Abstract
Background
Docetaxel is the mainline treatment approved by the FDA for castration-resistant prostate cancer (CRPC) yet its administration only increases median survival by two to four months. Docetaxel is metabolized in the liver by hepatic CYP3A4 activity. Piperine, a major plant alkaloid/amide, has been shown to inhibit the CYP3A4 enzymatic activity in a cell-free system. Thus, we investigated whether the co-administration of piperine and docetaxel could increase docetaxel’s pharmacokinetic activity in vitro and in vivo.
Methods
Liver CYP3A4 enzymatic activity was measured by fluorescence. In vivo docetaxel pharmacokinetic activity was analyzed by liquid chromatography. An in vivo xenograft model of human CRPC was utilized to assess the anti-tumor effect of docetaxel when co-administered with piperine.
Results
Inhibition of hepatic CYP3A4 activity resulted in an increased area under the curve (AUC), half-life and maximum plasma concentration of docetaxel when compared to docetaxel alone administration. The synergistic administration of piperine and docetaxel significantly improved the anti-tumor efficacy of docetaxel in a xenograft model of human CRPC.
Conclusions
Docetaxel is one of the most widely used cytotoxic chemotherapeutic agents and is currently the mainstay treatment for metastatic CRPC. Dietary constituents are important agents modifying drug metabolism and transport. In our studies, dietary consumption of piperine increases the therapeutic efficacy of docetaxel in a xenograft model without inducing more adverse effects on the treated mice.
Keywords: piperine, docetaxel, prostate, cancer
Introduction
Prostate cancer is the most common solid malignancy in men, accounting for an estimated 217,000 new cases and 32,000 deaths in 2010 [1]. Median survival for men with castration-resistant prostate cancer (CRPC) is 16.5 months, and treatment options for are limited. Docetaxel-based combination chemotherapy is the mainline therapy approved by the FDA for treatment of CRPC, extending survival by 2–4 months, however toxicities, especially hematopoietic side-effects, are significant and dose-limiting [2]. Docetaxel is primarily metabolized by the hepatic cytochrome P450 family of enzymes. Specifically, a member of the cytochrome P450 mixed-function oxidase system CYP3A4 is responsible for the breakdown of docetaxel, in particular isoforms CYP3A4 and CYP3A5, the latter of which has a 10-fold lower affinity for docetaxel [3].
Hepatic metabolism is the primary elimination pathway of docetaxel with urinary excretion accounting for less than 10% of the clearance of each administered dose. [4,5] Furthermore, metabolites of docetaxel are substantially less active than the parent drug and contribute very little to docetaxel activity. [3] Thus, manipulation of the hepatic metabolic pathway can considerably alter the pharmacokinetics of docetaxel. For example, docetaxel’s relative bioavailability was increased from 4% to 183% by the co-administration of the CYP3A4 inhibitor ritonavir, increasing systemic exposure by 50-fold [6]. Although studies using another potent CYP3A4 inhibitor ketoconazole demonstrated an inconsistent effect on docetaxel’s systemic levels [7,8], this inconsistency can potentially be explained by the large inter- and intra-individual variability in absorption seen with this drug. [7,9] Nevertheless, manipulation of CYP3A4 hepatic metabolism represents a potential therapeutic avenue to augment docetaxel’s efficacy.
Natural products are the most consistent source of drug development. Furthermore, new insights suggest that dietary constituents may play an important role in cancer prevention as well as in cancer management/therapy. These products may act synergistically with radiotherapy and chemotherapy to kill tumor cells by reducing angiogenesis, inflammation and the induction of metastasis. Recent studies demonstrate that piperine, a major plant alkaloid/amide present in black (Piper nigrum Linn) and long (P. longum Linn) peppers, inhibits the activity of the drug-metabolizing enzyme CYP3A4 in a cell-free system [10,11]. Also, by acting on hepatic enzymatic breakdown, piperine can enhance the bioavailability of several therapeutic compounds, including phenytoin, propranolol and theophylline in healthy volunteers and rifampin in patients with tuberculosis [12]. Furthermore, preliminary studies suggest that piperine itself can inhibit the development of breast cancer by inhibiting stem cell renewal [13] as well as inhibit antioxidant, anti-inflammatory, and anti-cancer activities in cancer cell models [14]. Taken together, these findings indicate that piperine not only can alter the metabolism and thereby the therapeutic effectiveness of many pharmaceutical compounds that are processed by the CYP3A4 pathway, including chemotherapeutic agents such as docetaxel, but also may promote anti-tumor effect either individually or in concert with traditional therapies.
