Abstract
Background
Rapid tolerance to the anxiolytic effects of ethanol appears to be an important factor in the development of alcoholism. Here, we investigated the involvement of amygdaloid histone deacetylases (HDAC)-induced epigenetic changes in rapid ethanol tolerance (RET).
Methods
RET in rats was induced by two ethanol injections administered 24 hrs apart. Both ethanol-tolerant and control rats were treated with the HDAC inhibitor, trichostatin A (TSA), and anxiety-like behaviors were measured. HDAC activity, histone (H3 & H4) acetylation, and neuropeptide Y (NPY) expression in the amygdala of these rats were also measured.
Results
A single ethanol exposure was able to produce an anxiolytic response, inhibit amygdaloid HDAC activity, and increase both histone acetylation and NPY expression (mRNA and protein levels) in the central nucleus of amygdala (CeA) and medial nucleus of amygdala (MeA) of rats. In contrast, two exposures of the same dose of ethanol (24 hrs apart) neither elicited a similar anxiolytic response nor modulated HDAC activity, histone acetylation, or NPY expression in the amygdala. However, exposure to a higher dose of ethanol on the second day was able to produce an anxiolytic response and also inhibit amygdaloid HDAC activity. TSA treatment caused the reversal of RET by inhibiting HDAC activity thereby increasing histone acetylation and NPY expression in the CeA and MeA.
Conclusions
Cellular tolerance to the initial acute ethanol-induced inhibition of HDAC activity and the subsequent up-regulation of histone acetylation and NPY expression in the amygdala may be involved in the mechanisms underlying rapid tolerance to the anxiolytic effects of ethanol.
Keywords: Amygdala, anxiety, HDAC, histone acetylation, neuropeptide Y, rapid ethanol tolerance
Alcohol tolerance is a functional phenomenon that can be defined as the need for a higher dose of alcohol to achieve the initial responses of alcohol exposure and may thereby play important roles in the development of dependence and in promoting alcohol drinking behaviors (Kalant 1998; Tabakoff et al., 1986). Alcohol tolerance is an important criterion used to clinically diagnose alcohol dependence, according to the Diagnostic and Statistical Manual of Mental Disorders IV (DSM IV) guidelines (American Psychiatric Association, 1994). Various forms of alcohol tolerance have been identified, such as acute functional tolerance (AFT), rapid ethanol tolerance (RET), and chronic ethanol tolerance (CET) (Kalant 1998; Tabakoff et al., 1986). AFT can develop during a single session of alcohol consumption (Mellanby 1919), whereas CET has been observed after long-term ethanol exposure (Kalant 1998; Tabakoff et al., 1986). RET develops between 8 and 24 hrs after the first ethanol exposure (Crabbe et al., 1979; Khanna et al., 1991, 1996; Koob et al., 1987) and can serve as a good predictor of CET (Khanna et al., 1991).
Tolerance to some of the behavioral effects of ethanol, such as hypothermia, ataxia, sedation, hypnosis, and related neurobiological mechanisms has been studied (Chandler et al., 1998; Crabbe et al., 1979; Gill and Deitrich, 1998; Kalant 1998; Khanna et al., 1991; Kurtz et al., 1996). However, few studies have been conducted in relation to rapid tolerance to the anxiolytic effects of ethanol in animal models (Debatin and Barbosa, 2006; Koob et al., 1987). Alcohol produces anxiolytic effects in humans and rapid tolerance to these anxiolytic effects may be involved in promoting alcohol drinking in alcoholics (Cloninger et al., 1988; Cooper et al., 1995; Debatin and Barbosa, 2006; Lipscomb et al., 1980; Moberg and Curtin, 2009). Thus, identifying the molecular mechanisms underlying rapid tolerance to the anxiolytic effects of ethanol is important in order to gain a better understanding of the processes of alcohol addiction.
Several studies indicate that epigenetic mechanisms, such as covalent histone modifications via acetylation, phosphorylation and methylation, and DNA methylation, appear to be involved in the pathophysiology of brain disorders including addictive behaviors (Feng and Fan, 2009; Grayson et al., 2010; Kazantsev and Thompson, 2008; Renthal and Nestler, 2008; Tsankova et al., 2007). Histone acetyltransferases and histone deacetylases (HDAC) can respectively add or remove acetyl groups from histones, thereby eliciting changes in the chromatin structure leading to altered gene expression (Kalkhoven 2004; Korzus et al., 2004; Smith 1991; Strahl and Allis, 2000). Amygdaloid structures, particularly the central nucleus of amygdala (CeA) and medial nucleus of amygdala (MeA), serve as major neuroanatomical substrates for anxiety and alcohol-drinking behaviors (Davis 1997; Koob 2003; McBride 2002; Pandey et al., 2006). The neuropeptide Y (NPY) system in these amygdaloid brain regions has been shown to be involved in anxiety and alcohol-drinking behaviors in various animal models (Gilpin et al., 2008; Heilig 1995; Heilig et al., 1989; Pandey 2004; Pandey et al., 2005, 2008; Primeaux et al., 2006; Thorsell et al., 2007; Zhang et al., 2010).
We have recently shown that attenuation of amygdaloid HDAC activity by trichostatin A (TSA), a potent HDAC inhibitor, was able to correct deficits in histone acetylation (H3-K9 & H4-K8) and NPY expression in the CeA and MeA, while simultaneously preventing anxiety-like behaviors in rats under going withdrawal after chronic ethanol exposure (Pandey et al., 2008). However, the role of amygdaloid chromatin remodeling in the development of rapid tolerance to the anxiolytic effects of ethanol is currently unknown. Therefore, we investigated the role of amygdaloid HDAC-induced histone modifications and related changes in NPY expression in the development of RET.
MATERIALS AND METHODS
Animals and pharmacological manipulations
Adult male Sprague Dawley rats (290–325 g) were used in the present study. All procedures were conducted in accordance with the NIH guidelines for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee. Ethanol was diluted to 200 mg/ml in n-saline, and was injected as 5 or 10 μl/g of body weight to achieve a dose of 1 or 2 g/kg of body weight, respectively. Trichostatin A (TSA; Sigma, St. Louis) was dissolved in DMSO to a 5 mg/ml concentration and was further diluted in phosphate buffered saline (PBS; 1:5), as described by us (Pandey et al., 2008) and other investigators (Korzus et al., 2004; Kumar et al., 2005). A TSA dose of 2 mg/kg was used and DMSO diluted in PBS (1:5) was used as vehicle.
