Abstract
Objective
To investigate risk factors associated with unilateral or bilateral decreased visual acuity in preschool children.
Design
Population-based cross-sectional prevalence study.
Participants
Population-based samples of 6504 children ages 30-72 months from California and Maryland.
Methods
Participants were preschool African-American, Hispanic, and non-Hispanic white children from Los Angeles, California, and Baltimore, Maryland. Data were obtained by a parental interview and a detailed ocular examination. Logistic regression models were used to evaluate the independent associations between demographic, behavioral and clinical risk factors with unilateral and bilateral decreased visual acuity.
Main Outcome Measures
Odds ratios (ORs) for various risk factors associated with inter-ocular difference (IOD) in visual acuity (VA) of 2 or more lines with 20/32 or worse in the worse eye, or bilateral decreased VA worse than 20/40 or worse than 20/50 if younger than 48 months of age.
Results
In multivariate logistic regression analysis, 2-line IOD with VA 20/32 or worse was independently associated with Hispanic ethnicity (OR 2.05); esotropia (OR 8.98); spherical equivalent (SE) anisometropia (ORs ranging between 1.5 and 39.7 for SE anisometropia ranging between 0.50 to <1.00 diopters and ≥2.00 diopters); and aniso-astigmatism in J0 or J45 (ORs ranging between 1.4 and ≥5.3 for J0 or J45 differences ranging between 0.25 to <0.50 D and ≥1.00 D). Bilateral decreased VA was independently associated with lack of health insurance (OR 2.9); lower primary caregiver education (OR 1.7); astigmatism (OR 2.3 and 17.6 for astigmatism 1.00 to <2.00 D and ≥2.00 D); and SE hyperopia ≥4.00 D (OR 10.8).
Conclusions
Anisometropia and esotropia are risk factors for IOD in visual acuity. Astigmatism and high hyperopia are risk factors for bilateral decreased visual acuity. Guidelines for the screening and management of decreased visual acuity in preschool children should be considered in light of these risk associations.
Amblyopia is a common cause of visual impairment.1-5 Unilateral amblyopia is commonly associated with strabismus and/or anisometropia, while bilateral amblyopia usually results from high bilateral refractive error. Other causes of unilateral or bilateral amblyopia, such as visual axis occlusion from congenital cataract, are much less common. The magnitude of risk of amblyopia associated with different types of strabismus and degrees of refractive error remains unclear. This information is of importance as various technologies (autorefractors, photoscreeners) permit identification of amblyopia risk factors at young ages, when obtaining optotype acuity is not possible, and when early treatment is most effective and may be preventative. Quantitative risk data would allow practitioners, parents and policy-makers to make informed judgments regarding treatment, and would help guide referral policies for preschool screening.
A number of studies in clinic populations, or children referred from screening programs on the basis of anisometropia, have found a relationship between the degree of anisometropia and the frequency6-9 or severity10-13 of amblyopia. However, these studies are conducted in selected samples and may not provide data on the risk associated with different levels of anisometropia in the general population.
Strabismus is a well-known risk factor for unilateral amblyopia, but the magnitude of risk and its relative importance compared to refractive error is unknown. Studies of screening-based and population-based samples of children have reported amblyopia in anywhere from 13% to over 40% of strabismic children.14-16 However, it is difficult to assess the independent relationship of strabismus with amblyopia in these studies as children with strabismus frequently also had anisometropia, which is itself a risk factor for amblyopia.
Bilateral ametropic amblyopia in the setting of very high hyperopia has been described in numerous clinical populations18-23 and one population-based screening sample.24 Studies of Native American children25-26 have explored bilateral decreased visual acuity associated with high astigmatism. However, the threshold at which hyperopia or astigmatism begins to confer a risk of bilateral amblyopia remains unknown.
Studies in clinic-based samples may overestimate amblyopia risk, because they may underrepresent children who have risk factors yet have normal vision; furthermore, studies using fixation preference testing to determine amblyopia status identify many subjects with normal vision as amblyopic, especially if they have strabismus.27-29 Clinic-based studies of amblyopia may also not be representative of children who have decreased visual acuity but no ocular abnormality.
Our purpose is to report ocular, demographic, clinical and behavioral risk factors for inter-ocular visual acuity differences and bilaterally decreased visual acuity in a population-based sample of Hispanic, non-Hispanic white, and African-American children from California and Maryland.
Methods
The study population, recruitment, cross-site standardization and certification and an overview of the interview and ocular examination, including details of cycloplegic refraction procedures, are described in a companion paper30 and prior publications.31-32
This report is limited to children aged 30 to 72 months. A parent or guardian of each participant gave written informed consent. Ocular alignment was tested by unilateral cover testing and alternate cover testing of standardized duration, at 6 m fixation and 40 cm fixation, with and without correction (if worn). Hirschberg testing at near was used when unilateral cover testing could not be performed. Monocular distance visual acuity (VA) was tested in children aged ≥30 months, without exclusions for developmental delay or disability, using single-surrounded HOTV optotypes on the electronic VA tester33 with the Amblyopia Treatment Study protocol34, with naming or matching of letters. Details of the VA protocol have been described elsewhere.3,35 Presenting VA was tested with correction, if worn. If VA was decreased (worse than 20/50 in either eye or worse than 20/40 in either eye in a child aged 48 months or more), or if there was a 2-line inter-ocular difference (IOD) with VA 20/32 or worse in the worse eye, VA was retested after cycloplegic refraction, with full refractive correction. Children who still had decreased VA in either eye using the best result from testing, or who had a 2-line IOD with VA of 20/32 or worse in the worse eye and a unilateral amblyopia risk factor (e.g., unilateral visual axis occlusion, strabismus, or at least 1.00 diopter (D) spherical equivalent (SE) aniso-hyperopia, 3.00 D SE aniso-myopia or 1.50 D aniso-astigmatism), were scheduled for return-visit retesting without cycloplegia, with full correction of myopia and astigmatism, and undercorrection of hyperopia by up to 1.50 D. Children untestable at the initial visit were scheduled for a return visit. The final, best-measured VA for each eye was the best of all test results recorded for that eye. Vector analysis36 was used to determine the J0 (Cartesian) and J45 (oblique) vector components of astigmatism, defined as: J0= −C/2(cos2α); J45= −C/2(sin2α), where C is the cylinder amount in sphero-cylindrical notation and α is the angle of astigmatism.
The analysis population consisted of all children able to complete VA measurements in both eyes. The outcome for unilaterally decreased VA was defined as a 2-line IOD in best-measured logMAR VA with VA 20/32 or worse in the worse eye. Bilateral decreased VA was defined as best VA worse than 20/50 in both eyes for children <48 months of age, or worse than 20/40 in both eyes for children ≥48 months of age. Risk factors for these outcomes were explored using univariate analysis; those showing at least marginally significant associations (P<0.1) were considered candidates for subsequent forward stepwise multiple logistic regression (except for Down syndrome and cerebral palsy, due to small numbers). Further details are provided in a companion paper,30 as are details of the demographic, clinical and behavioral factors evaluated (see also Tables 1 and 2), and the use of LOWESS plots (locally weighted polynomial regression) to examine independent relationships between continuous refractive variables and outcome prevalence. In the MEPEDS subgroup, ancillary data on preschool or daycare attendance (yes or no) was also evaluated for association with bilateral decreased VA, due to a previously observed association of preschool or daycare attendance with achieving VA of 20/32 or better in the right eye.37 Ocular risk factors evaluated for association with unilateral decreased VA were strabismus (esotropia, exotropia, or no horizontal strabismus); SE anisometropia (<0.50 D; 0.50 to <1.00 D; 1.00 to <2.00 D; ≥2.00 D); J0 anisometropia (IOD in J0 of <0.25 D; 0.25 to <0.50 D; 0.50 to <1.00 D; ≥1.00 D); and J45 anisometropia (IOD in J45 of <0.25 D; 0.25 to <0.50 D; 0.50 to <1.00 D; ≥1.00 D). We also analyzed the overall IOD in optical blur, herein termed overall anisometropia, and defined as the square root of ((SE anisometropia)2 + (J0 anisometropia)2 + (J45 anisometropia)2); this corresponds to the distance in 3-dimensional vector space between right and left eye points each defined by the 3-dimensional vector (SE refractive error, J0, J45), and is equal to the vector dioptric difference divided by the square root of 2.38 Ocular risk factors evaluated for bilateral decreased VA were bilateral SE refractive error (SE of less hyperopic eye <0.00 D; 0.00 to <1.00 D; 1.00 to <2.00 D; 2.00 to <3.00 D; 3.00 to <4.00 D; ≥4.00 D) and bilateral astigmatism (absolute astigmatism of less astigmatic eye <1.00 D; 1.00 to <2.00 D; ≥2.00 D).