In this study, we investigated the pharmacokinetic and anti-cancer effects of piperine when given in combination with docetaxel. We demonstrate that treatment with piperine inhibits hepatic CYP3A4 activity in vivo which coincides with an increased area under the curve (AUC), half-life, and maximum plasma concentration of docetaxel when administered in combination with piperine versus docetaxel alone. Importantly, the co-administration of docetaxel and piperine resulted in a statistically significant synergistic anti-tumor effect in a xenograft animal model of castration-resistant human prostate cancer.
Materials and Methods
Reagents
Piperine was obtained from Sigma (St. Louis, MO). Docetaxel was obtained from LC Laboratories (Woburn, MA).
Analysis of CYP3A4 activity
Six week-old male C.B17/Icr-scid mice were given piperine (100 mg/kg by mouth in vegetable oil) or vegetable oil only. The mice were sacrificed by CO2 asphyxiation one hour after piperine administration. After homogenizing mice livers, CYP3A4 activity was measured using CYP3A4 assay kit (Promega, Madison, WI) and normalized according to wet tissue weight. According to manufacturer’s instructions, CYP3A4 activity of control and piperine treated mice was determined by relative fluorescence and quantified by a Synergy HT Microplate Reader (Bio Tek, Winooski, VT).
Pharmacokinetic analysis
Six week-old male C.B17/Icr-scid mice were given piperine (100 mg/kg p.o. in vegetable oil) or vegetable oil only followed by intravenous administration of docetaxel (12.5 mg/kg in 5% ethanol, 5% TWEEN 80 and 5% glucose in PBS). At the termination of the experiment, blood was collected from the retro-orbital plexus under anesthesia from both experimental and control animals. The mice were then sacrificed by CO2 asphyxiation. Docetaxel was extracted from study plasma samples by liquid-liquid extraction using methyl tert-butyl ether (MTBE) fortified with 10 ng/mL paclitaxel as an internal standard (IS). Briefly, a 50 μL volume of plasma was diluted with 50 μL of water. An 800 μL volume of MTBE (with paclitaxel) was added and the sample was mixed for approximately 10 minutes. After mixing, the samples were centrifuged at 3,220 rcf for 10 minutes and approximately 500 μL of the MTBE was removed and dried under nitrogen. The residue was re-suspended in 100 μL of 50% methanol with 0.1% acetic acid. The re-suspension was gently vortexed for approximately five minutes and stored at 10°C. Docetaxel calibration standards in plasma were prepared by diluting a 10 μg/mL docetaxel stock solution in plasma to obtain a final range of standards from 10 to 5,000 ng/mL. The docetaxel plasma standards were extracted in duplicate using the same procedure as the study samples. A HPLC/MS/MS method utilizing electrospray ionization in positive mode was used for the analysis of docetaxel in mouse plasma using paclitaxel as IS. Docetaxel’s extraction from plasma samples and pharmacokinetic analysis were performed at Wolfe Laboratories (Watertown, MA).
Assessment of in vivo tumor growth
1 × 106 castration-resistant prostate cancer PC-3 cells were inoculated s.c. in the flank region of 6 week-old male C.B17/Icr-scid mice using a 27-gauge needle. All animal procedures were done in accordance with local guidelines on animal care and with appropriate institutional certification. Animals were fed an autoclaved AIN-93M diet (Harlan Teklad, Madison, WI) and water ad libitum. Two weeks after injection of tumor cells animals were randomly assigned to the control and experimental groups (n=5 mice/group). Mice were given piperine (100 mg/kg via oral gavage in vegetable oil) or vegetable oil only followed by intravenous administration of docetaxel (12.5 mg/kg in 5% ethanol, 5% TWEEN 80 and 5% glucose in PBS) or solvent solution 2 hours after ingestion of piperine. Administration of piperine with or without docetaxel occurred on a weekly basis. Tumors were measured twice weekly and their volumes were calculated by the formula: [volume = 0.52 × (width)2 × length]. Animals were sacrificed 14 days after treatment initiation.
