Abstract
Patients with corticobasal degeneration (CBD) pathology present with diverse clinical syndromes also associated with other neuropathologies, including corticobasal syndrome, progressive nonfluent aphasia, and an Alzheimer’s-type dementia. Some present with behavioral variant frontotemporal dementia (bvFTD), though this subtype still requires more detailed phenotypic characterization. All patients with CBD pathology and clinical assessment were reviewed (N=17) and selected if they initially met criteria for bvFTD [bvFTD(CBD): N=5]. Available bvFTD patients with Pick’s [bvFTD(Pick’s): N=5] were selected as controls. Patients were also compared to healthy older controls [N=53] on neuropsychological and neuroimaging measures. At initial presentation, bvFTD(CBD) showed few neuropsychological or motor differences from bvFTD(Pick’s). Neuropsychiatrically, they were predominantly apathetic with less florid social disinhibition and eating disturbances, and were more anxious than bvFTD(Pick’s) patients. Voxel-based morphometry revealed similar patterns of predominantly frontal atrophy between bvFTD groups, though overall degree of atrophy was less severe in bvFTD(CBD), who also showed comparative preservation of the frontoinsular rim, with dorsal > ventral frontal atrophy, and sparing of temporal and parietal structures relative to bvFTD(Pick’s) patients. Despite remarkable overlap between the two patient types, bvFTD patients with underlying CBD pathology show subtle clinical features that may distinguish them from patients with Pick’s disease neuropathology.
Keywords: Corticobasal degeneration, frontotemporal dementia, behavior, neuropsychiatry, neuropsychology, neuropathology
INTRODUCTION
Corticobasal degeneration (CBD) is a neurodegenerative disease affecting both cortical and subcortical areas, and manifesting in a variety of clinical syndromes. Since its original description (Reibiz, Kolodny, & Richardson, 1968), prediction of CBD neuropathology (i.e., “CBD”) has been based on motor disturbances that make up a corticobasal syndrome (CBS), classically involving a “dead” or “useless” limb due to rigidity, apraxia, akinesia, dystonia, or alien limb symptoms. While current diagnostic criteria for predicting CBD pathology emphasize these motor abnormalities (Litvan et al., 2003), other clinical presentations of CBD are gaining recognition. Though dementia was once thought to rule out CBD, recent studies suggest that cognitive impairment is not only a common feature, but is often an initial symptom in patients later confirmed to have CBD neuropathology (Grimes, Lang, & Bergeron, 1999). Both executive and visuospatial deficits have been reported in patients with presumed CBD (Graham, Bak, & Hodges, 2003; Tang-Wai et al., 2003; Hou, Carlin, & Miller, 2004) and a speech/language-predominant clinical syndrome is also increasingly recognized (Frattali, Grafman, Patronas, Makhlouf, & Litvan, 2000; Hou et al., 2004), usually presenting as a progressive nonfluent aphasia that later evolves into CBS (Kertesz, 1997; Boeve, Lang, & Litvan, 2003; Gorno-Tempini, Murray, Rankin, Weiner, & Miller, 2004; Knibb, Xuereb, Patterson, & Hodges, 2006; Seeley, Matthews et al., 2008).
While these cognitive syndromes resulting from CBD pathology have gained increasing attention, neuropsychiatric symptoms have also emerged as important predictors of underlying CBD. Depression, apathy, and irritability are common findings in CBS (Litvan, 1998; Wenning et al., 1998). Additionally, aggressiveness (Moretti, Torre, Antonello, Cattaruzza, & Cazzato, 2005), poor error monitoring (O'Keefe et al., 2007), inappropriate laughter or crying (Thumler et al., 2003), and obsessive-compulsive behavior (Rinne, Lee, Thompson, & Marsden, 1994; Rey et al., 1995; Geda et al., 2007) have been observed in patients with CBS. Hallucinations have been suggested as an exclusion criterion for predicting CBD pathology (Geda et al., 2007), though one case has been reported in the literature to date (Nagaoka, Ookawa, & Maeda, 2004).
The standards for clinical prediction of CBD pathology have been gradually broadening to include these common cognitive and neuropsychiatric features. However, some studies suggest that pathological CBD patients can present with more extreme behavioral syndromes. In particular, some CBD patients present with profoundly disordered personal and social behavior and loss of insight, with few or no motor symptoms, and are clinically diagnosed as behavioral variant frontotemporal dementia (bvFTD) (Wenning et al., 1998; Grimes et al., 1999; Josephs et al., 2006;Geda et al., 2007; Murray et al., 2007; Llado et al., 2008). One case described by Mathuranath demonstrated no CBD-type motor symptoms and was clinically diagnosed as bvFTD on the basis of drastic personality change, apathy, and cognitive deficits, but was found to have CBD neuropathology upon autopsy (Mathuranath, Xuereb, Bak, & Hodges, 2000). The same year, Kertesz reported on 35 patients who eventually developed clinical CBS, of whom seven were initially classified as bvFTD (Kertesz, Martinez-Lage, Davidson, & Munoz, 2000).
The ability to predict CBD pathology based on clinical presentation has been historically quite low, with a sensitivity averaging 35 percent (Litvan et al., 1997; Boeve et al., 1999; Josephs et al., 2006). Accuracy of early pathological predictions requires better incorporation of these divergent clinical presentations. Group-based neuroanatomic studies thus far have assumed that patients with CBD pathology share a common pattern of regional atrophy, or have divided patients according to whether they demonstrate either primarily motor symptoms or a dementia syndrome (Josephs et al., 2008). The subset of patients with CBD pathology who present with bvFTD has not been directly compared to bvFTD patients with other underlying pathologies. In this study, we examined these autopsy-proven patient groups using neuropsychological, neuropsychiatric, and quantitative anatomic methods to delineate distinct clinical profiles that might aid in the prediction of underlying CBD.
MATERIALS AND METHODS
Subjects
Seventeen consecutive patients (6 males and 11 females, all right-handed) with neuropathological CBD were identified and selected for review. All were seen at the Memory and Aging Clinic at the University of California, San Francisco between 1998 and 2007 and had undergone at least one neurological evaluation prior to death. All patients had an extensive dementia-oriented neuropathological diagnostic assessment at UCSF or the University of Pennsylvania, following a standard protocol described previously (Forman et al., 2006). Pathological CBD was diagnosed according to accepted research criteria (Dickson et al., 2002). Typical findings included neuronal loss, tau-positive neuronal and glial cytoplasmic inclusions, and thread pathology in both the cortex and subcortical white matter. Gliosis, astrocytic plaques and ballooned neurons were variably identified in the cortex. Five of the 17 patients presented with significantly disordered personality, emotion, and social cognition, and met Neary research criteria for bvFTD (Neary et al., 1998) at the initial visit. This group was designated by their initial clinical presentation, followed by their pathological diagnosis in parentheses, i.e., bvFTD(CBD). The remaining CBD patients were diagnosed with CBS (N=5), PNFA (N=4), PSP (N=2), and AD (N=1) and were excluded from further analysis because they did not meet criteria for bvFTD.
We then identified all consecutive patients with Pick’s disease neuropathology (N=11) identified according to consensus criteria (Zhukareva et al., 2002; Cairns et al., 2007). Of these, three had no clinical evaluation at the MAC prior to autopsy, two had only limited clinical data and had MRI scans incompatible with quantitative analysis, and one carried a clinical diagnosis of corticobasal syndrome and did not meet Neary criteria for bvFTD. The remaining five subjects met clinical criteria for bvFTD at initial presentation and had at least one full clinical evaluation, and were included as a comparison group, designated bvFTD(Pick’s).
Fifty-three healthy older control subjects (NC), recruited from the community, were included to provide a comparison for neuropsychological and imaging analyses. For inclusion, NCs were required to have a normal neurological examination, a Clinical Dementia Rating Scale (CDR) score=0, MMSE score equal to or greater than 28/30, and delayed memory performance equal to or greater than the 25th percentile in both verbal and visuospatial domains. An informant was required to corroborate subjects’ level of function.
All subjects and their informants signed an institutional review board-approved research consent form to participate in the study. Subjects’ demographic characteristics can be seen in Table 1.
Table 1.
