Abstract
Background
Adenoid cystic carcinoma (ACC), a rare and progressive salivary malignancy, is characterized by histogenetic, morphologic and clinical heterogeneity. Extensive efforts to characterize molecular events associated with these tumors include the identification of biomarkers for prognostication and post therapy assessment. Our previous study of genome-wide methylation screening identified a limited number of differentially methylated gene regions in ACC, and significant hypermethylation was found at the transcriptional start site (TSS) of genes that encode for transcription factor EN1. Our clinicopathologic correlation analysis showed that the EN1 methylation status correlated with the histological tumor grade, tumor location and final outcome of the patient.
Methods
To ascertain definitively whether aberrant EN1 expression accompanies human salivary ACC, we used an immunohistochemical technique to directly evaluate EN1 protein expression in ACC of the salivary gland.
Results
Our data show increased EN1 protein expression in solid type ACC, which correlated with a significantly lower survival rate.
Conclusions
we validated EN1 as a potential biomarker in a large cohort of salivary ACC. Immunohistochemical analysis of EN1 in biopsy specimens obtained for diagnostic purposes and/or surgically resected material may show that EN1 is a biological predictor of poor prognosis in salivary ACC patients.
Keywords: adenoid cystic carcinoma, methylation, EN1, cellular localization
Introduction
Adenoid cystic carcinoma (ACC), the second most frequent malignancy of the major and minor salivary glands, comprise of approximately 15-23% of all carcinomas at these locations1, 2. Despite resection with clear margins, up to 60% of patients have locoregional or distant metastases. The median survival duration in the presence of distant metastases is around 3 years, though surprisingly, up to 10% of these patients may survive 10 years or longer with their metastases1, 2. Extensive efforts to characterize molecular events associated with these tumors include the identification of biomarkers for prognostication and post therapy assessment. Relatively few studies of the epigenetic modifications in ACC have been published. Methylation analysis is an attractive line of research because of its therapeutic potential. Our previous study of genome-wide methylation screening identified a limited number of differentially methylated gene regions in ACC (32 hypermethylated, and 7 hypomethylated)3. Several of the affected genes were known to be methylated in cancer; and of known biological functions in tumor formation or promotion3. Other known targets of DNA methylation associated with carcinogenesis were also identified in our study. Significant hypermethylation was found at the transcriptional start site (TSS) of genes that encode for transcription factors, including homeobox genes: EN1, GBX2, MEIS1, LBX2, POU3F3, IRX3, NKX2-4, PITX1, NKX2-5, forkhead box genes: FOXE1, FOXL1, and other transcription factors: TBX4, NR2F2, TFAP2C3. EN1 (engrailed homeobox 1) plays an important role in the development of the central nervous system. Epigenetic suppression of EN1 is common to most colorectal cancers4, prostate cancers5 and in astrocytomas6. Furthermore, in our study differences in EN1 promoter hypermethylation were highest between tumors, with low variation in hypermethylation among the 9 different methylated CpG island amplification microarray (MCAM) probes located in the CpG island of the EN1 promoter; subsequent pyrosequencing validation yielded an average methylation difference of 59%3. Our clinicopathologic correlation analysis showed that the EN1 methylation status correlated with the histological tumor grade, tumor location and final outcome of the patient. We concluded that hypermethylation of EN1 is a potential biomarker for ACC in salivary gland tumors, a finding that could be further explored in clinical studies3.
All previous analyses concerning EN1 expression in cancer have been performed at the DNA level.
To ascertain definitively whether aberrant EN1 expression accompanies human salivary ACC, we used an immunohistochemical technique to directly evaluate EN1 protein expression in ACC of the salivary gland.
Materials and Methods
Archival formalin-fixed paraffin blocks of adenoid cystic carcinomas from 200 patients accessioned at the University of Texas, M.D. Anderson Cancer Center between 1988 and 2006 formed this study (using appropriate written informed consent obtained after approval by the institutional review board). The material for tissue microarray was created using two 1.0 mm diameter cores of spatially different areas of representative tumor from each paraffin block. Pathologic patterns and the phenotypic expression of the EN1 were recorded and were compared with clinical factors including gender, age and stage along with clinical outcomes.