Statistical analysis
Statistical analysis was performed using a two-sided Student t test. Results are expressed as the mean ± SEM. Non-compartmental pharmacokinetic analysis was performed using WinNonlin.
Results
The effect of piperine administration on the hepatic activity of CYP3A4 in vivo
Hepatic metabolism by CYP3A4 is the primary elimination pathway of docetaxel [4,5]. Given that piperine is able to inhibit CYP3A4 activity in a cell-free system [10,11] we examined the effect of piperine on CYP3A4 hepatic activity in an animal model. Piperine was administered through oral gavage in a homogeneous suspension mixed in vegetable oil at a concentration of 50 mg/kg/dose. Control animals received an equal volume of vegetable oil only. One hour after piperine administration animals were euthanized by CO2 asphyxiation, and CYP3A4 activity was measured from liver homogenates obtained from control and experimental animals and normalized according to wet tissue weight. As displayed in Figure 1, CYP3A4 activity was significantly reduced in hepatic cells obtained from mice treated with piperine compared with control animals, confirming piperine’s ability to inhibit hepatic CYP3A4 enzymatic activity.
Figure 1.
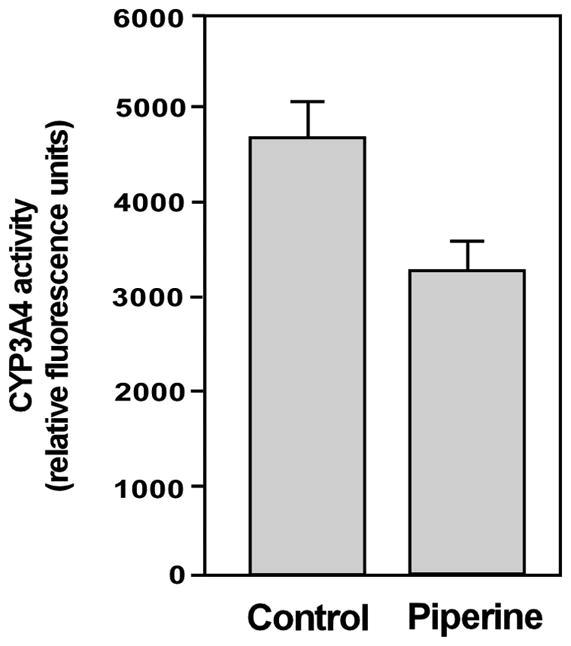
Piperine inhibits CYP3A4 hepatic activity in vivo. Six week-old male C.B17/Icr-scid mice were given piperine (50 mg/kg p.o.) or vegetable oil only. CYP3A4 activity was measured using the CYP3A4 assay kit (Promega, Madison, WI). Values represent means (n=3) ± SD.
Pharmacokinetic studies
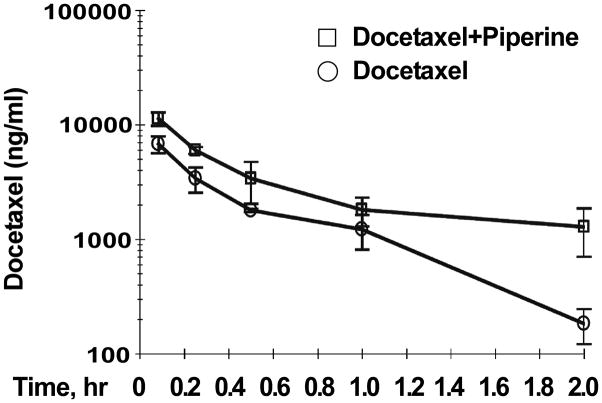
Next, we investigated the effect of piperine-mediated CYP3A4 inhibition on docetaxel’s pharmacokinetics in vivo. The administered doses of docetaxel selected were based on previously published results in animal studies showing that the maximally tolerated dose (MTD) of docetaxel ranges from 15 to 33 mg/kg/dose [15]. A summary plot comparing average docetaxel concentration in mice given piperine and mice not given piperine over time is presented in Figure 2. Administration of piperine significantly increased and prolonged the mouse plasma levels of docetaxel compared with mice which received docetaxel alone. Table 1 displays the corresponding mean docetaxel concentrations between the two groups over five time points. In mice that were fed piperine, mean docetaxel concentrations were uniformly almost double the mean docetaxel concentrations of mice not fed piperine. Most notably, the difference in docetaxel plasma levels was most pronounced between 1 and 2 hours after docetaxel administration, i.e, plasma docetaxel levels in the piperine/docetaxel group was 7-fold higher than in docetaxel only group.
