Abstract
Objective
Sleep spindles and K-complexes are EEG hallmarks of non-REM sleep. However, the brain regions generating these discharges and the functional connections of their generators to other regions are not fully known. We investigated the neuroanatomical correlates of spindles and K-complexes using simultaneous EEG and fMRI.
Methods
EEGs recorded during EEG-fMRI studies of 7 individuals were used for fMRI analysis. Higher-level group analyses were performed, and images were thresholded at Z≥2.3.
Result
fMRI of 106 spindles and 60 K-complexes was analyzed. Spindles corresponded to increased signal in thalami and posterior cingulate, and right precuneus, putamen, paracentral cortex, and temporal lobe. K-complexes corresponded to increased signal in thalami, superior temporal lobes, paracentral gyri, and medial regions of the occipital, parietal and frontal lobes. Neither corresponded to regions of decreased signal.
Conclusions
fMRI of both spindles and K-complexes depicts signal subjacent to the vertex, which likely indicates each discharges’ source. The thalamic signal is consistent with thalamic involvement in sleep homeostasis. The limbic region’s signal is consistent with roles in memory consolidation. Unlike the spindle, the K-complex corresponds to extensive signal in primary sensory cortices.
Significance
Identification of these active regions contributes to the understanding of sleep networks and the physiology of awareness and memory during sleep.
Keywords: electroencephalography (EEG), functional MRI (fMRI), spindles, K-complexes, sleep, non-REM
Introduction
Sleep is a brain state behaviorally suggesting brain inactivity, but actually due to active interactions of multiple brain networks related to different functions (Hobson and Pace-Schott, 2002). Although behavior is similar through the sleep period, sleep stages with distinct electrophysiological features are present that divide sleep into rapid eye movement (REM) sleep and a progression of deeper stages of non-REM (NREM) sleep (Kajimura et al., 1999). Spindles and K-complexes are ubiquitous NREM sleep EEG features and are signs of progression into stable sleep with the reaching of stage 2. They are well characterized by EEG features, but the network of generating and associated regions is poorly understood.
Spindles are spontaneous rhythmic bursts of waxing and waning waves occurring at the head’s vertex with a frequency typically between 12 – 14 Hertz and a duration typically between 0.5 – 1 second. They occur on a higher voltage, slow EEG background as isolated discharges or linked to a K-complex (Stern, 2005). The commonly accepted belief, corroborated by recent research, is that spindles may have a gating role for external sensory information during sleep, representing an EEG epiphenomenon of an underlying mechanism that maintains the sleep state. Despite great interest in spindles among sleep researchers, information about spindles’ brain networks remains preliminary (Hofle et al., 1997), (Gjedde, 1997), (Anderer et al., 2001), (Schabus et al., 2007), (Laufs et al., 2007). As complexes, K-complexes comprise more than one component. They are characterized by a short surface-positive wave followed by a larger surface-negative wave and then a positive wave. They typically are immediately followed by a spindle (Loomis et al., 1935), (Colrain, 2005), (Halasz, 2005), (Fisher and Cordova, 2006), (Chang et al., 2011). The common understanding is that they are a non-specific, multimodality-induced evoked potential during NREM sleep (Halasz, 2005).
After more than 70 years of sleep and EEG research, each discharge’s role and relationship to more widespread brain activity and networks remains unresolved. The purpose of this research is to identify the brain regions that are functionally related to spindles and K-complexes. Simultaneous recording of EEG and functional MRI (fMRI) provides such information by temporal identification of electrophysiologic activity with subsequent spatial localization of cerebral metabolic correlates of the activity (Stern, 2006).
Methods
EEGs recorded during EEG-fMRI studies of 7 participating individuals were reviewed to determine whether spindles and K-complexes were present. The EEG-fMRI studies were performed according to an IRB-approved protocol investigating the fMRI correlates of EEG activity from individuals with epilepsy and control individuals without neurologic disorders. The study group underwent uniform EEG and fMRI methods and each participant was partially sleep-deprived to half of a usual night’s sleep. No participant napped between morning awakening and imaging, which was performed between late morning and early afternoon. The partial sleep deprivation was intended to increase the likelihood of entering NREM stage 2 without increasing the risk that participants with epilepsy had seizures from more prolonged sleep deprivation. Antiepileptic medications were continued without a change to the participant’s usual regimen. No sleep inducing medication was administered.