Demographic, functional, and neuropsychological data at first examination for bvFTD(CBD) group (N=5), bvFTD(Pick's) (N=5), and healthy older Normal Control group (N=53). Due to the small bvFTD group sizes, data are represented as median(interquartile range).
| bvFTD(CBD) | bvFTD(Pick's) | NC | ||||
|---|---|---|---|---|---|---|
| Med(IQR) | Range | Med(IQR) | Range | Med(IQR) | ||
| Sex (M/F) | 3/2 | -- | 4/1 | -- | 24/29 | |
| Education | 14.5(5.0) | 12 – 19 | 16.0(2.0) | 12 – 20 | 18.0(4.0) | |
| Age at symptom onset | 58.0(11.0) | 53 – 74 | 60.0(7.0) | 49 – 71 | n/a | |
| Disease duration at first exam (years) | 3.4(2.0) | 3.1 – 9.4 | 3.7(1.8) | 2 – 8.2 | n/a | |
| Disease duration at death (years) | 7.6(1.3) | 3.8 – 12.7 | 7.2(3.3) | 3.3 – 9.8 | n/a | |
| GENERAL | ||||||
| MMSE | 22.0(11.0) | 9 – 26 | 27.0(1.0) | 22 – 27 | 30.0(1.0) | |
| Clinical Dementia Rating Scale | 1.0(1.5) | 0.5 – 2 | 1.0(0.0) | 1 – 2 | 0(0) | |
| CDR Sum of Boxes | 7.0(7.5) | 3 – 11.5 | 8.0(2.5) | 3.5 – 11 | 0(0) | |
| Functional Activities Questionnaire | 25.0(8.0) | 7 – 26 | 20.5(11.0) | 7 – 26 | n/a | |
| Neuropsychiatric Inventory Total | 35.0(7.0) | 18 – 41 | 47.5(19.5) | 36 – 58 | n/a | |
| NPI Distress Total | 12.0(9.0) | 0 – 18 | 20.5 (12.0) | 14 – 27 | n/a | |
| Geriatric Depression Scale (max=30) | 8.0(9.0) | 3 – 19 | 4.0(13.0) | 0 – 13 | 3.0(5.0) | |
| Calculations (max=5) | 2.0(3.0) | 0 – 4 | 5.0(1.0) | 1 – 5 | 5.0(0) | |
| Praxis Testing: | ||||||
| Cough (max = 2) | 2.0(0) | 2 – 2 | 2.0(0.5) | 1 – 2 | 2.0(0) | |
| Blow out a match (max=2) | 2.0(0) | 2 – 2 | 2.0(0.5) | 1 – 2 | 2.0(0) | |
| Praxis Total (max=14) | 14.0(1.5) | 11 – 14 | 13.5(3.0) | 9 – 14 | 14.0(0) | |
| SPEECH & LANGUAGE | ||||||
| Abbrev BNT (max=15) | 14.0(6.0) | 8 – 15 | 15.0(1.0) | 2 – 15 | 15.0(1.0) | |
| D-words/min | 3.0(2.0) | 0 – 11 | 4.0(2.0) | 2 – 10 | 16.0(6.0) | |
| Animals/min | 6.0(4.0) | 0 – 9 | 7.0(2.0) | 5 – 11 | 22.0(7.0) | |
| Syntax Comprehen. (max=7) | 4.0(2.0) | 2 – 4 | 4.0(0.0) | 2 – 4 | 4.0(0.0) | |
| Repetition (max=3) | 3.0(0) | 2 – 3 | 3.0(1.0) | 1 – 3 | 3.0(0) | |
| Write Sentence (pass/fail) | 3/2 | -- | 5/0 | -- | 50/0 | |
| Conversational Speech Ratings (max=4): | ||||||
| Prosody | 4.0(2.0) | 0 – 4 | 4.0(1.0) | 3 – 4 | 4.0(0) | |
| Phrase Length | 4.0(2.0) | 0 – 4 | 4.0(1.0) | 3 – 4 | 4.0(0) | |
| Paraphasic Errors | 4.0(0) | 4 – 4 | 4.0(0) | 4 – 4 | 4.0(0) | |
| Grammar | 4.0(1.5) | 1 – 4 | 4.0(0) | 4 – 4 | 4.0(0) | |
| Comprehension | 4.0(1.0) | 2 – 4 | 4.0(0) | 2 – 4 | 4.0(0) | |
| Word Finding | 4.0(2.0) | 0 – 4 | 4.0(0) | 4 – 4 | 4.0(0) | |
| VISUOSPATIAL | ||||||
| Pentagons (Pass/Fail) | 4/1 | -- | 4/0 | -- | 48/2 | |
| Modif. Rey-O copy (max=17) | 14.0 (2.0) | 12 – 17 | 15.0 (1.0) | 14 – 16 | 16.5(1.5) | |
| VOSP Number Location (max=10) | 7.5 (4.0) | 5 – 10 | 8.0 (3.0) | 6 – 9 | 10.0(1.0) | |
| Cube (max=2) | 1.0(1.0) | 0 – 2 | 2.0(1.0) | 1 – 2 | 2.0(0) | |
| MEMORY | ||||||
| CVLT-MS: | ||||||
| Total All Trials (max=36) | 10.0(12.0) | 5 – 22 | 20.0(12.0) | 9 – 24 | 30.0(6.0) | |
| Corr 30" (max=9) | 2.0(3.0) | 1 – 6 | 6.0(2.0) | 2 – 9 | 8.5(2.0) | |
| Corr 10' (max=9) | 1.0(3.0) | 0 – 4 | 5.0(1.0) | 0 – 8 | 8.0(3.0) | |
| Recog Hits (max=9) | 7.0(5.0) | 1 – 9 | 9.0(1.0) | 7 – 9 | 9.0(0) | |
| Recog False Positives (max=18) | 3.0(3.0) | 0 – 5 | 3.0(2.0) | 0 – 7 | 0(1.0) | |
| Modif. Rey-O 10' delay (max=17) | 6,0(8.0) | 1 – 11 | 6.0(7.0) | 2 – 14 | 13.0(4.5) | |
| Modif. Rey-O 10' delay Recog (pass/fail) | 2/3 | -- | 3/2 | -- | 45/3 | |
| EXECUTIVE/WORKING MEMORY | ||||||
| Span of digits backward | 2.0(5.0) | 0 – 5 | 4.0(1.0) | 0 – 6 | 5.0(2.0) | |
| World Backward (max=5) | 3.0(4.0) | 0 – 5 | 4.0(0.5) | 4 – 5 | 5.0(0) | |
| modified Trailmaking (#lines/min) | 4.5(4.5) | 0.5 – 7.1 | 3.3(2.8) | 2 – 7 | 30.0(20.5) | |
Neuropsychological/neuropsychiatric assessment
All subjects underwent one hour of cognitive testing upon their initial presentation to the clinic. Standard neuropsychological tests were administered to assess various aspects of cognition, while other cognitive features such as praxis, calculations, and conversational speech were rated using standardized screens developed for our clinic. For all but one bvFTD(Pick’s) patient, measures of functional and neuropsychiatric status were administered through an informant interview. See Table 1 for a detailed list of measures and results.
Image Acquisition
Structural imaging scans were obtained from 3/5 of bvFTD(CBD) patients and all bvFTD(Pick’s) and NC subjects using the same scanner and scanning protocol. One bvFTD(CBD) patient refused to participate in a research scan, though a separate 1.5T clinical scan was available for review. The other patient left the scanner prematurely in the midst of his T1 sequence, though T2 and FLAIR images were available for review. All magnetic resonance (MR) imaging studies were performed using 1.5 Tesla Magnetom VISION system (Siemens Inc., Iselin, NJ, USA) equipped with a standard quadrature head coil. Anatomic MR imaging sequences were obtained for each patient and included a volumetric magnetization prepared rapid gradient echo (MPRAGE, TR/TE/TI = 10/4/300 ms) in order to obtain T-1 weighted images, 15 degree flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm squared in-plane resolution and 1.5 mm slice thickness.
Voxel-Based Morphometry
The voxel-based morphometry (VBM) technique utilizes an image pre-processing step (spatial normalization, segmentation, modulation, and smoothing) followed by statistical analysis. Both stages were performed using the SPM5 software package (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm) running on Matlab 7.0.1 (MathWorks, Natick, MA). MRI images were pre-processed primarily using SPM5 default settings and tissue probability maps, though light cleanup of partitions was performed. Spatially normalized, segmented, and modulated grey matter images were then smoothed with a 12 mm FWHM isotropic Gaussian Kernel.
Covariate-by-condition statistical models were used to show subject group differences in voxel-wise gray matter volume. bvFTD(CBD), bvFTD(Pick’s), and NC subjects were entered into a single design matrix with age and sex as confounding covariates, and total intracranial volume (TIV) was used as a global covariate to correct for individual differences in head size. Regionally specific differences in grey matter volumes at each voxel were assessed using the general linear model, and the significance of each effect was determined using the theory of Gaussian fields.
RESULTS
Clinical Course and Severity
Due to the very small bvFTD group sizes, statistical comparisons were not attempted, though measures of central tendency (median and interquartile range).are represented in Table 1. Median age at symptom onset, disease duration before initial clinical assessment, and median symptom duration at death were very similar between the two bvFTD groups. At the time of initial assessment, bvFTD(CBD) subjects had lower median MMSE scores due to the inclusion of one bvFTD(CBD) subject with a more advanced disease process than the other patients.
Motor/Sensory
There was substantial variability in motor and sensory symptoms across all patients, and significant overlap between the CBD and Pick’s groups in the type and prevalence of these symptoms at initial evaluation. Few symptoms considered pathognomonic for corticobasal syndrome were seen in either group; in fact, 2/5 Pick’s and 2/5 CBD patients presented with no sensory or motor findings at all. No patient was found to have limb dystonia, myoclonus, alien limb, or mirror movements. No CBD and one Pick’s patient showed oral-bucco-facial apraxia, and 1/5 CBD and 2/5 Pick’s patients demonstrated mild limb apraxia, making body-part substitution errors on some transitive-limb praxis tasks. Three out of five patients in each group showed increased upper extremity tone, and patients in both groups were equally likely to demonstrate asymmetric versus symmetric rigidity. One of five patients in each group demonstrated axial rigidity, and 4/5 CBD and 2/5 Pick’s patients were noted to have decreased arm swing. One of five CBD and 2/5 Pick’s patients had slowed gait, while 2/5 CBD and 1/5 Pick’s patients showed overall bradykinesia. Two of five bvFTD(CBD) patients and 2/5 bvFTD(Pick’s) patients had either resting or postural tremor.
