Abstract
Listeria monocytogenes is a facultative intracellular bacterium that causes systemic infections in immunocompromised hosts. Early recruitment of myeloid cells, including inflammatory monocytes and neutrophils, to sites of L. monocytogenes infection is essential for the control of infection and host survival. Because previous experimental studies used depleting or blocking antibodies that affected both inflammatory monocytes and neutrophils, the relative contributions of these cell populations to defense against L. monocytogenes infection remain incompletely defined. Herein, we used highly selective depletion strategies to either deplete inflammatory monocytes or neutrophils from L. monocytogenes infected mice and demonstrate that neutrophils are dispensable for early and late control of infection. In contrast, inflammatory monocytes are essential for bacterial clearance during the innate and adaptive phases of the immune response to L. monocytogenes infection.
Introduction
Neutrophils and monocytes are distinct myeloid cell populations that contribute to innate immune defense against microbial pathogens (1, 2). Neutrophils constitute 50 to 60% of circulating white blood cells and are roughly 10 fold more numerous than circulating monocytes. Granulocytes are considered terminally differentiated cells that express a range of antimicrobial effector functions. On the other hand, circulating monocytes are pluripotent and, upon recruitment to sites of infection, can respond to the inflammatory context by differentiating into distinct effector populations expressing antimicrobial factors. The risk of microbial infection is markedly increased when mice or humans develop profound neutropenia (3). Upon recruitment to foci of infection, neutrophils phagocytose and kill microorganisms by generating reactive oxygen species and releasing granules that contain antimicrobial factors (4, 5). Neutrophil-mediated release of granules and chromatin generates neutrophil extracellular traps (NETs) that sequester and kill extracellular microbes (6–8). Neutrophils also secrete chemokines and recruit additional immune cells (9). In mice, circulating neutrophils specifically express the Ly6G cell surface protein (10).
Circulating monocytes enter the bloodstream from the bone marrow (11) and also contribute to antimicrobial defense. Monocytes are divided into subsets on the basis of chemokine receptor expression and the presence of specific surface markers (12). In mice, expression of high levels of Ly6C and CC-chemokine receptor 2 (CCR2) identifies a monocyte subset termed inflammatory or Ly6Chi monocytes. This population of monocytes represents approximately 2 to 5% of circulating white blood cells in an uninfected mouse and is rapidly recruited to sites of infection and inflammation. Inflammatory monocytes play essential roles in immune defense against various pathogens (2). Neutrophils and inflammatory monocytes share some antimicrobial effector functions, such as expression of phagocyte oxidase and secretion of TNF, and they are often recruited in tandem to sites of infection. However, the mechanisms and triggers inducing their recruitment and activation are distinct and their contributions to antimicrobial clearance are generally non-overlapping.
L. monocytogenes, a gram-positive facultative intracellular bacterium, causes severe disease in immunocompromised hosts and is acquired by ingestion of contaminated food (13, 14). Intravenous inoculation of mice with L. monocytogenes results in systemic infection and recruitment of neutrophils and monocytes to the spleen and liver. Early recruitment of inflammatory cells, mediated by CD11b, is essential for the control of L. monocytogenes infection (15) and sets the stage for adaptive immune responses, which ultimately clear the infection (16). Depletion of inflammatory cells by administering the depleting RB6-8C5 monoclonal antibody specific for Gr-1 markedly enhanced L. monocytogenes infection, and suggested that neutrophils play a major role in defense (17–21). At the time of these experiments, however, it was not appreciated that the RB6-8C5 monoclonal antibody associates with an epitope shared by the Ly6C and Ly6G proteins (22), and thus binds to and depletes neutrophils and inflammatory monocytes (2). Although a specific role for inflammatory monocytes in defense against Listeria monocytogenes has been revealed by studies in mice deficient for CCR2 (23, 24), a chemokine receptor that is required for monocyte recruitment (25), the relative contribution of neutrophils to defense against L. monocytogenes infection remains unresolved.
In this study, we specifically and selectively depleted inflammatory monocytes or neutrophils from mice during infection with Listeria monocytogenes. Inflammatory monocytes were depleted using a recently described CCR2-DTR mouse strain and neutrophils were depleted using the Ly6G-specific mAb 1A8 (10, 22), which deplete neutrophils but not Ly6Chi monocytes (26). We demonstrate that inflammatory monocytes but not neutrophils are essential in control L. monocytogenes infection.