Figure 2.
Effect of piperine co-administration on docetaxel plasma levels. Six week-old male C.B17/Icr-scid mice were given piperine (50 mg/kg p.o. in vegetable oil) or vegetable oil only followed by intravenous administration of docetaxel (12.5 mg/kg in 5% ethanol, 5% TWEEN 80 and 5% glucose in PBS). Blood was collected from the retro-orbital plexus under anesthesia from both experimental and control animals, and docetaxel plasma levels were examined as described above. Values represent means (n=3) ± SD.
Table 1.
Docetaxel levels in animal plasma samples
| Time (minutes) | Mean docetaxel concentration (ng/ml) | p value (two tail) | |
|---|---|---|---|
| Docetaxel | Docetaxel/Piperine | ||
| 5 | 6808±1140 | 11380±1528 | 0.0013 |
| 15 | 3403±841 | 5993±1969 | 0.0039 |
| 30 | 1793±55 | 3400±410 | 0.0003 |
| 60 | 1221±411 | 1810±508 | 0.0357 |
| 120 | 183±62 | 1284±580 | 0.0358 |
Table 2 compares the pharmacokinetics of docetaxel with and without administration of piperine. The area under AUC for docetaxel increased by 230% in the piperine/docetaxel group compared with the docetaxel alone group. Furthermore, docetaxel’s half-life increased from 0.44 hr to 1.14 hr when piperine was co-administered. Similarly, the mean values of docetaxel’s maximum concentration (Cmax) given in combination with piperine versus given alone were 11,380 ng/ml and 6,808 ng/ml, respectively. These pharmacokinetic data clearly demonstrate that inhibition of hepatic CYP3A4 activity by piperine results in meaningful prolongation of docetaxel’s half-life with an expected increase and prolongation of docetaxel’s serum concentration.
Table 2.
Summary of calculated PK parameters
| RoA | IV | IV | Units |
|---|---|---|---|
| Compound | Docetaxel | Docetaxel + Piperine | |
| Dose (docetaxel) | 12.5 | 12.5 | mg/kg |
| Tmax | 0.083 | 0.083 | hr |
| Cmax | 6808* | 11380* | ng/mL |
| Half-life | 0.440 | 1.14 | hr |
| AUC | 3756 | 8715 | hr* ng/mL |
| Vz | 2114 | 2368 | mL/kg |
| Cl | 3328 | 1434 | mL/hr/kg |
p-value=0.028
Piperine enhances anti-tumor efficacy of docetaxel in vivo
Given that docetaxel is metabolized by hepatic CYP3A4 enzymatic activity and that piperine inhibits this activity resulting in a meaningful increase and prolongation of docetaxel’s serum levels, we examined the effect of the addition of piperine to docetaxel on anti-tumor efficacy using a xenograft model of human prostate cancer. Castration-resistant PC-3 xenograft tumors were established in 6-week old male C.B17/Icr-scid mice. Two weeks after tumor cell implantation, at a time when baseline tumor volumes reached 200–400 mm3 across all mice, animals were randomly selected to the following groups (n=5 mice/group): (i) control; (ii) piperine p.o. weekly at 100 mg/kg/dose; (iii) docetaxel intravenously weekly at 12.5 mg/kg/dose; or (iv) piperine/docetaxel combination. There was no statistically significant difference in tumor volumes among these groups. Piperine was administered one hour prior to docetaxel injection.