EEG was recorded with an fMRI-compatible device (fEEG, Kappametrics, Inc., Virginia, US) that reduces fMRI and ballistocardiographic noise to allow visualization of cerebrally generated signals (Schachter et al., 2009). Each imaging session included multiple EEG-fMRI recordings with durations that ranged from 3.5 to 15 minutes. The total EEG-fMRI recording time for a participant was 45 to 60 minutes. Participants were instructed to relax with eyes closed during imaging and allow spontaneous sleep. No auditory stimulus was present except for the acoustic MRI noise.
The EEG recordings included 32 channels sampled at 2000 Hz from standard scalp electrode locations using carbon electrodes and wires and segmented RF filtering enclosures. Analog noise subtraction specific for each scalp location was followed by digital processing to yield a final gradient and ballistocardiographic noise reduction of 92 dB (McGlone et al., 2009). Analog subtraction was achieved by recording two channels of data from each electrode location. One channel included the cerebrally generated signal and the other was from the same scalp location but was shielded from the skull and included only the ambient noise. Digital processing used a real-time adaptive software algorithm that produced a noise template that was updated every 2 – 3 seconds and subtracted from the analog-corrected EEG signal. Ballistocardiographic artifact was digitally reduced for each channel individually using a timing signal based on the heartbeat and a subtracted ballistocardiographic template.
Imaging was performed with a 3T MRI system (Trio, Siemens, Erlangen, Germany). BOLD-sensitive functional imaging was performed using a gradient echo echo-planar sequence with parameters: TR=2000 ms, TE=30 ms, slice thickness 4 mm, 34 slices, 3.3 mm × 3.3 mm in-plane resolution. High-resolution structural images were obtained during the same imaging session using a 3-D spoiled gradient recalled (SPGR) sequence with parameters: TR=20 ms, TE=3 ms, slice thickness 1 mm, 160 slices, 1 mm × 1 mm in-plane resolution.
After noise cancellation post-processing, two fellowship-trained electroencephalographers (JMS and ZH) independently reviewed the EEGs and determined the occurrence time for each spindle, K-complex, and epileptiform discharge. The exact duration for each spindle also was determined. Spindles immediately following K-complexes were not considered separately; thus, spindles linked to a K-complex were not included in the spindle analysis group.
Image analysis was performed using FEAT (fMRI Expert Analysis Tool) version 5.98 within FSL (fMRIB Software Library) version 4.1.1 (Oxford, UK, www.fmrib.ox.ac.uk/fsl) (Forman et al., 1995), (Woolrich et al., 2001). Motion correction, non-brain removal and spatial smoothing (FWHM 5 mm) were performed prior to statistical analysis. Motion correction produced three rotation and three translation parameters that were modeled, unconvolved, as nuisance regressors along with their temporal derivatives (Friston et al., 1996). Onsets for both spindles and K-complexes were modelled using an impulse function convolved with a double-gamma hemodynamic response function with local autocorrelation correction using the FEAT FILM model. Spindle durations also were included in the modelling. Both the data and the design were high-pass filtered using Gaussian-weighted least squares straight-line fitting (σ= 50.0 sec). Analyses were performed using three models that differed in their regressors of interest. The models included: 1) spindles, 2) K-complexes, and 3) both spindles and K-complexes. The model including both spindles and K-complexes was analyzed with four separate contrasts: a) spindles, b) K-complexes, c) K-complexes greater than spindles, and d) spindles greater than K-complexes. The mean activity of the spindles and K-complex was also tested in models 1 and 2, however without partially accounting for the variance attributed to the other discharge through its inclusion in the model. This combination of models provides consideration of each discharge as independent of the other with multiple approaches.
The results were co-registered using FSL’s FLIRT module (FMRIB’s Linear Image Registration Tool). Functional images were first aligned to the participant’s coplanar, higher-resolution, T2-weighted image, and then the resulting image was aligned to the participant’s T1-weighted high-resolution structural image. After each of these first level analyses, the high-resolution structural image was aligned to MNI space using the standard MNI-152 image. Spindles and K-complexes were then separately processed in second level, fixed effects analyses that combined each participant’s multiple scans. A third level, mixed effects analysis was used to combine data across participants to obtain group results separately for both spindles and K-complexes. The mixed effects model treats the participants as random effects and was estimated using FSL’s FLAME module (FMRIB’s Local Analysis of Mixed Effects). Z (Gaussianized T) statistic images from the group analysis were thresholded using clusters determined by a Z ≥2.3 and a corrected cluster significant threshold of p≤0.05 (Worsley et al., 1992). Lastly, the statistical images were co-registered for display to a structural MRI that was a study-specific average anatomical image including each participant’s high- resolution structural image transformed to MNI space.
