Abstract
The details of the bifurcation of the lymphoid and myeloid lineages following commitment by multipotent progenitor cells (MPP) remain a topic of controversy. We report that the surface glycoprotein CD62L can be characterized as a novel marker of this and other stages of early hematopoietic differentiation. Cell isolation and transplant studies demonstrated CD62Lneg/low long-term hematopoietic stem cells and CD62Lhigh MPP within the traditionally defined c-kitposLinneg/lowSca-1pos (KLS) stem/progenitor cellpopulation. Within the MPP population previously defined as KLS-Thy-1.1negFlt3pos, Sca-1 and CD62L resolved 4 populations and segregated Sca-1highCD62Lneg/low MPP from Sca-1highCD62Lhigh leukocyte-biased progenitors. Utilizing a novel transplantation method that allows tracking of erythroid and platelet engraftment as an alternative to the classical method of in vitro colony formation, we characterized Sca-1highCD62Lneg/low cells as MPP based on transient engraftment of these lineages. These data establish CD62L as a useful tool in the study of early hematopoiesis, and emphasize the power of tri-lineage engraftment studies in establishing the lineage potential of MPP subsets.
Introduction
In adult mammals, all blood cells originate from a pool of hematopoietic stem cells (HSC)3 residing in the bone marrow. These adult stem cells possess the prototypical stem cell characteristics: the ability to self-renew through mitosis and the capacity to generate cells of all hematopoietic lineages (1). As HSC mature and differentiate into progeny cells, their self-renewal ability becomes limited and their multipotency is lost through lineage commitment. The early events of hematopoietic differentiation have been described to occur within a subset of immature cells in the bone marrow identified by a shared expression pattern of surface markers: co-expression of stem cell-associated markers c-kit and Sca-1 and no or only low-level expression of the mature cell markers collectively known as Lineage (Lin) (2, 3). This subset of hematopoietic stem and progenitor cells is routinely termed the KLS (c-kitposLinneg/lowSca-1pos) compartment.
Within the KLS compartment reside three distinct subpopulations that are considered to delineate early hematopoietic differentiation events. According to expression patterns of Flt3 and Thy1.1 surface markers4, the three subpopulations are designated as Thy1.1posFlt3neg long-term HSC (LT-HSC), Thy1.1posFlt3pos short-term HSC (ST-HSC), and Thy1.1negFlt3pos multipotent progenitor cells (MPP) (4–6). The LT-HSC subset includes the true HSC that initiates hematopoiesis. As LT-HSC differentiate, the Flt3 receptor is upregulated. Cells in the ST-HSC compartment are multipotent but possess a limited capacity for self-renewal, since transplantation studies have been shown the ST-HSC compartment to reconstitute the hematopoietic system of recipients only for approximately 6~12 weeks (5, 6). Finally, the last stage within the KLS compartment is the MPP stage that has lost self-renewal capability, accompanied by the loss of Thy1.1, but maintains multipotency.
The functional heterogeneity within the MPP compartment, as defined by Flt3-expressing KLS cells, has been the focus of recent discussions (7–11), mainly triggered by a study describing the existence of lymphoid-primed multipotent progenitors (LMPP) (7). The study identifies LMPP in the hematopoietic stem cell compartment as the population of cells that expresses the highest level of Flt3, constituting a significant fraction of MPP (approximately the top 25% of KLS cells for Flt3 expression). Unlike MPP cells, which have significant output in all hematopoietic lineages, LMPP cells generated insignificant numbers of platelets and red blood cells, suggesting the loss of erythro-megakaryocytic lineage (Meg/E) potential prior to cells exiting the hematopoietic stem cell compartment and demonstrating the existence of oligopotent progenitors within the pool of true MPP. A subsequent study by another group showed that while LMPP cells do have a detectable amount of Meg/E activity, it is significantly less than that of MPP, thereby contrasting the previous report’s claim of loss of Meg/E activity while confirming the existence of heterogeneity within the MPP population (9).
The MPP population has also been subfractionated using the vascular cell adhesion molecule-1 (VCAM-1). In these studies, VCAM-1pos MPP generated cells of all lineages similar to traditional MPP cells, while VCAM-1neg MPP failed to generate Meg/E potentially as robustly as MPP cells or VCAM-1pos MPP (10, 11). Consistent with the LMPP study, the investigators observed that VCAM-1neg MPP cells express high levels of Flt3, while VCAM-1pos MPP cells express both low and high levels of Flt3 (10). These observations suggest that Flt3 alone is insufficient to resolve committed subsets of MPP, and that additional markers will be required to help identify functionally distinct subpopulations within MPP (8).
One issue with previous studies of the Meg/E potential of MPP is the prevalent use of the CD45 allelic system in transplant models since its introduction in 1988 (3). This model allows tracing of donor contributions to nucleated cell lineages by flow cytometry, a major advance over classical techniques that utilized electrophoresis to trace the origin of erythroid cells in transplant studies (12). More recent studies of Meg/E engraftment have used surrogate markers or progenitor cell assays to infer platelet and erythrocyte engraftment since CD45 is not expressed by these lineages (13). The use of GFP transgenic mice allows lineage tracing of platelets (9), however application of the GFP transgenic model to erythroidchimerism has been problematic due to the failure of most GFP transgenic mouse strains to express the transgene in the erythroid lineage(14). As a result, the contributions by the MPP subsets to persistent erythroid engraftment in comparison to HSC in a transplant setting remain to be determined.