Materials and Methods
Mice and infections
All mice used in this study were bred at Memorial Sloan-Kettering Research Animal Resources Center. C57BL/6 and OT-1 TCR-Tg mice were purchased from the Jackson Laboratory. The CCR2-GFP and CCR2-DTR transgenic strain were previously described (27, 28). Mice were infected intravenously (IV) with 3 × 103 or 5 × 105 L. monocytogenes strain 10403S for primary or secondary challenges, respectively, or 1 × 105 LM-OVA, as described previously (29). At the indicated times following infection, livers were harvested and dissociated in PBS containing 0.05% Triton X-100, and bacterial CFU were determined by plating on brain-heart infusion agar plates.
mAb and DT treatment
Gr-1-specific RB6-8C5 and Ly6G-specific 1A8 monoclonal antibodies were purified from hybridoma supernatants. C57BL/6 mice were injected intra peritoneally (IP) with either 250ug per dose of 1A8 or RB6-8C5 antibodies following L. monocytogenes inoculation and daily during indicated periods after infection. CCR2 depleter mice were injected IP with 10 ng/g body weight dose of DT following L. monocytogenes inoculation and at indicated time points.
Flow cytometry
At various times points, blood, spleens and livers were collected and prepared as previously described (30). Non-parenchymal cells from livers were further purified using 40%/80% Percoll gradients. Cells at the interface of the Percoll layers were collected and subjected to Ab staining (28). The following fluorescent Abs were purchased from BD Pharmingen (San Diego, CA): anti–CD11b (M1/70), Ly6C (AL-21), Ly6G (1A8), CD4 (RM4-5), Thy1.1 (OX-7), Thy1.2 (30-H12), CD44 (IM7), IFN-γ (XMG1.2), and FoxP3 (FJK-16s). Anti–CD45 (30-F11), CD45.1 (A20), and CD62L (DREG-56) were purchased from BioLegend (San Diego, CA). Staining for intracellular IFN-γ, FoxP3 was performed using the foxp3 staining kit from ebioscience (San Diego, CA) according to the product manual. FACS was performed with a LSRII and data was analyzed with Flowjo software. For morphological examination, cell subsets were obtained by FACS sorting with the purity of >98%. Cells were spun onto glass slides and stained using Diff-Quik Satin Set (Dade Behring).
Adoptive transfers of OT1 cells and in vitro T cell re-stimulation
Naive CD8 OT1 TCR-tg cells were isolated from spleens of OT1 TCR-tg mice using a CD8+ isolation kit from Miltenyi Biotec followed by further FACS sorting for CD44loCD62Lhi population. 2 × 104 purified OT1 cells was injected intravenously into naive recipients 1 d before infection. T cells were isolated from spleens at d7.5 after infection, re-stimulated in vitro with peptide-pulsed DC in the presence of brefeldin A for 5h, and then subjected to intracellular cytokine staining.
Histology and immunofluorescence
The spleen or the left lobe of the liver were collected and fixed in 4% paraformaldehyde; 10-µm cryosections were prepared using a Leica CM1900UV microtome (Leica Microsystems, Wetzlar, Germany). Foci of infection were visualized by staining with L. monocytogenes antiserum (BD Biosciences, San Jose, CA), followed by staining with Alexa633-F(ab′)2-anti-rabbit IgG (Invitrogen). Neutrophils were visualized by anti-Ly6G (1A8) staining followed by staining with Alexa594-anti-rat IgG. Nuclei were visualized with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired with Leica TCS SP2 AOBS laser scanning confocal microscope (Leica Microsystems) with a 20× 0.7 NA objective lens. Acquired images were processed and analyzed with Volocity software (PerkinElmer Improvision, Waltham, MA).
Statistics
The unpaired Student's t test and log-rank (Mantel-Cox) test were used for statistical analyses with GraphPad Prism software. p values were calculated by t test; *p < 0.05, **p < 0.01, ***p < 0.001. p < 0.05 was considered statistically significant. All data are presented as the arithmetic mean ± SEM.