Table 1. Frequency distributions of demographic, behavioral, and clinical risk factors in children with and without an inter-ocular visual acuity difference of two or more lines and those with and without bilateral decreased visual acuity in the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study.
| Variable | With IOD (N=242) |
Without IOD (N=5455) |
P value** |
With BDVA (N=63) |
Without BDVA (N=5253) |
P value** |
|---|---|---|---|---|---|---|
| n (%)* | n (%)* | n (%)* | n (%)* | |||
| Age group (in months) | 0.003 | 0.0004 | ||||
| 60-72 | 64 (3.5) | 1772 (96.5) | 10 (0.6) | 1686 (99.4) | ||
| 48-59 | 66 (3.7) | 1737 (96.3) | 28 (1.7) | 1666 (98.4) | ||
| 36-47 | 92 (5.8) | 1487 (94.2) | 13 (0.9) | 1471 (99.1) | ||
| 30-35 | 20 (4.2) | 459 (95.8) | 12 (2.7) | 430 (97.3) | ||
| Sex: female | 128 (4.5) | 2726 (95.5) | 0.37 | 28 (1.1) | 2630 (99.0) | 0.38 |
| Race/ethnic group | <0.0001 | 0.006 | ||||
| Non-Hispanic White | 43 (2.9) | 1431 (97.1) | 4 (0.3) | 1216 (99.7) | ||
| African-American | 79 (3.3) | 2351 (96.7) | 32 (1.4) | 2315 (98.6) | ||
| Hispanic | 120 (6.7) | 1673 (93.3) | 27 (1.5) | 1722 (98.5) | ||
| Study site | <0.0001 | 0.001 | ||||
| MEPEDS | 216 (5.0) | 4076 (95.0) | 58 (1.5) | 3873 (98.5) | ||
| BPEDS | 26 (1.9) | 1379 (98.1) | 5 (0.4) | 1380 (99.6) | ||
|
Primary caregiver education:
high school diploma † |
172 (4.1) | 4013 (95.9) | 0.32 | 34 (0.9) | 3862 (99.1) | 0.0005 |
|
Family income below
$20,000/year † |
120 (4.8) | 2394 (95.2) | 0.16 | 35 (1.4) | 2444 (98.6) | 0.12 |
|
Health insurance within last
12 months † |
222 (4.3) | 4920 (95.7) | 0.25 | 57 (1.1) | 5029 (98.9) | 0.05 |
|
Vision insurance within last
12 months † |
92 (3.4) | 2655 (96.6) | 0.0001 | 24 (0.9) | 2703 (99.1) | 0.08 |
|
Last routine primary care visit
<2 years ago † |
228 (4.3) | 5061 (95.7) | 0.22 | 62 (1.2) | 5155 (98.8) | 1.00 |
| Difficulty accessing care † | 37 (5.2) | 671 (94.8) | 0.94 | 11 (1.6) | 687 (98.4) | 0.81 |
|
Maternal age at pregnancy
≥35 years † |
27 (4.1) | 629 (95.9) | 0.84 | 7 (1.1) | 639 (98.9) | 0.77 |
| Gestational age (weeks) † | 0.04‡ | 0.68‡ | ||||
| <33 | 11 (7.9) | 129 (92.1) | 2 (1.5) | 136 (98.5) | ||
| 33 to <37 | 14 (4.3) | 293 (95.7) | 4 (1.3) | 302 (98.7) | ||
| 37 to <42 | 183 (4.2) | 4149 (95.8) | 50 (1.2) | 4231 (98.8) | ||
| ≥42 | 10 (4.6) | 210 (95.4) | 2 (0.9) | 216 (99.1) | ||
| Small for gestational age † | 39 (4.3) | 876 (95.7) | 0.99 | 13 (1.4) | 893 (98.6) | 0.45 |
| Down syndrome † | 2 (28.6) | 5 (71.4) | 0.03 | 1 (14.3) | 6 (85.7) | 0.08 |
| Cerebral palsy † | 1 (20.0) | 4 (80.0) | 0.20 | 0 (0.0) | 5 (100.0) | 1.00 |
| Family history of strabismus † | 11 (3.3) | 318 (96.7) | 0.35 | 3 (0.9) | 326 (99.1) | 1.00 |
| Family history of amblyopia † | 6 (8.0) | 69 (92.0) | 0.14 | 1 (1.3) | 74 (98.7) | 0.59 |
| Smoked during pregnancy † | 19 (4.5) | 404 (95.5) | 0.95 | 8 (1.9) | 415 (98.1) | 0.21 |
|
Drank alcohol during
pregnancy † |
7 (4.9) | 135 (95.1) | 0.76 | 2 (1.4) | 139 (98.6) | 0.70 |
| Breastfed † | 151 (4.5) | 3182 (95.5) | 0.34 | 31 (0.9) | 3269 (99.1) | 0.02 |
IOD: inter-ocular visual acuity difference of 2 or more lines with visual acuity 20/32 or worse in the worse eye. BDVA: bilaterally decreased visual acuity, worse than 20/50, or worse than 20/40 in children aged ≥48 months. MEPEDS: Multi-Ethnic Pediatric Eye Disease Study. BPEDS: Baltimore Pediatric Eye Disease Study.
Denominator is the number of participants from both study centers having the stated level of the risk factor.
Chi square or fisher exact where applicable
Sum of n with (shown) and without (not shown) exposure for this variable differs from total N for one or both outcomes due to missing data.
P value reported is for dichotomous categorization of gestational age (<33 weeks; ≥33 weeks
Table 2. Frequency distributions of ocular risk factors in preschool children with and without inter-ocular visual acuity difference of two or more lines in the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study.
| Variable | With IOD (N=242) |
Without IOD (N=5455) |
P value** |
|---|---|---|---|
| n (%)* | n (%)* | ||
| Strabismus | ≤0.03† | ||
| No horizontal strabismus | 210 (3.8) | 5320 (96.2) | |
| Esotropia | 25 (27.5) | 66 (72.5) | |
| Exotropia | 7 (9.2) | 69 (90.8) | |
| SE anisometropia | <0.0001 | ||
| <0.50 D | 140 (3.1) | 4419 (96.9) | |
| 0.50 to <1.00 D | 52 (5.7) | 869 (94.3) | |
| 1.00 to <2.00 D | 25 (14.3) | 150 (85.7) | |
| ≥2.00 D | 25 (59.5) | 17 (40.5) | |
| J0 anisometropia | <0.0001 | ||
| <0.25 D | 170 (3.5) | 4709 (96.5) | |
| 0.25 to <0.50 D | 44 (6.6) | 620 (93.4) | |
| 0.50 to <1.00 D | 22 (16.5) | 111 (83.5) | |
| ≥1.00 D | 6 (28.6) | 15 (71.4) | |
| J45 anisometropia | <0.0001 | ||
| <0.25 D | 159 (3.4) | 4560 (96.6) | |
| 0.25 to <0.50 D | 46 (6.8) | 632 (93.2) | |
| 0.50 to <1.00 D | 27 (10.5) | 230 (89.5) | |
| ≥1.00 D | 10 (23.3) | 33 (76.7) |
IOD: inter-ocular visual acuity difference of 2 or more lines with visual acuity 20/32 or worse in the worse eye. SE: spherical equivalent. D: diopters. J0: J0 (Cartesian) power vector component of astigmatism. J45: J45 (oblique) power vector component of astigmatism.