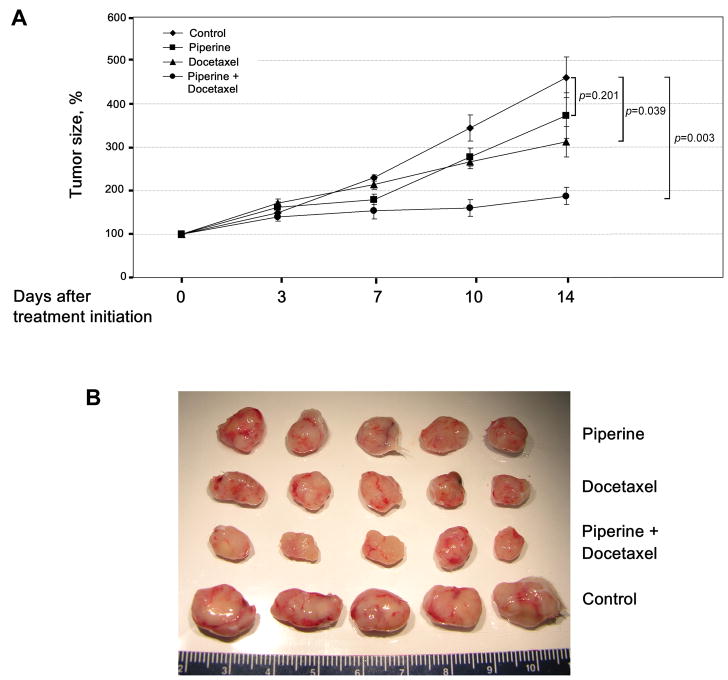
Two weeks after the initiation of treatment, mice treated only with docetaxel showed a significant decrease in the growth of their xenograft tumors (313% vs. 461%, P=0.039). Furthermore, as Figure 3 demonstrates, treatment with piperine alone also caused an observable decrease in tumor growth (373% vs. 461%), however this difference did not achieve statistical significance (P=0.201). Co-administration of piperine and docetaxel resulted in the most significant inhibition of tumor growth (188% vs. 461%, P=0.003) among the experimental groups. Importantly, the resulting increase in the mean plasma concentrations of docetaxel in mice fed piperine did not translate into an observed increase in mice toxicity during treatment. Also, none of the mice in the treatment groups died prematurely. Thus, by inhibiting hepatic clearance of docetaxel, piperine results in increased docetaxel serum levels without resulting in an increase in docetaxel-mediated toxicities. These findings indicate that piperine may represent a therapeutic strategy to enhance docetaxel’s efficacy in treating castration-resistant prostate cancer.
Figure 3.
Piperine enhances docetaxel’s anti-tumor efficacy in vivo. (A) PC-3 subcutaneous xenografts were established in 6 week old male C.B17/Icr-scid mice. Two weeks after tumor implantation animals were randomly assigned to the control and experimental groups (n=5 mice/group). Mice were given piperine (50 mg/kg p.o in vegetable oil) or vegetable oil only followed by intravenous administration of docetaxel (12.5 mg/kg) or solvent solution 2 hours after piperine ingestion. Administration of piperine with or without docetaxel occurred on a weekly basis. Tumors were measured twice weekly and their volumes were calculated by the formula: [volume = 0.52 × (width)2 × length]. Values are means (n=5) ± SEM. (B) Photographs of tumors from each group showing grossly a marked tumor response in mice treated with piperine and docetaxel compared to other experimental groups and control group.
Discussion
Docetaxel is one of the most widely used cytotoxic chemotherapeutic agents and has currently been approved for the treatment of breast cancer, non–small-cell lung cancer, squamous cell carcinoma of the head and neck, gastric adenocarcinoma, and CRPC). Despite its efficacy, docetaxel administration is limited by its toxicities, specifically myelosuppression, which can result in cessation of therapy. The pharmacokinetic profile of docetaxel is characterized by substantial inter-individual variability, with up to 10-fold differences in drug clearance even in patients with normal hepatic function. The importance of docetaxel’s clearance is highlighted by a previous study documenting that a 50% decrease in docetaxel clearance is associated with a greater than 430% increase in the odds of developing severe-grade 3 or 4-neutropenia [16]. Furthermore, a reduced AUC was associated with shorter survival in patients with non–small-cell lung cancer receiving docetaxel chemotherapy [17].