Results
Monocytes and neutrophils are recruited during L. monocytogenes infection
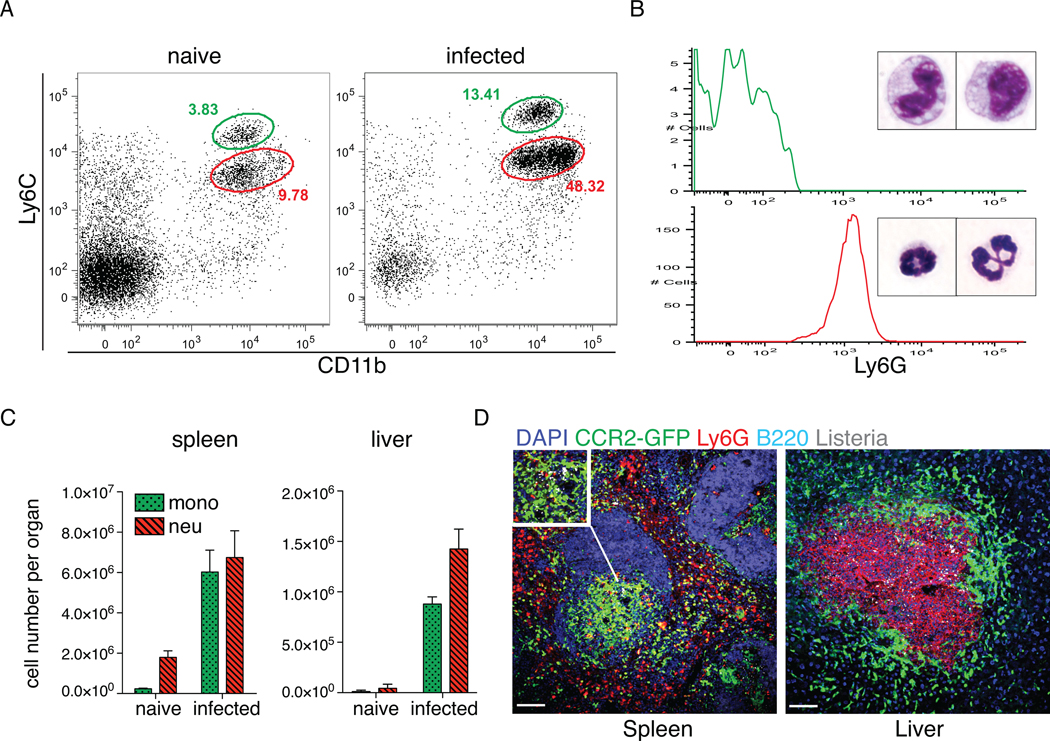
Systemic L. monocytogenes infection induces emigration of Ly6Chi monocytes and neutrophils from the bone marrow. Increased numbers of circulating monocytes, which are identified as CD11bintLy6ChiLy6G−, and neutrophils, which are identified as CD11bhiLy6CintLy6G+, are detectable within 1d of infection (Fig. 1A). As expected, monocytes and neutrophils differ in size and have distinct nuclear morphologies (Fig. 1B). Ly6Chi monocytes and neutrophils were recruited to the spleen and liver after infection of L. monocytogenes (Fig. 1C). To determine the localization of monocytes and neutrophils in the spleens and livers of infected mice, we used CCR2-GFP-reporter mice to detect inflammatory monocytes and staining with a Ly6G-specific monoclonal antibody to detect neutrophils (Fig. 1D). Whereas the majority of neutrophils localized to the red pulp of the spleen, Ly6Chi monocytes aggregated within the white pulp in areas of L. monocytogenes infection. In the liver, monocytes and neutrophils were also recruited to foci of infection. Neutrophils localized to the center of bacterial foci of infection, whereas monocytes remained more peripheral, forming a ring around the site of infection (Fig. 1D and (28)). The different localization of monocytes and neutrophils in hepatic foci of infection suggests that the two cell types make distinct contributions to innate defense against L. monocytogenes infection.
Figure 1. Monocytes and neutrophils are recruited to sites of L. monocytogenes infection.
(A). Increased numbers of circulating inflammatory monocytes (CD11bintLy6Chi) and neutrophils (CD11bhiLy6Cint) at d1 post infection with i.v. 3000 L. monocytogenes. Representative FACS plots are shown, with green color gate indicating Ly6Chi monocytes, and red color gate indicating neutrophils. (B). Morphology and Ly6G expression of FACS sorted CD11bintLy6Chi (upper) and CD11bhiLy6Cint (lower). (C). Numbers of Ly6Chi monocytes and neutrophils were numerated in spleens and livers of uninfected and d3 L. monocytogenes-infected mice. (D). Immunofluorescence staining was performed on spleen and liver sections of infected CCR2-GFP reporter mice at d3 p.i. GFP identifies Ly6Chi monocytes, and Ly6G identifies neutrophils. Data are presented as the arithmetic mean ± SEM. Scale bars represent 100 µm.