Percentage of participants from both study centers with stated level of risk factor
Chi square or fisher exact where applicable
P value for comparison of esotropia vs. no horizontal strabismus: <0.0001; esotropia vs. exotropia: 0.003; exotropia vs. no horizontal strabismus: 0.03.
The Institutional Review Board, ethics, privacy and study oversight statements for this report are identical to our statements in a companion paper.30
Results
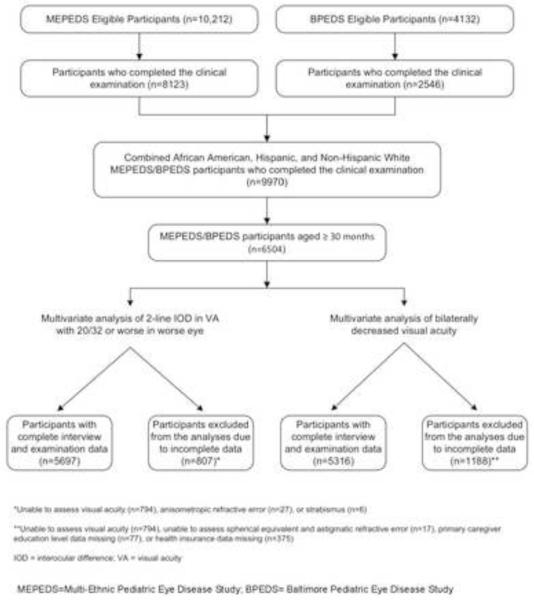
Eighty percent of eligible MEPEDS children and 62% of eligible BPEDS children were examined. Comparison of participants and nonparticipants is published elsewhere.32,39 Of a total of 9970 Hispanic, non-Hispanic white and African-American participants aged 6 to 72 months from both sites examined between 2003 and 2010, there were 6504 children ≥30 months of age, 5710 (88%) of whom were able to perform VA testing for both eyes (Fig 1). Testability as a function of age has been previously reported.32,35 Amblyopia was largely untreated in this population; a previous history of amblyopia diagnosis and resulting treatment using patching, cycloplegia, or glasses was reported in the analysis cohort for IOD in only 2.5% of children with unilateral decreased VA and 0.1% of children overall; no previous treatment was reported for any African-American or Hispanic children. Spectacle use was seen in only 1.7% of the analysis cohort, with no significant differences between race/ethnic groups (P=0.52). Interocular difference in VA assessed after the same-day VA retest with refractive correction was highly correlated with IOD assessed after the return visit retest in children who underwent both same-day and return visit retesting (Pearson’s r=0.88). There was no systematic tendency for IOD to resolve with return visit retesting, as the mean change in IOD after return visit retesting was a 0.2 line increase in the IOD (s.d. 1.35 lines), which was not significantly different from zero (P=0.27).
Figure 1.
Participant flowchart highlighting those children who were included and excluded from the final analysis sample for both outcomes – inter-ocular difference of 2 or more lines with visual acuity (VA) of 20/32 or worse in worse eye, and bilateral decreased VA, in preschool children from both the Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) and the Baltimore Pediatric Eye Disease Study (BPEDS).
The results of univariate analysis of associations between demographic, clinical, behavioral, and ocular risk factors and 2-line IOD (≤20/32 in the worse eye) are shown in Tables 1, and 2. Multivariate logistic regression resulted in a final model that included 5697 participants having complete data for all significant risk factors (Fig 1), 242 of whom had a 2-line IOD (VA ≤20/32 in the worse eye). In the final multivariate model (Table 3), the following demographic factors independently conferred increased risk for 2-line IOD (VA ≤20/32 in the worse eye): Hispanic ethnicity (OR 2.05 compared to non-Hispanic white children, the reference group); and age 36-47 months (OR 2.05 compared to the reference group of 60-72 months). Participation in BPEDS was associated with a reduced likelihood of having a 2-line IOD (VA ≤20/32 in the worse eye) (OR 0.45). Esotropia conferred an increased risk of having a 2-line IOD (VA ≤20/32 in the worse eye) (OR 8.98 compared to no horizontal strabismus), while exotropia did not confer a significantly increased risk. The following refractive errors were independent risk factors for 2-line IOD (VA ≤20/32 in the worse eye): SE anisometropia ≥0.50 D (OR 1.50 for SE anisometropia of 0.50 to <1.00 D, OR 4.48 for 1.00 to <2.00 D, and OR 39.76 for ≥2.00 D, compared to the reference level of <0.50 D); astigmatic anisometropia with regard to J45 ≥0.25 D (equivalent to 0.50 D inter-ocular difference in cylinder amount for a given axis of astigmatism) (OR 1.60 for J45 anisometropia of 0.25 to <0.50 D, OR 2.24 for 0.50 to <1.00 D, and OR 6.69 for ≥1.00 D, compared to the reference level of <0.25 D); and astigmatic anisometropia with regard to J0 ≥0.25 D (OR 1.49 for J0 anisometropia of 0.25 to <0.50 D, OR 2.36 for 0.50 to <1.00 D, and OR 5.31 for ≥1.00 D, compared to the reference level of <0.25 D). Subgroup analysis for MEPEDS alone produced a model including the same variables. Subgroup analysis of the smaller BPEDS sample gave similar results, but without significant associations with age or J0 anisometropia, and showing a significant association with gestational age <33 weeks (OR 4.32, CI 1.11-16.83). Only 4% of BPEDS children were of Hispanic ethnicity, and subgroup analysis did not show any association of race/ethnic group with unilaterally decreased VA in BPEDS. We also performed a secondary analysis in the combined MEPEDS/BPEDS population excluding children who wore glasses; the results were not substantially different, although the association with J45 anisometropia at the 0.25 to <0.50 D level became insignificant, and we saw a new association with age in the range of 30-35 months.
Table 3. Independent risk factors* for inter-ocular visual acuity difference of two or more lines in preschool children in the Multi-Ethnic Pediatric Eye Disease Study and Baltimore Pediatric Eye Disease Study.
| Variable | OR (95% CI) | P value |
|---|---|---|
| SE anisometropia | <0.0001 | |
| <0.5 D (reference) | 1.0 | |
| 0.5 to <1.0 D | 1.50 (1.06 – 2.11) | |
| 1.0 to <2.0 D | 4.48 (2.75 – 7.32) | |
| ≥2.0 D | 39.76 (19.59 – 80.71) | |
| Strabismus | <0.0001 | |
| No horizontal strabismus | Reference | |
| Exotropia | 1.23 (0.45 – 3.30) | |
| Esotropia | 8.98 (5.18 - 15.54) | |
| Race/ethnic group | <0.0001 | |
| Non-Hispanic White | Reference | |
| African-American | 0.97 (0.64 – 1.46) | |
| Hispanic | 2.05 (1.37 – 3.06) | |
| J45 anisometropia | <0.0001 | |
| <0.25 D | Reference | |
| 0.25 to <0.5 D | 1.60 (1.10 – 2.32) | |
| 0.5 to <1.0 D | 2.24 (1.37 – 3.66) | |
| ≥ 1.0 D | 6.69 (2.91 – 15.41) | |
| Age group (in months) | 0.0002 | |
| 60-72 | Reference | |
| 48-59 | 1.12 (0.76 - 1.63) | |
| 36-47 | 2.05 (1.44 - 2.92) | |
| 30-35 | 1.61 (0.94 – 2.76) | |
| J0 anisometropia | 0.0004 | |
| <0.25 D | Reference | |
| 0.25 to <0.50 D | 1.49 (1.02 – 2.17) | |
| 0.50 to <1.00 D | 2.36 (1.33 – 4.19) | |
| ≥ 1.00 D | 5.31 (1.81 – 15.63) | |
| Study site | 0.0006 | |
| MEPEDS | Reference | |
| BPEDS | 0.45 (0.29 – 0.71) |
IOD: inter-ocular visual acuity difference of 2 or more lines with visual acuity 20/32 or worse in the worse eye. SE: spherical equivalent. D: diopters. J0: J0 (Cartesian) power vector component of astigmatism. J45: J45 (oblique) power vector component of astigmatism. MEPEDS: Multi-Ethnic Pediatric Eye Disease Study. BPEDS: Baltimore Pediatric Eye Disease Study. OR: Odds ratio. 95%CI: 95% confidence interval.