Other classic parkinsonian features such as shuffling gait (1/5 CBD, 0/5 Pick’s), retropulsion (1/5 CBD, 0/5 Pick’s), history of fall (2/5 CBD, 1/5 Pick’s), and facial hypomimia (1/5 CBD, 0/5 Picks) may have occured somewhat more frequently in the CBD group. Oculomotor findings appeared about equally in both groups, including slowed saccades (0/5 CBD; 1/5 Pick’s) and restricted upgaze (1/5 CBD, 1/5 Pick’s). Primitive reflexes (snout, rooting, palmar grasp, Babinski, or Hoffman’s sign) were also seen about equally in both groups (1/5 CBD, 1/5 Pick’s).
Neuropsychology
Based on qualitative analysis, bvFTD patients in both groups performed worse than controls on most cognitive measures. Some patients in each group performed below expectations in the visuospatial domain, which is typically assumed to be preserved in bvFTD. Looking qualitatively at individual patients, neuropsychological task performance showed equally wide variability in both bvFTD groups, with scores ranging from adequate to severely impaired, depending on the individual and test. There were no clear differences between the two bvFTD groups’ performance on visuospatial or memory tasks, though bvFTD(CBD) patients appeared to have poorer auditory working memory than bvFTD(Pick’s) patients, as measured by their ability to spell the word “WORLD” backwards, their backwards digit span, and the total number of words immediately learned on the CVLT. .
There was a qualitative difference in written language between the bvFTD groups. While all of the bvFTD(Pick’s) patients (5/5) were able to correctly write a sentence on the MMSE, 2 of 5 bvFTD(CBD) patients failed this task. Examiners blind to diagnostic group rated all subjects’ spontaneous conversational speech at the end of the neuropsychological evaluation. These included ratings of the level of grammatical complexity of their speech, their ability to comprehend natural spoken speech and instructions, variability of vocal prosody, phrase length, and spontaneous word-finding. While the median scores were equal, bvFTD(CBD) patients showed more variability in this domain than bvFTD(CBD) on most of these measures. No bvFTD subject in either group made spontaneous paraphasic errors in their speech.
Neuropsychiatry
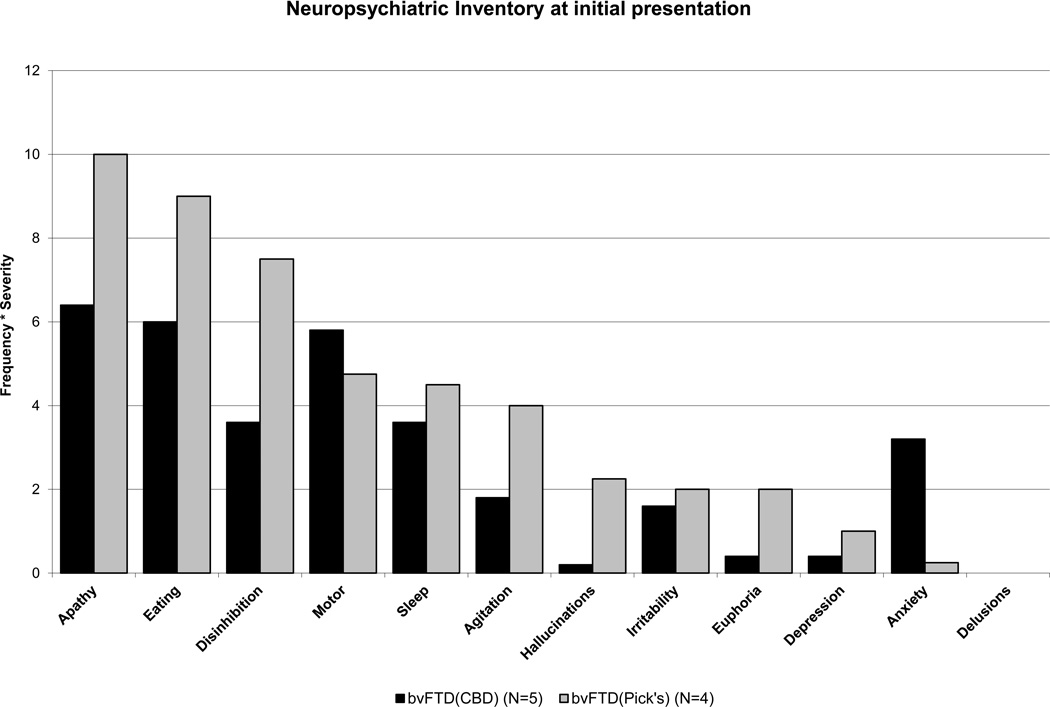
The array of behavioral manifestations within each patient group was complex and variable, and there was substantial overlap in symptoms between the two bvFTD groups. The bvFTD(Pick’s) group demonstrated higher frequency-by-severity product scores on the Neuropsychiatric Inventory (NPI)[43] than the bvFTD(CBD) group on nearly every symptom (Figure 4), resulting in much higher NPI Total and Distress Total scores (Table 1). In particular, bvFTD(Pick’s) showed greater magnitude of apathy, disinhibition, and aberrant eating behavior than bvFTD(CBD) patients. Anxiety was the one neuropsychiatric symptom with a higher mean frequency* severity product in bvFTD(CBD) than bvFTD(Pick’s) patients. Despite significant apathy, four out of five bvFTD(CBD) patients regularly displayed fearfulness or anxiety at the time of initial evaluation, while all but one of the bvFTD(Pick’s) patients were specifically described as having experienced a decrease in anxiety over the course of their illness. This difference also appeared in the patients’ responses to the GDS, a self-report measure of anxious and depressive symptoms (Yesavage, Brooks III, Taylor, & Tinkleberg, 1993), which bvFTD(CBD) patients endorsed at a level higher than both NCs and bvFTD(Pick’s) patients.. No differences in the frequency of angry, frustrated, or agitated behavior was noted between the two groups. Though the absence of hallucinations has previously been cited as possibly differentiating pathological CBD from other parkinsonian syndromes (Boeve et al., 2003; Geda et al., 2007), and were not seen in this study’s bvFTD(CBD) patients at the time of initial visit, complex visual hallucinations did develop in one bvFTD(CBD) patient within one year of his initial visit. This also did not provide good clinical differentiation between pathologies, as one bvFTD(Pick’s) patient had a significant hallucinatory syndrome.
FIGURE 4.
Behavioral and psychiatric symptoms reported on the Neuropsychiatric Inventory (NPI) for patients with bvFTD. Five bvFTD patients had CBD pathology (black bars) and 4 bvFTD patients had Pick’s pathology (gray bars).
Neuroanatomy
bvFTD(CBD) patients vs. Controls
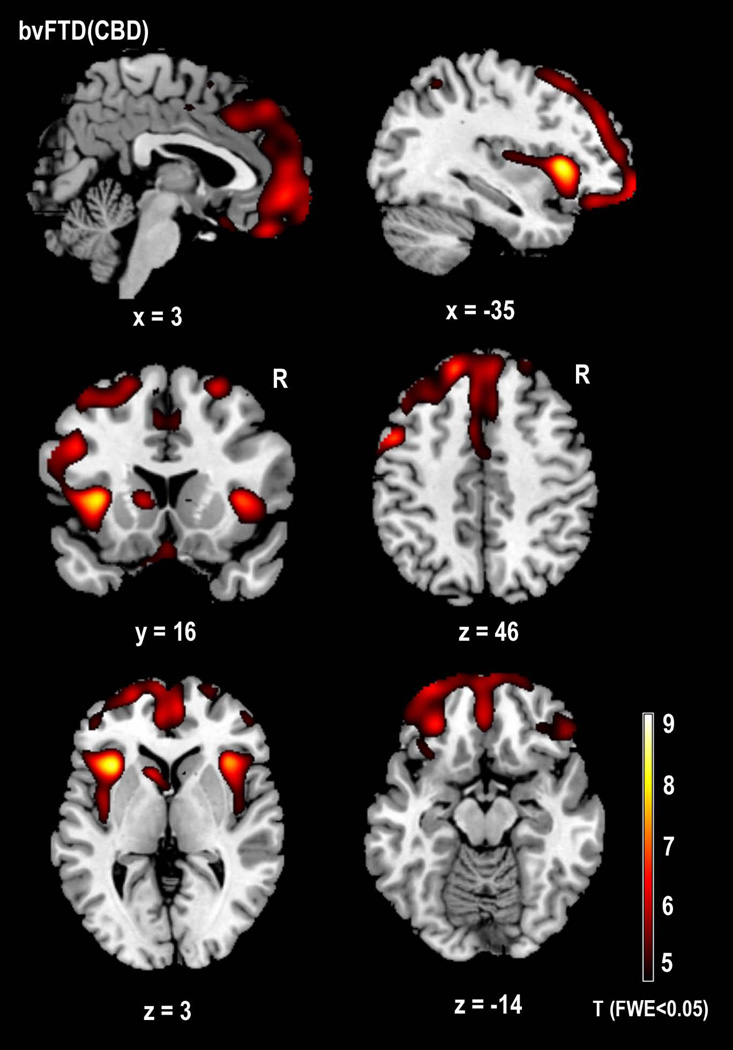
Whole-brain comparisons using VBM showed significant gray matter atrophy bilaterally in both anterior and posterior dorsal insula, caudate, superior frontal gyrii, and cerebral peduncles (FWE corrected: p<0.05). Unilateral atrophy was also seen in the right inferior and middle orbital gyrii, inferior temporal gyrus, and precentral gyrus, and in the left dorsal thalamus, gyrus rectus, superior orbital gyrus, middle frontal gyrus, supplementary motor area, postcentral gyrus, and inferior parietal lobule (Table 2, Figures 1 & 2).
Table 2.