Immunohistochemical and immunoreactivity analyses
Immunohistochemical analysis for EN1 was performed using the BOND MAX IHC staining protocol by Vision Biosystems (Norwell, Massachusetts) on 4-μm paraffin sections of the tissue microarray material. In brief, following dewaxing, washing and rehydration of the slides through xylene and graded alcohols washes, Tris-EDTA buffer was used for antigen retrieval. Slides were subsequently treated with 3% hydrogen peroxide to block endogenous peroxidase. Following incubation with the EN1 primary antibody, (AbCam, 1:25 dilution), the secondary conjugate antibody was applied. Finally, each specimen-containing slide was developed using the chromogen DAB and counterstained with hematoxylin.
EN1 immunohistochemistry was independently evaluated by 2 pathologists. For EN1, only nuclear staining was considered positive. Lesional tissues with strong staining in >50% of the neoplastic cells were scored as strongly positive. Weak staining or strong staining in <50% of the cells was scored as weakly positive. Less than 5% staining was scored as negative.
Statistical Analysis
Descriptive statistics for scaled values and frequencies of study patients within the categories for each of the parameters of interest were enumerated with the assistance of commercial statistical software. Correlations between biomarkers and between biomarkers and endpoints were assessed by Pearson’s Chi-square test or, where there were fewer than 10 subjects in any cell of a 2 × 2 grid, by the two-tailed Fisher’s exact test. Curves describing overall survival were generated by the Kaplan-Meier product limit method. The statistical significance of differences between the actuarial curves was tested by using the log rank test. Follow-up time was the time from first appointment at the University of Texas MD Anderson Cancer Center for the primary tumor of concern until the date of last contact or death. Calculated P values < 0.05 were considered significant. These statistical tests were performed with the assistance of the Statistica (StatSoft, Inc., Tulsa, OK) and SPSS (SPSS for Windows, SPSS Inc., Chicago IL) statistical software applications.
Results
Demographic and pathologic findings
The study cohort comprised of 101 women and 99 men, who ranged in age from 15 to 86 years, with the median age 52 years. Tumors sites included 29 in the parotid gland, 28 in the hard palate, 26 in the maxillary sinus, 20 in the submandibular and sublingual glands and 97 in various minor salivary glands sites. According to the AJCC staging criteria, 5 patients had stage I disease, 22 had stage II, 7 had stage III, and 31 had stage IV. For 122 patients staging was not available and 13 patients had no record of staging.
Histopathologic and Clinical Findings
Histopathologically,tissue cores show at least 2 distinctive patterns within and between cores of the same case. A predominant pattern was determined based on the presence of more than 50% of a given pattern in tumor. Among the 194 tumors for which a predominant type could be ascertained, 57 were tubular (30.0%), 109 (57.4%) had predominantly cribriform patterns (composed of epithelial and myoepithelial neoplastic cells) and 28 (12.6%) had a solid pattern (mostly devoid of myoepithelial cells). Sixty-seven (43%) patients developed distant metastasis, and 89 patients had no evidence of metastasis; 30 patients (20.4%) died in less than 3 years, and 117 patients survived more than 3 years; for 53 patients survival data were no available.
EN1 expression
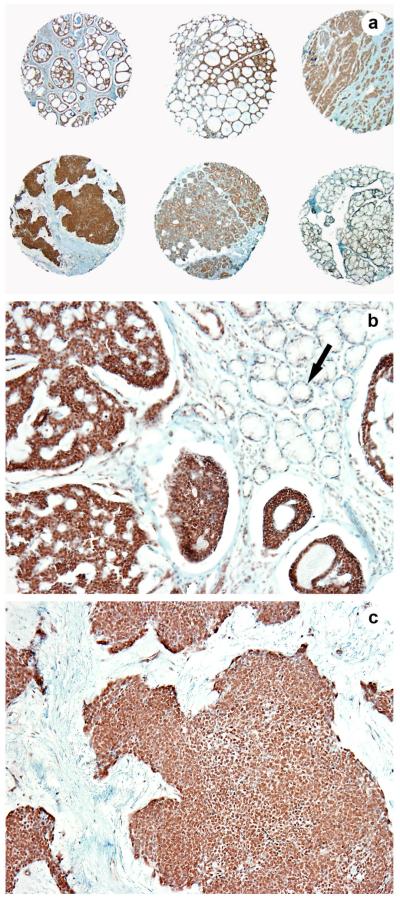
Salivary gland parenchyma: EN1 was faintly expressed in the cytoplasm of salivary ductal cells whereas no nuclear pattern was noted (Figure 1a, b arrow).
