Abstract
Regulatory T cells (Tregs) can suppress a wide variety of cell types, in diverse organ sites and inflammatory conditions. While Tregs possess multiple suppressive mechanisms, the number required for maximal function is unclear. Furthermore, whether any inter-relationship or cross-regulatory mechanisms exist to orchestrate and control their utilization is unknown. Here we assessed the functional capacity of Tregs lacking the ability to secrete both interleukin-10 (IL-10) and interleukin-35 (IL-35), which individually are required for maximal Treg activity. Surprisingly, IL-10/IL-35-double deficient Tregs were fully functional in vitro and in vivo. Loss of IL-10 and IL-35 was compensated for by a concurrent increase in cathepsin E (Ctse) expression, enhanced TRAIL (Tnfsf10) expression and soluble TRAIL release, rendering IL-10/IL-35-double deficient Tregs functionally dependent on TRAIL in vitro and in vivo. Lastly, while C57BL/6 Tregs are normally IL-10/IL-35-dependent, BALB/c Tregs, which express high levels of CTSE and enhanced TRAIL expression, are TRAIL-dependent by default. These data reveal that cross-regulatory pathways exist that control the utilization of suppressive mechanisms, thereby providing Treg functional plasticity.
INTRODUCTION
Regulatory T cells (Tregs) play a key role in maintaining immune tolerance, preventing autoimmune diseases and limiting inflammatory conditions (1–3). A unique and important feature of Tregs is the brevity and flexibility of their regulatory capacity. Tregs can suppress an array of different cells types [including CD4+ T cells [Th1/Th2/Th17] (4), CD8+ T cells (5), B cells (4, 6), dendritic cells (7) and osteoclasts (8)] in a variety of inflammatory conditions and in distinct tissue locations. The ability of Tregs to suppress a broad range of targets in a variety of scenarios can be attributed to the numerous mechanisms employed by Tregs to mediate their function (2, 9). However, it is not clear whether all these mechanisms are equally important or if they have non-redundant roles under different inflammatory settings. Indeed, it was recently reported that Tregs may have specialized mechanisms for controlling specific cell types, as Tregs appear to require IRF-4, T-bet and STAT3 to suppress Th2, Th1 and Th17 cells, respectively (10–12). However, it is unclear which Treg mechanisms are used under specific conditions, how many mechanisms are required for maximal Treg function and whether there is any crosstalk between the various regulatory mechanisms utilized. While it is well established that Foxp3 is a key transcription factor critical for the stability of Tregs (13), whether there is stability or plasticity in the regulatory mechanisms used by Tregs is unclear.
Tregs utilize multiple mechanisms to mediate their function, with the immunosuppressive cytokines TGF-β, IL-10 and IL-35 contributing significantly (14–16). IL-10 is important for Treg function in vitro and in vivo, especially in the gut (17, 18). IL-35 is a recently discovered heterodimeric cytokine composed of Ebi3 (also part of IL-27) and Il12a/p35 (also part of IL-12) that is uniquely expressed by Tregs, but not by Tconv cells, and is required for maximal Treg function (16). While the loss of either IL-10 or IL-35 significantly reduces Treg function, they do not become completely dysfunctional and deficient mice do not exhibit the lethal multiorgan inflammatory disease seen in Scurfy or Foxp3−/− mice that lack Tregs (19, 20). Thus, in the present study we speculated that Tregs that lacked both IL-10 and IL-35 might exhibit a more profound functional defect and that this approach could be used to assess the relative contributions of different suppressive mechanisms. Alternatively, given the importance of Tregs in the maintenance of immune homeostasis, as yet unknown compensatory mechanisms may be triggered that attempt to restore immune balance. These possibilities were tested in this study.