SPM5 voxel-based morphometry results for gray matter, comparing structural MRI scans of all subjects with VBM-compatible structural T1-weighted MRI scans: bvFTD(CBD) group (N=3), bvFTD(Pick's) group (N=5), healthy older control group (N=53). Results of patients compared to NCs are corrected for multiple comparisons at FWE p<0.05. Results of direct comparisons of the small bvFTD patient groups are presented at an uncorrected level of significance (p<0.01).
| x | y | z | T-score | ||
|---|---|---|---|---|---|
| bvFTD(CBD) < Normal Controls | |||||
| R dorsal anterior insula | 36 | 24 | 0 | 7.48 | |
| R medial orbitofrontal gyrus | 3 | 53 | −4 | 6.55 | |
| R middle frontal gyrus | 31 | 46 | 34 | 6.51 | |
| R middle frontal gyrus | 49 | 34 | 34 | 6.25 | |
| R superior frontal gyrus | 25 | 16 | 59 | 6.15 | |
| R dorsal posterior insula | 42 | −8 | 6 | 5.68 | |
| R inferior orbital gyrus | 50 | 36 | −16 | 5.49 | |
| R inferior orbital gyrus | 30 | 34 | −22 | 5.21 | |
| R inferior temporal gyrus | 42 | 4 | −48 | 5.05 | |
| R caudate head | 10 | 12 | 6 | 5.01 | |
| R precentral gyrus | 56 | 12 | 38 | 4.86 | |
| R middle orbital gyrus | 42 | 56 | −14 | 4.71 | |
| L dorsal anterior insula | −34 | 18 | 4 | 8.37 | |
| L middle frontal gyrus | −44 | 40 | 24 | 7.00 | |
| L superior frontal gyrus | −15 | 42 | 48 | 7.00 | |
| L superior orbital gyrus | −19 | 63 | −4 | 6.74 | |
| L caudate head | −10 | 13 | 6 | 6.55 | |
| L dorsal posterior insula | −38 | −4 | 8 | 6.52 | |
| L medial orbitofrontal gyrus | −2 | 52 | 7 | 6.48 | |
| L superior frontal gyrus | −21 | 62 | 14 | 6.48 | |
| L gyrus rectus | −1 | 17 | −21 | 5.40 | |
| L inferior pariental lobule | −36 | −55 | 52 | 5.34 | |
| L supplementary motor area | −2 | 8 | 60 | 5.31 | |
| bvFTD(Pick's) < Normal Controls | |||||
| R ventral anterior insula | 36 | 20 | −10 | 13.36 | |
| R caudate head | 12 | 16 | 2 | 12.50 | |
| R superior frontal gyrus | 22 | 46 | 38 | 10.96 | |
| R gyrus rectus | 6 | 38 | −26 | 9.48 | |
| R inferior frontal gyrus, triangularis | 54 | 32 | 18 | 8.99 | |
| R inferior frontal gyrus, triangularis | 48 | 22 | 28 | 8.50 | |
| R middle frontal gyrus | 42 | 10 | 56 | 7.63 | |
| R inferior temporal gyrus | 70 | −22 | −22 | 7.44 | |
| R inferior temporal gyrus | 54 | −48 | −24 | 5.14 | |
| R middle temporal gyrus | 64 | −4 | −30 | 6.94 | |
| R postcentral gyrus | 64 | −38 | 46 | 5.90 | |
| R inferior occipital gyrus | 48 | −70 | −20 | 5.28 | |
| R cerebellum | 8 | −92 | −22 | 5.03 | |
| L ventral anterior insula | −36 | 14 | −10 | 8.58 | |
| L caudate head | −8 | 14 | 2 | 7.98 | |
| L middle frontal gyrus | −28 | 12 | 60 | 7.10 | |
| L middle frontal gyrus | −42 | 16 | 52 | 6.79 | |
| L inferior frontal gyrus, opercularis | −48 | 14 | 24 | 6.87 | |
| L supramarginal gyrus | −64 | −38 | 38 | 6.18 | |
| L postcentral gyrus | −54 | −16 | 56 | 5.72 | |
| L inferior occipital gyrus | −50 | −66 | −22 | 5.71 | |
| L cerebellum | −46 | −52 | −56 | 5.12 | |
| bvFTD(Pick's) < bvFTD(CBD) | |||||
| R ventral anterior insula | 37 | 11 | −10 | 4.43 | |
| R ventral anterior insula | 32 | 13 | −17 | 4.35 | |
| R parahippocampal gyrus | 22 | 4 | −34 | 4.31 | |
| R superior temporal pole | 42 | 20 | −24 | 4.00 | |
| R caudate head | 14 | 16 | 0 | 4.21 | |
| R inferior frontal gyrus, opercularis | 53 | 12 | 6 | 3.45 | |
| R superior frontal gyrus | 20 | 34 | 46 | 3.40 | |
| R inferior temporal gyrus | 54 | −6 | −34 | 3.20 | |
| L ventral anterior insula | −33 | 9 | −15 | 3.32 | |
| L fusiform gyrus | −26 | −5 | −42 | 3.26 | |
| bvFTD(CBD) < bvFTD(Pick's) | |||||
| L cerebral peduncle | −6 | −30 | −22 | 4.10 | |
| L precuneus | −14 | −68 | 52 | 3.59 | |
| L precuneus | −16 | 66 | 38 | 2.99 | |
| L inferior parietal lobule | −30 | −48 | 50 | 2.89 | |
FIGURE 1.
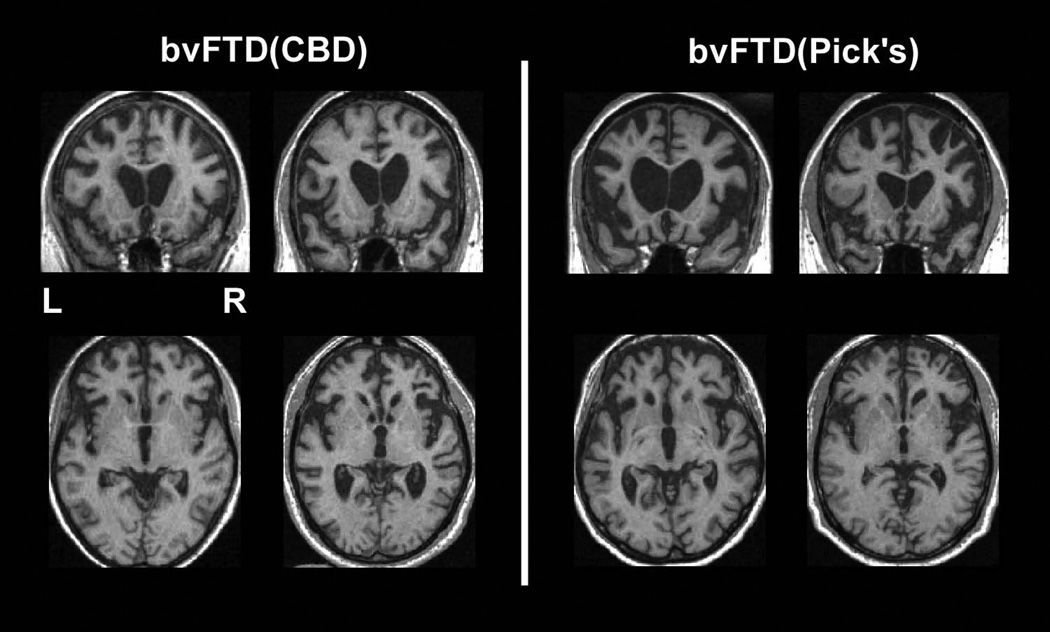
Selected coronal and axial slices demonstrating the atrophy patterns upon first clinical evaluation for 4 patients with behavioral variant frontotemporal dementia syndrome. Each coronal slice and the axial slice below it belong to a single patient. The two patients on the left were found to have corticobasal degeneration neuropathology upon autopsy; the two patients on the right were found to have Pick’s neuropathology.
FIGURE 2.
Voxel-based morphometry (SPM5) comparison of structural anatomy in 3 patients with bvFTD syndrome and CBD neuropathology versus 53 healthy older controls. Images are shown with a FWE-corrected lower threshold of p<0.05, corresponding to T=4.51. Regions in yellow/red show areas of volume loss in bvFTD(CBD) patients relative to healthy controls.