Results
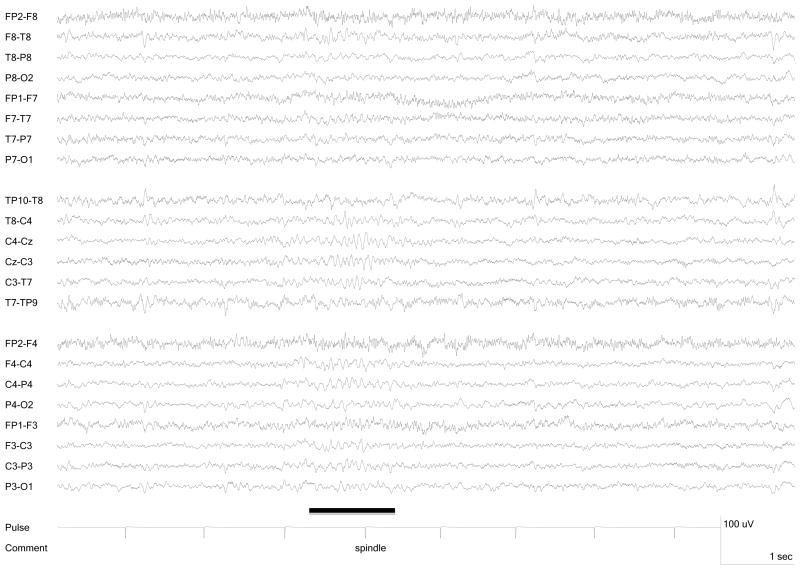
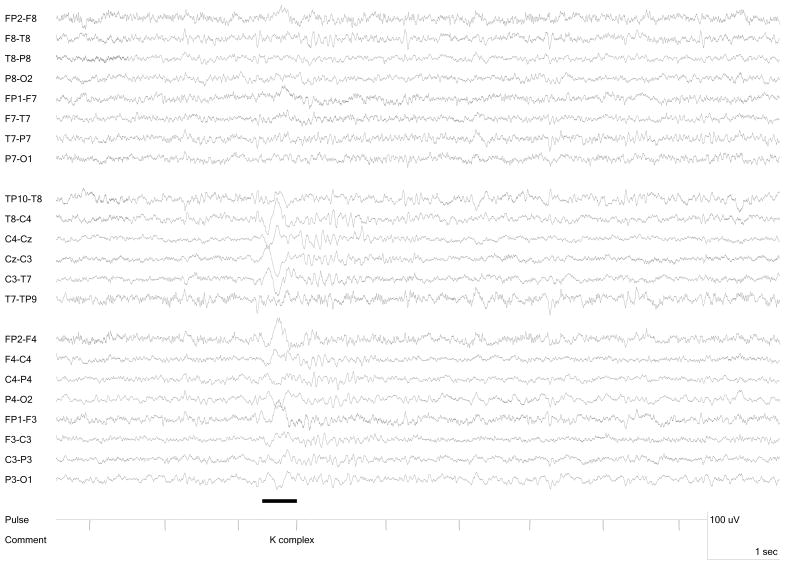
Seven participants (5 female, 2 male, age range 22 – 35 years) were included in the study group. Five had epilepsy and two were healthy volunteers without a history of neurologic illness or current use of neurologic medications (Table 1). Each participant had spontaneous sleep during the recording session, showing at least one spindle (Figure 1) and one K-complex (Figure 2). None reached slow wave or REM sleep. Overall, 86% of the scan time for this group was sleep with a range for the individuals from 58% to 100%. Scans including greater wakefulness were excluded from the data analysis because spindles and K-complexes were absent. Collectively among the 7 individuals, 106 central spindles and 60 K-complexes were present. (Figures 1 and 2) Frontal spindles were not present. The spindles had a total duration of 91.9 seconds. The total of 18 scans had a combined duration of 206 minutes. Interictal epileptiform discharges did not co-occur with any spindles or K-complexes. No behavioral or electrographic seizures occurred during any of the recordings.
Table 1.