Dietary constituents are important agents modifying drug metabolism and transport and thereby can contribute to great variability in drug disposition among individuals [18,19]. The induction or inhibition of drug-metabolizing enzymes or transporters could contribute to the underlying mechanism of altered drug concentrations [10,20]. Piperine, a major constituent of black pepper, has been shown to inhibit human P-glycoprotein transporters and CYP3A4 enzymatic activity in a cell-free system [10]. It has been well established that CYP3A4-mediated metabolism is the major route of docetaxel inactivation in humans [3]. Our present study demonstrates that treatment with piperine inhibits hepatic CYP3A4 activity in vivo which correlated with an increased AUC, half-life and maximum plasma concentration of docetaxel. Moreover, synergistic administration of piperine and docetaxel significantly enhanced the anti-tumor efficacy of docetaxel in an animal model of castration-resistant human prostate cancer.
Prior work has shown an anti-neoplastic effect of piperine [14]. The antitumor activity of piperine appears to be related to its immunomodulatory properties, which involves the activation of cellular and humoral immune responses [21]. In fact, piperine possesses only weak cytotoxic activity [21,22], which indicates that its anti-tumor activity is indirect and not related to anti-proliferative or pro-apoptotic effects on tumor cells. Some authors have shown that piperine’s anti-cancer effects may be due to its ability to inhibit stem cell renewal [13]. Although our data indicate piperine itself demonstrated a minor anti-tumor efficacy in vivo, dietary consumption of piperine might increase the therapeutic efficacy of drugs that are substrates for CYP3A4.
Polypharmacy is a very effective regimen to increase the therapeutic efficacy of docetaxelfor the treatment of certain solid malignancies such as breast cancer [23]. In a clinical study, the combination of docetaxel and adriamycin showed a better cure rate against metastatic breast cancer than other regimens [24]. However, the combination of docetaxel and adriamycin induces severe dose-limiting toxicity [24]. Therefore, increased antitumor effect of docetaxel could be compromised by adverse effects. The increased serum half-life of docetaxel due to the inactivation of CYP3A4 might potentially result in undesirable toxicity in vivo.
Daily dietary consumption of black pepper varies considerably within the population[25]. In the United States, average daily intake of black pepper has been estimated at 359 mg [26]. Piperine accounts for between 5% and 9% of black pepper content, implying a daily intake of approximately 60 to 110 μmol of piperine. Results of our study demonstrate that such an amount of piperine could potentially modulate pharmacokinetics of docetaxel in cancer patients. Information on dietary consumption of black pepper should be taken into consideration in patients receiving docetaxel and treatment outcome should be considered and assessed prospectively.
Acknowledgments
This work was supported in part by National Institutes of Health Grants (RO1 CA134463, CCSG, P30 CA006927) to VMK; American Institute for Cancer Research Grant (09A023) to RGU; and Department of Defense Physician Research Training Award (PC094474) to AK.
Footnotes
None of the authors listed have any significant or perceived conflicts of interest relating to the publishing of this manuscript.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Makhov P, Kutikov A, Golovine K, Uzzo RG, Canter DJ, Kolenko VM. Docetaxel-mediated apoptosis in myeloid progenitor TF-1 cells is mitigated by zinc: Potential implication for prostate cancer therapy. Prostate. 2011 doi: 10.1002/pros.21357. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels FK, Sparreboom A, Mathot RA, Verweij J. Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. Br J Cancer. 2005;93(2):173–177. doi: 10.1038/sj.bjc.6602698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel : recent developments. Clin Pharmacokinet. 2006;45(3):235–252. doi: 10.2165/00003088-200645030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet. 1999;36(2):99–114. doi: 10.2165/00003088-199936020-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bardelmeijer HA, Ouwehand M, Buckle T, Huisman MT, Schellens JH, Beijnen JH, van Tellingen O. Low systemic exposure of oral docetaxel in mice resulting from extensive first-pass metabolism is boosted by ritonavir. Cancer Res. 2002;62(21):6158–6164. [PubMed] [Google Scholar]