Differential depletion of monocytes and neutrophils
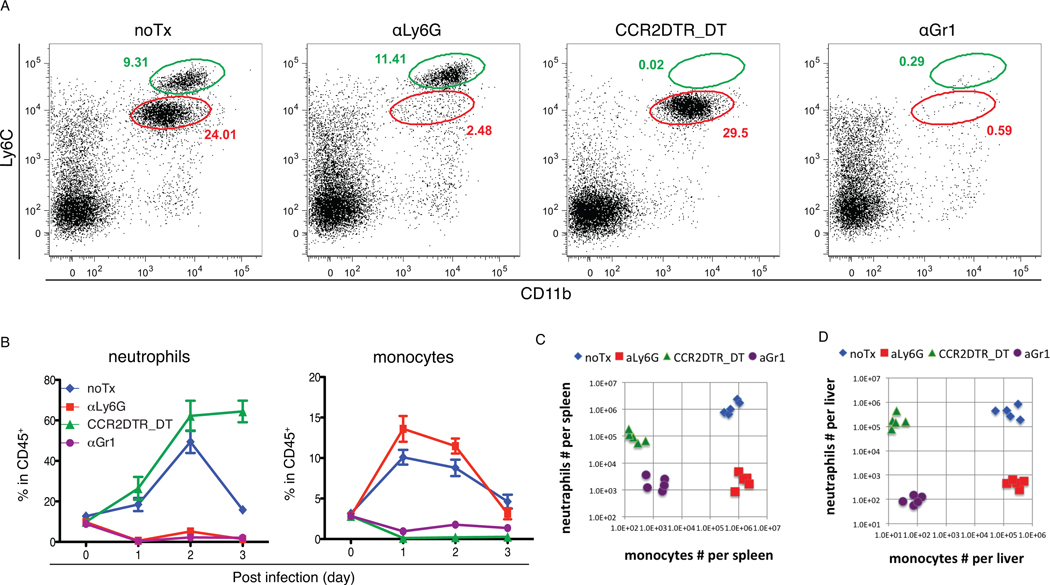
In order to characterize the roles of neutrophils and monocytes during L. monocytogenes infection, we developed an experimental strategy to selectively deplete these cell populations from infected mice. The anti-granulocyte receptor 1 (Gr1) monoclonal antibody (mAb) RB6-8C5 has been used extensively to deplete neutrophils in mice and to investigate the role of these cells in host defense to various infections (31–35). Since RB6-8C5 depletes both neutrophils and inflammatory monocytes (10), it is impossible to attribute phenotypic changes to one or the other cell population. Although blood monocytes can be depleted by i.v. injection of clodronate liposomes (36), this approach also depletes tissue macrophages and often increases circulating neutrophils due to reagent induced inflammation (32). Recently, the specific depletion of inflammatory monocytes using a CCR2-DTR mouse strain has been described (27). Administration of diphtheria toxin to CCR2-DTR mice results in 99% depletion of inflammatory monocytes that persists for 3 to 4 days. Specific depletion of neutrophils is achieved by injecting a Ly6G-specific mAb 1A8 (37). No overt toxicity is observed in RB6-8C5, 1A8 or DT-treated uninfected mice, and, in the absence of infection, treated mice remained healthy for at least 14-days. To test the effectiveness of neutrophil and monocyte depletion strategies, 1A8, RB6-8C5, and DT were injected into C57BL/6 mice and CCR2-DTR mice, respectively, following L. monocytogenes inoculation and during active infection. Circulating monocytes and neutrophils in the blood were monitored daily to measure the extent of cellular depletion (Fig. 2A and B). The accumulation of monocytes and neutrophils to spleens and livers was abolished by respective depletions of these cell populations (Fig. 2C and D). Of note, the recruitment of monocytes is not diminished by αLy6G treatment, suggesting neutrophils do not contribute to monocyte recruitment. Decreased numbers of neutrophils were recovered from the spleens of mice in which monocytes were depleted. This likely reflects increased cell death resulting from high bacterial burdens. Recruitment and positioning of monocytes or neutrophils at sites of infection in the spleen and liver are not affected by the depletion of either population (Fig. 3).
Figure 2. Differential depletion of monocytes and neutrophils.
C57BL/6 or CCR2-DTR mice were infected with 3000 L. monocytogenes. αLy6G 1A8 ab or αGr1 RB6-8C5 ab was injected i.p. upon infection and daily post infection. DT was injected upon infection and at d2 post infection. (A). Blood was obtained from infected mice with indicated treatment, and analyzed by flow cytometry. FACS plots show CD45+ nucleated cells in the circulation at d1. (B). Frequencies of Ly6Chi monocytes and neutrophils in CD45+ nucleated cells were monitored at the indicated time points after infection. Numbers of Ly6Chi monocytes and neutrophils were numerated in spleens (C) and livers (D) from mice with indicated treatment 3d after infection. Data are presented as the arithmetic mean ± SEM.