Summary of participants and data collection
| Participant | Age | Gender | Epilepsy | MRI | AEDs | Scans | Total Wake Time/Total Scan Time (min) | Total spindles | Spindles total duration (sec) | Total K- complexes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | F | left TLE | left MTS | LEV | 2 | 4/13.5 | 6 | 8.5 | 6 |
| 2 | 33 | F | right TLE | right MTS | LTG, LEV | 1 | 0/10 | 9 | 6 | 5 |
| 3 | 22 | F | left TLE | left MTS | PHT | 4 | 8/37.5 | 24 | 23 | 11 |
| 4 | 27 | F | left TLE | left MTS | LEV, PHT | 3 | 0/35 | 46 | 35.6 | 5 |
| 5 | 35 | F | right TLE | right MTS | LTG, LEV | 3 | 2.5/40 | 3 | 1.5 | 5 |
| 6 | 26 | M | none | normal | none | 3 | 12.5/30 | 3 | 2.5 | 18 |
| 7 | 31 | M | none | normal | none | 2 | 1.5/40 | 15 | 14.8 | 10 |
| Combined | 18 | 28.5/206 | 106 | 91.9 | 60 |
KC: K-complex, TLE: temporal lobe epilepsy, MTS: mesial temporal sclerosis, LEV: levetiracetam, LTG: lamotrigine, PHT: phenytoin
Figure 1.
Spindle recorded during fMRI.
Figure 2.
K-complex recorded during fMRI
Note spindle follows K-complex (indicated by line).
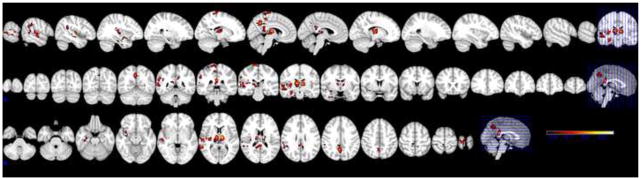
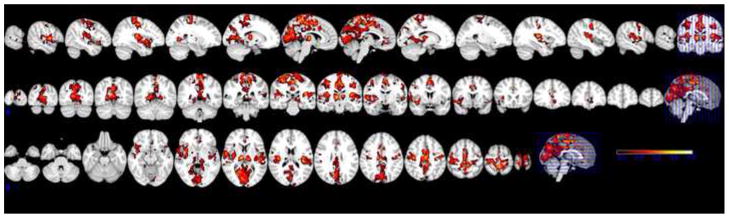
Functional statistical images corresponding to spindles’ occurrences indicated increased BOLD signal in bilateral thalami and posterior cingulate cortex, and right paracentral cortex, putamen, precuneus, and temporal lobe. (Figure 3) Analysis of the K-complexes identified increased signal in bilateral thalami, temporal lobes including hippocampi and amygdalae, paracentral gyri, posterior cingulate cortex, and the precuneus. (Figure 4) The maximum Z values for the voxel clusters corresponding to spindles ranged between 2.8 and 3.3. For the K-complexes, the maximum Z values ranged between 3.4 and 3.7. The locations of local activation clusters and their maximum Z values are listed in Tables 2 and 3. Neither spindles nor K-complexes corresponded to a region of signal decrease. In the spindle imaging, the regions of signal change were the same in both the model of spindles alone and the model that included K-complexes and contrast a (K-complexes as regressors of no interest). In the K-complex imaging, the regions of signal change were the same in the model of K-complexes alone and in two contrast analyses using the model of both spindles and K-complexes. These were contrast b (spindles as regressors of no interest) and contrast c (K-complexes greater than spindles). Contrast d (spindles greater than K-complexes) did not produce any regions of significant signal change.
Figure 3.
fMRI of 106 spindles from 7 individuals. Threshold Z ≥2.3.
Increased signal is within bilateral thalamus, posterior cingulate cortex, precuneus, right pre-central gyrus, right supramarginal gyrus, right superior temporal gyrus, right putamen, and right hippocampus. No regions of decreased signal are present. Model did not include K-complexes.
Figure 4.
fMRI of 60 K-complexes from 7 individuals. Threshold Z ≥2.3.
Increased signal is within bilateral thalamus, central and posterior midline cortex, bilateral pre-central gyri, bilateral superior temporal gyri, and bilateral insula. No regions of decreased signal are present. Model did not include spindles.
Table 2.