Our laboratory has previously reported that the CD62L adhesion molecule can be used to fractionate the Thy1.1neg subset of KLS to identify a T cell-biased CD62Lhigh MPP and a CD62Lneg/low MPP with more generally distributed multipotency(15). These findings have led us to hypothesize that CD62L is useful as an early marker of hematopoietic development. Our data demonstrate that in a transplant setting, the CD62Lneg/low fraction of KLS contains highly enriched HSC, while the CD62Lhigh fraction contains MPP with a limited duration of output. We show that the CD62Lneg/low fraction contains HSC in both Sca-1high as well as Sca-1low subsets of KLS, with less HSC activity in the Sca-1low subset, indicating a gradual population shift as Sca-1 is downregulated. We also present evidence that the primary source of HSC resides in the CD62Lneg/low fraction of the now widely accepted Flt3negThy1.1pos KLS population. Furthermore, we present evidence that CD62L and Sca-1 can be used to isolate distinct subpopulations within the traditional MPP compartment, the Thy1.1negFlt3pos KLS population. Within this MPP compartment, the CD62Lneg/lowSca-1high KLS population contains the most primitive progenitor population while the CD62LhighSca-1low population contains the most mature progenitor population based on transplant studies that resolve tri-lineage engraftment. Fractionation of Meg/E potential from progenitors of nucleated lineages was achieved at the CD62LhighSca-1high stage of development. These data indicate that CD62L is an effective marker for isolating functionally distinct MPP subpopulations, particularly in light of the restricted strain distribution of the Thy-1.1 allele (16) and the difficulty in confirming specificity of Flt3 staining due to the absence of sufficient numbers of Flt3pos cells in normal mouse tissues.
Materials and Methods
Mice
Mice carrying homozygous Thy1a and Ly5a alleles on the C57BL background were generated and maintained in our animal facilities previously described (14). GFP transgenic mice, generated by microinjection of C57BL/6 oocytes, were kindly provided by Dr. Masaru Okabe (Osaka University, Osaka, Japan) (17). These two strains were mated to generate C57BL mice with the GFP transgene on a Thy1a/bLy5a/b background, which served as transplant donors in all experiments except as shown in Figure 3, where the donor strain had homozygous Thy1a and Ly5a alleles on the C57BL background but lacked the GFP transgene. B6.Cg-Gpi1aHbbd H1b/DehJ mice (18) were kindly provided by Dr. David Harrison (Jackson Laboratory, Bar Harbor, ME, USA) and were used as transplant recipients. All mice were kept in the animal resources center at the University of Utah under the institutional animal care and use committee approved protocols.
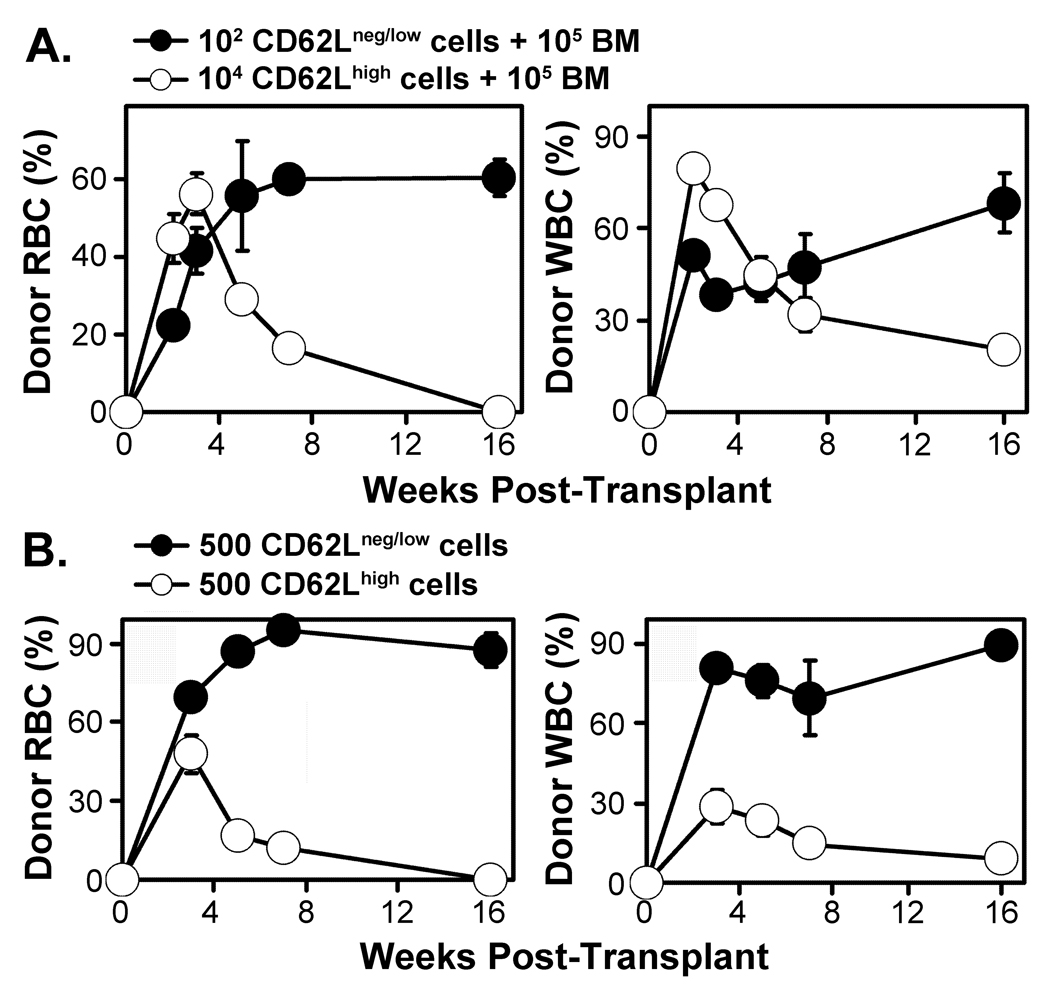
Figure 3. LT-HSC are enriched in the CD62Lneg/low fraction and MPP in the CD62Lhigh fraction of KLS.