Rapid ethanol tolerance and dose-dependent effects
For the development of RET, we employed a consecutive acute ethanol exposure paradigm, as reported earlier (Debatin and Barbosa, 2006). On the first day, rats were intraperitoneally (I.P.) injected with either n-saline (5 μl/g) or ethanol (1 g/kg) and were not subjected to behavioral measurements. On the following day (24 hrs after the first injection), n-saline treated rats were injected with either n-saline (Control group; n=7) or ethanol (1 g/kg) (Ethanol group; n=7), whereas ethanol-treated rats were injected with either 1 g/kg [Tolerant (1g) group; n=7] or 2 g/kg [Tolerant (2 g) group; n=7] doses of ethanol. One hour post-injection, rats were subjected to measurements of anxiety-like behaviors using the light/dark box (LDB) exploration test, as described below. Immediately after the behavioral measurements, animals were anesthetized with pentobarbital (50 mg/kg) and decapitated. The brains were taken out to dissect the amygdaloid tissues, which were quickly frozen and stored at −80 °C until used to measure HDAC activity.
Effect of TSA on rapid tolerance to the anxiolytic effects of ethanol
On the first day, rats were I.P. injected with either n-saline (5 μl/g) or ethanol (1 g/kg) and were not subjected to behavioral measurements. On the following day, n-saline injected rats (24 hrs after the first injection) were divided into: 1) control+ vehicle group [received I.P. injection with vehicle followed by n-saline injection after 1 hr; n=19], 2) control+ TSA group [received I.P. injection with TSA (2 mg/kg) followed by n-saline injection after 1 hr; n=16], and 3) ethanol+ vehicle group [received I.P. injection with vehicle followed by ethanol injection (1 g/kg) after 1 hr; n=19]. The next two groups were derived from the ethanol-injected rats (24 hrs after the first injection) and divided into: 4) tolerant+ vehicle group [received I.P. injection with vehicle followed by ethanol injection (1 g/kg) after 1 hr; n=19] and 5) tolerant+ TSA group [received I.P. injection with TSA (2 mg/kg) followed by ethanol injection (1 g/kg) after 1 hr; n=16]. In another batch of rats, we examined the effects of ethanol in TSA pre-treated rats. For this experiment, rats were treated on the first day with n-saline (5 μl/g) and on the second day after 24 hr, one group of rats was injected with vehicle followed by n-saline after 1 hr (Control + vehicle group; n=6), a second group of rats was injected with vehicle followed by ethanol (1g/kg) after 1 hr (Ethanol + vehicle group; n=6), and a third group of rats was first injected with TSA followed by ethanol (1g/kg) after 1 hr (Ethanol + TSA group; n=6). In all experiments, on day 2, one hour after the n-saline or ethanol injections and 2 hrs after the vehicle or TSA treatments, anxiety-like behaviors of the rats were measured using either the LDB or elevated plus maze (EPM) exploration tests, as described below.
Rats were anesthetized with pentobarbital (50 mg/kg) immediately after the behavioral measurement. Amygdaloid tissues of some rats, primarily containing CeA and MeA, were dissected out, quickly frozen and then stored at − 80 °C until used to measure HDAC activity. Some rats were perfused with n-saline followed by ice-cold 4% paraformaldehyde (PFA, pH 7.4) prepared in 0.1 M phosphate buffer (pH 7.4). After perfusion, brains were dissected out, postfixed overnight in 4% PFA, followed by a sucrose gradient (10, 20, and 30%) prepared in 0.1M phosphate buffer. Brains were frozen and stored at −80 °C until used for gold immunolabeling and in situ reverse transcription (RT)-PCR. Just prior to brain collection, blood was obtained from all rats injected with ethanol for measurement of blood ethanol levels using the Analox Alcohol Analyzer (Lunenburg, MA).
Elevated plus maze exploration test
Anxiety-like behavior was measured with EPM, as previously described by us and other investigators (File 1993; Pandey et al., 2006, 2008). Briefly, each rat was placed on the central platform of the plus maze and the number of entries and the time spent in each arm (open or closed) during the 5-min test period was recorded. The results are represented as percent open arm entries and the time spent in the open arm. The total number of closed arm entries was used to represent general activity of rats (File 1993; Pandey et al., 2006).
Light dark box exploration test
The LDB exploration test for anxiety-like measurement was performed, as described previously by us (Pandey et al., 2008; Zhang et al., 2010). The light and dark compartments of the LDB were connected to infrared beam and rat activity in each compartment was monitored by the computer for the 5-min test session. The percent time spent in either the light or dark compartment by each rat was calculated. Total ambulations in the light and dark compartments were recorded as the general activity of the rat.
HDAC activity in the amygdala
HDAC activity in amygdaloid tissues was measured, as described by us previously (Pandey et al., 2008). In brief, total cell lysates (cytosolic plus nuclear fractions) were prepared using a nuclear extraction kit (Sigma, St. Louis, MO). HDAC activity (class I and II HDACs) was assayed using the colorimetric HDAC activity assay kit (BioVision Research, Mountain View, CA). Optical density (O.D.) was measured using an ELISA plate reader at 405 nm (Spectra MR; DynexTechnologies, Chantilly, VA). The results are represented as the mean percent of controls.
Gold immunolabeling for acetylated histones H3 and H4, and NPY
Coronal sections of brains (20 μm) were used for the gold immunolabeling histochemical procedure, as described previously by us (Pandey et al., 2008; Zhang et al., 2010). In brief, sections were washed and incubated in RPMI 1640 medium containing L-Glutamine (Life Technologies, Grand Island, NY) for 30 min. Sections were incubated with 10% normal goat serum (NGS) diluted in PBS containing 0.25% Triton X-100 (PBST) for 30 min at room temperature. After blocking the sections with 1% BSA in PBST, they were incubated with antibodies against acetylated histone H3-Lys 9 (1:500), acetylated histone H4-Lys8 (1:500) (Millipore, Billerica, MA) or NPY (1:500; Immunostar, Hudson, WI) diluted in 1% BSA prepared in PBST for 18 h at room temperature. Sections were then washed with PBS followed with 1% BSA in PBS, followed by incubation with gold particle (1.4 nm)-conjugated anti-rabbit secondary antibody (1:200 dilution in 1% BSA in PBS; Nanoprobes, Inc., Yaphank, NY) for 1 hr at room temperature. After washing the sections with 1% BSA in PBS, they were washed with distilled water. Gold particles were then silver enhanced (Ted Pella Inc., Redding, CA) for 12 to 20 min and washed several times with tap water. The sections were then mounted on slides and examined under a light microscope. Quantification of the immunolabeled gold particles was performed by the computerized Image Analyzer (Loats Associates, Westminster, MD), as described previously by us (Pandey et al., 2006, 2008; Zhang et al., 2010). The results are represented as the number of immunogold particles per 100 μm2 area.