bvFTD(Pick’s) patients vs Controls
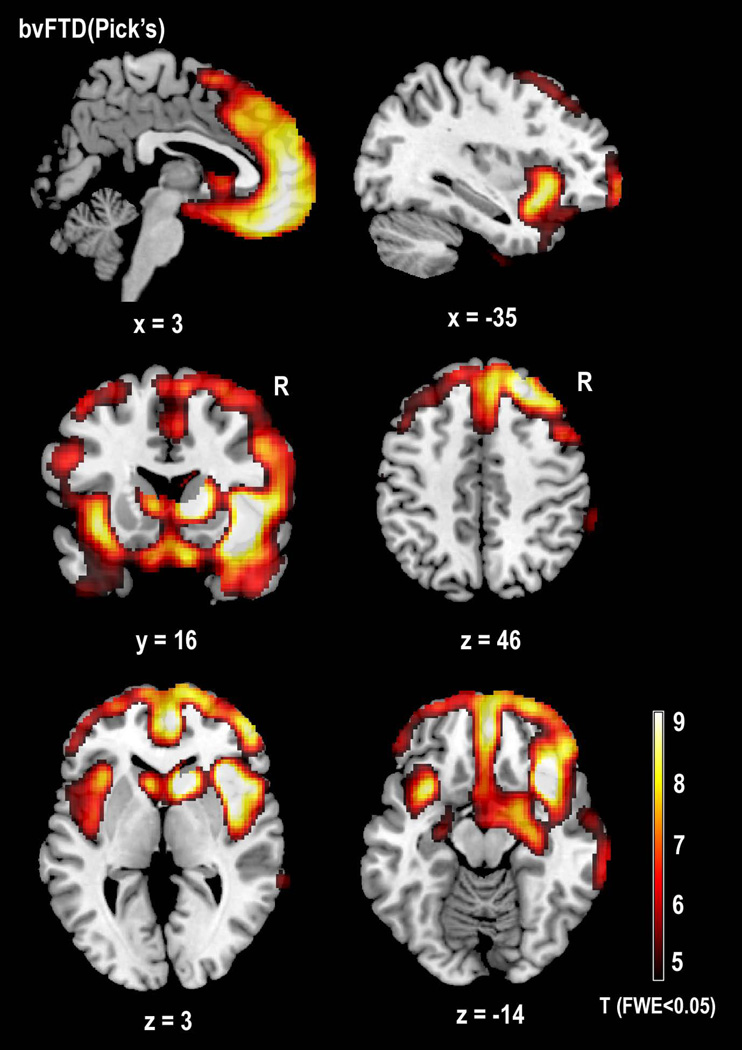
VBM of gray matter atrophy showed significant loss bilaterally in the ventral anterior insulas, caudates, middle frontal gyrii, postcentral gyrii, inferior occipital gyrii, and cerebellum (p<0.05 FWE) (Figures 1 & 3, Table 2). Atrophy was more prominent on the right in the gyrus rectus, the pars triangularis of the inferior frontal gyrus, the superior frontal gyrus, and the inferior and middle temporal gyrii. Predominantly left-sided atrophy was seen in the pars opercularis of the inferior frontal gyrus, and in the supramarginal gyrus.
FIGURE 3.
Voxel-based morphometry (SPM5) comparison of structural anatomy in 5 patients with bvFTD syndrome and Pick’s neuropathology versus 53 healthy older controls. Images are shown with a FWE-corrected lower threshold of p<0.05, corresponding to T=4.51. Regions in yellow/red show areas of volume loss in bvFTD(Pick’s) patients relative to healthy controls.
bvFTD(CBD) vs. bvFTD(Pick’s)
Accepting an uncorrected level of significance due to small patient group sizes (p<0.01), VBM analysis revealed significantly greater gray matter atrophy to the left cerebral peduncle, precuneus, and inferior parietal lobule, in bvFTD(CBD) patients compared to the bvFTD(Pick’s) group (Table 2).
In the reverse comparison, gray-matter VBM analysis showed bvFTD(Pick’s) to have significantly greater atrophy to the right ventral anterior insula, parahippocampal and inferior temporal gyrus, superior temporal pole, caudate head, pars opercularis of the inferior frontal gyrus, and superior frontal gyrus (Table 2, Figures 1–3).
DISCUSSION
In this series of seventeen consecutive patients referred to a dementia specialty clinic who were later found to have CBD neuropathology, five presented with behavioral-variant frontotemporal dementia. The neuropsychological, neuropsychiatric, sensory/motor, and neuroanatomic features of this bvFTD(CBD) subgroup showed substantial overlap with findings in a comparison group of bvFTD patients with Pick’s disease neuropathology.
While CBD and Pick’s disease are both tauopathies, and can both result in a bvFTD clinical syndrome, they are neuropathologically distinct diseases. CBD is a 4-repeat (4R) tauopathy featuring swollen achromatic neurons, tau-positive neurofibrillary tangles, and tau-immunoreactive astrocytic plaques, coiled bodies, and threads unique to CBD, while Pick’s disease involves neuronal aggregates of hyperphosphorelated 3R tau known as Pick’s bodies (Dickson et al., 2002; Cairns et al., 2007; Ludolph et al., 2009). Clinically, CBD is known to present as anatomically and behaviorally distinct syndromes, including classical CBS, PNFA, Alzheimer’s disease, and bvFTD. This demonstrates that CBD-related tau misfolding and aggregation can compromise and spread within several closely related networks, with preferred targets in the opercular and premotor cortices, but also involving divergent regions in both hemispheres. This flexibility in the neuronal vulnerability pattern drives the diverse clinical syndromes manifested in CBD. Preferred but flexible vulnerability is well-established for other neurodegenerative diseases, such as Alzheimer’s disease, in which patients most often develop an amnestic syndrome but can also present with predominant language, visuospatial, or even behavioral deficits (Alladi et al., 2007). This ability of CBD to affect distinct networks implicated in other diseases raises the question of how closely CBD-based clinical syndromes mimic those resulting from non-CBD neuropathologies, because as treatments targeting specific neuropathological mechanisms appear, accurate prediction of underlying pathology from clinical syndrome becomes increasingly critical. Though CBD presenting as bvFTD has been described elsewhere (Kertesz et al., 2000; Mathuranath et al., 2000), cases occur infrequently enough to impede large scale studies of this subgroup.
Timing of disease onset and course did not help differentiate bvFTD patients with CBD versus Pick’s disease in our sample. In fact, the two bvFTD groups were nearly identical in age at symptom onset (CBD: median age 58, range 53–74; Pick’s: 60, 49–71), disease duration before presenting to a dementia specialist (CBD: 3.4 years ; Pick’s: 3.7 years), and disease duration at death (CBD: 7.6 years, range 3.8 – 12.7 ; Pick’s: 7.2, 3.3–9.8), emphasizing the similarity of the disease mechanism underlying these distinct tauopathies. Other studies have shown similar, fulminant courses from first symptom to death for patients with CBD pathology presenting with any clinical syndrome: 64.9 months (Murrary, et al., 2007); 6.1 years (Grimes, Lang, & Bergeron, 1999); 7.0 years (SD 3.0) (Josephs, et al., 2006); and 7.9 years (SD 2.6) (Wenning, et al., 1998), though two studies report a slightly longer symptom duration (10±4 years (Llado, et al., 2008), 11.8±3.9 years (Roberson, et al., 2005).
Clinical asymmetry is a factor often hypothesized to differentiate patients with CBD from those with other underlying pathologies. Existing diagnostic criteria suggest that asymmetry tilts the scales towards a clinical diagnosis of CBD and away from PSP or another tauopathy (Litvan, et al., 2003). While this has clearly been supported in the case of patients presenting with a PNFA syndrome (Gorno-Tempini et al., 2004; Knibb et al., 2006), results have been mixed for patients with CBS or AD syndromes. While some quantitative imaging studies report asymmetric (L>R) atrophy and hypoperfusion (Soliveri et al., 1999; Boxer et al., 2006), others have found nearly symmetric involvement of left and right hemispheres (Groschel et al., 2004; Josephs et al., 2008). One confound is that studies that rely on clinical, not pathological findings will tend to be biased towards finding asymmetry if a CBD diagnosis was made more likely by asymmetry. Also, these studies group together all clinical variants with either suspected or proven CBD pathology, essentially washing out right versus left differences.
In a recent study that carefully characterized the different clinical syndromes of CBD patients and performed a laterality analysis, PNFA patients showed marked L>R hemispheric asymmetry, but patients with executive-motor predominant and bvFTD behavior-dominant syndromes showed consistently bilateral damage [Lee et al, in press, Archives of Neurology]. Our study includes the same subset of bvFTD(CBD) patients, who might have been expected to demonstrate an asymmetrically right-predominant disease pattern, perhaps mirroring left-sided patients with predominantly language symptoms (Gorno-Tempini et al., 2004). Yet the laterality analysis in Lee et al suggests that the relative degree of frontal asymmetry ranged widely in these bvFTD(CBD) patients.. Thus, asymmetric involvement of the language-dominant hemisphere may remain a useful diagnostic criterion for predicting CBD pathology in some clinical presentations like PNFA or CBS; however, the absence of asymmetry should not be used to rule out CBD, particularly in cases with a bvFTD syndrome. These data do suggest that bvFTD patients with greater than expected left-sided relative to right-sided atrophy may be more likely to have underlying CBD pathology, since this pattern was seen in two bvFTD(CBD) patients and in none of the bvFTD(Pick’s) patients.
Minimal Cognitive and Motor Distinctions
Standard neuropsychological testing revealed no clear differences between the two bvFTD groups. There was a wide range of performance on all tasks across patients in both groups. This pattern is frequently seen when bvFTD patients undergo cognitive testing, because behavior problems such as apathy, inattention, disorganization, and stimulus-boundedness can cause haphazard, artificially diminished scores on tasks in otherwise unaffected domains.
Constructional difficulty, i.e., difficulty copying or drawing, has long been associated with clinical CBS (Graham, Bak, & Hodges, 2003). Preservation of visuospatial functioning, on the other hand, is a secondary, supportive diagnostic feature of bvFTD (Neary et al., 1998). However, a recent study of FTD-spectrum disorders found that a group of patients with tauopathies, 55% of whom had CBD pathology, were more likely than other tau-negative subtypes to have visuospatial deficits (Grossman et al., 2007). While it is notable that some patients in both bvFTD groups in the present study performed at an impaired level on visuoconstruction testing, the fact that only one patient failed to correctly copy the pentagons on the MMSE suggests that these patients’ constructional impairment was mild at best, and may have been attributable in part to the executive disorganization or behavioral impulsiveness often seen in bvFTD. Both bvFTD groups in this study demonstrated some scattered parietal damage on VBM analysis, though only the bvFTD(Pick’s) group showed significantly lower volumes in parietal ROIs than controls, specifically in the inferior parietal and paracentral regions. Other tests of parietal functions showed a similarly variable pattern; e.g., only the bvFTD(Pick’s) group showed even mild apraxia, but the bvFTD(CBD) group was more likely to exhibit early acalculia. These mixed results in this small sample suggest that additional study of the etiologic mechanisms underlying this tauopathy-related visuoconstruction deficit is warranted.