Panel A The CD62Lneg/low fraction (102 cells) or the CD62Lhigh fraction (104 cells) of the KLS compartment were each transplanted into groups of 5 lethally-irradiated recipient mice along with 105 normal bone marrow cells. Donor animals in this experiment lacked the GFP transgene, therefore only erythrocytes (RBC) and leukocytes (WBC) were tracked over time by peripheral blood sampling based on analysis of HBB and CD45 allelic markers. Panel B Each CD62L fraction of the KLS compartment was transplanted into 4 lethally-irradiated mice at a dose of 500 cells per mouse without added competitive bone marrow cells. Donor cells lacked the GFP transgene. All transplant recipient mice survived, however only recipients of CD62Lneg/low cells showed persistent engraftment of both RBC and WBC lineages. Error bars indicate SEM.
Antibodies
Monoclonal antibodies against CD2 (Rm2.2), CD3 (KT3-1.1), CD5 (53-7.3), CD8 (53-6.7), CD11b (M1/70), Ly-6G (RB6-8C5), TER119, B220 (CD45R; RA3-6B2), and CD19 (1D3) were purified from the media of cultured hybridoma cell lines. PE-conjugated Sca-1 monoclonal antibody was purchased from PharMingen (San Diego, CA, USA). c-kit (3C11) monoclonal antibody was purified and conjugated to Alexa Fluor 647 in our laboratory. CD4 and CD8 monoclonal antibodies were purified and conjugated to allophycocyanin (APC) in our laboratory. Biotinylated Flt3, CD62L APC-AF750, Thy1.1 PerCP-Cy5.5, Mac-1 PE and Gr-1 PE antibodies were purchased from eBioscience (San Diego, CA, USA). Rat monoclonal antibody 4A5, specific for mouse blood platelets (19), was kindly provided by Dr. S.A. Burstein (University of Oklahoma) and was purified and conjugated to AlexaFluor 647 in our laboratory.
Isolation of hematopoietic progenitor and stem cells
Bone marrow cells were harvested from young adult (6–12 week old) donor mice and incubated with a cocktail of rat antibodies to mature cell markers (CD2, CD3, CD5, CD8, CD11b, Ly-6G, TER119, B220 and CD19). Magnetic depletion of mature cells was performed by two successive incubations with magnetic bead-coupled sheep anti-rat antibodies (Dynal, Oslo, Norway). Lineage-depleted cells were stained with various fluorochrome-conjugated antibodies as indicated in the figures to electronically visualize and sort using a FACS Aria instrument (BD Immunocytometry Systems, San Jose, CA, USA). A 0.3 µM solution of DAPI was used to discriminate dead cells from live cells.
Bone marrow transplantation
Recipient mice were lethally irradiated one day prior to transplant, using a 137Cs source (Model Mark I-30, JL Shepherd & Associates, San Fernando, CA) to deliver 13 Gy at a rate of 75 cGy/min delivered in two doses separated by 3 hours. Isolated donor cells (genotype GFPtg/− Thy1a/bLy5a/bHbbs/s) were injected into recipients (genotype Thy1bLy5bHbbd/d) retro-orbitally. The recipient mice were anesthetized with isoflurane using the E-Z Anesthesia system (Euthanex Corp., Palmer, PA) for injections.
Peripheral blood analysis
For post-transplant analysis, mice were anesthetized with isoflurane using the E-Z Anesthesia system (Euthanex Corp., Palmer, PA) and peripheral blood samples were collected into acid citrate dextrose anticoagulant solution from the retro-orbital sinus using heparinized capillary tubes. Immediately after the collection of blood samples, a volume of 10 µl blood per sample was added to diluted AlexaFluor-647-conjugated 4A5 antibody, and an additional 10 µl sample was diluted in 10 ml PBS for differential cell counting using a Serono System 9010+CP hematology counter (Serono Diagnostics, Allentown, PA). The remainder of each sample was mixed with 500 µl of 2% Dextran T500 (Amersham Biosciences, Piscataway, NJ) in PBS and incubated at 37°C for 30 min to separate the RBC and WBC fractions. WBC were stained with PE-conjugated antibodies against Mac-1 and Gr-1 for myeloid WBC detection, biotinylated B220 or CD19 antibody with a subsequent labeling with Alexa Fluor 750-conjugated avidin for B cell detection, and CD4 and CD8 conjugated with APC for T cell detection. Platelets and WBC were analyzed by FACScan flow cytometer (BD Biosciences, San Jose, CA; modified by Cytek Development, Fremont, CA). Platelet analysis was performed by increasing forward and side scatter parameter gains until the platelet population, identified by 4A5 staining, could be gated to exclude debris. Donor WBC and platelets were identified by GFP fluorescence. Data are reported as percent donor cells of the indicated lineages (Figures 1–4) or as calculated absolute cell numbers (Figure 5). Significance was determined by using a one-tailed T-test with equal variance (Excel, Microsoft Corporation, Bellevue, WA).
Figure 1. CD62L expression pattern in early hematopoietic progenitors.