Regions of maximal, local signal change corresponding to spindles
| Region | Maximum Z-value | MNI Coordinates (x,y,z) | Cluster Index (Voxel Count) |
|---|---|---|---|
| Right thalamus | 3.26 | 11, −12, 12 | 1 (7335) |
| Left thalamus | 3.23 | −11, −14, 9 | 1 (7335) |
| Right temporal | 3.17 | 48, −15, 4 | 2 (6694) |
| Posterior cingulate cortex | 3.12 | 0, −38, 22 | 3 (6003) |
| Right hippocampus | 2.94 | 30, −18, −18 | 4 (2221) |
| Right middle temporal gyrus | 2.93 | 63, −48, 7 | 5 (4501) |
| Right pre-central gyrus | 2.89 | 5, −34, 54 | 6 (3669) |
| Right supramarginal gyrus | 2.80 | 45, −41, 24 | 5 (4501) |
Table 3.
Regions of maximal, local signal change corresponding to K-complexes
| Region | Maximum Z-value | MNI Coordinates (x,y,z) | Cluster Index (Voxel Count) |
|---|---|---|---|
| Right post-central gyrus | 3.74 | 1, −43, 62 | 1 (170159) |
| Right pre-central gyrus | 3.64 | 3, −32, 53 | 1 (170159) |
| Left pre-central gyrus | 3.62 | −1, −25, 54 | 1 (170159) |
| Right thalamus | 3.64 | 8, −15, 8 | 2 (32472) |
| Right insular cortex | 3.56 | 33, −24, 11 | 2 (32472) |
| Right superior temporal gyrus | 3.41 | 51, −2, 1 | 2 (32472) |
Discussion
The image analysis is based solely on statistical association between the discharges’ occurrence times and changes in local blood oxygenation (BOLD signal), which is understood to indicate metabolic changes related to local field potentials (Logothetis et al., 2001). As such, fMRI images depict regions encompassing the discharge’s generator, other active regions that are either downstream or upstream from the generator, and also active regions that are functionally integrated with these other regions. Therefore, regions of increased signal reflect the respective generators of spindles and K-complexes and regions functionally correlated with their occurrence that are not evident with EEG.
Spindles
The paracentral region of activity is subjacent to the spindle EEG localization, so it most likely indicates a neocortical source for this discharge. The thalamic activity is too deep to be evident at the scalp, but it is expected based on intracerebral electrophysiologic recordings of the thalamus indicating spindle generation includes a critical interaction between GABAergic neurons of the nucleus reticularis, which function as pacemakers, and glutamatergic thalamo-cortical projections that mediate their synchronized propagation to cortical regions (Steriade et al., 1985), (Steriade et al., 1987). As such, the spindle generator is not the source of spindles’ evident EEG discharge. The neuronal activity within the thalamus during spindles produces an increase in inhibition for external sensory experience during spindles, and supports the understanding that spindles have a role in sleep maintenance (Elton et al., 1997), (Cote et al., 2000), (Steriade et al., 1990). Overall, the region of greatest fMRI signal change is the thalamus, and this also is the region of neuronal generation and principal function for spindles.
The temporal lobe fMRI signal is consistent with the understanding that spindles have a role in memory function. NREM sleep benefits the consolidation of hippocampus-dependent (declarative) memory, and studies in rats and humans have shown increases in spindle density and activity during NREM sleep following memory tasks learned during the previous awake period (Maquet, 2001), (Gais et al., 2002). Moreover, thalamo-cortical spindles are believed to prime cortical networks for the long-term storage of memory representation (Diekelmann and Born, 2010). Memory formation in the brain has been conceptualized as the process in which neuronal activity reverberates in specific circuits that were active during the information encoding process to promote the consolidation of synaptic changes (Hebb, 1949); as such, the BOLD correlates for spindles may represent the process of re-activation and reverberating of neuronal circuits for the encoding of the sensory information and consolidation for memory storage. The unilateral signal is due to the right temporal lobe signal surpassing the threshold Z≥2.3, and the left temporal lobe signal not meeting threshold with its Z=2.0.
The precuneus and posterior cingulate regions of activity also may correspond with memory consolidation networks, as these regions have considerable connectivity with the anterior temporal lobes (Parvizi et al., 2006). The precuneus and posterior cingulate regions also are the hub of the default mode network and the spindle association finding may have implications for the understanding of the default mode network during sleep (Hagmann et al., 2008).