Based on a multivariate step-wise logistic regression model.
SE anisometropia ≥0.50 D, ≥1.00 D and ≥2.00 D was seen in 20.0%, 3.8% and 0.7% of the analysis group, respectively. Frequency distributions show that 2-line IOD with 20/32 or worse in the worse eye was seen in 5.7% of children with 0.50 to <1.00 D of SE anisometropia, 14.3% of children with 1.00 to <2.00 D, and 59.5% of children with ≥2.00 D, compared to a baseline frequency of 3.1% in children with <0.50 D SE anisometropia (Table 2). This level of IOD was seen in 9.0% of children with ≥0.50 D and 23.0% of children with ≥1.00 D of SE anisometropia. After taking into account the baseline frequency of 2-line IOD, approximately 67 cases of 2-line IOD may be considered to be attributable to SE anisometropia ≥0.50 D (23, 20 and 24 cases, respectively, in children with 0.50 to <1.00 D, 1.00 to <2.00 D, and ≥2.00 D of anisometropia). We also examined the prevalence of 3-line or greater IOD in VA in children with different levels of SE anisometropia. This level of IOD occurred in 1.6% of children with 0.50 to <1.00 D of SE anisometropia, 5.1% of those with 1.00 to <2.00 D, and 45.2% of those with ≥2.00 D SE anisometropia, compared to 0.7% of children with <0.50 D SE anisometropia. IOD of 3 or more lines was seen in 3.8% of children with ≥0.50 D and 12.9% of children with ≥1.00 D of SE anisometropia. After taking into account the baseline frequency of 3-line IOD, 36 cases of 3-line IOD may be considered to be attributable to SE anisometropia ≥0.50 D (9, 8 and 19 cases, respectively, in children with 0.50 to <1.00 D, 1.00 to <2.00 D, and ≥2.00 D of anisometropia).
We also analyzed a multivariate model in which the three descriptors of anisometropia (SE, J0 and J45) were replaced with a single variable describing overall anisometropia (see methods), analyzed as a continuous variable. In this model, a 1.00 D increase in overall anisometropia produced a greater than 5-fold increase in the odds of having a 2-line IOD with 20/32 or worse in the worse eye (OR 5.51; 95% CI 4.30-7.07). Figure 2 shows the LOWESS plot demonstrating the independent relationship between overall anisometropia and the adjusted prevalence of 2-line IOD with 20/32 or worse in the worse eye, controlling for all other significant risk factors based on the multivariate model. The relationship is strongly linear over the range from 1.00 to 5.00 D of overall anisometropia, and at 5.00 D the prevalence approaches 100%. LOWESS plots showing the prevalence of 2-line IOD with 20/32 or worse in the worse eye (adjusted for all other significant covariates) as a function of SE, J0 and J45 anisometropia also illustrate a steady rise in risk of IOD with higher refractive error over the range of refractive error represented in the population, for each of the three types of anisometropia (“Figure 3, available at http://aaojournal.org”).
Figure 2.
Locally weighted regression line illustrating the independent relationship between level of overall anisometropia and the estimated prevalence of inter-ocular visual acuity (VA) difference of 2 or more lines with VA 20/32 or worse in the worse eye, in preschool children in the Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) and the Baltimore Pediatric Eye Disease Study (BPEDS), after controlling for other risk factors. The estimated prevalence of having an inter-ocular VA difference of 2 or more lines with VA 20/32 or worse in the worse eye was obtained using the stepwise logistic regression procedure. VA: visual acuity.
Results of univariate analysis of associations between demographic, clinical, behavioral, and ocular risk factors and bilateral decreased VA are shown in Tables 1, and 3. In addition, preschool or daycare attendance was analyzed in the MEPEDS subgroup and was not associated with bilaterally decreased visual acuity at the univariate level (P=0.19). Multivariate logistic regression resulted in a final model that included 5316 participants with complete data for all significant risk factors (Fig 1), 63 of whom had bilateral decreased VA. There were no significant differences in characteristics of children included in the analysis compared to children excluded for missing data. The following were identified as independent factors conferring a greater risk for bilateral decreased VA (Table 5): age 30-35 months or 48-59 months (OR 8.83 and 3.29, respectively, compared to the reference age group of 60-72 months); lack of health insurance (OR 2.91); lack of primary caregiver high school education (OR 1.72); astigmatism in the less astigmatic eye ≥1.00 D (OR 2.34 for astigmatism of 1.00 to <2.00 D, and OR 17.57 for ≥2.00 D, compared to the reference level of <1.00 D of astigmatism); and SE hyperopia in the less hyperopic eye of at least 4.00 D (OR 10.83 compared to the reference level of plano to <1.00 D). Sample sizes did not permit subclassification of hyperopia greater than 4.00 D. We tested for a statistically significant interaction between SE refractive error and astigmatism by including a product term in the model, and no significant interaction was found. Subgroup analysis for MEPEDS confirmed significant associations with the risk factors identified in the combined analysis, except that primary caregiver education was not significant in the final model, whereas there were significant independent associations with lack of breastfeeding (OR=1.89; 95% CI 1.05-3.42), and active maternal smoking during pregnancy (OR=2.75; 95% CI 1.13-6.68). No stable multivariate model that included SE refractive error could be derived in subgroup analysis of the BPEDS sample alone, due to sample size. We also performed a secondary analysis in the combined MEPEDS/BPEDS population excluding children who wore glasses. In this analysis the association with caregiver education just failed to achieve significance (95% CI for OR 0.99-3.13), and we saw an additional association with age in the range of 36-47 months; however, the associations with refractive errors were not substantially altered.
Table 5. Independent risk factors* for bilateral decreased visual acuity in preschool children in the Multi-Ethnic Pediatric Eye Disease Study and Baltimore Pediatric Eye Disease Study.
| Variable | OR (95% CI) | P value |
|---|---|---|
| Astigmatism † | <0.0001 | |
| <1.00 | Reference | |
| 1.00 to <2.00 D | 2.34 (1.13 – 4.86) | |
| ≥2.00 D | 17.57 (8.96 – 34.46) | |
| SE refractive error † | <0.0001 | |
| ≥0.00 to <+1.00 D | Reference | |
| <0.00 D | 1.72 (0.81 – 3.62) | |
| +1.00 to <+2.00 D | 0.43 (0.17 – 1.11) | |
| +2.00 to <+3.00 D | 0.96 (0.34 – 2.69) | |
| +3.00 to <+4.00 D | 2.13 (0.73 – 6.20) | |
| ≥+4.00 D | 10.83 (4.83 – 24.29) | |
| Age group (in months) | <0.0001 | |
| 60-72 | Reference | |
| 48-59 | 3.29 (1.52 – 7.14) | |
| 36-47 | 1.94 (0.81– 4.66) | |
| 30-35 | 8.83 (3.52 – 22.14) | |
| Health insurance | 0.02 | |
| Yes | Reference | |
| No | 2.91 (1.16 – 7.33) | |
| Primary caregiver education: H.S.** diploma | 0.0495 | |
| Yes | Reference | |
| No | 1.72 (1.001 – 2.96) |
SE: spherical equivalent. D: diopters. OR: Odds ratio. 95% CI: 95% confidence interval.