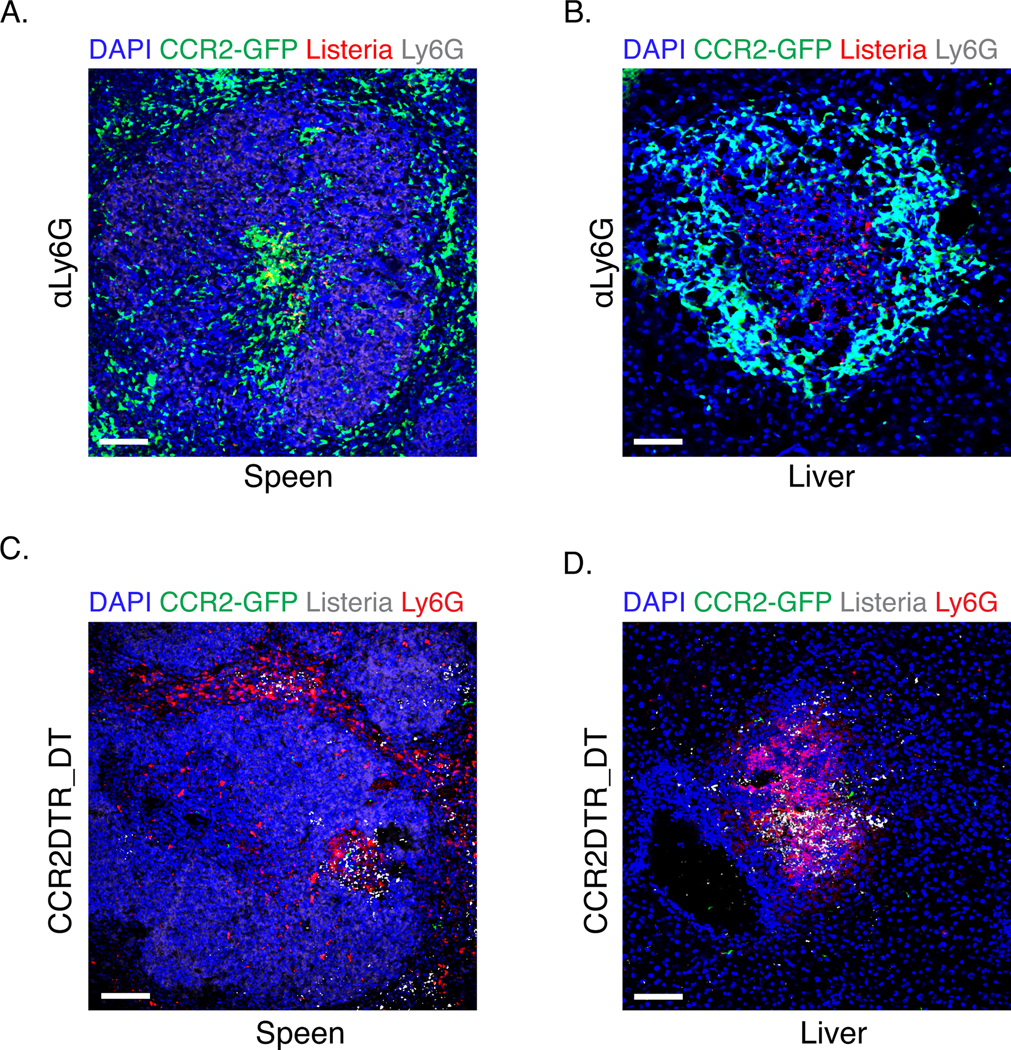
Figure 3. Immunofluorescence staining of the spleen and liver after the depletion of monocytes or neutrophils.
C57BL/6 or CCR2-DTR mice were infected with 3000 L. monocytogenes. αLy6G 1A8 ab was injected i.p. upon infection and daily post infection. DT was injected upon infection and at d2 post infection. Organs were collected at d3 p.i. Panels (A) and (B) are spleen and liver sections from mice treated with αLy6G; panels (C) and (D) are spleen and liver sections from CCR2DTR mice having received DT. Scale bars represent 100 µm.