Panel A Four independent preparations of lineage-depleted bone marrow cells were labeled with antibodies specific for c-kit, Sca-1, CD62L, Thy-1.1, and Flt3 conjugated to the indicated fluorochromes for flow cytometric analysis of the KLS hematopoietic stem cell compartment. Percentages are means ± SD derived from applying identical gates to the four independent data files. The KLS cells are gated and displayed with respect to CD62L expression in the histogram display. Shaded histograms indicate unstained spleen cells (light shaded histogram) and spleen cells stained for CD62L (dark shaded histogram). Panel B KLS cells gated as shown in Panel A are displayed to illustrate expression of the indicated antigens. The Flt3 versus Thy-1.1 panel indicates LT-HSC, ST-HSC, and MPP subsets as previously defined (5, 9). Panel C The two subsets of KLS cells defined by CD62L staining as shown in Panel A were sorted and 103 cells of each subset were transplanted into five lethally irradiated recipients along with 105 recipient genotype bone marrow cells as competitors. The donor-derived cells in peripheral blood were tracked via GFP expression for platelets and white blood cells by flow cytometry. Red blood cells were tracked through the hemoglobin variant Hbbs using HPLC. Values indicate mean ± SEM of the percentage of donor-derived cells of the indicated lineages in peripheral blood samples.
Figure 4. The CD62Lneg/low phenotype adds additional resolution to LT-HSC as defined by Thy-1.1 and Flt3 expression.
Panel A KLS cells were gated to select for the Flt3negThy-1.1pos LT-HSC subset. CD62L expression by the Thy-1.1posFlt3neg fraction of KLS (KLSFneg) is shown in the histogram. The percentages indicate the frequency of the KLSFneg subset of KLS, and the frequencies of CD62Lneg/low and CD62Lhigh cells within the KLSFneg compartment, determined as described in the legend to Figure 1. Panel B The CD62Lneg/low and CD62Lhigh fractions of the KLSFneg population were sorted, and 2 × 103 cells per fraction were transplanted into each of five lethally irradiated mice along with 105 competitor cells. Peripheral blood analysis was performed at the indicated times. Error bars indicate SEM.
Figure 5. CD62L and Sca-1 expression levels define functionally-distinct subsets of the MPP compartment.
Panel A The KLS population was gated to isolate MPP based on the surface phenotype of Flt3pos and Thy1.1neg. These cells are shown with respect to the expression of Sca-1 and CD62L. Four separate populations were identified and isolated by cell sorting, as indicated. Similar levels of Flt3 were expressed by the progenitor populations subfractionated by CD62L (data not shown). Panel B For each of the four populations shown in Panel A, 3 × 103 sorted cells were transplanted along with 2.5 × 105 normal bone marrow cells as competitors into groups of 4 to 5 lethally irradiated recipients. Beginning 2 weeks after transplantation, peripheral blood was sampled from each animal and complete blood counts plus the percentage of donor-derived cells in each lineage were determined. The absolute number of donor-derived cells in each lineage is plotted. Error bars represent SEM. Significance values refer to the p value of the indicated data set compared to the data set of the nearest point of lower value, as calculated using a one-sided T-test with equal variance. Three of 5 mice transplanted with CD62LhighSca-1low cells had no platelet engraftment at any time after transplant, while 2 mice showed wide variation in platelet engraftment. For this reason no data is plotted for this population. Panel C For engraftment in the nucleated lineages, the activity of the CD62Lneg/low and CD62Lhigh subsets did not differ. These data were therefore pooled to limit the analysis to the subsets resolved by Sca-1low and Sca-1high expression levels. Other details of the analysis are as in Panel B.
HPLC analysis of hemoglobin variants
A HPLC cation exchange protocol was developed in our laboratory to discriminate and quantify Hbbd and Hbbs in the peripheral blood samples (14). A stock solution of 100 mM 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) (Sigma-Aldrich, St. Louis, MO) was prepared by dissolving 100 mg of DTNB in 2.5 ml of DMSO and stored at −20°C. The RBC fraction was derivatized by adding 5 µl of the RBC fraction into 250 µl of 40 mM NaCl and 2 mM DTNB and incubating at room temperature for 30 minutes. Following centrifugation at 12,000 g for 2 minutes, the supernatant was analyzed using a VARIANT hemoglobin testing system (Bio-Rad Laboratories, Hercules, CA) with an optimized β–thalassemia short program.
Repopulating Unit (RU) calculation
The RU calculation is a commonly used method to quantify the frequency of repopulating cells in comparison to a known quantity, often competitor cells of whole bone marrow (12, 20). The formula for RU calculation is: donor RU = % donor cells * C/(100 − % donor cells), where C = the number of competing RU(12). One competing RU is assumed to be equivalent to 105 whole bone marrow cells (21, 22).
Results
CD62L and Flt3 expression patterns in the KLS pool are analogous
In order to test the hypothesis that CD62L is a useful marker of the early stages of hematopoietic differentiation, the KLS pool of early hematopoietic progenitors was analyzed for CD62L expression (Figure 1). The expression pattern (Figure 1A) revealed a bimodal distribution of CD62L among KLS cells, with one subset expressing an intensity of CD62L expression equal to or exceeding that seen on spleen cells (CD62Lhigh hereafter, representing 61 ± 2% of KLS cells) and a second subset ranging from negative to low (CD62neg/low hereafter, representing 39 ± 2% of KLS cells). Multiparameter flow cytometry comparing the distribution of CD62L with respect to Thy-1.1, which has been previously been shown to segregate Thy-1.1pos HSC from Thy-1.1neg progenitor cells (23, 24), validated the distinction between the CD62neg/low and CD62Lhigh subsets (Figure 1B). Visual examination of the data indicated a clear discrimination of four distinct populations, analogous to what is seen using Thy1.1 and Flt3 expression for the traditionally defined LT-HSC, ST-HSC and MPP subsets (Figure 1B). The fourth subset resolved by both marker sets as Thy-1.1negCD62Lneg/low or Thy-1.1negFlt3neg comprised about 15% of KLS cells and was not further evaluated in these studies, however we have previously characterized the Thy-1.1negCD62Lneg/low KLS subset as primarily a B lymphocyte progenitor population with some T lymphocyte and myeloid engraftment potential (15).
