healthy sexual development with these youth as they age. Some youth were already engaging in risky sexual behavior, and for PHIV+ youth with HIV− caregivers, we may see significant increases in sexual risk behavior as they age. HIV prevention interventions implemented before a significant increase in sexual behaviors are warranted, particularly for sexually-active PHIV+ youth, a subset of who are engaging in unprotected sexual behavior. A noteworthy challenge is developing interventions that foster healthy sexual development and promote safe sexual practices without inadvertently heightening PHIV+ youth’s perceived stigma. Undoubtedly, sex positive interventions for PHIV+ youth will require us to have a greater understanding of how HIV infection impacts PHIV+ youth’s sexual development, both physically and emotionally. Future research in this area is warranted. Finally, our findings suggest that families affected by HIV may possess substantial strengths upon which HIV prevention interventions can build. Family-based programs that involve HIV infected caregivers may be particularly effective as they tackle critical issues related to communication about sexual risk behavior and potential transmission.
In sum, with four comparison groups, we were able to examine prospectively differences in cumulative odds of sexual initiation over time comparing PHIV+ youth to their HIV-counterparts, while also examining the role of caregiver HIV status. Our findings support past research suggesting that PHIV+ youth may be more likely to delay their sexual onset as compared to HIV− youth (Bauermeister et al., 2009). As PHIV+ become sexually active, however, they may require assistance to decrease HIV transmission risks as they explore their sexuality. Future research examining how youth’s and caregiver’s HIV infection may influence family processes is needed.
Table 3.
Logistic age-centered growth curves for youth’s sexual behaviors across adolescence (N = 417)
| Oral Sex | Penetrative Sex | Unsafe Sex | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | CI (Lower) | CI (Upper) | OR | CI (Lower) | CI (Upper) | OR | CI (Lower) | CI (Upper) | |
| Change Over Time | |||||||||
| Intercept | 2.25 | 1.62 | 3.12*** | 2.07 | 1.55 | 2.76*** | 2.58 | 1.61 | 4.13*** |
| CG is Bioparent | 1.37 | .99 | 1.89 | 1.41 | 1.05 | 1.90* | 1.49 | 1.02 | 2.18* |
| Female | .97 | .77 | 1.22 | 1.15 | .93 | 1.41 | 1.13 | .86 | 1.47 |
| Hispanic/Latino | .92 | .72 | 1.17 | .94 | .76 | 1.16 | .89 | .69 | 1.15 |
| Other Race/Ethnicity | .82 | .55 | 1.23 | .91 | .54 | 1.52 | .89 | .54 | 1.50 |
| In Poverty | .80 | .60 | 1.06 | 1.05 | .86 | 1.29 | .84 | .62 | 1.13 |
| NegY-PozCg | .89 | .63 | 1.26 | .68 | .50 | .93* | .64 | .43 | .96* |
| PozY-PozCg | .72 | .50 | 1.04 | .64 | .45 | .92* | .45 | .31 | .67*** |
| PozY-NegCg | 1.22 | .84 | 1.76 | 1.06 | .77 | 1.47 | 1.05 | .59 | 1.85 |
p < .05,
p < .01,
p < .001
Notes. African American youth serve as referent group for race/ethnicity comparisons; Males serve as referent group for sex comparisons; Negative Caregivers with Negative Youth (NegY-NegCg) serve as referent group for caregiver-youth categories. Poverty is defined as earning less than $25,000. Caregivers’ age, gender, educational attainment, and work status were not statistically-significant and are excluded from the final models.
Acknowledgments
This research was supported by several grants from the National Institute of Mental Health (R01MH069133, PI: C. Mellins; R01MH63636, PI: C. Mellins, a supplement under the American Recovery and Reinvestment Act (ARRA); P30MH43520; PI: A. Ehrhardt) and a grant from the WT Grant Foundation (97-1807-97; PI C. Mellins). Drs. Bauermeister and Elkington are supported by Career Development Awards from the National Institute of Mental Health (K01MH087242; PI: J. Bauermeister; K01MH089832; PI: K. Elkington). Dr. Robbins is supported by a NRSA grant (T32MH19139, PI: T. Sandfort).