Inflammatory monocytes but not neutrophils are essential for control of L. monocytogenes infection
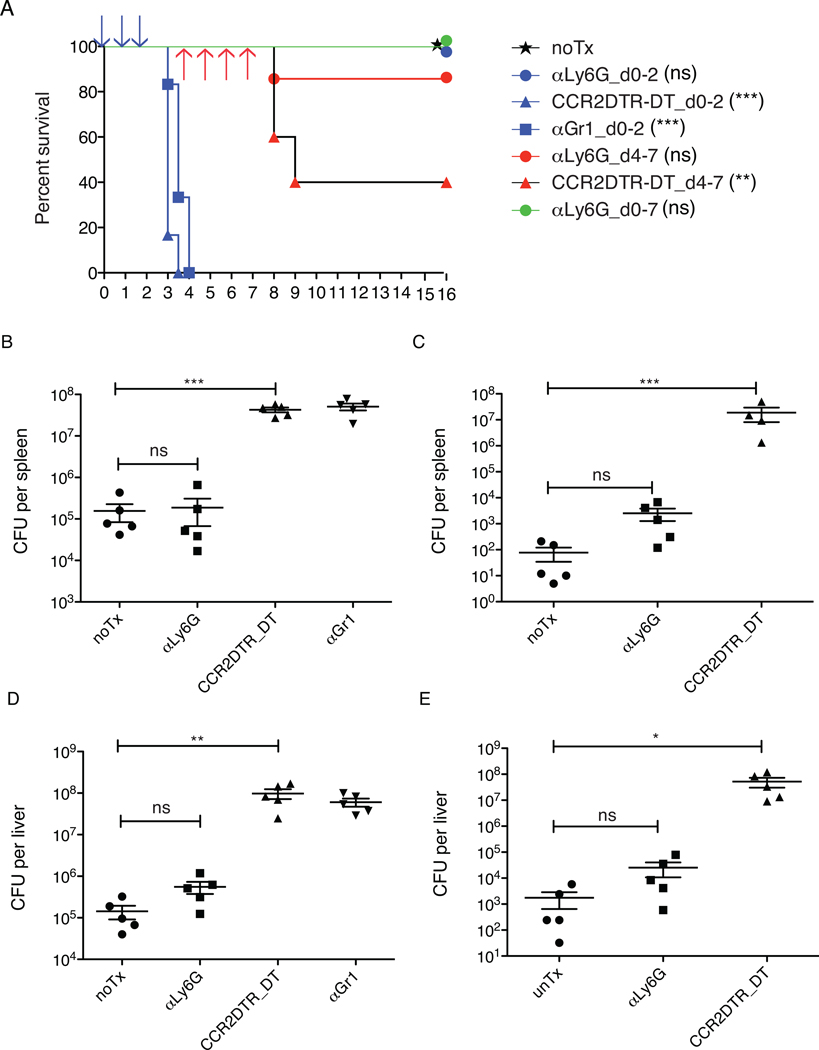
While previous studies have suggested that neutrophils play an essential role in early immune defense against L. monocytogenes infection (17–21), we found that monoclonal antibody 1A8-mediated depletion of Ly6G+ neutrophils did not adversely affect survival of infected mice (Fig. 4A). Furthermore, depletion of neutrophils 4 to 7 days after infection also did not significantly impact survival (Fig. 4A). Cultures of spleens and livers from neutrophil depleted mice at the end of the 16-day observation period revealed complete clearance of L. monocytogenes. In contrast, mice rapidly succumbed to L. monocytogenes infection when monocytes were depleted by DT administration to CCR2-DTR mice or αGr1 Ab administration to C57BL/6 mice in the early stage of infection (Fig. 4A), with markedly increased bacterial counts in spleens (Fig. 4B) and liver (Fig. 4D) at day 3 after infection. Treatment of CCR2-DTR mice with DT on days 4 through 7 following L. monocytogenes inoculation also resulted in increased mortality (Fig. 4A) and significantly higher numbers of bacteria in the spleen (Fig. 4C) and liver (Fig. 4E), as determined on day 8 after infection. These results indicate that Ly6Chi monocytes and monocyte-derived cells, but not neutrophils, are essential for effective immune defense against L. monocytogenes infection.
Figure 4. Inflammatory monocytes but not neutrophils are essential in control L. monocytogenes infection.
C57BL/6 or CCR2-DTR mice were infected with 3000 L. monocytogenes. αLy6G 1A8 ab or αGr1 RB6-8C5 ab was injected i.p. upon infection and daily during the periods indicated. DT was injected upon infection and at every other day during the periods indicated.
(A). Survival of infected mice under three different depletion regimens. Ab or DT was administrated at periods of d0-3(blue), d4-7(red), d0-7(green). 10–12 mice per group were used. p values were derived from the comparisons of each treatment group to untreated group, and are indicated in parentheses. Viable L. monocytogenes from spleens (B) and livers (D) was quantified at d3 after infection, when Ab or DT was administrated at d0-2. Viable L. monocytogenes from spleens (C) and liver (E) was quantified at d8 after infection, when Ab or DT was administrated at d4-7. Each symbol represents one mouse. Data are presented as the arithmetic mean ± SEM. **, P ≤ 0.01; ***, P ≤ 0.001.