The CD62Lneg/low fraction of KLS contains the LT-HSC population
In order to investigate the presence of HSC in the CD62Lhigh and CD62Lneg/low fractions in vivo, we performed a transplant experiment. GFPtg/−Hbbs/s mice were used as donors to allow for the tracking of platelets and white blood cells (WBC) produced from the transplanted populations via flow cytometric analysis. Red blood cells (RBC) were tracked by the hemoglobin variant Hbbs using HPLC analysis. For each transplant recipient, 103 donor KLS cells were sorted according to CD62L expression alone (Figure 1A), without selection for Thy-1.1 or Flt3 expression. The donor cells were transplanted into lethally irradiated Hbbd/d recipient mice along with 105 recipient bone marrow cells serving as competitors.
The transplant data showed that CD62Lneg/low cells reconstituted all three lineages of the hematopoietic system of the recipient mice strongly and persistently (Figure 1C). In contrast, CD62Lhigh cells failed to engraft persistently. While cells of all three blood lineages were generated by CD62Lhigh cells, the donor-derived cells diminished significantly during the weeks post-transplant. Donor-derived platelets were undetectable shortly after the transplant, while donor-derived RBC were not detected after week 9. WBC diminished to a very low level after initial engraftment, but persisted throughout the entire observation period. The maximum number of RBC peaked prior to week 3 for CD62Lhigh cells, however, CD62Lneg/low cells did not peak in RBC production until week 5 (Figure 1C). Altogether, the data suggest that LT-HSC are restricted to the CD62Lneg/low and are not present in the CD62Lhigh population.
To confirm the utility of the CD62Lneg/low phenotype as a marker of LT-HSC, the bone marrow of the recipient mice was examined following the termination of the transplant experiment. Bone marrow samples harvested from recipients of CD62Lhigh or CD62Lneg/low cells were lineage-depleted and stained with c-kit and Sca-1 antibodies in order to analyze the KLS compartment for GFP+ donor cells. Bone marrow cells of the CD62Lneg/low recipients showed a significant level of GFP+ cells (54 ± 24% of KLS, range 35–89%) while in recipients of CD62Lhigh cells only trace numbers of GFP+ cells were found (1.7±0.6% of KLS, range 1.1–2.4%, Figure 2A). The GFP+ KLS cells were then isolated by FACS sorting and transplanted into another set of lethally irradiated hosts. Since CD62Lhigh recipients generated only trace numbers of GFP+ cells in their KLS fractions, all GFP+ cells were pooled into one injection and given to one recipient. Five weeks later, peripheral blood samples were analyzed for donor-derived cells. Only mice receiving CD62Lneg/low donor-derived GFP+ KLS cells produced GFP+ progenies in the secondary transplant recipients (Figure 2B). The one mouse transplanted with CD62Lhigh donor-derived KLS GFP+ cells did not produce a detectable number of GFP+ cells in any lineage. These secondary transplantation results demonstrate that LT-HSC are restricted to the CD62Lneg/low fraction and are not present in appreciable numbers within the CD62Lhigh fraction.
Figure 2. LT-HSC are confined to the CD62Lneg/low fraction of the KLS compartment.
The transplant recipients shown in Figure 1C were sacrificed 13 weeks post-transplant and their bone marrow cells analyzed for the presence of donor-derived cells within the KLS population. Panel A Lineage-depleted bone marrow prepared from each of the 5 transplant recipients in each group was evaluated for GFP+ cells within the KLS subset. One representative animal from each group is shown. Numbers indicate mean ± SD values of each group. Panel B GFP+ KLS cells from each of 5 CD62Lneg/low transplant recipients were sorted and transplanted into one lethally irradiated secondary recipient. The trace numbers of GFP+ KLS cells in CD62Lhigh transplant recipients were pooled together and transplanted into one lethally irradiated secondary recipient. Five weeks post-transplant, peripheral blood analysis was performed. The average percentages of donor-derived cells are shown. “UD” indicates that donor-derived cells were undetectable.
The CD62Lhigh fraction of KLS contains the MPP population
In the primary transplant of the experiment shown in Figure 2, we transplanted 105 bone marrow cells along with an equal number of 103 cells for both CD62Lneg/low and CD62Lhigh fractions. To evaluate the presence of MPP as defined by our ability to discriminate donor-derived cells in the peripheral blood of transplant recipients over time, we transplanted CD62high cells at a dose 10-fold higher than in the previous experiment (104 cells per recipient). To evaluate the differential frequency of LT-HSC in the CD62Lneg/low fraction, these cells were transplanted at a limiting dose of 102 cells. As before, both populations were transplanted competitively with 105 bone marrow cells. Analysis of RBC engraftment in this experiment confirmed and extended our previous findings. Transplantation of 104 CD62Lhigh cells in competition with 105 BM cells produced a wave of donor-derived RBC that diminished over time to a negligible percentage at week 16 (Figure 3A). This indicates that the CD62Lhigh subset of KLS, which comprises less than 0.1% of the bone marrow, includes about 95% of the transient erythroid progenitor potential based on competition with 105 bone marrow cells. CD62Lhigh progenitors also peaked in RBC and WBC production earlier than CD62Lneg/low cells. WBC analysis showed results similar to the previous experiment, consistent with the interpretation that the CD62Lneg/low and CD62Lhigh populations contain LT-HSC and MPP, respectively (Figure 3A).