Our study obtained very detailed neuropsychological evaluation of speech and language symptoms, including examiner ratings of many elements of spontaneous speech. While some patients in both bvFTD groups performed normally on every other speech and language measure, all patients performed in the impaired range on both lexical and category verbal fluency tests. Four out of five CBD patients and 3/5 Pick’s patients had reduced overall speech output by history, and two of the bvFTD(CBD) patients, who exhibited very impoverished spontaneous speech at the time of the initial evaluation, typically spoke only in stereotyped phrases or using placeholder words. Though patients in the bvFTD(Pick’s) group also used pat phrases, none of them showed this level of impoverishment. The degree to which these language deficits were due to involvement of the motor speech system, versus other aspects of the language system, is unclear. A literature review by Graham (Graham, Bak, & Hodges, 2003) suggests that 63% of pathologically-proven CBD patients, regardless of syndromic diagnosis, are aphasic at presentation. In one study of clinically diagnosed CBD patients, every patient performed in the impaired range on lexical and category fluency tests, and had significant deficits in spelling and oral phoneme blending and segmentation (Graham, Bak, Patterson, & Hodges, 2003). Alternatively, poor performance on fluency tasks in these bvFTD patients may also have resulted from an apathy-related reduction of speech.
In a finding that may implicate the motor system in at least part of the bvFTD(CBD) patients’ impairment on language testing, some patients in both bvFTD groups were described as having difficulty writing by history, particularly with spelling and word retrieval. However, only the bvFTD(CBD) patients (2/5, compared to 0/5 in the Pick’s group) failed to correctly write a full sentence on the MMSE (which was given full credit if it had a subject and a verb, regardless of whether there were spelling or grammatical errors). Also, 3/5 bvFTD(CBD) patients were found to have micrographia, while no bvFTD(Pick’s) patients exhibited this symptom. Micrographia has been associated with white matter lesions (Scolding & Lees, 1994; Ishihara et al., 2006) and with basal ganglia lesions (Kuoppamaki et al., 2005; Gangadhar, Joseph, & Chakravarthy, 2008), though such lesions were seen in both bvFTD groups in this study. There was also a trend towards bvFTD(CBD) patients having more typical features of parkinsonism, but the numbers were so small it was not clear whether this was truly a signal.
Overall, our data suggest that in a bvFTD patient, neither the absence of “typical” corticobasal symptoms (such as dystonia, alien limb, ideomotor apraxia, and myoclonus), nor even the absence of any motor symptoms at all, can rule out the presence of CBD pathology. However, they do suggest that suspicion of CBD in a bvFTD patient should increase with greater number and severity of symptoms consistent with parkinsonism.
Divergent neuroanatomic patterns in bvFTD
Both the CBD and Pick’s pathology groups met Neary clinical criteria for behavioral variant frontotemporal dementia (Neary, et al., 1998), demonstrating a primarily behavioral syndrome consisting of apathy, disinhibition, aberrant personal behavior, and social behavior that was both insensitive and inappropriate. Quantitative neuroanatomic analysis demonstrated substantial overlap between bvFTD(CBD) and bvFTD(Pick’s) groups, and showed that the regions affected in bvFTD(CBD) patients are quite different from the left-sided, dorsolateral frontal-parietal pattern observed in studies of other CBD-based clinical syndromes. This involvement of “bvFTD-specific” networks in bvFTD(CBD) explains many of the behavioral features observed in this study. However, in both anatomy and quantitative neuropsychiatry, the overall clinical severity in the bvFTD(CBD) group appeared milder than that of the bvFTD(Pick’s) group, despite the fact that the two groups were nearly perfectly matched for disease duration, and there was an equal mix of mildly to severely impaired patients across both groups.
Close examination of atrophy patterns revealed that despite substantial damage to the inferior portions of the frontal cortex in bvFTD(CBD), the most medial and caudal portions of the OFC and subgenual cingulate, along with the ventral part of the insula and much of the frontoinsular rim, were relatively spared. This finding contrasts with the pattern observed in the bvFTD(Pick’s) patients, in which these regions were among the most severely affected, both in this study and in others (Brambati et al., 2007; Seeley et al., 2008). Many clinical researchers have noted that bvFTD patients can often be categorized into two groups: those with a predominantly disinhibited behavioral syndrome, with ventral > dorsal frontal atrophy, and those presenting with an apathy-predominant syndrome, typically involving dorsal > ventral frontal atrophy (Snowden et al., 2001). In both anatomy and behavior, these bvFTD(CBD) patients uniformly present with the apathetic subtype of bvFTD. Apathy was their most severe symptom on the NPI, followed by motor, eating, and sleep disturbances. While all of the bvFTD(CBD) patients demonstrated some social disinhibition, caregivers rated these behaviors as relatively mild in comparison to those observed in the bvFTD(Pick’s) group. The relatively spared regions of the medial OFC are known to be involved in evaluation of both positive and negative reinforcers, emotion interpretation (Hornak et al., 2004), and social disinhibition (Rosen et al., 2006).
Despite the relative mildness of their disinhibition compared to the more florid bvFTD(Pick’s) patients, the bvFTD(CBD) patients did lose social comportment and the ability to recognize and adhere to social norms. This may be attributable to the substantial damage to other frontal structures seen in these bvFTD(CBD) patients, including the lateral orbitofrontal cortex and the most anterior, polar portions of the OFC. Damage to these regions has long been associated with bvFTD-like behavior change including decreased socialization, impulsivity, and impaired judgment (Rosen et al., 2005). The lateral OFC is involved with assigning and evaluating negative behavioral reinforcers (Kringelbach & Rolls, 2004). An inability to alter behavior to avoid potential punishment is consistent with the symptoms seen in the bvFTD(CBD) group, such as reckless driving, impulsive and irresponsible investments, spending, and donations, and extramarital promiscuity. Like the bvFTD(Pick’s) group, the bvFTD(CBD) group also showed substantial damage to more anterior structures, particularly the medial, polar regions of the OFC bilaterally. The frontal pole’s involvement in social cognition has been gaining recognition, and it has been implicated in complex processes such as interpersonal perspective taking (Gallagher & Frith, 2003; Decety & Jackson, 2004), goal-directed behavior (Kreuger, Barbey, & Grafman, 2009), and prosocial cognition involving compassion, embarrassment, guilt, indignation, and emotional moral reasoning (Moll et al., 2007; Moll, De Oliveira-Souza, & Zahn, 2008). Failure to correctly reason about cognitive and emotional states of the self and other, coupled with a loss of automatic social sensitivity and responsiveness, are the hallmarks of the bvFTD syndrome. bvFTD results in such drastic, pervasive social and emotional deficits because brain regions mediating primitive emotionality and those mediating higher-order social cognition are both affected early in the disease. The bilateral damage to the frontal polar cortex seen in this study likely contributed to the impoverished social cognition seen in these bvFTD(CBD) patients, including loss of embarrassment over public incontinence and compulsive nose-picking, making rude and racially prejudiced observations in public, and habitually ignoring others in one’s social vicinity.
The fact that these bvFTD(CBD) patients demonstrate ventral < dorsal damage may also help explain the milder eating disturbances seen in the bvFTD(CBD) group relative to the bvFTD(Pick’s) patients. The region most directly associated with binge eating in bvFTD patients is the ventral portion of the anterior insula (Woolley et al., 2007), a region showing significantly greater damage in bvFTD(Pick’s) patients than in bvFTD(CBD). Review of the bvFTD(CBD) cases suggests that only two of the four cases engaged in binge eating, and that it was rated as severe only in one of these patients at the time of the initial evaluation. While 4/5 of the bvFTD(Pick’s) patients’ altered eating behavior had resulted in substantial weight gain, this was true of only 2/5 of the bvFTD(CBD) patients. The anterior insula contains primary and secondary gustatory cortices and is connected with the olfactory bulb and OFC, implicating it in taste preference (Mesulam, 1991; Rousseaux, Godefroy, Cabaret, & Bernati, 1996). Increased propensity for sweet foods may also correspond to the decreased function of deep structures like the ventral hypothalamus (Sparks et al., 1994).
Though the ventral insula was comparatively spared, bvFTD(CBD) patients did evidence significant early damage to the right dorsal insula. While the posterior portion of the insula is a terminal part of the pathway receiving bodily sensations, the more anterior portions of the insula integrate those sensations into conscious awareness (i.e., interoception) (Craig, 2009). The frontal insula participates in emotional processing and leads the brain’s response to salient emotional stimuli (Sridharan, Levitin, & Menon, 2008), functions critical to adaptive behavior in social contexts. Disproportionate right insular involvement may also explain why a number of these bvFTD(CBD) patients had obsessions, compulsions, or delusions involving their own bodies, particularly involving urination and sexual behavior. One bvFTD(CBD) patient had compulsions involving repeated toilet flushing, hand washing, and showering, and vacillated between thinking he was constipated or had diarrhea. He had delusions and complex visual hallucinations that were focused upon his genitalia. A second case also exhibited highly excessive trips to the restroom as well as eating compulsions, which continued despite emesis. Damage to the right insula may have interfered with patients’ ability to perceive and correctly process visceral, autonomic information (Craig, 2002; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004), which in turn may have led to disturbed and at times delusional beliefs about bodily functions, a finding also common in bvFTD patients with other neuropathologies. When these disturbed somatic signals became the object of patients’ obsessive-compulsive behavior, they formed rigid dysfunctional behavior patterns centered on hygiene, voiding, and sexual behavior (Perneczky et al., 2008).