In situ reverse transcription (RT)-PCR for NPY mRNA measurement
In situ RT-PCR of NPY mRNA was performed in 40 μm thick coronal brain sections, as described by us previously (Pandey et al., 2008; Zhang et al., 2010). Briefly, brain sections (40 μm) were treated with proteinase K and then digested with DNase. Sections after washing with PBS, were transferred to PCR tubes containing 100 μl of PCR reaction mixture (Applied Biosystems, Foster City, CA) to reverse transcribe for 1 h at 42 °C in the presence of oligo d(T)16 using reverse transcriptase (RT) enzyme. Negative control sections were also reverse transcribed, however, without RT enzyme. PCR was performed (94°C for 3 min; 94°C for 45 sec; 60°C for 45 sec; 72°C for 45 sec; for a total of 25 cycles and then 72°C for 7 min) using Taq DNA polymerase enzyme and 100 pmol of each NPY primer (Primers 5′-TAGGTAACAAACGAATGGGG-3′ and 5′-AGGATGAGATGAGATGTGGG-3′) and 1 mM of each NTP (except that dTTP was replaced by digoxigenin (DIG)-11-dUTP) in 100 μl reaction mixture. Once the PCR amplification process was complete, sections were carefully mounted on slides and NPY mRNA-positive cells were detected by an alkaline phosphatase conjugated anti-DIG antibody employing NBT/BCIP as the specific substrate (Roche Molecular Biochemical, Mannheim, Germany). The optical density (O.D.) of NPY-positive cell bodies from three fields in the amygdaloid structures of each of the three brain sections from each rat was calculated and values were then averaged. The results are represented as mean O.D./100 pixels of area.
Confocal microscopy for the localization of acetylated histones H3 and H4, and NPY in neurons (NeuN) in amygdala
We used confocal microscopy to examine the cell-type specific [neuron-specific nuclear protein (NeuN)] localization of acetylated histones H3 and H4, and NPY, according to the procedure previously reported by us (Zhang et al., 2010). Coronal brain sections (20 μm) were incubated with antibodies against acetylated histones (H3-K9 or H4-K8) or NPY and co-incubated with NeuN (Millipore, Billerca) antibody. Negative control sections were incubated in 2% normal goat serum diluted in PBST. Sections were then incubated with AlexaFluor-488 dye-conjugated goat anti-rabbit or AlexaFluor-568 dye-conjugated goat anti-mouse secondary antibodies (Molecular Probes Inc., Eugene, OR) to co-localize the acetylated histones H3 or H4, or NPY with NeuN, respectively. Sections were mounted on microscope slides and viewed using a confocal microscope (LSM 510, Zeiss, Thornwood).
Statistical analysis
The differences between the various groups were tested for significance using a one way ANOVA test. Tukey’s test was applied for Post hoc multiple comparisons. The p values less than 0.05 were considered to be significant.
RESULTS
Rapid tolerance to anxiolytic effects and HDAC activity: ethanol dose-response
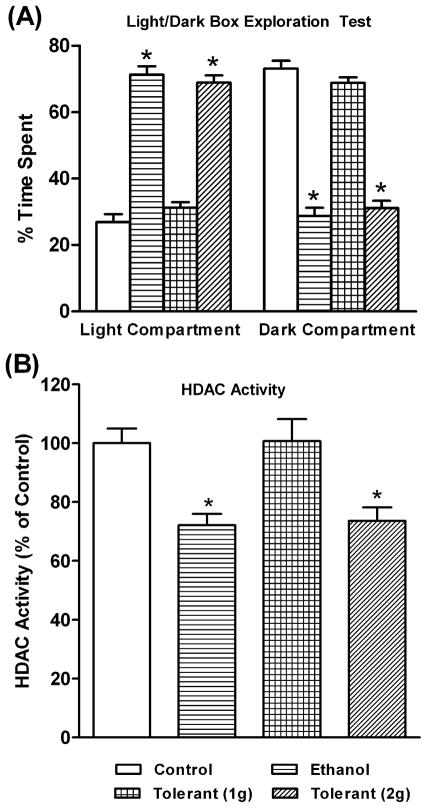
We examined the development of rapid tolerance to the anxiolytic effects of ethanol (Figure 1A) and ethanol-induced changes in HDAC activity in the amygdala (Figure 1B) of rats. We found that one dose of ethanol (1 g/kg; Ethanol group) produced anxiolytic effects, as the rats spent significantly (p<0.001) less time in the dark compartment and more time in the light compartment of the LDB (Figure 1A). Interestingly, two doses of 1 g/kg ethanol (24 hrs apart) elicited no anxiolytic effects in rats [Tolerant (1g) group]. However, a 1 g/kg injection of ethanol on the first day, followed by a 2 g/kg ethanol injection on the second day [Tolerant (2g) group] significantly (p<0.001) resulted in anxiolytic effects, as observed during LDB exploration test. The blood ethanol levels (mg %) of the animals in various groups (mean± SEM; n=7) were 85.13±2.15 (Ethanol), 85.36±2.95 [Tolerant (1g)], and 169.24±4.55 [Tolerant (2g)].
Figure 1.
The effects of acute ethanol exposure [Ethanol group (1 g/kg; I.P.)] and tolerance (1 g/kg or 2 g/kg) [Tolerant (1g) group or Tolerant (2g) group], on the light/dark box (LDB) exploration test of anxiety-like behavior (A) and HDAC activity in the amygdala of rats (B). Values are the mean ± SEM of 7 rats in each group. *Significantly different from their respective control groups [p<0.05-0.001; ANOVA (F 3, 24 =112, p<0.001 for LDB; F3, 24=8.6, p<0.001 for HDAC activity) followed by Tukey’s test].
We also found that two exposures of ethanol (1 g/kg on the first day followed by 1 g/kg on the second day) did not significantly modify HDAC activity in the amygdala, whereas HDAC activity was significantly (p<0.01) inhibited by a single ethanol exposure (Figure 1B). In addition, tolerance to ethanol-induced HDAC inhibition in the amygdala was reversed (p<0.05) by treatment with high doses of acute ethanol (1 g/kg ethanol on the first day followed by 2 g/kg ethanol on the second day). These results suggest that rapid tolerance to the anxiolytic effects of acute ethanol may be related to the development of cellular tolerance at the level of HDAC activity in the amygdala of rats. The second high dose (2 g/kg) but not low dose (1 g/kg) of ethanol exposure, elicited anxiolytic effects and inhibited HDAC activity, as observed after the first lower dose (1 g/kg) of ethanol exposure, suggesting that higher concentrations of ethanol are needed to achieve initial responses of ethanol exposure.