To compare relative activity of the CD62Lneg/low and CD62Lhigh populations in the absence of normal marrow competitors, and to investigate if both populations of cells conferred radioprotection, 500 cells per CD62L fraction were transplanted. The results (Figure 3B) show that donor cells of both fractions successfully rescued all transplanted mice. However, persistent engraftment of donor-derived cells was observed only following transplantation of CD62Lneg/low cells, while CD62Lhigh cells provided only transient engraftment of erythroid and leukocyte lineages that was eclipsed by endogenous HSC activity by 16 weeks post-transplant. Collectively, the data shown in Figures 1–3 illustrate the utility of CD62L expression levels as a useful biomarker for separation of LT-HSC from MPP within the KLS bone marrow population.
The CD62Lneg/low fraction of the KLSFneg compartment includes most LT-HSC activity
Thy-1.1 and Flt3 have previously been characterized as markers for FACS-sorting of LTHSC. To evaluate the distribution of CD62L expression relative to LT-HSC potential among KLS cells in the context of Thy-1.1 and Flt3, we performed multiparameter flow cytometry to evaluate the Thy-1.1posFlt3neg subset of KLS, previously shown to include LT-HSC, with respect to CD62L expression. Electronic gating on the Thy-1.1posFlt3neg fraction of KLS (KLSFneg) showed that the frequencies of the CD62Lneg/low (78 ± 3%) and CD62Lhigh (22 ± 3%) populations reversed with respect to that seen in the complete KLS population (Figure 4A; compare to Figure 1A). To evaluate LT-HSC activity, we transplanted 2 × 103 sorted cells (KLSFneg CD62Lneg/low and KLSFneg CD62Lhigh) into lethally irradiated recipients along with 105 normal bone marrow competitor cells.
Peripheral blood analysis for engraftment activity showed strong tri-lineage engraftment by the KLSFneg CD62Lneg/low fraction, reconstituting approximately 90% of platelets, erythrocytes, and leukocytes in spite of competition from a 50-fold excess of unseparated bone marrow cells (Figure 4B). In contrast, the KLSFneg CD62Lhigh fraction showed a persistent but weak engraftment. Platelet and WBC engraftment diminished to approximately 10% at the end of the analysis period, while RBC engraftment stabilized at approximately 20%. These results indicate that the KLSFnegCD62Lneg/low fraction includes the majority of LT-HSC.
To allow a direct quantitative comparison of LT-HSC activity between the CD62Lneg/low and CD62Lhigh subsets of KLSFneg cells, we calculated repopulating unit (RU) values for each subset. Since platelet life-span is shorter than erythrocyte or lymphocyte life-span, we utilized the platelet data for RU calculation. Platelet engraftment at the end of analysis period was 7.7% for KLSFnegCD62Lhigh cells and 85% for KLSFnegCD62Lneg/low cells, resulting in calculated RU values of 0.083 and 5.7, respectively. Adjusting for the frequency of each subset, we calculate that the frequency of LT-HSC within the KLSFneg population is approximately 240-fold higher in the KLSFnegCD62Lneg/low population relative to the KLSFnegCD62Lhigh population. Therefore, we conclude that the KLSFnegCD62Lneg/low population includes the majority of LT-HSC in mouse bone marrow.
Phenotypically distinct subpopulations suggest heterogeneity within the MPP population
In order to test the hypothesis that CD62L-based subfractionation can demonstrate heterogeneity within the MPP population, we analyzed the MPP subset of KLS using flow cytometry. The KLS cells were labeled with Flt3 and Thy1.1 antibodies to display the traditional division of KLS into LT-HSC-, ST-HSC- and MPP-enriched subsets (Figures 1B and 5A). Combined labeling of Flt3 and CD62L revealed a high degree of co-expression, since 23 ± 1% of cells expressed neither marker and 50 ± 2% of cells expressed both markers (Figure 5A). This high degree of co-expression pattern is particularly interesting considering previous evidence demonstrating that variable expression levels of Flt3 correlated with variable capacity for multipotency among Flt3+ MPP(9, 10). Also, the presence of cells that are mutually exclusive for the expression of CD62L and Flt3 (27 ± 1% of KLS, Figure 5A) demonstrates distinct populations that would not be identifiable without the use of CD62L as a marker.
To proceed with the isolation of subpopulations for functional analysis, the Thy1.1neg Flt3pos MPP subset of KLS cells was gated to subdivide the cells into 4 subsets based on Sca-1 and CD62L levels (Sca-1low/Sca-1high, CD62Lneg/low/CD62Lhigh, Figure 5A). Based on previous studies, we would expect the least mature of these subsets to be Sca-1high and CD62Lneg/low and the most mature subset to be Sca-1low and CD62Lhigh. The 4 subsets were isolated from GFPtg/−Hbbs/s donor mice by cell sorting, reanalyzed to confirm purity, and 3 × 103 cells of each population were transplanted along with 2.5 × 105Hbbd/d whole bone marrow cells into lethally irradiated Hbbd/d mice. Peripheral blood samples of the transplant recipients were periodically analyzed to identify and quantify progenies of specific lineages.
Over the course of 9 weeks, the transplanted populations displayed varying amounts Meg/E potential. CD62Lneg/lowSca-1high cells, suspected to include the least mature progenitor population among the four, generated significantly more erythrocytes and platelets relative to the other three populations (Figure 5B). As expected, all four progenitor populations generated platelets and erythrocytes only temporarily. The CD62LhighSca-1low population, suspected to include the most mature progenitors, generated the lowest number of erythrocytes and platelets among the four progenitor populations, and 3 of 5 recipients showed no platelet engraftment at any time after transplant (data not shown). CD62Lneg/lowSca-1low and CD62LhighSca-1high cells generated intermediate numbers of platelets and erythrocytes compared to the other two populations, suggesting that they represent transitional populations between the less mature CD62Lneg/lowSca-1high and the more mature CD62LhighSca-1low populations. The dramatic reduction of Meg/E potential observed as progenitors shifted from CD62Lneg/lowSca-1high to CD62LhighSca-1low is consistent with the idea that the CD62Lneg/lowSca-1high phenotype is uniquely associated with progenitor cells that retain Meg/E potential. This observation parallels other studies that also showed the reduction of Meg/E potential with the changing expression of other developmental antigens (Flt3 and VCAM-1) (7, 10).