Striatal structures, including the head of the caudate, were damaged early in these bvFTD(CBD) patients, similar to other varieties of bvFTD patients. There is evidence that the right caudate and putamen are integrally involved in social and emotional behaviors, including empathy (Rankin et al., 2007), emotion processing (Phan, Wager, Taylor, & Liberzon, 2002), and interpretation of emotional voice prosody (Cancelliere & Kertesz, 1990). At a lower threshold medial temporal structures such as the amygdala were also significantly atrophied in the bvFTD(CBD) patients. Bilateral damage to this structure can result in a Kluver-Bucy syndrome involving symptoms such as hyperorality, changes in sexual behavior or diet, and docility (Hayman, Rexer, Pavol, Strite, & Meyers, 1998). With their connections to the hypothalamus, insula, and OFC, these subcortical structures play a key role in emotion processing and are likely involved in many bvFTD-like behaviors.
Almost every behavioral symptom that appeared in the bvFTD(Pick’s) group also appeared in the bvFTD(CBD) group, if to a milder degree. The one apparent exception was that more of the bvFTD(CBD) patients exhibited a greater degree of anxious self-concern than was seen in the bvFTD(Pick’s) patients. This distinction was seen on quantitative ratings of anxiety, including the caregiver-rated NPI and the patient self-reported GDS, on which bvFTD(CBD) patients were much more likely than bvFTD(Pick’s) patients to personally endorse feeling fearful, worried, or dysphoric. Review of the clinical reports also supports this distinction, suggesting that 4/5 of the bvFTD(CBD) patients often experienced heightened anxiety, which at times was specific but sometimes took on a more generalized form. Even the bvFTD(CBD) patient who presented quite late in her course was described as often “frightened,” and intermittently recognized that something was wrong with her, occasionally saying vaguely, “I think I’m in trouble.” In contrast, despite mention of a phase of increased anxiety in one bvFTD(Pick’s) patient at initial symptom onset, none of the bvFTD(Pick’s) patients demonstrated the capacity for fearfulness and anxious self-concern at the time of their initial evaluation. This lack of anxiety (fear) is distinct from lack of frustration (anger), which increased in 2/5 Pick’s and 3/5 CBD patients after disease onset. It is also distinct from mild depression or sadness, which was reported in two bvFTD(Pick’s) and 3/5 bvFTD(CBD) patients at initial presentation.
The relative ventral frontal sparing seen in the bvFTD(CBD) patients may account for this ability to still feel self-concern. There is evidence that anxiety involves a primarily ventral network, including the inferior insula, medial orbitofrontal, and inferior temporal cortex (Liotti et al., 2000). In direct comparison with bvFTD(CBD) patients, bvFTD(Pick’s) patients showed significantly greater damage to structures in the ventral “anxiety” network, including right medial temporal and inferior insular cortex. It is possible that the comparative sparing of this network in bvFTD(CBD) patients allows them to retain some rudimentary self-concern, manifesting in the ability to feel fearful over the sense that “something is wrong,” and to self-report anxious symptoms, while this capacity is lost in the more ventral-predominant, “disinhibited-subtype” patients like the bvFTD(Pick’s) group.
Summary and Conclusions
Given the substantial clinical overlap between bvFTD presentations arising from different pathologies, it is not surprising that expert neurologists still fail to predict CBD neuropathology in patients who present with bvFTD. At initial presentation, all patients in both bvFTD(CBD) and bvFTD(Pick’s) groups met Neary research criteria for bvFTD (Neary et al., 1998), showing insidious onset and progression, with disordered personal and interpersonal conduct, and profound loss of insight and emotional blunting. Though some patients in both groups had normal sensory-motor evaluations, several motor findings were seen in both groups, including slowed gait, decreased arm swing, rigidity, tremor, restricted upgaze, and primitive reflexes. The severity and frequency of typical parkinsonism was marginally greater in bvFTD(CBD) than bvFTD(Pick’s) patients; however, no specific motor feature clearly differentiated the groups, and none of the patients demonstrated symptoms consistent with a typical corticobasal syndrome. Neuropsychological testing revealed that both bvFTD groups have equivalent deficits in most cognitive domains, including learning and memory, executive functioning, visuoconstruction, and language skills such as naming and word generation. However, micrographia appeared more frequently in the bvFTD(CBD) patients. Formal neuropsychiatric assessment of the two bvFTD groups showed that while both evidenced substantial apathy, bvFTD(CBD) patients had less severe social disinhibition, as well as less pronounced eating and sleep disturbances, agitation, and irritability. Potentially due to their comparative preservation of ventral frontoinisular rim structures, bvFTD(CBD) patients appeared to have greater retention of the capacity for self-referential anxiety, while this appeared largely absent in the bvFTD(Pick’s) patients. Analysis of the regional atrophy patterns in the two bvFTD groups revealed no significant differences at a corrected level of analysis. Both groups had substantial damage to inferior frontotemporal regions, particularly to the frontal poles, bilateral insula, orbitofrontal and superior medial frontal cortex, and bilateral caudate, areas involved in the “salience network” (Seeley, 2007) central to the control of eating, self-awareness, and social behavior. However, the bvFTD(CBD) patients could all have their pattern of frontal damage classified as dorsal > ventral, and their particular behavioral pattern was predominantly apathetic rather than disinhibited.
Our findings add to an emerging recognition that classical definitions of CBD-associated clinical features, which emphasize parietal dysfunction and atrophy with extrapyramidal deficits, will miss many patients with pathological CBD. bvFTD is a major mode of presentation of CBD, affecting almost one-third of the cases at our dementia speciality clinic found to have CBD at autopsy. CBD patients with bvFTD present in a manner almost indistinguishable from bvFTD due to Pick’s disease. Milder social disinhibition, and rudimentary preservation of anxious self-concern may help distinguish bvFTD from CBD from other bvFTD etiologies. This behavioral phenotype may result from the fact that bvFTD(CBD) patients may lack the degree of ventral frontoinsular damage that emerges early in most bvFTD (Pick’s) patients.
Disease-modifying treatments focused upon abnormal tau aggregation are becoming available in the next year. These therapies, if successful, may exert better efficacy for patients with CBD than for those with tau-negative FTLD pathologies. To maximize success of these trials, clinicians and researchers must continue to refine their sensitivity to pathology-predictive clinical features that occur early in the disease course, when interventions still stand to most positively impact patient lives.
ACKNOWLEDGEMENTS
NIA-PPG P01-AG1972403;GCRC-M01-RR00079; AG19724-01A1; ARCC 01-154-20; T32AG23481, P50AG023501, State of California DHS 04-33516.