Rapid tolerance to anxiolytic effects of acute ethanol and HDAC activity: effects of TSA
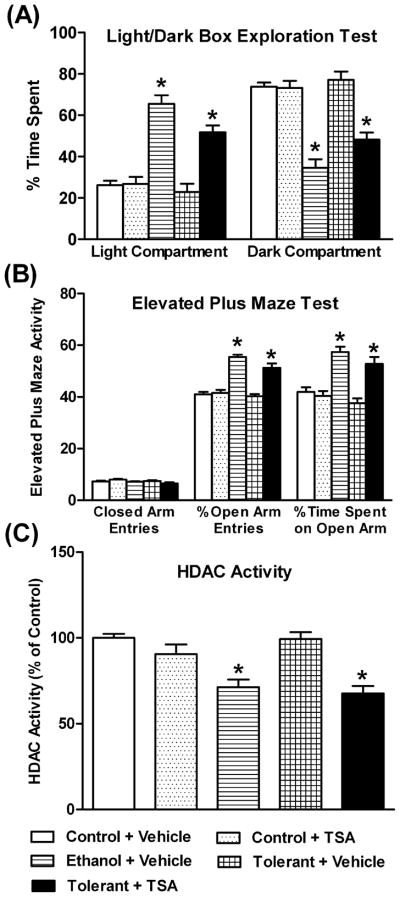
We examined the effects of TSA on rapid tolerance to the anxiolytic effects of ethanol and on HDAC activity in the amygdala of rats. We found that a single injection of ethanol produced anxiolytic effects as measured by the LDB (Figure 2A) and EPM (Figure 2B) tests, while two injections of ethanol (24 hrs apart) were unable to elicit anxiolytic effects. Results of the LDB test demonstrated that ethanol-treated rats (Ethanol+ Vehicle) spent significantly (p<0.001) more time in the light compartment and less time in the dark compartment, compared to control rats. No significant differences were found in the time spent by ethanol-tolerant rats (Tolerant+ Vehicle) in either compartment, as compared with control rats. However, TSA treated ethanol-tolerant rats spent significantly more time in the light compartment and significantly less time in the dark compartment, compared to vehicle treated tolerant (p<0.001) or control (p<0.001) rats. TSA treatment did not significantly affect the time spent in either the light or dark compartments by the control rats (Figure 2A). The blood ethanol levels (mg %) of the animals in various groups (mean± SEM; n=6–9) were 100.08±5.38 (Ethanol+ Vehicle), 97.87±3.06 (Tolerant+ Vehicle), and 99.88±4.14 (Tolerant+ TSA).
Figure 2.
The effects of acute ethanol exposure [Ethanol group (1 g/kg; I.P.)] and tolerance (1 g/kg) with (Tolerant+ TSA) or without TSA (Tolerant+ Vehicle) treatment on anxiety-like behavior in rats using the light/dark box exploration (LDB) test (A) and elevated plus maze (EPM) test (B), and HDAC activity in the amygdala (C). Values are the mean ± SEM of 6–9 rats for the LDB exploration test and HDAC activity measurement, whereas 10 rats per group for the EPM test. *Significantly different from their respective control groups [p<0.01-0.001; ANOVA (F4, 34=29.9, p<0.001 for LDB; F4, 45=16.8, p<0.001 for EPM % open arm time spent; F4, 45=36.5, p<0.001 for EPM % open arm entries; F4, 34=13.9, p<0.001 for HDAC activity) followed by Tukey’s test].
We also examined anxiety-like behaviors using the EPM test (Figure 2B). Ethanol-treated rats (Ethanol+ Vehicle) spent more time on the open arms (p<0.001) and had more open arm entries (p<0.001) compared to control rats (Control+ Vehicle). However, two doses (24 hrs apart) of ethanol (Tolerant+ Vehicle) did not produce anxiolytic effects, since we did not observe any significant differences in the time spent on the open arms or in the percent open arm entries of tolerant rats compared to the control rats. On the other hand, TSA-treated ethanol-tolerant rats (Tolerant+ TSA) showed significantly higher open arm entries and the time spent on the open arms compared to vehicle-treated tolerant rats (p<0.001) or control rats (p<0.01). TSA treatment did not significantly affect the entries or time spent on open and closed arms of the EPM by control rats (Figure 2B). The blood ethanol levels (mg %) of the animals in various groups (mean± SEM; n=10) were 101.33±5.27 (Ethanol+ Vehicle), 103.33±6.75 (Tolerant+ Vehicle), and 101.09±5.43 (Tolerant+ TSA). The general activity of rats, as measured by either the number of closed arm entries in EPM (Figure 2B) or the total ambulations in the LDB exploration test (data not shown), was not significantly different for various groups compared to the control group.
HDAC activity in the amygdala of control, ethanol, and ethanol-tolerant rats treated with either vehicle or TSA was also determined (Figure 2C). HDAC activity was significantly (p<0.001) inhibited after a single acute ethanol exposure (Ethanol+ Vehicle) compared to control rats (Control+ Vehicle). However, this ethanol-induced inhibition of HDAC activity was not seen after two exposures of ethanol, i.e. after the onset of tolerance (Tolerant+ Vehicle). Surprisingly, the expression of tolerance at the level of HDAC activity by two consecutive doses of ethanol was prevented by TSA pretreatment (Tolerant+ TSA; 1 hr before the second ethanol injection). HDAC activity was significantly (p<0.001) inhibited in the amygdala of the tolerant rats treated with TSA compared to control rats. Interestingly, HDAC activity in the amygdala was not significantly modulated by TSA treatment in control rats (Figure 2C). These results suggest that rapid tolerance to the anxiolytic effects of ethanol may be due to development of tolerance to acute ethanol-induced inhibition of HDAC activity in the amygdala of rats.
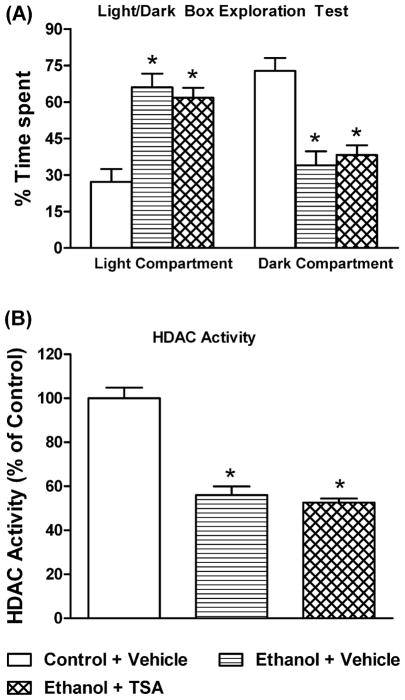
We also examined the effects of TSA pretreatment on acute ethanol (single injection)-induced anxiolytic effects and inhibition of HDAC activity in the amygdala of rats (Figure 3A, B). Blood ethanol levels (mg %) in ethanol treated rats, with or without TSA pretreatment (mean± SEM; n=6), were 79.8±2.9 (Ethanol+ Vehicle) and 87.2±2.6 (Ethanol+ TSA), respectively. It was found that ethanol significantly (p<0.001) inhibited HDAC activity in the amygdala and produced anxiolytic effects, as demonstrated by significant increases in time spent in the light compartment of the LDB. These effects of ethanol were not changed by pretreatment with TSA (Figure 3A, B). These results suggest that TSA pretreatment did not modulate the anxiolytic effects of ethanol or ethanol-induced inhibition of HDAC activity in the amygdala of rats.
Figure 3.
The effects of TSA pretreatment on acute ethanol exposure (1g/kg; I.P.)-mediated anxiolytic effects in rats using the light/dark box exploration (LDB) test (A) and inhibition of HDAC activity in the amygdala (B). Values are the mean ± SEM of 6 rats. *Significantly different from control group [p<0.001; ANOVA (F 2, 15 =18.0, p<0.001 for LDB; F2, 15=49.9, p<0.001 for HDAC activity) followed by Tukey’s test].