Based on a step-wise multiple regression model
Level of SE refractive error defined by less hyperopic eye and level of astigmatism defined by less astigmatic eye, or by eye with refractive error data if data missing for one eye.
H.S.: high school.
Frequency distributions show that bilateral decreased VA was seen in 2.5% of children having 1.00 to <2.00 D of astigmatism, and 13.6% of children with ≥2.00 D of astigmatism, compared to 0.7% of children with <1.00 D of astigmatism (Table 4). Bilateral decreased VA was seen in 10.0% of children with hyperopia ≥4.00 D, compared to 0.8% of children with plano to <1.00 D of hyperopia (Table 4). Bilateral decreased VA was seen in 2.4% of children with 3.00 to <4.00 D of hyperopia, but the trend toward increased risk in this category was not statistically significant in the multivariate logistic regression analysis.
Table 4. Frequency distributions of ocular risk factors in preschool children with and without bilateral decreased visual acuity in the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study.
| Variable | With BDVA (N=63) |
Without BDVA (N=5253) |
P value** |
|---|---|---|---|
| n (%)* | n (%)* | ||
| Astigmatism † | <0.0001 | ||
| <1.00 | 33 (0.7) | 4706 (99.3) | |
| 1.00 to <2.00 D | 11 (2.5) | 426 (97.5) | |
| ≥2.00 D | 19 (13.6) | 121 (86.4) | |
| SE refractive error † | <0.0001 | ||
| <0.00 D | 16 (2.2) | 703 (97.8) | |
| ≥0.00 to <+1.00 D | 16 (0.8) | 1885 (99.2) | |
| +1.00 to <+2.00 D | 6 (0.3) | 1771 (99.7) | |
| +2.00 to <+3.00 D | 5 (0.9) | 552 (99.1) | |
| +3.00 to <+4.00 D | 5 (2.4) | 207 (97.6) | |
| ≥+4.00 D | 15 (10.0) | 135 (90.0) |
BDVA: bilaterally decreased visual acuity, worse than 20/50, or worse than 20/40 in children aged ≥48 months. SE: spherical equivalent. D: diopters.
Percentage of participants from both study centers with stated level of risk factor
Chi square or fisher exact where applicable
Level of SE refractive error defined by less hyperopic eye and level of astigmatism defined by less astigmatic eye, or by eye with refractive error data if data missing for one eye.
LOWESS plots of the adjusted prevalence of bilateral decreased VA as a function of SE refractive error and astigmatism are shown in Figure 4a and 4b, showing that greater SE hyperopic refractive error and greater astigmatism are both associated with a higher prevalence of bilateral decreased VA.
Figure 4.
(A) Locally weighted regression lines illustrating the independent relationship between level of spherical equivalent refractive (SE) error ; (B) or absolute cylindrical power (astigmatism) and the estimated prevalence of bilateral decreased visual acuity (VA) in preschool children in the Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) and the Baltimore Pediatric Eye Disease Study (BPEDS) after controlling for other risk factors. The estimated prevalence of having any bilateral decreased VA was obtained using the stepwise logistic regression procedure. The plot for Figure 3a excludes 2 children with highly hyperopic refractions (>11.00 D SE in both eyes) due to aphakia. VA: visual acuity. SE: spherical equivalent.
Discussion
We evaluated the association of risk factors with decreased VA in two population-based samples of preschool children. The major factors associated with an IOD in VA were anisometropia and esotropia. Hispanic ethnicity also posed an increased risk independent of the ocular factors. The major risk factors associated with bilaterally decreased VA were hyperopia of at least 4.00 D, and astigmatism; lack of health insurance and lower primary caregiver education were also associated with decreased vision.
Our data reveal that the threshold level of spherical anisometropia at which the risk of IOD increases is lower than previously reported, with a significantly increased odds ratio seen even for anisometropia in the range of 0.50 to <1.00 D, although the absolute risk at this level of anisometropia is low. Our study supports previous observations that anisometropia of 1.00 D SE or greater significantly increase amblyopia risk7-8,40 and reinforces the severity-dependent nature of the risk. Previous work reported that IOD was 13 times more common in children with aniso-hyperopia in the range of 1.00 to 2.00 D than in children with 0.00 to <1.00 D of anisometropia.7,8 However, this work was clinic-based and excluded children with strabismus, both potential sources of selection bias; furthermore, amblyopia was defined as a one-line difference in VA. The present analysis finds an OR for 2-line IOD of 4.5 for 1.00 to <2.00 D of SE anisometropia; since the study is population-based, controls for strabismus using multivariate analysis, and uses a more stringent definition of IOD, it provides stronger evidence than previously of the risk associated with moderate anisometropia.
Similar observations were made for astigmatic anisometropia: a clear severity-dependent response is evident, and inter-ocular J0 or J45 differences as low as 0.25-0.50 D (equivalent, in the absence of differences of cylinder axis, to cylinder differences of 0.50 to 1.00 D) are associated with a higher risk of having unilateral decreased VA, although the absolute risk is low at this level of aniso-astigmatism. The J0 and J45 components of astigmatic anisometropia confer similar magnitudes of risk. Our data suggest that the threshold at which aniso-astigmatism may be amblyogenic is lower than previously thought: studies by Weakley7-8 and Dobson40 reported that IOD risk began to increase with aniso-astigmatism in the range of 1.50 to 2.00 D, or 2.00 to 3.00 D in the case of Native-American children.
Although levels of anisometropia as low as 0.50 to <1.00 D SE increase the risk of unilateral decreased VA, most children with these small and relatively common levels of anisometropic refractive error have normal VA: IOD of 2 or more lines was seen in <6% of these children, compared to a baseline frequency of 3% in children with <0.50 D SE anisometropia. By contrast, 60% of children with ≥2.00 D SE anisometropia had 2 or more lines of IOD in VA. Considering a more stringent definition of unilateral decreased VA, IOD of 3 lines or more was seen in <2% of children with 0.50 to <1.00 D SE anisometropia (baseline frequency of 0.7%), whereas 45% of children with ≥2.00 D SE anisometropia had 3 or more lines of IOD.
To further explore the relationship between anisometropia and unilateral VA deficits, we used vector analysis to study the overall inter-ocular blur difference (overall anisometropia) expressed as a single variable, rather than treating spherical equivalent, J0 and J45 as independent components of anisometropia. Overall anisometropia is proportional to the vector dioptric difference. For example, 1.00 D of overall anisometropia is equivalent to 1.00 D of SE anisometropia alone, or 2.00 D of difference in astigmatism of equal axis in the two eyes without SE anisometropia. The relationship of overall anisometropia to the estimated prevalence of IOD in VA (adjusted for all other significant covariates) is particularly strong and linear over the range of 1.00 to 5.00 D of overall anisometropia, with prevalence of IOD approaching 100% at 5.00 D of overall anisometropia. This use of a single variable to describe inter-ocular blur differences illustrates the utility and versatility of power vectors for the analysis of refractive error.38
Bilateral decreased VA, like unilateral deficits in VA, was closely related to refractive error, as previously reported.18-26 We observed a severity-dependent relationship in the association between astigmatism and bilateral decreased VA. A significant association with bilateral decreased VA was observed for levels of astigmatism as low as 1.00 to <2.00 D, which is lower than those levels considered to be associated with increased risk of ametropic amblyopia in consensus guidelines and clinical studies.45 However, VA deficits occur in only a small proportion of these children: bilaterally decreased VA was seen in <3% of children having 1.00 to <2.00 D of bilateral astigmatism, but in over 13% of children with ≥2.00 D astigmatism. Hyperopia was the other major refractive risk factor for bilateral decreased VA, the odds of which increased substantially only for levels of hyperopia ≥4.00 D in the less ametropic eye, although there was a trend toward increased risk with 3.00 to <4.00 D of hyperopia that did not reach statistical significance. Bilateral decreased VA was seen in 10% of children with hyperopia ≥4.00 D. Children with lesser degrees of hyperopia, especially <3.00 D, may be better able to compensate adequately through their accommodative efforts, whereas children with even moderate amounts of astigmatism experience astigmatic defocus at all times. On the other hand, it should be noted that lower levels of hyperopia confer a significantly elevated risk of esotropia, as reported in a companion paper.46
The fact that amblyopia is related to refractive error supports the use of refractive error-based screening instruments to identify children at risk of having amblyopia; however, our data highlight some difficulties inherent in this approach. In the case of anisometropia, for instance, it is difficult to identify a threshold level of anisometropia that is both sensitive and specific for existing IOD in VA, because low (lower-risk) refractive errors are much more common than high (higher-risk) refractive errors. For example, of children with ≥1.00 D SE anisometropia, nearly 1 in 4 has at least a 2-line IOD with VA 20/32 or worse in the worse eye; however, this threshold misses over one third of the IOD cases in our study population that are attributable to SE anisometropia ≥0.50 D. Raising the threshold would miss more cases. Lowering the threshold to 0.50 D would capture a greater proportion of cases, but at the cost of targeting 20% of the population, >90% of whom would not have a 2-line IOD. Changing the definition of decreased VA to 3 lines of IOD does not eliminate these issues. Close to half of children with ≥2.00 D SE anisometropia have a 3-line IOD, but this threshold misses nearly half of the cases of 3-line IOD that are attributable to SE anisometropia ≥0.50 D. Lowering the anisometropia threshold to 1.00 D would capture more of the cases, but nearly 90% of the children targeted would not have a 3-line IOD.