Comparison to previous functional imaging studies of spindles
Spindles have been investigated with EEG-fMRI by Laufs and colleagues, Schabus and colleagues, and Tyvaert and colleagues with mostly similar results (Laufs, Walker, 2007), (Schabus, Dang-Vu, 2007), (Tyvaert et al., 2008). All three groups identified increased signal in bilateral thalamus and no regions of decreased signal, which is consistent with our results, and both Schabus et al. and Laufs et al. identified paracentral increased signal, which also is consistent with our results. No other group identified the signal change we observed in the posterior midline cortex; however, Schabus et al. and Laufs et al. both identified increased signal in more anterior midline cortex. In summary, three out of four groups reported spindles to be associated with increased signal within the thalamus, cingulate, and paracentral gyrus.
Both the Laufs and Schabus groups also identified increased signal in bilateral superior temporal lobes. This contrasts with increased signal in only the right temporal lobe in our results and no temporal lobe signal change in the results by Tyvaert et al. The discrepancy may be due methodological differences, including image analysis and participant characteristics. The unilateral temporal lobe signal in our results would have been bilateral if a slightly lower threshold was chosen. The participant groups differed with the Schabus and Laufs groups including only healthy volunteers, and the Tyvaert group including only participants with epilepsy. Within the Tyvaert et al. group, 13 of the 15 total had temporal lobe abnormality, and all 5 of the epilepsy participants in our group had temporal lobe epilepsy. The other divergence in results is the identification of increased signal in bilateral putamen by Tyvaert et al. and in the right putamen in our results. Overall, this suggests the possibility that temporal lobe epilepsy may have an effect on temporal lobe and putamen activity during spindles, which would have implications toward the understanding of the commonly present memory dysfunction associated with MTLE.
The thalamic correlation is consistent with PET research showing a change in regional cerebral blood flow (rCBF) in the thalamus related to sleep spindles (Hofle, Paus, 1997). The PET result is decreased rCBF, which is interpreted as indicating the inhibitory neurotransmission that occurs in the reticular nucleus during sleep (Gjedde, 1997). However, inhibitory neurotransmission is still the result of a neuronal firing, which produces a metabolic change that may affect and the fMRI BOLD signal (Vanzetta and Grinvald, 2008). Although PET rCBF and fMRI BOLD are highly correlated, the relationship is non-linear, especially with decreases in rCBF (Ramsey et al., 1996), (Mechelli et al., 2001).
Whole-head magnetoencephalography examined the cortical regions involved in maximal spindle activity in healthy subjects during daytime naps, and identified multiple cortical sources encompassing frontal, parietal and temporal regions (Gumenyuk et al., 2009). However, averaging the sleep spindles’ amplitude from all of these regions identified a region of maximal activity in the paracentral region. This was interpreted as the neocortical generator, which is consistent to our fMRI findings.
K-complexes
Our K-complex results comprise the regions of fMRI signal during spindles and the regions of fMRI signal during vertex sharp transients (Stern et al., 2011). EEG-fMRI of vertex sharp transients has identified regions of activity in the primary sensorimotor cortices, which may be interpreted as indicating a role for vertex sharp transients in gating non-specific, multimodality sensorimotor function during sleep. Therefore, K-complexes may be producing a more expansive effect for sleep maintenance that combines the role of vertex sharp transients and sleep spindles. This is consistent with K-complexes typically occurring later in NREM sleep than the other two discharges. The question of whether a K-complex is truly a vertex sharp transient that has been linked to a spindle cannot be fully addressed with these results because fMRI temporal resolution cannot separate the K-complex and spindle that occur in succession. Therefore, fMRI cannot compare K-complexes independent of the after going spindle to vertex sharp transients or to spindles that occur separate from K-complexes. However, the same regions of signal change were present in model of K-complexes alone as in the model that included spindles and contrasted the spindles as either events of no interest or events that are less than the K-complex. This suggests that the K-complex results are not including regions of signal change that are entirely due to spindle activity, assuming isolated spindles are identical to spindles that follow K-complexes.
The functional significance of K-complexes has long been a source of debate, dividing researchers according to consideration of them as a cortical response to an arousing stimulus or a marker of a brain state conducive to the production of delta EEG activity for slow wave sleep (Colrain, 2005). Animal studies by Amzica and Steriade highlighted the link between K-complexes and delta sleep EEG activity with K-complexes representing the EEG expression of the mechanism underlying the rhythmic slow wave synchronization (Amzica and Steriade, 2002). A role for K-complexes in sleep-specific endogenous information processing for external or internal stimuli has been proposed, based on works using “oddball” paradigm experiments in normal sleeping humans (Colrain et al., 1999). An additional role for K-complex also has been proposed, which is in memory consolidation and similar to spindles (Diekelmann and Born, 2010). Overall, the principal functions of K-complexes are proposed to be the processing of external stimuli during sleep and the consolidation of sensory and possibly also emotional memory. The regions identified in our results are consistent with both of these functions.