Effects of TSA on rapid ethanol tolerance-associated changes in histone acetylation in the amygdala
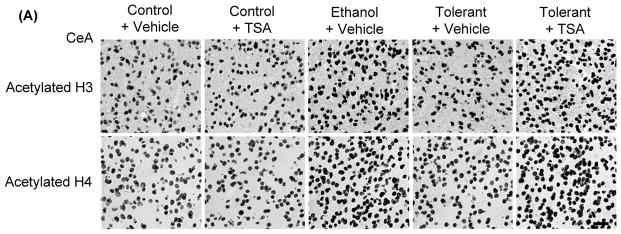
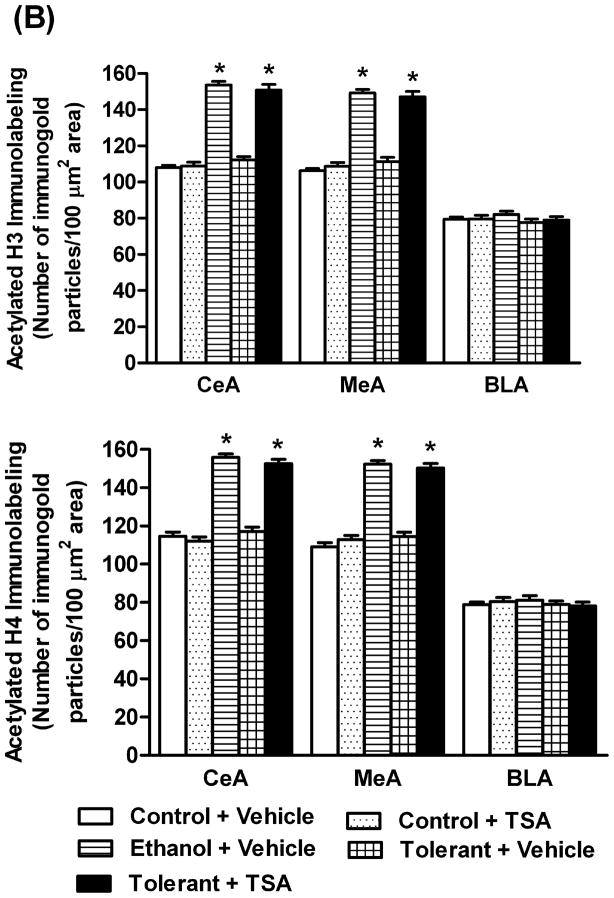
In order to examine if tolerance-induced changes in HDAC activity may be associated with changes in histone acetylation, we employed gold immunolabeling to measure the protein levels of acetylated histones H3 (K9) and H4 (K8) in the amygdala of rats. The levels of acetylated histones significantly (p<0.001) increased in the CeA and MeA, but not in BLA (Figures 4A, B) of a single ethanol injected rats (Ethanol+ Vehicle) compared to control (Control+ Vehicle) rats. However, this acute ethanol-induced increase in histone acetylation was not observed in rats after two exposures (24 hrs apart) of the same dose of ethanol (Tolerant+ Vehicle). TSA treatment also caused the reversal of tolerance at the level of histone acetylation and significantly (p<0.001) increased protein levels of acetylated histones (H3-K9 & H4-K8) in the CeA and MeA of ethanol-tolerant (Tolerant+ TSA) rats compared to vehicle treated control and ethanol-tolerant rats. TSA treatment did not significantly affect histone acetylation in any of the amygdaloid structures of control rats (Figures 4A, B).
Figure 4.
A) Representative low-magnification photomicrographs of acetylated histones H3 (Lys 9) and H4 (Lys 8) gold-immunolabeling in central amygdaloid (CeA) structures of control rats with (Control+ TSA) or without TSA (Control+ Vehicle) treatments, ethanol-treated rats (Ethanol+ Vehicle) or ethanol-tolerant rats with (Tolerant+ TSA) or without TSA (Tolerant+ Vehicle) treatments (Scale bar = 40 μm). B) Changes in the acetylation of histones H3 and H4 in various amygdaloid (CeA, MeA, and BLA) structures of control rats with (Control+ TSA) or without (Control+ Vehicle) TSA treatments, ethanol-treated rats (Ethanol+ Vehicle) and ethanol-tolerant rats with (Tolerant+ TSA) or without (Tolerant+ Vehicle) TSA treatments. Values are the mean ± SEM of 5–6 rats per group. *Significantly different from their respective control groups [p<0.001; ANOVA (acetylated histone H3: CeA, F4, 25=117.7, p<0.001; MeA, F4, 25=95, p<0.001; Acetylated histone H4: CeA, F4, 20=104.8, p<0.001; MeA, F4, 20=98.8, p<0.001) followed by Tukey’s test)].
Effects of TSA on rapid ethanol tolerance-associated changes in NPY expression in the amygdala
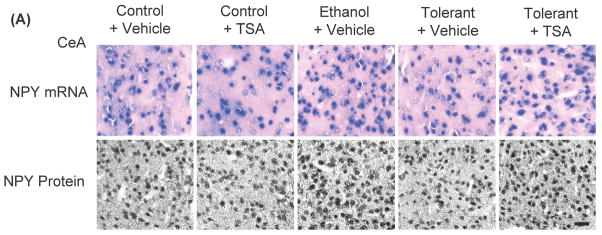
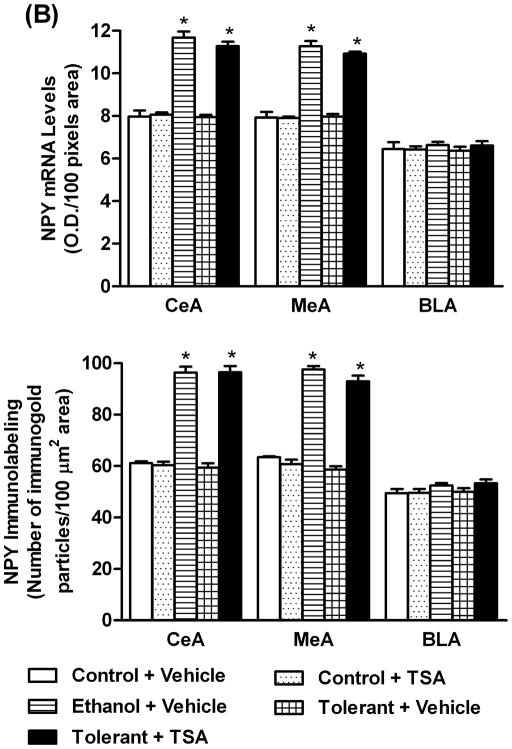
To examine the role of NPY in RET, changes in the levels of NPY in the amygdala during ethanol tolerance were also investigated (Figures 5A, B). It was found that a single ethanol exposure (Ethanol+ Vehicle) was able to significantly (p<0.001) increase NPY mRNA and protein levels in the CeA and MeA, but not in the BLA of rats. However, two ethanol exposures (Tolerant+ Vehicle) had no effect on NPY expression in any amygdaloid structures of rats compared to control rats (Control+ Vehicle). In addition, it was found that NPY protein and mRNA levels in the CeA and MeA of ethanol-tolerant rats treated with TSA (Tolerant+ TSA) were significantly (p<0.001) higher compared to vehicle treated control and ethanol-tolerant rats. These results suggest that RET at the level of NPY expression in the amygdala can be reversed by TSA treatment.