The peripheral blood analysis of the transplant recipients also revealed differences in the numbers of white blood cell progenies generated from the subfractionated populations of the KLS MPP, however these were only significant between subsets discriminated by Sca-1 expression levels and not by CD62L expression levels. Both Sca-1high populations generated robust numbers of myeloid and B lymphocyte cells two weeks after transplantation, with B lymphocytes persisting to a greater extent compared to myeloid lineage cells (Figure 5C). A similar pattern was observed for T cell development, although donor-derived T cell numbers were low and not significant until 9 weeks post-transplant (data not shown). At this time, the Sca-1high subset generated 0.33 ± 0.09 × 103 T cells/µL of peripheral blood while the Sca-1low subset generated 0.07 ± 0.02 T cells/µL (p = 0.007). The observed difference in lineage potential seen in Figures 5B and 5C suggest that the CD62Lneg/lowSca-1high subset includes true MPP, but that upregulation of CD62L expression is accompanied by a decrease in Meg/E potential and a maintenance of white blood cell potential. Subsequently, a further loss of progenitor cell potential is identified by downregulation of Sca-1 expression.
Discussion
In this report, we characterize the utility of CD62L as a useful marker of hematopoietic differentiation. While numerous markers for isolation of HSC subsets have previously been described, additional markers of differentiation add to our understanding of the complexity of the hematopoietic hierarchy. New advances in fluorescent probe and instrument technologies allow a deeper and more detailed view into the stages of development previously defined by a relatively small subset of surface antigens. The depth of our understanding of early hematopoietic development will depend on the availability and specificity of various markers. Additionally, the utilization of robust methods for distinction of transplanted donor cells from recipient cells is critical, since the inability to visualize engraftment in erythroid and platelet lineages using the CD45 allelic system has resulted in a general lack of good experimental evidence regarding the progenitor cells for these lineages. In vitro colony-forming assays have been used for this purpose, but these experiments are prone to artifacts and lack the in vivo relevance inherent in transplantation assays (25, 26).
The expression profile of Flt3 in conjunction with Thy1.1 has been useful in resolving the three early hematopoietic compartments (LT-HSC, ST-HSC and MPP) that form the foundation of our current understanding of early hematopoietic events in adult mice (4–6). We have found that few commercially-available anti-Flt3 reagents are suitable for high-quality resolution of LT-HSC from MPP, and the use of Flt3 is further complicated by the lack of suitable positive-staining control cell populations. As an additional marker, Thy1.1 is effective for the isolation of HSC within the KLS population, but is limited to the few Thy1.1-expressing mouse strains since the more common Thy-1.2 allele is not expressed by most HSC (23). These issues emphasize the need for ongoing investigation of known markers as well as exploration for new markers to advance our ability to isolate HSC and to understand HSC biology. We have presented in vivo evidence for CD62L as an attractive alternative as well as a useful supplemental antigen for use with Flt3 in order to improve isolation of HSC subsets. Antibodies against CD62L are available in a wide variety of conjugates, and expression of CD62L in normal mouse spleen is robust (Figure 1A). Our transplant data demonstrate that CD62L fractionation of the KLS population yields a CD62Lneg/low population containing LT-HSC and a CD62Lhigh population devoid of LTHSC and containing MPP. Further fractionation of MPP as defined by the phenotype KLS-Thy-1.1neg Flt3pos convincingly separates CD62Lneg/low MPP from more restricted progenitor subsets lacking Meg/E potential that are CD62Lhigh. Differential temporal kinetics and persistence of engraftment are also consistent with the utility of CD62L expression as a marker of LT-HSC versus MPP and later stages of progenitor cells.
In this report, we have expanded upon previous findings describing CD62L as an effective marker for dividing the lymphoid progenitor subset (KLS-Thy-1.1neg) of bone marrow into a CD62Lhigh fraction, which resembled an early T-lineage progenitor, and a CD62Lneg/low fraction, which resembled the traditional MPP (15). Bone marrow transplant results from previous studies were restricted to the characterization of white blood cell engraftment only, since the approach used to differentiate the donor-derived hematopoietic cells from the cells of the recipient mice was allele-specific antibody labeling of CD45. As a result, previous studies could not discriminate identify Meg/E potential in the two fractions of MPP as we have shown here. The data presented in this study demonstrate subfractions of KLS MPP that are distinguishable by surface expression levels of CD62L and Sca-1 and are functionally distinct. Our in vivo transplant experiments have demonstrated that the CD62Lneg/lowSca-1high KLS MPP population includes the most robust engraftment activity in all lineages, while the CD62highSca-1low population exhibited significantly less production of all lineages. Interestingly, platelet and erythrocyte potential segregated from white blood cell potential at the CD62LhighSca-1high stage of development within the KLS MPP population. This finding is consistent with previous reports demonstrating that subpopulations within KLS MPP vary with respect to Meg/E activity.