MATERIALS AND METHODS
Mice
Ebi3−/− mice (C57BL/6: now 100% C57BL/6 by microsatellite analysis performed by Charles River) were provided by R. Blumberg and T. Kuo, Il10−/− mice were provided by T. Geiger, Tnfsf10−/− mice were provided by D. Green, and Foxp3−/− were provided by J. Ihle with permission from A. Rudensky. TGFβRII.DN, Il12a−/−, Rag1−/−, C57BL/6, BALB/c and B6.PL mice were purchased from the Jackson Laboratory. All animal experiments were performed in American Association for the Accreditation of Laboratory Animal Care-accredited, Helicobacter-free, MNV-free, specific-pathogen-free facilities in the St. Jude Animal Resource Center following national, state and institutional guidelines. Animal protocols were approved by the St Jude Animal Care and Use Committee. Spleens and lymph nodes from Tnfrsf10b−/− (DR5−/−) mice were provided by T. Ferguson, with approval of the Washington University Animal Care and Use Committee.
Cell purification, staining and flow cytometric analysis
Tconv (CD4+CD25− CD45RBhi) cells and Tregs (CD4+CD25+CD45RBlo) from spleens and lymph nodes of either knockout or wild type C57BL/6 mice were positively sorted by FACS following staining with fluorochrome-conjugated antibodies: anti-mouse CD4, anti-mouse CD45RB and anti-mouse CD25 (Biolegend, San Diego, CA). The cells were sorted on a Reflection (i-Cyt, Champaign, IL) or on a MoFlo (Dako-Cytomation, Fort Collins, CO). For flow cytometric analysis, purified Tregs were cultured as described and stained with anti-TRAIL-PE antibody (eBioscience, San Diego, CA) at the indicated time points after culture in presence of rIL2 (1000IU/ml). CTSE intracellular staining was performed as previously described (21). Briefly, freshly isolated Tregs were fixed with formaldehyde and permeabilized with Triton X-100 for 2 min prior to staining with anti-CTSE antibody (R&D). Cells were analyzed on a FACS Calibur (Beckman Coulter, Brea, CA) and data analyzed using FlowJo.
Messenger RNA isolation, cDNA synthesis and quantitative PCR
Purified Tregs from wild type C57BL/6 mice or knockout mice were activated in the presence of anti-CD3 and anti-CD28 coated beads or cultured in combination with Tconv cells at 4:1 (Tconv: Tregs) ratio for 48h. Where indicated for TRAIL expression, cells were activated in presence of IL2 (1000 IU/ml) and cells collected from RNA isolation at indicated time points. Cells were resorted based on their congenic markers where indicated. RNA was isolated using Qiagen micro or mini RNA kit following manufacturer's instructions. RNA was quantified using a nanodrop spectrophotometer, and equal amount of total RNA in each sample was reverse-transcribed with the high capacity cDNA reverse-transcription kit (Applied Biosystems) following the manufacturer's guidelines. TaqMan primers and probes were designed with Primer Express software and were synthesized by the St Jude Hartwell Center for Biotechnology and Bioinformatics. The primers for CTSE were CTSE forward, CAACCTCTGGGTCCCTTC, CTSE reverse, TGATTCCCTACCTCCGTG, CTSE probe, CATGCAAGGCACACCCAG. TRAIL-R2 (DR5) forward primer, TGCTGCTCAAGTGGCGC, DR5 Reverse primer, GGCATCCAGCAGATGGTTG, Trail forward primer, CCTCTCGGAAAGGGCATTC, TRAIL reverse primer, TCCTGCTCGATGACCAGCT Actin forward, ACCCACACTGTGCCCATCTAC, Actin Reverse, AGCCAAGTCCAGACGCAGG and Actin probe, AGGGCTATGCTCTCCCTCACGCCA. Equal volume of cDNA samples were used in a qPCR reaction with primer probes and amplified for 40 cycles using an ABI prism 7900 Sequence Detection System instrument according to the manufacturer's protocol. Relative quantification of mRNA expression was carried out using the comparative CT (critical threshold) method as described in the ABI User Bulletin number 2 (http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf). Here the amount of target mRNA is normalized to the endogenous beta-actin expression and is calculated by the formula 2ΔΔCT.
Immunoprecipitation and western blotting
Immunoprecipitation and immunoblotting for CTSE were performed as previously described (21). Tconv, wild type or knockout Treg were purified from spleens as described. Equal numbers of nTregs and Tconv cells were lysed with lysis buffer. To all supernatants, lysis buffer containing 0.1% Tween 20, 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, and 1 complete protease inhibitor tablet (Roche, Indianapolis, IN) per 50 ml lysis buffer, was added. Supernatants were immunoprecipitated with anti-mouse CTSE (R&D systems) and Protein G-sepharose beads. Immunoprecipitates were resolved by 10 % SDS-PAGE (Invitrogen Life Technologies), and blots were probed with a anti-goat HRP secondary antibody (Amersham Biosciences). Blots were developed using ECL (Amersham Biosciences) and autoradiography.