In our multivariate models adjusting for anisometropia, esotropia was independently associated with a nine fold increase in the odds of IOD in visual acuity, while no measurable increase was seen with exotropia. This is consistent with clinical experience and data41 indicating that amblyopia is rare or very mild in the setting of the preserved binocularity typically seen with intermittent exotropia, the most common form of exotropia.39,42
One previous population-based study in 6-year-old Australian children15,43,44 examined the associations between amblyopia and anisometropia and between amblyopia and strabismus. The authors reported an odds ratio of 29 (adjusted for strabismus) for the association between amblyopia and SE anisometropia ≥1.00 D, and an odds ratio of 8 for the association between amblyopia and cylindrical anisometropia ≥1.00 D43. Amblyopia was present in 20% of children with exotropia, and in 48% of those children with esotropia, with an odds ratio of 65 for the association between strabismus and amblyopia.15,44 Although the study was similar to our own in finding strong associations, the magnitude of odds ratios between the two studies cannot be compared because in the Australian study, the definition of amblyopia was expanded beyond the a priori definition based on VA, to include children with milder decreases in VA who had amblyogenic risk factors together with a parent-reported history of “lazy eye” or of amblyopia treatment (including strabismus surgery). The inclusion of risk factors in the definition of amblyopia may have inflated the strength of the reported associations.
We did not observe any clear trend in the association between age and IOD, despite previous observations suggesting that amblyopia is more frequent in older children with a given level of anisometropia.16,47 Previous studies may have been confounded by the use of non-optotype measures of VA in younger children, as well as by referral/selection biases; or the children in our study may not be old enough to reveal such an age-related pattern.
We found a higher risk of IOD and bilateral decreased VA in some younger age groups relative to the oldest reference group. IOD was more likely to occur in children aged 36-47 months than in children aged 60-72 months; a similar trend was seen, but was not significant, in the 30-35 month-old group, which had a smaller sample size and lower proportion of children able to complete visual acuity testing. Previously, we have demonstrated that a higher proportion of normal children had 2-line VA differences in 36-47 month-olds compared to older children.37 This is likely related to more variable test performance in younger children, who may either perform better on the second eye due to practice, or perform worse on the second eye due to short attention spans. Bilateral VA worse than 20/40 (used to define decreased VA over 48 months) was more likely to occur in children aged 48-59 months than in the reference group of children aged 60-72 months. Similarly, in children under 48 months, for whom bilateral decreased VA was defined as VA worse than 20/50, there was an increased likelihood of decreased VA in 30- to 35-month-old children, but not in 36- to 47-month-old children. This again is likely a consequence of age-related improvements in VA test performance, since VA test performance in the right eye of normal children is steeply related to age, suggesting that separate norms should apply to every age group.37 For future studies, one approach to controlling for age-related changes in test performance might be to apply different definitions of subnormal VA a priori to every age category to be used in the analysis;37 an alternative would be to use a single definition of decreased VA, as long as the analysis adjusts for age. However, the analysis in the present study is committed to using the MEPEDS and BPEDS a priori definitions of decreased VA, which only distinguish between children under or over 48 months, because these thresholds were integral in determining whether or not a participant was required to undergo VA retesting with correction.
Hispanic ethnicity was related to a higher risk of having IOD in VA in this study. Two-fold greater odds of unilateral decreased VA in children of Hispanic ethnicity were seen as compared to VA in non-Hispanic white children, independent of other amblyopia risk factors. While it is possible that this reflects differences in frequency of prior amblyopia treatment, past treatment for a diagnosis of amblyopia was rare overall, with none reported for any African-American or Hispanic children. Furthermore, although spectacles might constitute amblyopia treatment even in the absence of an explicit diagnosis of amblyopia, spectacle wear was rare in this population, and did not differ by race/ethnic group. Hispanic ethnicity might be associated with some aspect of anisometropia or esotropia that is not captured by the corresponding variables in the cross-sectional analysis, such as age of onset (i.e., duration) of anisometropia, or type (i.e., infantile or accommodative) of esotropia. Genetic factors that vary with race/ethnic group may also predispose toward amblyopia in the setting of high-risk refractive error.
Additional demographic risk factors associated with bilateral decreased VA were lack of health insurance and lack of primary caregiver high school education. The impact of lacking health insurance is unlikely to reflect decreased access to early interventional measures such as spectacle correction, because spectacle use was infrequent in this population overall, and lack of health insurance remained a significant risk factor even after excluding children with glasses. Health insurance may instead be a surrogate indicator of other demographic or environmental factors that might impact vision outcomes (e.g. dietary or other lifestyle factors). As for the association of bilateral decreased VA with lower caregiver education levels, it is not possible to determine whether this reflects true differences in visual function associated (directly or indirectly) with parental education, or simply better test performance in children of more educated parents.
Active maternal smoking during pregnancy and lack of breastfeeding were also associated with bilateral decreased VA in our study, although these associations were only seen in the MEPEDS subgroup analysis, not in our primary analysis. Since both of these behavioral factors may be themselves associated with parental education, which was associated with bilateral decreased VA in the primary analysis but not in the MEPEDS subgroup analysis, we cannot rule out the possibility that these variables are indicators of caregiver education, rather than being causally related to VA deficits. On the other hand, the finding of decreased VA in children who were not breastfed is consistent with previous work showing that visual outcomes are better in breastfed children than in children fed commercial infant formulas, in the absence of long-chain polyunsaturated fatty acid supplementation.48,49 Other studies have found associations between maternal smoking and vision outcomes: the Avon Longitudinal Study of Parents and Children reported an association between maternal smoking and amblyopia after adjustment for concurrent visual problems,50 and a borderline association between gestational smoking exposure and amblyopia was seen in a population-based study of Australian children.44 It should be noted that since active maternal smoking during pregnancy increases the risk of astigmatism and hyperopia (see companion papers), gestational tobacco exposure may also contribute to decreased VA indirectly, through its effect on refractive error; any such impact would not be evident in the present analysis, because all our models control for refractive error.
Interocular differences in visual acuity were less likely in BPEDS participants than in MEPEDS participants after adjustment for all other associated covariates. The reasons for this are not clear. Study protocols were standardized across study sites, and the use of the Amblyopia Treatment Study visual acuity testing protocol with the electronic VA tester leaves little room for subjectivity in determination of VA. Although we controlled for a range of demographic and behavioral variables in this study, a number of factors that could be hypothesized to impact VA were not evaluated, such as genetic differences, nutrition, or environmental pollution. Geographic location may be a surrogate marker for significant genetic, demographic or environmental factors that were not directly represented in our multivariate model.