Additional markers have previously been reported to subfractionate the KLS compartment into distinct subpopulations. Several of the lineage markers that are typically used for depletion of mature hematopoietic cells have been applied in subfractionation strategies (27). Another set of markers, the Slam family proteins (CD150, CD244 and CD48) have also been reported to be fractionate KLS into HSC and MPP (28, 29). A ligand for CD62L, the CD34 molecule, has also been used to isolate HSC and to fractionate KLS (30, 31). The co-expression patterns of these markers with CD62L is unknown. Future investigations into the relationship of CD62L expression with these other markers would be interesting, and may yield new subpopulations that may represent acute transition stages, granting a better understanding of early hematopoietic events at higher resolution.
Collectively, our data demonstrate that CD62L, despite having an expression pattern that is similar to that of Flt3, is able to identify functionally distinct subpopulations of MPP that would be impossible to resolve using Flt3 alone. Furthermore, the data support the model of heterogeneous KLS MPP that sheds Meg/E potential, marked by the upregulation of CD62L, prior to the lymphoid and myeloid lineage separation through the formation of lymphoid- and myeloid-restricted progenitor cells.
Footnotes
Authorship Contribution: SC and GJS jointly designed the research, performed experiments, analyzed results, generated figures, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
This work was funded by grants R01DK57899, RC1AI086238 (co-funded by the Office of the Director and NIAID), and T32DK007115 from the National Institutes of Health. Additional funding from the Brian Rooney Fund of The Lymphoma Foundation is gratefully acknowledged. The flow cytometry core facility used in these studies is partially supported by P30CA042014 awarded to the Huntsman Cancer Institute.
Abbreviations used in this article: APC, allophycocyanin; DTNB, 5,5’-dithiobis-(2-nitrobenzoic acid; HSC, hematopoietic stem cell; KLS, c-kitposLinneg/lowSca-1pos bone marrow stem/progenitor population; Lin, lineage antigens that define mature cells; LMPP, lymphoid-primed multipotent progenitor cells; LT-HSC, long-term HSC; Meg/E, erythro-megakaryocytic lineage; MPP, multipotent progenitor; RU, repopulating unit; ST-HSC, short-term HSC.
Historically, Thy-1.1 expression levels have been termed Thy-1.1neg, Thy-1.1low, and Thy-1.1high to distinguish stem/progenitor cells (Thy-1.1neg and Thy-1.1low) from mature T cells (Thy-1.1high). To avoid confusion due to this nomenclature, we here refer only to Thy-1.1neg and Thy-1.1pos cells since in this study it is not necessary to distinguish Thy-1.1low stem/progenitor cells from Thy-1.1high T cells.
References
- 1.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 4.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 5.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 7.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Akashi K, Traver D, Zon LI. The complex cartography of stem cell commitment. Cell. 2005;121:160–162. doi: 10.1016/j.cell.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 13.Scheid MP, Triglia D. Further description of the Ly-5 system. Immunogenetics. 1979;9:423–433. [Google Scholar]
- 14.Spangrude GJ, Cho S, Guedelhoefer O, Vanwoerkom RC, Fleming WH. Mouse models of hematopoietic engraftment: limitations of transgenic green fluorescent protein strains and a high-performance liquid chromatography approach to analysis of erythroid chimerism. Stem Cells. 2006;24:2045–2051. doi: 10.1634/stemcells.2006-0013. [DOI] [PubMed] [Google Scholar]
- 15.Perry SS, Wang H, Pierce LJ, Yang AM, Tsai S, Spangrude GJ. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood. 2004;103:2990–2996. doi: 10.1182/blood-2003-09-3030. [DOI] [PubMed] [Google Scholar]
- 16.Spangrude GJ, Brooks DM. Phenotypic analysis of mouse hematopoietic stem cells shows a Thy-1-negative subset. Blood. 1992;80:1957–1964. [PubMed] [Google Scholar]
- 17.Nakanishi T, Kuroiwa A, Yamada S, Isotani A, Yamashita A, Tairaka A, Hayashi T, Takagi T, Ikawa M, Matsuda Y, Okabe M. FISH analysis of 142 EGFP transgene integration sites into the mouse genome. Genomics. 2002;80:564–574. doi: 10.1006/geno.2002.7008. [DOI] [PubMed] [Google Scholar]
- 18.Harrison DE, Astle CM, Lerner C. Number and continuous proliferative pattern of transplanted primitive immunohematopoietic stem cells. Proc Natl Acad Sci U S A. 1988;85:822–826. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burstein SA, Friese P, Downs T, Mei RL. Characteristics of a novel rat anti-mouse platelet monoclonal antibody: application to studies of megakaryocytes. Exp Hematol. 1992;20:1170–1177. [PubMed] [Google Scholar]
- 20.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Harrison DE, Jordan CT, Zhong RK, Astle CM. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 22.Yuan R, Astle CM, Chen J, Harrison DE. Genetic regulation of hematopoietic stem cell exhaustion during development and growth. Exp Hematol. 2005;33:243–250. doi: 10.1016/j.exphem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Spangrude GJ, Brooks DM. Phenotypic analysis of mouse hematopoietic stem cells shows a Thy-1-negative subset. Blood. 1992;80:1957–1964. [PubMed] [Google Scholar]
- 24.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin− Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak JY, Cho S, Spangrude GJ. Gradients of antigen expression and developmental potential in hematopoiesis. Ann N Y Acad Sci. 2007;1106:82–88. doi: 10.1196/annals.1392.002. [DOI] [PubMed] [Google Scholar]
- 26.Richie Ehrlich LI, Serwold T, Weissman IL. In vitro assays misrepresent in vivo lineage potentials of murine lymphoid progenitors. Blood. 2011;117:2618–2624. doi: 10.1182/blood-2010-05-287102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 28.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1 doi: 10.1371/journal.pgen.0010028. e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3-short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]