Figure 5.
A) Representative low-magnification photomicrographs of NPY in situ RT-PCR (mRNA levels) and NPY gold-immunolabeling (protein levels) in central amygdaloid (CeA) structures of control rats with (Control+ TSA) or without TSA (Control+ Vehicle) treatments, ethanol-treated (Ethanol+ Vehicle) or ethanol-tolerant rats with (Tolerant+ TSA) or without TSA (Tolerant+ Vehicle) treatments (Scale bar= 40 μm). B) Changes in mRNA and protein levels of NPY in CeA, MeA, and BLA of control rats with (Control+ TSA) or without TSA (Control+ Vehicle) treatments, ethanol-treated (Ethanol+ Vehicle) or ethanol-tolerant rats with (Tolerant+ TSA) or without TSA (Tolerant+ Vehicle) treatments. Values are the mean ± SEM of 5 rats in each group. *Significantly different from their respective control groups (p<0.001; ANOVA (NPY protein: CeA, F4, 20=122.1, p<0.001; MeA, F4, 20=159.8, p<0.001; NPY mRNA: CeA, F 4, 20=83.1, p<0.001; MeA, F4, 20=89, p<0.001) followed by Tukey’s test)].
Double-immunofluorescence labeling of histones and NPY with NeuN
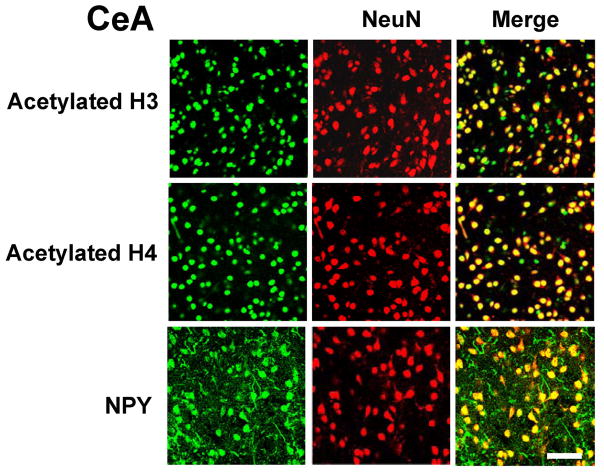
Double-immunofluorescence labeling was performed in order to determine neuronal localization of acetylated histones H3 and H4, and NPY in the amygdaloid structures of rats. We found that acetylated histones (H3 & H4), as well as NPY, were predominantly expressed in neurons, since these proteins were highly localized in NeuN-positive cells in the CeA of rats (Figure 6). Similar observations were also observed in the MeA and BLA of rats (data not shown). These results suggest that changes in the histone acetylation and NPY expression in the CeA and MeA by ethanol and/or TSA treatment (Figures 4, 5) may be neuron-specific, since these proteins were observed to be predominantly localized in neuronal cells (Figure 6).
Figure 6.
Representative photomicrographs showing immunofluorescence staining of acetylated histones H3 or H4, or NPY (green), and NeuN (red) in the cells of the central nucleus of amygdala (CeA). The yellow color represents localization of acetylated histones and NPY in NeuN-positive cells. Acetylated histones H3 and H4, and NPY are predominantly expressed in NeuN-positive cells. Scale bar = 50 μm.
DISCUSSION
The results of the present investigation show that cellular tolerance to the ethanol-induced inhibition of HDAC and increased histone acetylation and NPY expression in the amygdala of rats may be operative in the development of rapid tolerance to the anxiolytic effects of ethanol (Figure 7). Several clinical and preclinical studies indicate a relationship between anxiety and alcoholism, suggesting that the anxiolytic properties of ethanol may be crucial in promoting drinking in an attempt to self-medicate (Carrigan and Randall, 2003; Cloninger et al., 1988; Cooper et al., 1995; Conway et al., 2006; Debatin and Barbosa, 2006; Lipscomb et al., 1980; Merikangas et al., 1998; Pandey et al., 2005; Primeaux et al., 2006; Schuckit and Hesselbrock, 1994; Zhang et al., 2010). The phenomenon of rapid tolerance to the anxiolytic effects of ethanol has been shown in both humans and animal models (Cloninger et al., 1988; Debatin and Barbosa, 2006; Koob et al., 1987; Libscomb et al., 1980). Here, we established a novel role for HDAC-induced histone modifications in the amygdala in the molecular mechanisms of RET. Tolerance to both the inhibitory properties of HDACs and to the anxiolytic effects of ethanol were observed after two consecutive days of ethanol (1 g/kg) exposure. However, treatment with a higher dose (2 g/kg) of ethanol on the second day produced anxiolytic effects and inhibited HDAC activity in the amygdala. This finding supports the notion that rapid tolerance to the anxiolytic effects of ethanol may promote drinking in order to achieve a response similar to that obtained from an initial ethanol exposure. Using Drosophila melanogaster as a model, it was found that covalent histone modifications and induction of BK-type Ca2+-activated K+ channel gene may be linked to behavioral tolerance to the sedative effects of benzyl alcohol (Wang et al., 2007). Thus, covalent histone modifications represent an attractive mechanism that may regulate gene expression during the process of alcohol tolerance.
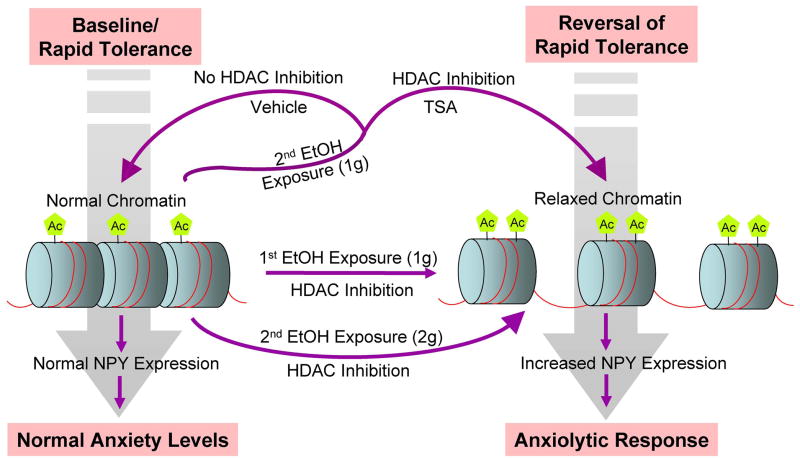
Figure 7.