T cell responses are not affected by depletion of neutrophils
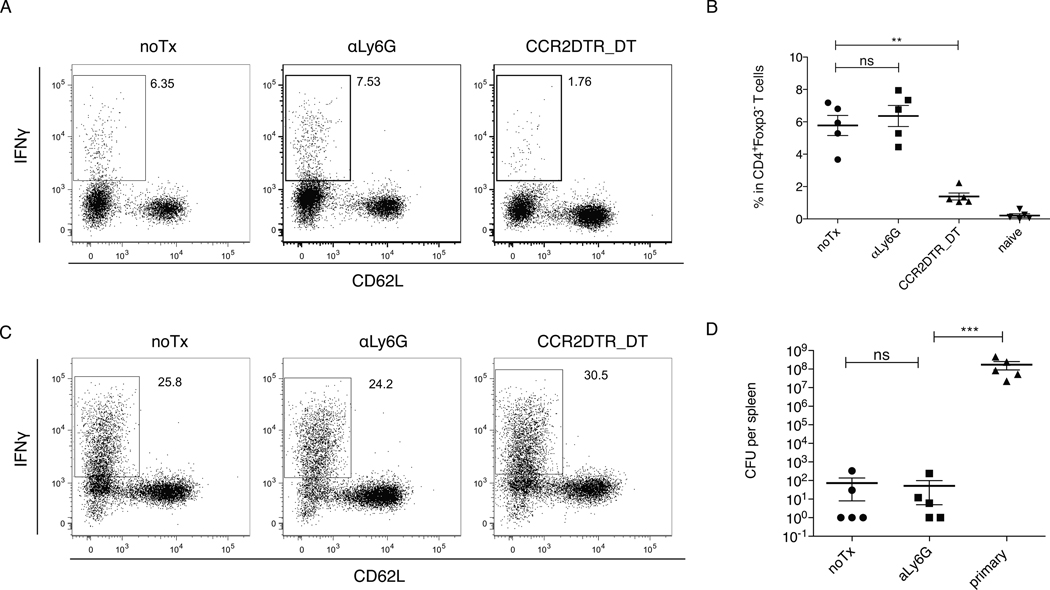
Early inflammatory responses influence T cell responses to L. monocytogenes. Although previous studies have implicated neutrophils in CD8 T cell priming following L. monocytogenes infection (38), the in vivo depletion approaches that were used could not distinguish between neutrophils and monocytes. To determine whether depletion of Ly6Chi monocytes or neutrophils affects T cell responses, we depleted inflammatory monocytes or neutrophils 4–7 days following infection and L. monocytogenes-specific CD4 and CD8 T cells responses were measured. CD4 T cell responses were measured by intracellular cytokine staining after stimulation of T cells from the spleen with DCs coated with the immunodominant LLO190–201 epitope. CD8 T cell responses were measured by adoptively transferring OT-1 TCR transgenic cells into recipient mice followed by infection with a strain of L. monocytogenes that secretes ovalbumin (LM-OVA). Treatment with αLy6G Ab did not change the frequency of IFNγ producing, LLO190–201 specific CD4 T cells, or IFNγ production by adoptively transferred OT1 cells. In contrast, monocyte depletion markedly impaired IFNγ expression by CD4 T cells while the OVA-specific CD8 T cell response remained intact (Fig. 5A–C). Mice with continuous depletion of neutrophils from d0 to d7 resolved the infection, and were protected 21 days following primary infection from re-challenge with a lethal dose (Fig. 5D), indicating that T cell-mediated protective immunity against L. monocytogenes does not depend on neutrophils.
Figure 5. T cell responses are not affected by depletion of neutrophils.
OT1 TCR-tg cells were adoptively transferred into C57BL/6 or CCR2-DTR mice 1d before infection with 1e5 LM-OVA. αLy6G 1A8 ab or αGr1 RB6-8C5 ab was injected i.p. daily during the period of d4-7. DT was injected i.p. at every other day during the period of d4-7. T cells were isolated from spleens at d7.5 after infection, and were re-stimulated in vitro with peptide-pulsed DC in the presence of brefeldin A for 5h, followed by intracellular cytokine staining.
(A). Representative FACS plots of CD4+Foxp3− T cells recovered from spleens of infected mice that have undergone treatments as indicated. T cells were re-stimulated with DC plus LLO190–201 peptides. (B). Frequencies of IFNγ producing cells among CD4+Foxp3− T cells. (C). Representative FACS plots of OT1 TCR-tg cells recovered from spleens of recipient mice that have undergone treatments as indicated. T cells were re-stimulated DC plus OVA (SIINFEKL) peptides. (D). C57BL/6 or CCR2-DTR mice were infected with 3000 WT L. monocytogenes. αLy6G 1A8 ab was injected i.p. daily during the period of d4-7. Mice were re-challenged with 5 × 105 WT L. monocytogenes at d21 after the first infection. Viable Listeria from spleens were quantified 3d after secondary infection, and compared with that from primary infection. Data are presented as the arithmetic mean ± SEM. **, P ≤ 0.01; ***, P ≤ 0.001.