Figure 1.
EN1 expression: In the benign salivary gland, EN1 was faintly expressed in the cytoplasm of salivary ductal cells whereas no nuclear pattern was noted (Fig 1a, b arrow). In ACCs, EN1 expression was limited to the inner ductal cells (with a nuclear pattern of staining) and was negative in the myoepithelial cells in both tubular and cribriform patterns of ACCs (Fig 1a, b). The solid pattern type tumors were significantly more likely to be EN1 positive than were tumors with tubular or cribriform patterns (Fig 1a, c).
ACCs: EN1 expression was limited to the inner ductal cells (with a nuclear pattern of staining) and was negative in the myoepithelial cells in both tubular and cribriform patterns of ACCs (Figure 1a, b). The solid pattern type tumors were significantly more likely to be EN1 positive than were tumors with tubular or cribriform patterns (Figure 1a, c). Of the solid type tumors 24 (85.7%) of 28 were EN1 positive while 85 (57.1%) of 149 of the tubular plus cribriform tumors were positive (17 tumors were not evaluable for EN1 staining). This difference was significant by 2-tailed Fisher exact test (p = 0.00517). Among the tubular tumors, 29 (58.8%) of 50 were positive and, among the cribriform tumors, 56 (56.6%) of 99 were positive which, is very close to the same proportions for each (Table 1).
Table 1.
EN1 marker expression in different ACC patterns.
| ACC histologic pattern | |||
|---|---|---|---|
| EN1 (IHC) | Tubular | Cribriform | Solid |
| (+) | 58.8% (29) | 56.6% (56) | 85.7% (24) |
| (−) | 41% (21) | 43.4% (43) | 14.3% (4) |
ACC- adenoid cystic carcinoma
Clinicopathologic Correlations
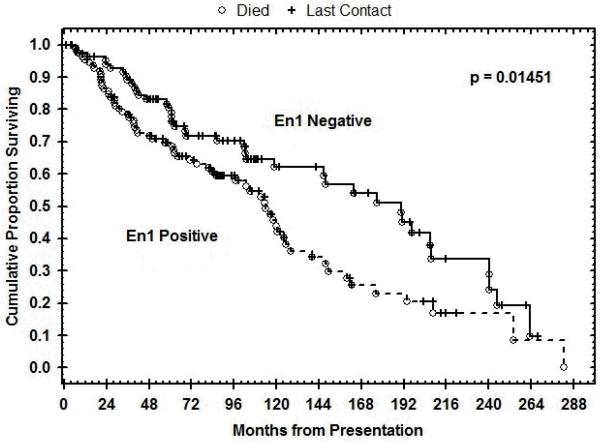
Epithelial EN1 positivity in ACC correlated significantly with poor survival (p= 0.014). The Kaplan-Meier plot of the overall survival of the population is shown in Figure 2.
Figure 2.
The Kaplan-Meier plot of the overall survival of the population shows epithelial EN1 positivity in ACC correlated significantly with poor survival (p= 0.014).
Discussion
To ascertain definitively whether aberrant EN1 expression accompanies human salivary adenoid cystic carcinoma, in the present study, we used an immunohistochemical technique to directly examine EN1 protein expression in this condition.
Nuclear EN1 immunoreactivity was not observed in normal salivary parenchyma. Although EN1 is expressed and exerts its function in normal cells, the EN1 protein expression level in normal tissue does not reach the threshold that can be detected by immunohistochemic staining. Nuclear EN1 immunoreactivity was limited to the inner ductal cells and was negative in the myoepithelial cells in both tubular and cribriform patterns of ACCs. The solid type ACCs were significantly more likely to be EN1 positive than were tubular or cribriform tumors, showing strongly diffuse nuclear immunoreactivity. Moreover, our study demonstrated that patients with ACC who had positive results for EN1 protein expression had a significantly lower survival rate than patients with EN1-negative tumors (p= 0.014). Also, if patients had lymph node metastasis, they were more likely to have positive results for EN1. These results are in keeping with the morphological findings, where the solid variant is characterized by a more aggressive clinical course. We tested in parallel the EN1 immunoreactivity on a breast TMA constructed from 35 cases of triple negative breast carcinomas. Results similar to those for solid salivary ACC were noticed for the high grade basaloid breast carcinoma tumors. We did not further investigate or correlate with clinicopathologic parameters, since this was not the purpose of the present study.