A schematic model depicting the possible molecular mechanisms of rapid tolerance to the anxiolytic effects of ethanol, within the amygdaloid neurocircuitries (particularly central and medial amygdaloid structures). First acute ethanol exposure (1 g/kg) inhibits histone deacetylases (HDAC), thereby increasing histone acetylation (Ac). Increased histone acetylation opens the chromatin structure (relaxed chromatin), making it more accessible to transcriptional machinery, thereby increasing neuropeptide Y (NPY) gene expression, and ultimately eliciting an anxiolytic response. The second ethanol exposure using the same dose (1 g/kg) as the first ethanol exposure does not inhibit HDAC activity or increase histone acetylation, maintaining normal chromatin structure and no change in NPY expression or anxiety levels, i.e. development of rapid tolerance. However, treatment with the HDAC inhibitor, trichostatin A (TSA), prior to the second ethanol exposure (1 g/kg), prevents the expression of rapid ethanol tolerance by inhibiting HDAC and increasing both histone acetylation and NPY levels. On the other hand, a second ethanol exposure using a higher dose of ethanol (2 g/kg) elicited a similar response (inhibition of HDACs and anxiolytic effects) observed after the first ethanol exposure using a lower dose (1 g/kg), suggesting that higher concentrations of ethanol are needed during a second exposure to ethanol in order to overcome the onset of tolerance and experience the anxiolytic effects of ethanol. This may lead to development of alcohol addiction.
Eleven different zinc-dependent HDAC isoforms have been identified and categorized as either class I (HDAC 1–3, 8), class II (HDAC 4–7, 9, 10), or class IV (HDAC 11) HDACs. Class III HDACs are known as sirtuins and require NAD+ co-factor for activation (Abel and Zukin, 2008; Chuang et al., 2009; Lee et al., 2008; Xu et al., 2007). It has been shown that repeated ethanol exposure has no effect on histone (H3 & H4) acetylation in several brain regions, such as the frontal cortex or nucleus accumbens (NAc) of rats (Pascual et al., 2009). Our previous studies (Pandey et al., 2008) showed that chronic ethanol exposure had no significant effect on HDAC activity, histone acetylation, or NPY expression in the amygdala. Chronic ethanol exposure also did not elicit anxiety-like behaviors in rats, whereas these behaviors appeared during withdrawal. HDAC activity significantly was increased in the amygdala during withdrawal after chronic ethanol exposure, which correlated with decreased acetylation of histones (H3 & H4) and decreased expression of NPY in the CeA and MeA, but not in BLA of rats. Treatment with a pan-HDAC inhibitor, TSA, attenuated the development of anxiety-like behaviors during ethanol withdrawal and corrected ethanol withdrawal-induced deficits in both histone acetylation and NPY expression in amygdaloid structures of rats (Pandey et al., 2008). Acute ethanol treatment also inhibited HDAC activity in the amygdala and produced anxiolytic effects in rats (Pandey et al., 2008). We extended these studies and found that HDAC-induced histone modifications may be involved in the process of RET (Figure 7). Furthermore, the lack of changes in HDAC activity, histone acetylation, and NPY expression in the amygdala during chronic ethanol exposure (Pandey et al., 2008) and RET suggest that similar epigenetic mechanisms may exist in the process of CET and RET. It is important to mention that the majority of acetylated histones H3 and H4, as well as NPY proteins, were co-localized with NeuN-positive neurons in amygdaloid structures. Therefore, it is possible that ethanol-induced chromatin remodeling may orchestrate different patterns of gene expression profiling within neuronal networks in the amygdala that may contribute to the development of RET.
HDACs have been emerging as potential therapeutic targets in the treatment of neurodegenerative diseases and psychiatric disorders, including addictive behaviors (Abel and Zukin, 2008; Kazantsev and Thompson, 2008; Kumar et al., 2005; Renthal and Nestler, 2008; Romieu et al., 2008; Tsakova et al., 2007). Recently, studies involving manipulation of specific HDAC isoforms suggest the involvement of HDAC 2, HDAC 4, and HDAC 5 in the pathogenesis of several brain disorders. For example, the hippocampal HDAC 5 isoform and HDAC 2 isoform in the NAc were both shown to play roles in depressive behaviors and in the mechanism of action of antidepressant drugs (Covington et al., 2009; Tsankova et al., 2006). In addition, the HDAC 2 isoform was found to negatively regulate synaptic plasticity related to memory formation (Guan et al., 2009), while the HDAC 4 isoform may be involved in neuronal cell death and cocaine addiction (Bolger and Yao, 2005; Kumar et al., 2005). These results clearly display distinct roles for specific HDAC isoforms in various psychiatric diseases. Future studies are needed to investigate the specific roles of HDAC isoforms in chromatin remodeling during alcoholism. Nonetheless, perturbing HDACs (class I & II) by pharmacological means has revealed a critical role for HDAC-induced histone modifications in the amygdala during the development of RET. It is important to point out that TSA treatment had no effect on HDAC activity, histone acetylation, or NPY expression in control rats, similar to findings from our previous study (Pandey et al., 2008). However, when HDACs were perturbed, as in ethanol tolerant and dependent rats, TSA was able to reverse RET and block the development of anxiety-like behaviors during withdrawal after chronic ethanol exposure in rats (Pandey et al., 2008). These findings are also supported by the observations that TSA pretreatment did not modulate the acute ethanol-induced anxiolytic effects or inhibition of HDAC activity in the amygdala of rats. Similarly, other investigators (Kumar et al., 2005) have shown that HDAC inhibitors alone have no effects on striatal histone phospho-acetylation or histone acetylation-mediated changes in c-fos expression in control rats, but significantly modulated the cocaine-mediated responses on these measures.
RET may be mediated by histone modification-induced gene expression in the amygdala or other brain regions. One such mechanism could involve changes in NPY expression in the CeA and MeA. NPY is an endogenous anxiolytic compound and higher levels of NPY in the amygdala, particularly in the CeA, have been shown to be anxiolytic, whereas lower levels of NPY have been shown to be anxiogenic in various animal models (Gilpin et al., 2008; Heilig 1995; Heilig and Widerlov, 1995; Heilig et al., 1989; Pandey 2004; Pandey et al., 2005, 2008; Primeaux et al., 2006; Thorsell et al., 2007; Zhang et al., 2010). We found that acute ethanol increased NPY expression in the CeA and MeA, whereas NPY levels were not altered in ethanol-tolerant rats. However, TSA treatment was able to increase NPY expression in the CeA and MeA of ethanol-tolerant, but not of control rats. These results suggest that rapid induction of NPY by acute ethanol and an observed lack of response in NPY expression during ethanol tolerance may be regulated via HDAC-induced epigenetic changes in the amygdala. Several other mechanisms related to neurotransmitters such as glutamate, gamma aminobutyric acid, and arginine-vasopressin, and cyclic-AMP signaling systems have been implicated in acute ethanol tolerance (Hoffman and Tabakoff, 1989; Kalant 1998; Kumar et al., 2009; Yang et al., 2003). A recent genomic study showed that genes involved in synaptic plasticity may also play a role in AFT (Hu et al., 2008). Future studies are needed to examine if HDAC-induced histone modifications in the amygdala during ethanol exposure is responsible for changes in the expression of genes, other than NPY, and their possible roles in RET.