References
- Abrams EJ. Prevention of mother-to-child transmission of HIV: Successes, controversies and critical questions. AIDS Reviews. 2004;6:131–143. [PubMed] [Google Scholar]
- Bauermeister JA, Elkington KS, Brackis-Cott E, Dolezal C, Mellins C. Sexual behavior and perceived peer norms: comparing perinatally infected and affected youth. Journal of Youth & Adolescence. 2009;38:1110–22. doi: 10.1007/s10964-008-9315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto MT, Garrett JM, Dugliss MA, Daeschner CW, Johnson CA, Leigh MW, Majure JM, Schultz WH, Konrad T. Risky behavior in teens with cystic fibrosis or sickle cell disease: A multicenter study. Pediatrics. 1998;101:250–256. doi: 10.1542/peds.101.2.250. [DOI] [PubMed] [Google Scholar]
- Brogly SB, Watts H, Ylitalo N, Franco E, Seage G, Oleske J, Eagle N, Van Dyke R. Reproductive Health of Adolescent Girls Perinatally Infected With HIV. American Journal of Public Health. 2007;97:1047–1052. doi: 10.2105/AJPH.2005.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Paikoff R. Sexuality and developmental transitions during adolescence. In: Schulenberg J, Maggs JL, Hurrelman K, editors. Health risks and developmental transitions during adolescence. Cambridge, UK: Cambridge University Press; 1997. pp. 190–219. [Google Scholar]
- Brouwers P, Belman AL, Epstein LG. Central nervous system involvement: Manifestations and evaluation. In: Pizzo P, Wilfert C, editors. Pediatric AIDS: The challenge of HIV infection in infants, children and adolescents. Baltimore, MD: Williams and Wilkins; 1991. pp. 318–335. [Google Scholar]
- Brown LK, Schultz JR, Parsons JT, Butler R, Forsberg A, Kocik S, King G, Manco-Johnson M, Aledort L. Sexual Behavior Change among Human Immunodeficiency Virus-Infected Adolescents with Hemophilia. Pediatrics. 2000;106:e22–e28. doi: 10.1542/peds.106.2.e22. [DOI] [PubMed] [Google Scholar]
- Buchacz K, Rogol AD, Lindsey JC, Wilson CM, Hughes MD, Seage GR, Oleske J, Rogers AS. Delayed onset of pubertal development in children and adolescents with perinatally acquired HIV infection. JAIDS. 2003;33:56–65. doi: 10.1097/00126334-200305010-00009. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Trends in HIV− and STD-related risk behaviors among high school students – United States, 1991–2007. MMWR. 2008;57:814–822. [PubMed] [Google Scholar]
- Donenberg GR, Pao M. Youth and HIV/AIDS: Psychiatry’s role in a changing epidemic. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:728–747. doi: 10.1097/01.chi.0000166381.68392.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Lim C, Brener ND, Wechsler H Youth Risk Behavior Surveillance – United States. MMWR. 2007;57:1–131. [PubMed] [Google Scholar]
- Elkington KS, Bauermeister JA, Brackis-Cott E, Dolezal C, Mellins CA. Substance use and sexual risk behaviors in perinatally human immunodeficiency virus-exposed youth: The role of caregivers, peers and HIV status. Journal of Adolescent Health. 2009;45:133–141. doi: 10.1016/j.jadohealth.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington KS, Robbins R, Bauermeister JA, Abrams E, McKay M, Mellins CA. Mental health in youth infected with or affected by HIV: The role of caregiver HIV infection. Journal of Pediatric Psychology. 2011;36:360–373. doi: 10.1093/jpepsy/jsq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeanolue EE, Wodi AP, Patel R, Dieudonne A, Oleske JM. Sexual behaviors and procreational intentions of adolescents and young adults with perinatally acquired human immunodeficiency virus infection: Experience of an urban tertiary center. Journal of Adolescent Health. 2006;38:719–725. doi: 10.1016/j.jadohealth.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Fielden SJ, Sheckter L, Chapman GE, Alimenti A, Forbes JC, Sheps C, Cadell S, Frankish JC. Growing up: Perspectives of children, families and service providers regarding the needs of older children with perinatally-acquired HIV. AIDS Care. 2006;18(8):1050–1053. doi: 10.1080/09540120600581460. [DOI] [PubMed] [Google Scholar]