Preparation of anti-CD3/CD28-coated latex beads
4µM sulfate latex beads (Molecular Probes) were incubated overnight at room temperature with rotation in a 1:4 dilution of anti-CD3 and anti-CD28 antibody mix (13.3 µg/ml anti-CD3 (murine clone # 145-2c11, and 26.6 µg/ml anti-CD28 murine clone # 37.51, eBioscience). Beads were washed 3 times with 5mM phosphate buffer pH 6.5 and resuspended at 5×107/ml in sterile phosphate buffer with 2mM BSA.
In vitro Treg suppression assay and Transwell™ Treg assay
In vitro Treg suppression assays were performed as described previously (16, 17, 22). Anti-CD3 and anti-CD28 coated beads used for T cell stimulation in these assays were prepared as described previously (17, 22). Briefly, Tconv and Treg cells from wild type C57BL/6, Ebi3−/−, Il10−/−, Ebi3−/−Il10−/− and Il12a−/−Il10−/− mice were purified by FACS. Purified Tregs were titrated in to a 96-well round bottom plate starting at a 2:1 ratio (Tconv:Tregs) with 5×104 Tconv cells. The cells were stimulated with anti-CD3/anti-CD28-coated latex beads for 72h. Cultures were pulsed with 1 µCi of [3H]-thymidine for the final 8h of the 72h assay and harvested with a Packard harvester. The counts per minute (cpm) were determined using a Packard Matrix 96 direct counter (Packard Biosciences).
In vitro Transwell™ suppression assays were performed as described previously to assess the ability of Tregs to suppress via soluble mediators (17, 22). Tconv and Treg cells from wild type C57BL/6 mice, and Tregs from Ebi3−/−, Il10−/−, Ebi3−/− Il10−/− mice were purified by FACS. Wild type Tconv and wild type or knock out Treg cells were cultured at a 2:1 ratio in the Transwell™ insert with a pore size of 0.4µM (Millipore, MA). Target wild type Tconv or Tnfrsf10b−/− (DR5−/−) Tconv cells were activated in the bottom compartment of the Transwell™ plate with anti-CD3 and anti-CD28-coated latex beads for 72 h. Where indicated, neutralizing IL-10 mAb (JES5-2A5; BD Biosciences), neutralizing IL-35 mAb (V1.4C4.22), isotype control or DR5-Fc were added to standard Treg assays and Transwell™ experiments at the concentrations indicated. Where indicated, Tconv cells were fixed at a 1:5 dilution of 20% formaldehyde in culture medium, incubated at room temperature for 20 min, and washed 3 to 5 times with medium prior to culture. After 64h in culture, top Transwell™ inserts were removed and [3H]-thymidine was added directly to the responder Tconv in the bottom chambers of the Transwell™ plate for the final 8h of the 72h assay. Cultures were harvested with a Packard harvester. The cpm were determined using a Packard Matrix 96 direct counter (Packard Biosciences).
CTSE/TRAIL transfection assay
For in vitro assays with transfected 293T cells co cultured with Tconv cells, 293T cells were transfected with Ctse (murine CTSE in pIYneo [pCIneo (Clontech) with an IRES-YFP expression cassette]; provided by Dr. Paul Kayser) or Tnfsf10 (murine TRAIL in pIGneo [pCIneo with IRES-GFP]; provided by Dr Thomas Griffith) alone or in combination. Post-transfection (48h), the cells were irradiated (3000 rads) and seeded at a density of 7×103 cells per well in the 96-well flat bottom plate. Purified C57BL/6 Tconv cells were added to the seeded plate at 8×104 per well and stimulated with anti-CD3 and anti-CD28 coated beads for 72 h with [3H]-thymidine added during the last 8h of culture. T cell proliferation was calculated by subtracting the basal [3H]-thymidine incorporation of irradiated 293T plus unstimulated Tconv cells.