This study has several limitations. First, the cross-sectional nature of the study design does not allow us to determine whether the refractive errors we observe actually preceded the associated deceased visual acuity. Furthermore, some of the younger children (who did not have VA deficits at the time of our examination) may in fact be at risk of amblyopia even though they had not yet developed it, while conversely, among the older children, some with normal refractions may in fact have had anisometropic refractive errors when they were younger. The temporal relationship between the risk factors and decreased VA deserves further study to clearly illuminate the relationship between early refractive error (the target of screening efforts) and VA outcomes, and can best be addressed by a prospective study. Second, prior successful treatment of amblyopia could, in principle, partially mask associations with refractive and other risk factors, in which case the associations could be even stronger than reported here; however, the extremely low reported rate of prior amblyopia treatment in this study (2.5% among children with unilateral decreased VA), along with the very low frequency of appropriate spectacle correction for refractive errors in the population studied,3 make it unlikely that treatment effects substantially reduce the observed strength of the associations with refractive error relative to their true magnitude. To further address this issue we performed a secondary analysis excluding children with spectacles; the similarity of the findings to the primary analysis further indicates that our findings are unlikely to be impacted by prior treatment in this population. On the other hand, although it is not known whether early spectacle correction of refractive errors can prevent unilateral and bilateral visual acuity deficits, it must be acknowledged that our findings might not be applicable to preschool populations with very high rates of appropriate spectacle wear. Third, because the etiology of esotropia was not known for all esotropic children in this cross-sectional study, we are unable to determine whether the risk of amblyopia is different (e.g., possibly lower) in infantile esotropia as opposed to acquired, accommodative esotropia. Fourth, in our protocol, certain children with a 2-line IOD with VA of 20/32 or worse in the worse eye and levels of anisometropia below the a priori definition of a unilateral amblyopia risk factor did not undergo return visit VA retesting; some of these cases might have resolved with return visit retesting. However, we do not think this biased our study toward finding an association of IOD with low levels of anisometropia because these children did undergo same-day retesting with correction, and return visit retesting was shown to have no systematic effect on IOD outcomes in children who underwent both same-day and return visit retesting. Finally, we excluded a sizeable number of children from the analysis of risk factors for bilateral decreased VA due to missing data for risk factors that remained significant in the final model; but we did not find significant differences in the characteristics of children included in the analysis compared to those who were excluded.
The strengths of this study are its size and design. A population-based study allows us to generalize our results to other similar populations and is less likely to have the referral and selection biases that many clinic-based or screening studies suffer. The multivariate analysis allows us to evaluate the independent contributions of different risk factors to amblyopia, despite the frequent coexistence of different types of refractive error and strabismus. Our subgroup analyses showed our main findings to be replicated in two sites. The pooling of MEPEDS and BPEDS data has provided us with the power to detect statistically significant associations of decreased visual acuity with low levels of refractive error previously not known to be significant.
In conclusion, our data support the strong association of anisometropic and bilateral refractive errors with unilateral and bilateral decreased best-corrected VA, respectively. We suggest an expanded range of refractive errors that should be considered as potentially contributing to amblyopia. We have confirmed the high risk of unilateral VA deficits associated with esotropia and the low risk associated with exotropia. Prospective, longitudinal observation is required to establish the prognostic significance of risk factors that are present at an early age, and clinical trials are still needed to establish whether early treatment of refractive error or strabismus might improve long-term visual acuity outcomes. In the meantime, our data may help practitioners and parents make more informed decisions regarding the management of these conditions in preschool children. Our data highlight the limits of using refractive error thresholds as predictors of VA deficits and may thus help inform vision screening policies in preschool children.
Supplementary Material
Acknowledgements
The MEPEDS-BPEDS Investigators would like to acknowledge the helpful advice and support of the members of the National Eye Institute’s Data Monitoring and Oversight Committee comprising of: Jonathan M. Holmes, MD (Chair), Eileen Birch, PhD, Karen Cruickshanks, PhD, Natalie Kurinij, PhD, Maureen Maguire, PhD, Joseph Miller, MD, MPH, Graham Quinn, MD, and Karla Zadnik, OD, PhD.
Support: Supported by the National Eye Institute, National Institutes of Health, Bethesda, MD (grant nos. EY14472, EY03040 and EY14483), and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Dr. Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no proprietary or commercial interest in any materials discussed in the manuscript.
“This article contains online-only material. The following should appear online-only: Figure 3”
References
- 1.Robaei D, Rose K, Ojaimi E, et al. Visual acuity and the causes of visual loss in a population-based sample of 6-year-old Australian children. Ophthalmology. 2005;112:1275–82. doi: 10.1016/j.ophtha.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 2.Robaei D, Huynh SC, Kifley A, Mitchell P. Correctable and non-correctable visual impairment in a population-based sample of 12-year-old Australian children. Am J Ophthalmol. 2006;142:112–8. doi: 10.1016/j.ajo.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 3.Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) Group Prevalence and causes of visual impairment in African-American and Hispanic preschool children: the Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology. 2009;116:1990–2000. doi: 10.1016/j.ophtha.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessel L, Hougaard JL, Mortensen C, et al. Visual acuity and refractive errors in a suburban Danish population: Inter99 Eye Study. Acta Ophthalmol Scand. 2004;82:19–24. doi: 10.1111/j.1395-3907.2004.0179.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang JJ, Foran S, Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2000;28:268–73. doi: 10.1046/j.1442-9071.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 6.Tanlamai T, Goss DA. Prevalence of monocular amblyopia among anisometropes. Am J Optom Physiol Opt. 1979;56:704–15. doi: 10.1097/00006324-197911000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Weakley DR., Jr. The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108:163–71. doi: 10.1016/s0161-6420(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 8.Weakley DR. The association between anisometropia, amblyopia, and binocularity in the absence of strabismus. Trans Am Ophthalmol Soc. 1999;97:987–1021. [PMC free article] [PubMed] [Google Scholar]
- 9.Leon A, Donahue SP, Morrison DG, et al. The age-dependent effect of anisometropia magnitude on anisometropic amblyopia severity. J AAPOS. 2008;12:150–6. doi: 10.1016/j.jaapos.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Townshend AM, Holmes JM, Evans LS. Depth of anisometropic amblyopia and difference in refraction. Am J Ophthalmol. 1993;116:431–6. doi: 10.1016/s0002-9394(14)71400-x. [DOI] [PubMed] [Google Scholar]
- 11.Rutstein RP, Corliss D. Relationship between anisometropia, amblyopia, and binocularity. Optom Vis Sci. 1999;76:229–33. doi: 10.1097/00006324-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Zaka-Ur-Rab S. Evaluation of relationship of ocular parameters and depth of anisometropic amblyopia with the degree of anisometropia. Indian J Ophthalmol. 2006;54:99–103. doi: 10.4103/0301-4738.25830. [DOI] [PubMed] [Google Scholar]