- Frederick T, Thomas P, Mascola L, Hsu HW, Rakusan T, Mapson C, Weedon J, Bertolli J. Human immunodeficiency virus-infected adolescents: a descriptive study of older children in New York City, Los Angeles County, Massachusetts and Washington, DC. The Pediatric Infectious Disease Journal. 2000;19:551–555. doi: 10.1097/00006454-200006000-00012. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Chernoff M, Williams PL, Brouwers P, Morse E, Heston J, Hodge J, Di Poalo V, Deygoo NS, Nachman S. Co-occurring psychiatric symptoms in children perinatally infected with HIV and peer comparison sample. Journal of Developmental and Behavioral Pediatrics. 2010;31:116 –128. doi: 10.1097/DBP.0b013e3181cdaa20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JF, Mellins CA. Psychiatric aspects of HIV/AIDS in childhood and adolescence. In: Rutter M, Bishop DVM, Pinge S, Scott S, Stevenson J, Taylor E, Thapar A, editors. Child and Adolescent Psychiatry. Oxford, UK: Blackwell; 2008. pp. 945–955. [Google Scholar]
- Jennings JM, Ellen JM, Deeds BG, Harris DR, Muenz LR, Barnes W, Lee S, Auerswald CL the Adolescent Trials Network for HIV/AIDS Interventions. Youth living with HIV and partner-specific risk for the secondary transmission of HIV. Sexually Transmitted Diseases. 2009;36:439–444. doi: 10.1097/OLQ.0b013e3181ad516c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E, Mellins CA, Ng WYK, Robinson LG, Abrams EA. Standing between two worlds in Harlem: A developmental psychopathology perspective of perinatally acquired human immunodeficiency virus and adolescence. Journal of Applied Developmental Psychology. 2008;29:227–237. [Google Scholar]
- Kirshenbaum SB, Hirky EA, Correale J, Goldstein R, Johnson M, Rotheram-Borus MJ, Ehrhardt AA. “Throwing the dice”: Pregnancy decision-making among HIV-positive women in four U.S. cities. Perspectives on Sexual and Reproductive Health. 2004;36:106–113. doi: 10.1363/psrh.36.106.04. [DOI] [PubMed] [Google Scholar]
- Koenig LJ, Pals S, Sulachni C, Hodge K, Abramowitz S, Barnes W, D’Angelo L. Sexual transmission risk behavior of adolescents with HIV acquired perinatally or through risk behaviors. Journal of Acquired Immune Deficiency Syndromes. 2010;55:380–390. doi: 10.1097/QAI.0b013e3181f0ccb6. [DOI] [PubMed] [Google Scholar]
- Lee MB, Lester P, Rotheram-Borus MJ. The relationship between adjustment of mothers with HIV and their adolescent daughters. Clinical Child Psychology and Psychiatry. 2002;7:71–84. [Google Scholar]
- Leonard NR, Gwadz MV, Cleland CM, Vekaria PC, Ferns B. Maternal substance use and HIV status: Adolescent risk and resilience. Journal of Adolescence. 2008;31:389–405. doi: 10.1016/j.adolescence.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhefka S, Valentin C, Pinto R, Demetriou N, Wiznia A, Mellins CA. “I feel like I’m carrying a weapon”. Information and motivations related to sexual risk among girls with perinatally acquired HIV. AIDS Care. doi: 10.1080/09540121.2010.532536. (in press) Published ahead online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S, Lester P, Ilardi M, Rotheram-Borus MJ. Childbearing among daughters of parents with HIV. American Journal of Health Behavior. 2006;30:72–84. doi: 10.5555/ajhb.2006.30.1.72. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Brackis-Cott E, Dolezal C, Meyer- Bahlburg HFL. Behavioral risk in early adolescents with HIV-positive mothers. Journal of Adolescent Health. 2005;36:342–351. doi: 10.1016/j.jadohealth.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Brackis-Cott E, Dolezal C, Leu CS, Valentin C, Meyer-Bahlburg HFL. Mental health of early adolescents from high-risk neighborhoods: The role of maternal HIV and other contextual, self-regulation, and family factors. Journal of Pediatric Psychology. 2008;33:1065–1075. doi: 10.1093/jpepsy/jsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins CA, Brackis-Cott E, Leu CS, Elkington K, Dolezal C, Wiznia A, McKay M, Bamji M, Abrams EJ. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. Journal of Child Psychology and Psychiatry. 2009;50:1131–1138. doi: 10.1111/j.1469-7610.2009.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins CA, Dolezal C, Brackis-Cott E, Nicholson O, Warne P, Meyer-Bahlburg HFL. Predicting the onset of sexual and drug risk behaviors in HIV-negative youths with HIV-positive mothers: the role of contextual, self-regulation, and social-interaction factors. Journal of Youth and Adolescence. 