Treg-mediated control of homeostatic expansion
Homeostasis assays were performed as described previously (16, 23). Briefly, naïve Thy1.1+ Tconv cells from B6.PL mice, which were used as “target” cells, and Thy1.2+ wild type or knockout Tregs were purified by FACS. Tconv cells (2×106) and Tregs (5×105) were resuspended in 0.5 ml PBS plus 2% FBS and injected i.v. into Rag1−/− mice. Where indicated the mice were injected on day 0 and 3 with anti-TRAIL antibody (0.3mg) (provided by Thomas Griffith, University of Iowa) or isotype control antibody (0.3mg) (R&D systems). Mice were euthanized 7 days post transfer, and splenocytes were counted, stained and analyzed by flow cytometry using antibodies against CD4, Thy1.1, Thy1.2 (Biolegend) and Foxp3 (BD Biosciences). For each group, 6–8 mice were analyzed.
Inflammatory bowel disease model
A recovery model of colitis/IBD was used, with some modifications (14, 23). Briefly, Rag1−/− mice were injected i.v. with 0.5×106 of wild type or DR5−/− (CD4+CD45RBhiCD25−) naïve Tconv cells to induce IBD. Mice were weighed at the time of injection (time 0) and every week on the same day. At the onset of clinical symptoms of colitis (approximately 4 weeks post Tconv cell transfer), the mice were divided into Treg recipient or no Treg control groups. Purified Tregs from wild type, Ebi3−/−, Il10−/− or Ebi3−/−Il10−/− were injected i.p. All mice were weighed weekly and euthanized 32 days-post the initial T cell transfer. A recovery model of colitis/IBD was set up as described in the methods section. In experimental mice, the colons were collected, fixed in 10% neutral-buffered formalin 4 weeks after Treg injection. The tissues were further processed, 4µm sections cut and stained with H&E. Pathology of the large intestine was scored in a blinded manner using a semi-quantitative scale as described previously (23). In summary, grade 0 was assigned when no changes were observed; grade 1, minimal inflammatory infiltrates present in the lamina propria with or without mild mucosal hyperplasia; grade 2, mild inflammation in the lamina propria with occasional extension into the submucosa, focal erosions, minimal to mild mucosal hyperplasia and minimal to moderate mucin depletion; grade 3, mild to moderate inflammation in the lamina propria and submucosa occasionally transmural with ulceration and moderate mucosal hyperplasia and mucin depletion; grade 4 marked inflammatory infiltrates commonly transmural with ulceration, marked mucosal hyperplasia and mucin depletion, and multifocal crypt necrosis; grade 5, marked transmural inflammation with ulceration, widespread crypt necrosis and loss of intestinal glands.
Foxp3−/− rescue model
The Foxp3−/− rescue model was performed as described previously (23). Briefly, wild type or knockout Tregs purified by FACS were injected (106) i.p. into 2–3 day old Foxp3−/− mice. Recovery from disease was monitored weekly and reported as a clinical score. Five macroscopic categories were utilized to generate a 6 point scoring system. Mice were scored on the first 4 categories based on whether they showed (score of 1) or did not show (score of 0) the following characteristics: (1) body size runted, (2) tail is scaly and/or with lesions, (3) ears small, scaly with or without lesions, and (4) eyelids scaly and/or not fully open. The final scoring parameter was monitoring the activity level of the mouse. A score of 0 was assigned if the mouse was normal. A score of 1 was assigned if the mouse’s activity was moderately impaired, and a score of 2 assigned if the mouse was immobile. A combined score of 4 or greater was assigned moribund for longevity. Mice were euthanized 25 days post-transfer, spleen cells counted, stained and cell numbers determined by flow cytometry. Lung, liver and ear pinna were prepared for H&E analysis and the severity of inflammation was assessed and scored in a blinded manner by an experienced veterinary pathologist. The scoring system used for assessing inflammation was based on a simple algorithm for expressing inflammatory infiltrates in the lungs, liver and ear. The scores allotted to these three tissues were 0–9, 0–11 and 0–8, respectively, giving a maximum possible total of 28. Scoring criteria for each organ was as follows. The lung score was based upon inflammation in the peribronchiolar region, perivascular region, or interstitium. A score of 0–3 was assigned to each category with 0 being minimal or no inflammation, and scores of 1, 2, or 3 indicative