- 13.Caputo R, Frosini R, De Libero C, et al. Factors influencing severity of and recovery from anisometropic amblyopia. Strabismus. 2007;15:209–14. doi: 10.1080/09273970701669983. [DOI] [PubMed] [Google Scholar]
- 14.Chia A, Dirani M, Chan YH, et al. Prevalence of amblyopia and strabismus in young Singaporean Chinese children. Invest Ophthalmol Vis Sci. 2010;51:3411–7. doi: 10.1167/iovs.09-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robaei D, Rose KA, Kifley A, et al. Factors associated with childhood strabismus: findings from a population-based study. Ophthalmology. 2006;113:1146–53. doi: 10.1016/j.ophtha.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Donahue SP. The relationship between anisometropia, patient age, and the development of amblyopia. Trans Am Ophthalmol Soc. 2005;103:313–36. [PMC free article] [PubMed] [Google Scholar]
- 17.Calcutt C, Murray AD. Untreated essential infantile esotropia: factors affecting the development of amblyopia. Eye (Lond) 1998;12:167–72. doi: 10.1038/eye.1998.42. [DOI] [PubMed] [Google Scholar]
- 18.Werner DB, Scott WE. Amblyopia case reports--bilateral hypermetropic ametropic amblyopia. J Pediatr Ophthalmol Strabismus. 1985;22:203–5. doi: 10.3928/0191-3913-19850901-09. [DOI] [PubMed] [Google Scholar]
- 19.Schoenleber DB, Crouch ER., Jr. Bilateral hypermetropic amblyopia. J Pediatr Ophthalmol Strabismus. 1987;24:75–7. doi: 10.3928/0191-3913-19870301-06. [DOI] [PubMed] [Google Scholar]
- 20.Fern KD. Visual acuity outcome in isometropic hyperopia. Optom Vis Sci. 1989;66:649–58. doi: 10.1097/00006324-198910000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Edelman PM, Borchert MS. Visual outcome in high hypermetropia. J AAPOS. 1997;1:147–50. doi: 10.1016/s1091-8531(97)90056-2. [DOI] [PubMed] [Google Scholar]
- 22.Klimek DL, Cruz OA, Scott WE, Davitt BV. Isoametropic amblyopia due to high hyperopia in children. J AAPOS. 2004;8:310–3. doi: 10.1016/j.jaapos.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Ziylan S, Yabas O, Zorlutuna N, Serin D. Isoametropic amblyopia in highly hyperopic children. Acta Ophthalmol Scand. 2007;85:111–3. doi: 10.1111/j.1600-0420.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 24.Friedman Z, Neumann E, Abel-Peleg B. Outcome of treatment of marked ametropia without strabismus following screening and diagnosis before the age of three. J Pediatr Ophthalmol Strabismus. 1985;22:54–7. doi: 10.3928/0191-3913-19850301-05. [DOI] [PubMed] [Google Scholar]
- 25.Dobson V, Miller JM, Harvey EM, Mohan KM. Amblyopia in astigmatic preschool children. Vision Res. 2003;43:1081–90. doi: 10.1016/s0042-6989(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 26.Harvey EM, Dobson V, Miller JM, Clifford-Donaldson CE. Amblyopia in astigmatic children: patterns of deficits. Vision Res. 2007;47:315–26. doi: 10.1016/j.visres.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakim OM. Association between fixation preference testing and strabismic pseudoamblyopia. J Pediatr Ophthalmol Strabismus. 2007;44:174–7. doi: 10.3928/0191-3913-20070301-11. [DOI] [PubMed] [Google Scholar]
- 28.Friedman DS, Katz J, Repka MX, et al. Lack of concordance between fixation preference and HOTV optotype visual acuity in preschool children: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2008;115:1796–9. doi: 10.1016/j.ophtha.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotter SA, Tarczy-Hornoch K, Song E, et al. Multi-Ethnic Pediatric Eye Disease Study Group Fixation preference and visual acuity testing in a population-based cohort of preschool children with amblyopia risk factors. Ophthalmology. 2009;116:145–53. doi: 10.1016/j.ophtha.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joint Writing Committee for the Multi-Ethnic Pediatric Eye Disease Study and Baltimore Pediatric Eye Disease Study Groups Risk factors for hyperopia and myopia in preschool children: the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2011 doi: 10.1016/j.ophtha.2011.06.030. Accepted for publication on June 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varma R, Deneen J, Cotter S, et al. Multi-Ethnic Pediatric Eye Disease Study Group The Multi-Ethnic Pediatric Eye Disease Study: design and methods. Ophthalmic Epidemiol. 2006;13:253–62. doi: 10.1080/09286580600719055. [DOI] [PubMed] [Google Scholar]
- 32.Friedman DS, Repka MX, Katz J, et al. Prevalence of decreased visual acuity among preschool-aged children in an American urban population: the Baltimore Pediatric Eye Disease Study, methods, and results. Ophthalmology. 2008;115:1786–95. doi: 10.1016/j.ophtha.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the Amblyopia Treatment Study visual acuity testing protocol. Am J Ophthalmol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 34.Holmes JM, Beck RW, Repka MX, et al. Pediatric Eye Disease Investigator Group The Amblyopia Treatment Study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 35.Cotter SA, Tarczy-Hornoch K, Wang Y, et al. Multi-Ethnic Pediatric Eye Disease Study Group Visual acuity testability in African-American and Hispanic children: the Multi-Ethnic Pediatric Eye Disease Study. Am J Ophthalmol. 2007;144:663–7. doi: 10.1016/j.ajo.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–75. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Pan Y, Tarczy-Hornoch K, Cotter SA, et al. Multi-Ethnic Pediatric Eye Disease Study Group Visual acuity norms in pre-school children: the Multi-Ethnic Pediatric Eye Disease Study. Optom Vis Sci. 2009;86:607–12. doi: 10.1097/OPX.0b013e3181a76e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller JM. Clinical applications of power vectors. Optom Vis Sci. 2009;86:599–602. doi: 10.1097/OPX.0b013e3181a6a211. [DOI] [PubMed] [Google Scholar]
- 39.Multi-ethnic Pediatric Eye Disease Study Group Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months: the Multi-ethnic Pediatric Eye Disease Study. Ophthalmology. 2008;115:1229–36. doi: 10.1016/j.ophtha.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobson V, Miller JM, Clifford-Donaldson CE, Harvey EM. Associations between anisometropia, amblyopia, and reduced stereoacuity in a school-aged population with a high prevalence of astigmatism. Invest Ophthalmol Vis Sci. 2008;49:4427–36. doi: 10.1167/iovs.08-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romanchuk KG, Dotchin SA, Zurevinsky J. The natural history of surgically untreated intermittent exotropia-looking into the distant future. J AAPOS. 2006;10:225–31. doi: 10.1016/j.jaapos.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116:2128–34. doi: 10.1016/j.ophtha.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huynh SC, Wang XY, Ip J, et al. Prevalence and associations of anisometropia and aniso-astigmatism in a population based sample of 6 year old children. Br J Ophthalmol. 2006;90:597–601. doi: 10.1136/bjo.2005.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robaei D, Rose KA, Ojaimi E, et al. Causes and associations of amblyopia in a population-based sample of 6-year-old Australian children. Arch Ophthalmol. 2006;124:878–84. doi: 10.1001/archopht.124.6.878. [DOI] [PubMed] [Google Scholar]
- 45.Wallace DK, Chandler DL, Beck RW, et al. Pediatric Eye Disease Investigator Group Treatment of bilateral refractive amblyopia in children three to less than 10 years of age. Am J Ophthalmol. 2007;144:487–96. doi: 10.1016/j.ajo.2007.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joint Writing Committee for the Multi-Ethnic Pediatric Eye (MEPEDS) Disease Study and Baltimore Pediatric Eye Disease Study (BPEDS) Groups Risk factors associated with childhood strabismus: the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2011 doi: 10.1016/j.ophtha.2011.06.032. Accepted for publication on June 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donahue SP. Relationship between anisometropia, patient age, and the development of amblyopia. Am J Ophthalmol. 2006;142:132–40. doi: 10.1016/j.ajo.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 48.Birch EE, Castaneda YS, Wheaton DH, et al. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am J Clin Nutr. 2005;81:871–9. doi: 10.1093/ajcn/81.4.871. [DOI] [PubMed] [Google Scholar]
- 49.Birch EE, Garfield S, Castaneda Y, et al. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid- supplemented infant formula. Early Hum Dev. 2007;83:279–84. doi: 10.1016/j.earlhumdev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Williams C, Northstone K, Howard M, et al. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. Br J Ophthalmol. 2008;92:959–64. doi: 10.1136/bjo.2007.134700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.