2007;36:265–278. doi: 10.1007/s10964-006-9129-3. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Elkington KE, Bauermeister JA, Brackis-Cott E, Dolezal C, McKay M, Wiznia A, Bamji M, Abrams EJ. Sexual and Drug Use Behavior in Perinatally HIV-Infected Youth: Mental Health and Family Influences. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:810–819. doi: 10.1097/CHI.0b013e3181a81346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins CA, Tassiopoulos K, Malee K, Moscicki AB, Patton D, Smith R, Usitalo A, Allison S, Van Dyke R, Seage G. Behavioral health risks in perinatally HIV-exposed youth: Co-occurrence of sexual and drug use behavior, mental health problems, and non-adherence to antiretroviral treatment. AIDS Patient Care and STDs. doi: 10.1089/apc.2011.0025. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Ehrhardt AA, Exner TM, Gruen RS, Dugan T. Sexual Risk Behavior Assessment Schedule Youth, Depressed Females, Baseline (SERBAS-Y-DEPR-F-1) 1995 1995. [Google Scholar]
- Murphy DA. HIV-positive mothers’ disclosure of their serostatus to their young children: A review. Clinical Child Psychology and Psychiatry. 2008;13:105–122. doi: 10.1177/1359104507087464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Herbeck DM, Marelich WD, Schuster MA. Predictors of Sexual Behavior among Early and Middle Adolescents Affected by Maternal HIV. International Journal of Sexual Health. 2010;22:195–204. doi: 10.1080/19317611003800614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Steers WN, Dello Stritto ME. Maternal disclosure of mother’s HIV serostatus to their young children. Journal of Family Psychology. 2001;15:441–450. doi: 10.1037//0893-3200.15.3.441. [DOI] [PubMed] [Google Scholar]
- New York City Department Health and Mental Hygiene. HIV epidemiology & field services program: Semiannual report. 2007;2(1) Available at: www.nyc.gov. [Google Scholar]
- Nozyce ML, Lee SS, Wiznia A, Nachman S, Mofenson LM, Smith ME, Yogev R, McIntosh K, Stanley K, Pelton S. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- O’Sullivan LF, Dolezal C, Brackis-Cott E, Traeger L, Mellins CA. Communication about HIV and risk behaviors among mothers living with HIV and their early adolescent children. The Journal of Early Adolescence. 2005;25:148–167. [Google Scholar]
- Prendergast ML, Urada D, Podus D. Meta-analysis of HIV risk-reduction interventions within drug abuse treatment programs. Journal of Consulting & Clinical Psychology. 2001;69:389–405. doi: 10.1037//0022-006x.69.3.389. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks, California: Sage; 2002. [Google Scholar]
- Reyland SA, Higgins-D’Alessandro A, McMahon TJ. Tell them you love them because you never know when things could change: voices of adolescents living with HIV-positive mothers. AIDS Care. 2002;14:285–294. doi: 10.1080/09540120120076977. [DOI] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Draimin BH, Reid HM, Murphy DA. The impact of illness disclosure and custody plans on adolescents whose parents live with AIDS. AIDS. 1997;11:1159–1164. doi: 10.1097/00002030-199709000-00012. [DOI] [PubMed] [Google Scholar]
- Santamaria EK, Dolezal C, Marhefka S, Hoffman S, Ahmed Y, Elkington K, Mellins CA. Psychosocial implications of HIV serostatus disclosure to youth with perinatally-acquired HIV. AIDS Patient Care and STDs. 2011;25(4):257–263. doi: 10.1089/apc.2010.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Malee K, Charurat M, Magder L, Mellins C, Macmillan C, Hittleman J, Lasky T, Llorente A, Moye J. Timing of perinatal human immunodeficiency virus type 1 and rate of neurodevelopment. Pediatric Infectious Disease Journal. 2000;19:862–871. doi: 10.1097/00006454-200009000-00010. [DOI] [PubMed] [Google Scholar]
- Tompkins TL, Henker B, Whalen CK, Axelrod J, Comer LK. Motherhood in the context of HVI infection: Reading between the numbers. Cultural Diversity and Ethnic Minority Psychology. 1999;5:197–208. [Google Scholar]
- Wiener LS, Battles HB, Wood LV. A Longitudinal Study of Adolescents with Perinatally or Transfusion Acquired HIV Infection: Sexual Knowledge, Risk Reduction Self-efficacy and Sexual Behavior. AIDS & Behavior. 2007;11:471–478. doi: 10.1007/s10461-006-9162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]