Comparison to previous functional imaging studies of K-complexes
Laufs and colleagues investigated K-complexes as well as spindles (Laufs, Walker, 2007). Their results contrast strongly with ours as they identified widespread decreased signal, encompassing the thalamus, frontal, central, temporal, and, to a lesser extent, occipital cortices. The difference may be due to the group size difference (1 participant in their investigation) and analysis methods. Their results support the cortical down-state concept for K-complexes, a cellular level change that corresponds to hyperpolarizing potassium currents during which neuronal discharges are impeded. This is consistent with the K-complex as a response to external stimulation; however, our group-based results are also consistent with this understanding of the K-complex (Cash et al., 2009).
Study limitation and methodological issues
The study group included 5 patients with epilepsy being treated with antiepileptic drugs, which raises the question whether either the disorder or treatment affected our results. In particular, the patient participants all had mesial temporal lobe epilepsy (MTLE), which indicates limbic system dysfunction in a majority of the group. This potential confound is theoretical because individuals with MTLE have normal spindles, K-complexes, and other EEG features of NREM sleep, and the continuous EEG confirms that no seizures occurred during image acquisition and that no interictal epileptiform discharges co-occurred with spindles or K-complexes. Furthermore, the participants had different treatment regimens, and no one medication was present across the whole group. Nevertheless, the epilepsy and healthy groups differed in their contributions of the two discharge types to the dataset. The epilepsy patients accounted for 83% of the spindles but only 53% of the K-complexes. This was consistent with the healthy volunteers reaching deeper sleep during imaging. To better address whether the subgroups differed in their fMRI results, each was analyzed separately for spindles and K-complexes. Although the epilepsy and control groups were not large enough for an informative statistical comparison of them, the spindle and K-complex analyses produced similar regions of signal change. Larger participant groups would be needed to determine whether the subgroups differ in temporal lobe and putamen activity during spindles, as was observed by comparing our results to those reported by the Laufs et al., Schabus et al., and Tyvaert et al. Additional participants in both the healthy and epilepsy groups would provide the power to determine whether of temporal lobe abnormality during spindles is reliably present.
Conclusions
Active brain regions during both spindles and K-complexes include the bilateral thalami, paracentral regions, and temporal lobes, but K-complexes have larger regions of activity that comprise primary sensorimotor cortices similar to the regions active during vertex sharp transients (Stern et al., 2011). The thalamic signal may be partially due to the spindle-like component of the K-complex because it is absent during vertex sharp transients, but a regression analysis suggests at least part of the thalamic signal is not due to the after going spindle. Spindles and K-complexes both correspond to limbic system and paralimbic activity, which supports their proposed roles in memory consolidation. These results also may support a role for spindles and K-complex in sleep maintenance because the regions that are active during their occurrence are also integral to the processing of external, sensory stimuli.
Highlights.
Sleep spindles and K-complexes are features specific to non-REM sleep that may be related to sleep homeostasis and memory consolidation.
The full extent of brain regions active during their occurrences is not known.
Simultaneous EEG and functional MRI identified activity in the thalamus and temporal lobe for both discharges and additional regions in sensory cortex for the K-complex.
Acknowledgments
Funding was provided by NIH-NINDS K23 grant (NS044936), the Leff Family Foundation, and the Vradenburg Family Foundation.
Footnotes
Research was performed at UCLA, Los Angeles, California.