REFERENCES
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb JA, Patterson K, Hodges JR. Focal cortical presentations of alzheimer's disease. Brain. 2007;130(10):2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54 Suppl 5:S15–S19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, Petersen RC. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53(4):795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Lisberger SG, Rankin KP, Hellmuth J, Dean D, Miller B. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. Journal of Neuroscience. 2006;26(23):6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Renda NC, Rankin KP, Rosen HJ, Seeley WW, Ashburner J, Gorno-Tempini M. A tensor based morphometry study of longitudinal gray matter contraction in FTD. NeuroImage. 2007;35(3):998–1003. doi: 10.1016/j.neuroimage.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ Consortium for Frontotemporal Lobar Degeneration. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathologica. 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancelliere AEB, Kertesz A. Lesion localization in acquired deficits of emotional expression and comprehension. Brain and Cognition. 1990;13:133–147. doi: 10.1016/0278-2626(90)90046-q. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel -- now? the anterior insula and human awareness. Nature Reviews in Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3(2):71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Litvan I. Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61(11):935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, Kimonis VE. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containin protein gene mutations. Journal of Neuropathology and Experimental Neurology. 2006;65(6):571–581. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]
- Frattali CM, Grafman J, Patronas N, Makhlouf F, Litvan I. Language disturbances in corticobasal degeneration. Neurology. 2000;54(4):990–992. doi: 10.1212/wnl.54.4.990. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of 'theory of mind'. Trends Cogn Sci. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gangadhar G, Joseph D, Chakravarthy VS. Understanding parkinsonian handwriting through a computational model of basal ganglia. Neural Comput. 2008;20(10):2491–2525. doi: 10.1162/neco.2008.03-07-498. [DOI] [PubMed] [Google Scholar]
- Geda YE, Boeve BF, Negash S, Graff-Radford N, Knopman DS, Parisi JE, Petersen RC. Neuropsychiatric features in 36 pathologically confirmed cases of corticobasal degeneration. J Neuropsychiatry Clin Neurosci. 2007;19(1):77–80. doi: 10.1176/jnp.2007.19.1.77. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M, Murray RC, Rankin KP, Weiner MW, Miller BL. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: A case report. Neurocase. 2004;10(6):426–436. doi: 10.1080/13554790490894011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NL, Bak TH, Hodges JR. Corticobasal degeneration as a cognitive disorder. Mov Disord. 2003;18(11):1224–1232. doi: 10.1002/mds.10536. [DOI] [PubMed] [Google Scholar]
- Graham NL, Bak T, Patterson K, Hodges JR. Language function and dysfunction in corticobasal degeneration. Neurology. 2003;61(4):493–499. doi: 10.1212/01.wnl.0000081230.09863.ed. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology. 1999;53(9):1969–1974. doi: 10.1212/wnl.53.9.1969. [DOI] [PubMed] [Google Scholar]
- Groschel K, Hauser TK, Luft A, Patronas N, Dichgans J, Litvan I, Schulz JB. Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. NeuroImage. 2004;21(2):714–724. doi: 10.1016/j.neuroimage.2003.09.070. [DOI] [PubMed] [Google Scholar]
- Grossman M, Libon DJ, Forman MS, Massimo L, Wood E, Moore P, Trojanowski JQ. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Archives of Neurology. 2007;64(11):1601–1609. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- Hayman LA, Rexer JL, Pavol MA, Strite D, Meyers CA. Kluver-bucy syndrome after bilateral selective damage of amygdala and its cortical connections. J Neuropsychiatry Clin Neurosci. 1998;10(3):354–358. doi: 10.1176/jnp.10.3.354. [DOI] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hou CE, Carlin D, Miller BL. Non-alzheimer's disease dementias: Anatomic, clinical, and molecular correlates. Can J Psychiatry. 2004;49(3):164–171. doi: 10.1177/070674370404900303. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Ozawa T, Otsuki M, Shimbo J, Tanaka K, Nishizawa M. Atypical micrographia associated with corticostriatal white matter lesions in systemic lupus erythematosus. J Neurol Neurosurg Psychiatry. 2006;77(8):993–994. doi: 10.1136/jnnp.2005.083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, Dickson DW. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66(1):41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, Jack CR. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiology of Aging. 2008;29(2):280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Frontotemporal dementia, pick disease, and corticobasal degeneration. Arch Neurol. 1997;54LP:1427–1429. doi: 10.1001/archneur.1997.00550230090024. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55(920540734) doi: 10.1212/wnl.55.9.1368. 1368-175. [DOI] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59(1):156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Kreuger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends in Cognitive Sciences. 2009 doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kuoppamaki M, Rothwell JC, Brown RG, Quinn N, Bhatia KP, Jahanshahi M. Parkinsonism following bilateral lesions of the globus pallidus: Performance on a variety of motor tasks shows similarities with parkinson's disease. J Neurol Neurosurg Psychiatry. 2005;76(4):482–490. doi: 10.1136/jnnp.2003.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biological Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Litvan I. Progressive supranuclear palsy: Staring into the past, moving into the future. The Neurologist. 1998;4:13–20. [Google Scholar]
- Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, Bartko JJ. Accuracy of the clinical diagnosis of corticobasal degeneration: A clinicopathologic study. Neurology. 1997;48(1):119–125. doi: 10.1212/wnl.48.1.119. [DOI] [PubMed] [Google Scholar]
- Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Wenning GK. Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18(5):467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- Llado A, Sanchez-Valle R, Rey MJ, Ezquerra M, Tolosa E, Ferrer I, Molinuevo JL. Clinicopathological and genetic correlates of frontotemporal lobar degeneration and corticobasal degeneration. Journal of Neurology. 2008;255(4):488–494. doi: 10.1007/s00415-008-0565-8. [DOI] [PubMed] [Google Scholar]
- Ludolph AC, Kassubek J, Landwehrmeyer BG, Mandelkow E, Mandelkow EM, Burn DJ Reisensburg Working Group for Tauopathies With Parkinsonism. Tauopathies with parkinsonism: Clinical spectrum, neuropathologic basis, biological markers, and treatment options. European Journal of Neurology : The Official Journal of the European Federation of Neurological Societies. 2009;16(3):297–309. doi: 10.1111/j.1468-1331.2008.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathuranath PS, Xuereb JH, Bak T, Hodges JR. Corticobasal ganglionic degeneration and/or frontotemporal dementia? A report of two overlap cases and review of literature. J Neurol Neurosurg Psychiatry. 2000;68(3):304–312. doi: 10.1136/jnnp.68.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Psychol Aging. 1991;6(1):28–35. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Garrido GJ, Bramati IE, Caparelli-Daquer E, Paiva ML, Grafman J. The self as a moral agent: Linking the neural bases of social agency and moral sensitivity. Soc Neurosci. 2007;2(3–4):336–352. doi: 10.1080/17470910701392024. [DOI] [PubMed] [Google Scholar]
- Moll J, De Oliveira-Souza R, Zahn R. The neural basis of moral cognition: Sentiments, concepts, and values. Ann N Y Acad Sci. 2008;1124:161–180. doi: 10.1196/annals.1440.005. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G. Cognitive impairment in the lateralized phenotype of corticobasal degeneration. Dement Geriatr Cogn Disord. 2005;20(2–3):158–162. doi: 10.1159/000087299. [DOI] [PubMed] [Google Scholar]
- Murray R, Neumann M, Forman MS, Farmer J, Massimo L, Rice A, Grossman M. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68(16):1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- Nagaoka K, Ookawa S, Maeda K. A case of corticobasal degeneration presenting with visual hallucination. Rinsho Shinkeigaku. 2004;44(3):193–197. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(699071142):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- O'Keefe FM, Murray B, Coen RF, Dockree PM, Bellgrove MA, Garavan H, Robertson IH. Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain. 2007;130:753–764. doi: 10.1093/brain/awl367. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Drzezga A, Boecker H, Wagenpfeil S, Forstl H, Kurz A, Haussermann P. Right prefrontal hypometabolism predicts delusions in dementia with lewy bodies. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Phan K, Wager T, Taylor S, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Liu AA, Howard SM, Slama H, Hou CE, Shuster K, Miller B. A case-controlled study of altered visual art production in alzheimer's and FTLD. Cognitive and Behavioral Neurology. 2007;20(1):48–61. doi: 10.1097/WNN.0b013e31803141dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibiz JJ, Kolodny EH, Richardson EP. Corticodentoniagral degeneration with neuronal achromasia. Archives of Neurology. 1968;18:20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- Rey GJ, Tomer R, Levin BE, Sanchez-Ramos J, Bowen B, Bruce JH. Psychiatric symptoms, atypical dementia, and left visual field inattention in corticobasal ganglionic degeneration. Mov Disord. 1995;10(1):106–110. doi: 10.1002/mds.870100117. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lee MS, Thompson PD, Marsden CD. Corticobasal degeneration. A clinical study of 36 cases. Brain. 1994;117(Pt 5):1183–1196. doi: 10.1093/brain/117.5.1183. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Hesse JH, Rose KD, Slama H, Johnson JK, Yaffe K, Forman MS, Miller CA, Trojanowski JQ, Kramer JH, Miller BL. Frontotemporal dementia progresses to death faster than Alzheimer's disease. Neurology. 2005;65:719–725. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini M, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Wilson MR, Schauer GF, Allison S, Gorno-Tempini M, Pace-Savitsky C, Miller BL. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44(3):365–373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Rousseaux M, Godefroy O, Cabaret M, Bernati T. Dysexecutive syndrome and disorders of motor control in prefrontal mediobasal and cingulate lesions. Rev Neurol (Paris) 1996;152(8–9):517–527. [PubMed] [Google Scholar]
- Scolding NJ, Lees AJ. Micrographia associated with a parietal lobe lesion in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1994;57(6):739–741. doi: 10.1136/jnnp.57.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini M. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini M, Foti D, Mackenzie IR, Miller BL. Unravelling bolero: Progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131:39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70(30):323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveri P, Monza D, Paridi D, Radice D, Grisoli M, Testa D, Girotti F. Cognitive and magnetic resonance imaging aspects of corticobasal degeneration and progressive supranuclear palsy. Neurology. 1999;53(3):502–507. doi: 10.1212/wnl.53.3.502. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Danner FW, Davis DG, Hackney C, Landers T, Coyne CM. Neurochemical and histopathologic alterations characteristic of pick's disease in a non-demented individual. J Neuropathol Exp Neurol. 1994;53(1):37–42. doi: 10.1097/00005072-199401000-00005. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Wai D, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology. 2003;61(8):1134–1135. doi: 10.1212/01.wnl.0000086814.35352.b3. [DOI] [PubMed] [Google Scholar]
- Thumler BH, Urban PP, Davids E, Siessmeier M, Schreckenberger T, Benz P, Hopf HC. Dysarthria and pathological laughter/crying as presenting symptoms of corticobasal-ganglionic degeneration syndrome. J Neurol. 2003;250(9):1107–1108. doi: 10.1007/s00415-003-0075-7. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Litvan I, Jankovic J, Granata R, Mangone CA, McKee A, Pearce RK. Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry. 1998;64(2):184–189. doi: 10.1136/jnnp.64.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini M, Seeley WW, Rankin KP, Lee SS, Matthews BR, Miller BL. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69(14):1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brooks JO, III, Taylor J, Tinkleberg J. Development of aphasia, apraxia, and agnosia and decline in alzheimer's disease. Am J Psychiatry. 1993;150:742–747. doi: 10.1176/ajp.150.5.742. [DOI] [PubMed] [Google Scholar]
- Zhukareva V, Mann D, Pickering-Brown S, Uryu K, Shuck T, Shah K, Lee VM. Sporadic pick's disease: A tauopathy characterized by a spectrum of pathological tau isoforms in gray and white matter. Ann Neurol. 2002;51(6):730–779. doi: 10.1002/ana.10222. [DOI] [PubMed] [Google Scholar]