These results for the first time suggest that the treatment with HDAC inhibitors, such as TSA, was able to reverse rapid tolerance to the anxiolytic effects of ethanol via HDAC-induced epigenetic changes in the amygdala (Fig. 7). These findings, along with our previous report on alcohol dependence (Pandey et al., 2008), suggest that HDAC-induced histone modifications in the amygdala may be involved in the process of alcoholism.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA-016690, AA-019971, AA-010005, and AA-013341 and by the Department of Veterans Affairs (Merit Review Grant; Research Career Scientist award) to S.C.P.
References
- Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington DC, USA: 1994. [Google Scholar]
- Bolger TA, Yao TP. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J Neurosci. 2005;25:9544–9553. doi: 10.1523/JNEUROSCI.1826-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan MH, Randall CL. Self-medication in social phobia: a review of alcohol literature. Addict Behav. 2003;28:269–284. doi: 10.1016/s0306-4603(01)00235-0. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Gilligan SB, von Knorring AL, Reich T, Bohman M. Genetic heterogeneity and the classification of alcoholism. Adv Alcohol Subst Abuse. 1988;7:3–16. doi: 10.1300/J251v07n03_02. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Rigter H, Uijlen J, Strijbos C. Rapid development of tolerance to the hypothermic effect of ethanol in mice. J Pharmacol Exp Ther. 1979;208:128–133. [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Debatin T, Barbosa AD. Effect of isopregnanolone on rapid tolerance to the anxiolytic effect of ethanol. Rev Bras Psiquiatr. 2006;28:18–23. doi: 10.1590/s1516-44462006000100005. [DOI] [PubMed] [Google Scholar]
- Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. Int Rev Neurobiol. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- Gill K, Deitrich RA. Acute tolerance to the ataxic effects of ethanol in short-sleep (SS) and long-sleep (LS) mice. Psychopharmacology (Berl) 1998;136:91–98. doi: 10.1007/s002130050543. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Kundakovic M, Sharma RP. Is there a future for histone deacetylase inhibitors in the pharmacotherapy of psychiatric disorders? Mol Pharmacol. 2010;77:126–135. doi: 10.1124/mol.109.061333. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic-like action of NPY in amygdala and paradoxically increases feeding. Regul Pept. 1995;59:201–205. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- Heilig M, Söderpalm B, Engel JA, Widerlöv E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heilig M, Widerlöv E. Neurobiology and clinical aspects of neuropeptide Y. Crit Rev Neurobiol. 1995;9:115–136. [PubMed] [Google Scholar]
- Hoffman PL, Tabakoff B. Mechanisms of alcohol tolerance. Alcohol Alcohol. 1989;24:251–252. [PubMed] [Google Scholar]
- Hu W, Saba L, Kechris K, Bhave SV, Hoffman PL, Tabakoff B. Genomic insights into acute alcohol tolerance. J Pharmacol Exp Ther. 2008;326:792–800. doi: 10.1124/jpet.108.137521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H. Research on tolerance: what can we learn from history? Alcohol Clin Exp Res. 1998;22:67–76. doi: 10.1111/j.1530-0277.1998.tb03618.x. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E. CBP and p300: HATs for different occasions. Biochem Pharmacol. 2004;68:1145–1155. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Chau A, Shah G. Characterization of the phenomenon of rapid tolerance to ethanol. Alcohol. 1996;13:621–628. doi: 10.1016/s0741-8329(96)00083-3. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Shah G, Weiner J. Rapid tolerance as an index of chronic tolerance. Pharmacol Biochem Behav. 1991;38:427–432. doi: 10.1016/0091-3057(91)90302-i. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Wall TL, Schafer J. Rapid induction of tolerance to the antipunishment effects of ethanol. Alcohol. 1987;4:481–484. doi: 10.1016/0741-8329(87)90090-5. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacol (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz DL, Stewart RB, Zweifel M, Li TK, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53:585–591. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Kim YS, Kummar S, Giaccone G, Trepel JB. Histone deacetylase inhibitors in cancer therapy. Curr Opin Oncol. 2008;20:639–649. doi: 10.1097/CCO.0b013e3283127095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb TR, Nathan PE, Wilson GT, Abrams DB. Effects of tolerance on the anxiety-reducing function of alcohol. Arch Gen Psychiatry. 1980;37:577–582. doi: 10.1001/archpsyc.1980.01780180091011. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- Mellanby E. Alcohol: its absorption into and disappearance from blood under different conditions. Medical Research Committee Special Report. 1919;31:1–48. [Google Scholar]
- Merikangas KR, Stevens DE, Fenton B, Stolar M, O’Malley S, Woods SW, Risch N. Co-morbidity and familial aggregation of alcoholism and anxiety disorders. Psychol Med. 1998;28:773–788. doi: 10.1017/s0033291798006941. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable vs. predictable threat. J Abnorm Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC. The gene transcription factor cyclic AMP-responsive element binding protein: role in positive and negative affective states of alcohol addiction. Pharmacol Ther. 2004;104:47–58. doi: 10.1016/j.pharmthera.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in Rats. J Neurosci. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Hesselbrock V. Alcohol dependence and anxiety disorders: what is the relationship? Am J Psychiatry. 1994;151:1723–1734. doi: 10.1176/ajp.151.12.1723. [DOI] [PubMed] [Google Scholar]
- Smith MM. Histone structure and function. Curr Opin Cell Biol. 1991;3:429–437. doi: 10.1016/0955-0674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Cornell N, Hoffman PL. Alcohol tolerance. Ann Emerg Med. 1986;15:1005–1012. doi: 10.1016/s0196-0644(86)80119-6. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O’Dell LE, Chen SA, King AR, Lekic D, Koob GF, Sanna PP. Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain. 2007;130:1330–1337. doi: 10.1093/brain/awm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Wang Y, Krishnan HR, Ghezzi A, Yin JC, Atkinson NS. Drug-induced epigenetic changes produce drug tolerance. PLoS Biol. 2007;5:2342–2353. doi: 10.1371/journal.pbio.0050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- Yang X, Oswald L, Wand G. The cyclic AMP/protein kinase A signal transduction pathway modulates tolerance to sedative and hypothermic effects of ethanol. Alcohol Clin Exp Res. 2003;27:1220–1225. doi: 10.1097/01.ALC.0000081626.02910.19. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sakharkar AJ, Shi G, Ugale R, Prakash A, Pandey SC. Neuropeptide Y signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol Clin Exp Res. 2010;34:451–461. doi: 10.1111/j.1530-0277.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]