No conflicts of interest were present.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amzica F, Steriade M. The functional significance of K-complexes. Sleep Med Rev. 2002;6:139–49. doi: 10.1053/smrv.2001.0181. [DOI] [PubMed] [Google Scholar]
- Anderer P, Klosch G, Gruber G, Trenker E, Pascual-Marqui RD, Zeitlhofer J, et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001;103:581–92. doi: 10.1016/s0306-4522(01)00028-8. [DOI] [PubMed] [Google Scholar]
- Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, et al. The human K-complex represents an isolated cortical down-state. Science. 2009;324:1084–7. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BSDLS, Niedermeyer E. Normal EEG and Sleep: Adults and Elderly. In: Schomer DL, Lopes da Silva FH, editors. Niedermeyer’s Electroencephalography. 6. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- Colrain IM. The K-complex: a 7-decade history. Sleep. 2005;28:255–73. doi: 10.1093/sleep/28.2.255. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J Sleep Res. 1999;8:273–80. doi: 10.1046/j.1365-2869.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Cote KA, Epps TM, Campbell KB. The role of the spindle in human information processing of high-intensity stimuli during sleep. J Sleep Res. 2000;9:19–26. doi: 10.1046/j.1365-2869.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Elton M, Winter O, Heslenfeld D, Loewy D, Campbell K, Kok A. Event-related potentials to tones in the absence and presence of sleep spindles. J Sleep Res. 1997;6:78–83. doi: 10.1046/j.1365-2869.1997.00033.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Cordova S. EEG for Beginners. In: Krauss GL, Fisher RS, editors. The Johns Hopkins Atlas of Digital EEG. Baltimore: The Johns Hopkins University Press; 2006. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–55. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–4. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A. The relation between brain function and cerebral flow and metabolism. In: Hunt Batjer H, editor. Cerebrovascular Disease. Philadelphia: Lippincott-Raven; 1997. pp. 23–40. [Google Scholar]
- Gumenyuk V, Roth T, Moran JE, Jefferson C, Bowyer SM, Tepley N, et al. Cortical locations of maximal spindle activity: magnetoencephalography (MEG) study. J Sleep Res. 2009;18:245–53. doi: 10.1111/j.1365-2869.2008.00717.x. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz P. K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev. 2005;9:391–412. doi: 10.1016/j.smrv.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: A Neurophysiological Theory. New York: John Wiley & Sons; 1949. [Google Scholar]
- Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–93. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, et al. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci. 1997;17:4800–8. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura N, Uchiyama M, Takayama Y, Uchida S, Uema T, Kato M, et al. Activity of midbrain reticular formation and neocortex during the progression of human non-rapid eye movement sleep. J Neurosci. 1999;19:10065–73. doi: 10.1523/JNEUROSCI.19-22-10065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Walker MC, Lund TE. ‘Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study’--its limitations and an alternative approach. Brain. 2007;130:e75. doi: 10.1093/brain/awm084. author reply e6. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Loomis AL, Harvey EN, Hobart G. Potential Rhythms of the Cerebral Cortex during Sleep. Science. 1935;81:597–8. doi: 10.1126/science.81.2111.597. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- McGlone F, Dunseath R, Stern JM. Simultaneous EEG and functional MRI employing novel noise reduction. Epilepsia. 2009;50:82. [Google Scholar]
- Mechelli A, Price CJ, Friston KJ. Nonlinear coupling between evoked rCBF and BOLD signals: a simulation study of hemodynamic responses. Neuroimage. 2001;14:862–72. doi: 10.1006/nimg.2001.0876. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A. 2006;103:1563–8. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, Kirkby BS, Van Gelderen P, Berman KF, Duyn JH, Frank JA, et al. Functional mapping of human sensorimotor cortex with 3D BOLD fMRI correlates highly with H2(15)O PET rCBF. J Cereb Blood Flow Metab. 1996;16:755–64. doi: 10.1097/00004647-199609000-00001. [DOI] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–9. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter SC, Guttag J, Schiff SJ, Schomer DL. Advances in the application of technology to epilepsy: the CIMIT/NIO Epilepsy Innovation Summit. Epilepsy Behav. 2009;16:3–46. doi: 10.1016/j.yebeh.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Deschenes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54:1473–97. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–73. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, Llinas RR. Thalamic Oscillations and Signalling. New York: Wiley; 1990. [Google Scholar]
- Stern JM. Atlas of EEG Patterns. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Stern JM. Simultaneous electroencephalography and functional magnetic resonance imaging applied to epilepsy. Epilepsy Behav. 2006;8:683–92. doi: 10.1016/j.yebeh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Stern JM, Caporro M, Haneef Z, Yeh HJ, Buttinelli C, Lenartowicz A, et al. Functional imaging of sleep vertex sleep transients. Clin Neurophysiol. 2011;122:1382–1386. doi: 10.1016/j.clinph.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyvaert L, Levan P, Grova C, Dubeau F, Gotman J. Effects of fluctuating physiological rhythms during prolonged EEG-fMRI studies. Clin Neurophysiol. 2008;119:2762–74. doi: 10.1016/j.clinph.2008.07.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Coupling between neuronal activity and microcirculation: implications for functional brain imaging. HFSP J. 2008;2:79–98. doi: 10.2976/1.2889618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]