Discussion
Neutrophils efficiently and expeditiously clear extracellular pathogens by releasing bactericidal granules and producing NETs (5, 6). Patients with severe neutropenia are particularly vulnerable to infections caused by extracellular pathogens but not infection by intracellular bacteria. Thus, it has been difficult to reconcile the clinically and experimentally defined role for neutrophils in defense against extracellular bacterial infection with experimental studies suggesting a requirement for neutrophils in defense against Listeria monocytogenes infection. Recent studies have demonstrated that depletion of Gr-1+, but not Ly6G+ myeloid cell populations exacerbates infections caused by intracellular pathogens, including Toxoplasma gondii (37), mycobacteria (39), herpes simplex virus type 1 (40) and certain strain of influenza (41). Our studies extend the spectrum of infections for which neutrophils are dispensable to include L. monocytogenes and also demonstrate, through the use of CCR2-depleter mice, that the susceptibility phenotype associated with Gr1-depletion, is attributable exclusively to inflammatory monocyte depletion. Our study, together with others’ using different intracellular microbial pathogens, suggest that microbial escape from the extracellular environment into intracellular compartments of host cells is an effective strategy to evade neutrophil-mediated killing.
Acknowledgments
This work is supported by National Institutes of Health Grants R37 AI039031 and PO1 CA023766.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703–1714. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 4.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 5.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 7.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 8.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 9.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 10.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 11.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 13.Gellin BG, Broome CV, Bibb WF, Weaver RE, Gaventa S, Mascola L. The epidemiology of listeriosis in the United States--1986. Listeriosis Study Group. Am J Epidemiol. 1991;133:392–401. doi: 10.1093/oxfordjournals.aje.a115893. [DOI] [PubMed] [Google Scholar]
- 14.Gellin BG, Broome CV. Listeriosis. JAMA. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 15.Rosen H, Gordon S, North RJ. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 17.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 19.Czuprynski CJ, Brown JF, Wagner RD, Steinberg H. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect Immun. 1994;62:5161–5163. doi: 10.1128/iai.62.11.5161-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakhmilevich AL. Neutrophils are essential for resolution of primary and secondary infection with Listeria monocytogenes. J Leukoc Biol. 1995;57:827–831. doi: 10.1002/jlb.57.6.827. [DOI] [PubMed] [Google Scholar]
- 21.Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagendra S, Schlueter AJ. Absence of cross-reactivity between murine Ly-6C and Ly-6G. Cytometry A. 2004;58:195–200. doi: 10.1002/cyto.a.20007. [DOI] [PubMed] [Google Scholar]
- 23.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 25.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 26.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 27.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi C, Velazquez P, Hohl TM, Leiner I, Dustin ML, Pamer EG. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J Immunol. 2010;184:6266–6274. doi: 10.4049/jimmunol.0904160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauvau G, Vijh S, Kong P, Horng T, Kerksiek K, Serbina N, Tuma RA, Pamer EG. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294:1735–1739. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- 30.Shi C, Jia T, Mendez-Ferrer S, Hohl T, Serbina N, Lipuma L, Leiner I, Li M, Frenette P, Pamer E. Bone Marrow Mesenchymal Stem and Progenitor Cells Induce Monocyte Emigration in Response to Circulating Toll-like Receptor Ligands. Immunity. 2011 doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun. 2001;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis. 2009;200:647–656. doi: 10.1086/600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Zhang ZH, Watanabe T, Yamashita T, Kobayakawa T, Kaneko A, Fujiwara H, Sendo F. The involvement of neutrophils in the resistance to Leishmania major infection in susceptible but not in resistant mice. Parasitol Int. 2005;54:109–118. doi: 10.1016/j.parint.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Watanabe T, Watanabe H, Sendo F. Neutrophil depletion exacerbates experimental Chagas' disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur J Immunol. 2001;31:265–275. doi: 10.1002/1521-4141(200101)31:1<265::AID-IMMU265>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Zhang Z, Sendo F. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin Exp Immunol. 2000;120:125–133. doi: 10.1046/j.1365-2249.2000.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 37.Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun. 2010;78:1564–1570. doi: 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tvinnereim AR, Hamilton SE, Harty JT. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J Immunol. 2004;173:1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761–771. doi: 10.1016/j.immuni.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Wojtasiak M, Pickett DL, Tate MD, Londrigan SL, Bedoui S, Brooks AG, Reading PC. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J Gen Virol. 2010;91:2158–2166. doi: 10.1099/vir.0.021915-0. [DOI] [PubMed] [Google Scholar]
- 41.Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The Role of Neutrophils during Mild and Severe Influenza Virus Infections of Mice. PLoS ONE. 2011;6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]