- 7.Engels FK, Mathot RA, Loos WJ, van Schaik RH, Verweij J. Influence of high-dose ketoconazole on the pharmacokinetics of docetaxel. Cancer Biol Ther. 2006;5(7):833–839. doi: 10.4161/cbt.5.7.2839. [DOI] [PubMed] [Google Scholar]
- 8.Van Veldhuizen PJ, Reed G, Aggarwal A, Baranda J, Zulfiqar M, Williamson S. Docetaxel and ketoconazole in advanced hormone-refractory prostate carcinoma: a phase I and pharmacokinetic study. Cancer. 2003;98(9):1855–1862. doi: 10.1002/cncr.11733. [DOI] [PubMed] [Google Scholar]
- 9.JamisDow CA, Pearl ML, Watkins PB, Blake DS, Klecker RW, Collins JM. Predicting drug interactions in vivo from experiments in vitro - Human studies with paclitaxel and ketoconazole. Am J Clin Oncol-Canc. 1997;20(6):592–599. doi: 10.1097/00000421-199712000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302(2):645–650. doi: 10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 11.Volak LP, Ghirmai S, Cashman JR, Court MH. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab Dispos. 2008;36(8):1594–1605. doi: 10.1124/dmd.108.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S. Herb-drug interactions: a literature review. Drugs. 2005;65(9):1239–1282. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122(3):777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Yadev VR, Aggarwal BB, Nair MG. Inhibitory effects of black pepper (Piper nigrum) extracts and compounds on human tumor cell proliferation, cyclooxygenase enzymes, lipid peroxidation and nuclear transcription factor-kappa-B. Nat Prod Commun. 5(8):1253–1257. [PubMed] [Google Scholar]
- 15.Dykes DJ, Bissery MC, Harrison SD, Jr, Waud WR. Response of human tumor xenografts in athymic nude mice to docetaxel (RP 56976, Taxotere) Invest New Drugs. 1995;13(1):1–11. doi: 10.1007/BF02614214. [DOI] [PubMed] [Google Scholar]
- 16.Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16(1):187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 17.Bruno R, Olivares R, Berille J, Chaikin P, Vivier N, Hammershaimb L, Rhodes GR, Rigas JR. Alpha-1-acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non-small cell lung cancer treated with docetaxel. Clin Cancer Res. 2003;9(3):1077–1082. [PubMed] [Google Scholar]
- 18.Scheen AJ. Drug-drug and food-drug pharmacokinetic interactions with new insulinotropic agents repaglinide and nateglinide. Clin Pharmacokinet. 2007;46(2):93–108. doi: 10.2165/00003088-200746020-00001. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson GR. The effects of diet, aging and disease-states on presystemic elimination and oral drug bioavailability in humans. Adv Drug Deliv Rev. 1997;27(2–3):129–159. doi: 10.1016/s0169-409x(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 20.Evans AM. Influence of dietary components on the gastrointestinal metabolism and transport of drugs. Ther Drug Monit. 2000;22(1):131–136. doi: 10.1097/00007691-200002000-00028. [DOI] [PubMed] [Google Scholar]
- 21.Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine J Ethnopharmacol. 2004;90(2–3):339–346. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Bezerra DP, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, Costa-Lotufo LV. Antiproliferative effects of two amides, piperine and piplartine, from Piper species. Z Naturforsch C. 2005;60(7–8):539–543. doi: 10.1515/znc-2005-7-805. [DOI] [PubMed] [Google Scholar]
- 23.To H, Shin M, Tabuchi M, Sakaguchi H, Takeuchi A, Matsunaga N, Higuchi S, Ohdo S. Influence of dosing schedule on toxicity and antitumor effects of a combination of adriamycin and docetaxel in mice. Clin Cancer Res. 2004;10(2):762–769. doi: 10.1158/1078-0432.ccr-1000-03. [DOI] [PubMed] [Google Scholar]
- 24.Nabholtz JM, Smylie M, Mackey JR, Noel D, Paterson AH, al-Tweigeri T, Au D, Sansregret E, Delorme F, Riva A. Docetaxel/doxorubicin/cyclophosphamide in the treatment of metastatic breast cancer. Oncology (Williston Park) 1997;11(8 Suppl 8):37–41. [PubMed] [Google Scholar]
- 25.Sung B, Kunnumakkara AB, Sethi G, Anand P, Guha S, Aggarwal BB. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol Cancer Ther. 2009;8(4):959–970. doi: 10.1158/1535-7163.MCT-08-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kindell S. PhD thesis. Drexel University; Philadelphia, PA: 1984. Studies on Selected Genotoxic and Toxic Properties of Piperine. [Google Scholar]