The immunohistochemical findings are at variance with our prior methylation studies in a cohort of 16 ACC patients, where MCAM and pyrosequencing revealed a 59% difference in methylation between tumor and matched normal tissue3. The mechanisms underlying the protein overexpression of EN1 in neoplastic tissues of ACC are not clear. Possible causes may include increased expression of trans-acting transcriptional activators, or decreased expression, or dysfunction of transcriptional suppressors of EN1.
Apparently, not all genes accumulated CpG methylation at an equal rate and similar onset. The inverse correlation between the EN1 markers’ levels of methylation and EN1 levels of protein expression across the neoplastic stages suggests several possibilities, including a feedback mechanism. One hypothesis is that in ACC tissue of salivary glands, the sequela of a high EN1 RNA transcription, and its protein expression, which accumulates to its peak concentration about 8 hours later, triggers at high EN1 protein concentration a feed-back regulation that silences EN1 gene expression via CpG methylation, (as shown in our previous study). When, after a long period of time the EN1 protein degradation reaches a low- threshold concentration, the cycle starts again, and for a very short period of time the gene silencing will be removed, and EN1 RNA and protein will be highly expressed, until enough protein has accumulated to once again trigger the gene silencing via DNA methylation. Therefore, in our previous study we found nearly all of the EN1 genes in ACC in their silenced state owing to strong CpG methylation. The short lifetime of the EN1 mRNA compared to the long livespan of the EN1 protein explains why we find these high levels of EN1 protein in the ACC tumor cells at times when its gene has already been silenced by CpG-methylation, and its mRNA has long been degraded. It is further very likely that certain EN1 target genes, which might be essential for survival, or for subtype formation of ACC may depend on the presence of additional factors besides EN1 to activate their gene expression.
In summary, we validated EN1 as a potential biomarker in a large cohort of salivary ACC. Our data show increased EN1 protein expression in solid type ACC, which correlated with a significantly lower survival rate and higher incidence of lymph node metastasis. Immunohistochemical analysis of EN1 in biopsy specimens obtained for diagnostic purposes and/or surgically resected material may show that EN1 is a biological predictor of poor prognosis in salivary ACC patients.
Acknowledgments
This study was supported in part by the National Institutes of Health National Institute of Dental and Craniofacial Research and Rare Disease Research grant number U01DE019756 (A.K.E.N). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or National Institutes of Health.
Footnotes
Conflict of interest disclosures-none
References
- 1.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125(2):149–52. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 2.Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck--a 20 years experience. Int J Oral Maxillofac Surg. 2004;33(1):25–31. doi: 10.1054/ijom.2003.0448. [DOI] [PubMed] [Google Scholar]
- 3.Bell A, Bell D, Weber RS, El-Naggar AK. CpG Island Methylation Profiling in Human Salivary Gland Adenoid Cystic Carcinoma. Cancer. 2011 doi: 10.1002/cncr.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayor R, Casadome L, Azuara D, et al. Long-range epigenetic silencing at 2q14.2 affects most human colorectal cancers and may have application as a non-invasive biomarker of disease. Br J Cancer. 2009;100(10):1534–9. doi: 10.1038/sj.bjc.6605045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devaney J, Stirzaker C, Qu W, et al. Epigenetic deregulation across chromosome 2q14.2 differentiates normal from prostate cancer and provides a regional panel of novel DNA methylation cancer biomarkers. Cancer Epidemiol Biomarkers Prev. 2011;20(1):148–59. doi: 10.1158/1055-9965.EPI-10-0719. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Rauch TA, Zhong X, et al. CpG island hypermethylation in human astrocytomas. Cancer Res. 2010;70(7):2718–27. doi: 10.1158/0008-5472.CAN-09-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]