of <10%, 10–50%, or >50%, respectively. The liver was scored based on 3 criteria. First, was the degree of portal tract inflammation with a score of 0 assigned to minimal or no inflammation. A score of 1, 2 or 3 was assigned if inflammation was associated with <25%, 25–75%, or >75% of the liver portal tracts, respectively. The second criteria related to portal/periseptal interface hepatitis. A focus of interface hepatitis associated with either a few or most of the portal tracts were scored 1 and 2, respectively. Two or more foci of interface hepatitis surrounding <50% or > 50% of the portal tracts or periseptae was scored 3 and 4 respectively. Third, foci of granulocytes and/or lymphocytes with or without necrotic hepatocytes that expand the sinusoid were considered foci of inflammation. The number of inflammatory foci in 10 contiguous 10X objective fields was counted and recorded as the average number of foci per 10X field and given a score of 0 to 4. A score of 0 was assigned when sinusoidal foci of inflammatory cells was absent. One focus or less per 10X field, two to four foci per 10X field, five to ten foci per 10X field and more than ten foci per 10X field was scored 1, 2, 3 and 4 respectively. The ear pinna was similarly scored based on 2 parameters; the percent of the ear dermis with inflammatory infiltrates and the intensity of the dermal inflammation. For percent analysis, a score of 0 was assigned when the inflammatory cells in all segments were not beyond that of normal background level. A score of 1, 2, 3 or 4 was assigned when the average percent for the segments was <25%, 25–50%, 51–75% or >75%, respectively. The intensity of the inflammatory infiltrate in the dermis was assessed as being of a loose or dense nature. A score of 0 was assigned when inflammatory cells in the dermis was not beyond the normal background level. When all the inflammation was of the loose nature, a score of 1 was assigned. When there was a mixture of loose and dense inflammatory cell infiltrates a score of 2 was assigned when the loose form was dominant. A score of 3 was assigned when the dense form was dominant. A score of 4 was assigned when all of the inflammation was of a dense nature.
Affymetrix array and analysis
Wild type or knock out Tregs were purified by FACS and mRNA isolated using the Qiagen micro RNA kit (Qiagen). Quality was confirmed by UV spectrophotometry and by analysis on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Total RNA (100ng) was processed in the Hartwell Center for Biotechnology & Bioinformatics according to the Affymetrix eukaryote two-cycle target labeling protocol and arrayed on a Mouse-430v2 GeneChip array. The expression data from the Affymetrix U133 plus 2 arrays was analyzed as MAS 5.0 signal log-start transformed using the following formula: log signal = ln (signal +20). This transform improves data dispersion, normality and stabilizes the variance of the data (24). Statistical tests and batch effect removal was performed using Partek Genomics Suite (St Louis, MO). The log2ratio of Ebi3−/−Il10−/− Treg to wild type Treg was calculated and the 20 most positively induced named genes were selected. The log2 ratios are calculated in STATA/SE 11.0 (College Station, TX) by the following formula: logratio A over B = log(exp(mean log signal A)/exp(mean log signal b))/log(2). Minimum selected gene had a log ratio of 1.65 which is 3.14 fold induced. Log ratios of the Il10−/− Treg and the Ebi3−/− Treg with respect to wild type were also defined and plotted with the logratio of Ebi3−/−Il10−/− Treg to wild type as a heat map using Spotfire Decision Site software (Figure 3A). T tests were then applied to each probeset to compare the Ebi3−/−Il10−/− Treg to wild type Treg and single knock out Treg samples and log2ratios were calculated. The p-value from the t tests were then −log10 transformed to create the significance score seen in the X-axis of the volcano plot Figure 3 B. A second series of t tests were performed to compare Treg to Tconv and to develop a Treg signature. Probe sets that had a p-value < 10−5, an absolute value log ratio of Treg versus Tconv of at least 3 (log2), and a defined gene name were selected for each category in the signature the mean was found. If a gene name appeared more than once then the mean data was averaged for that gene. The scores were calculated by finding the maximum and minimum values for each gene and then rescaling them from 0 to 1 by the following formula: scoreg = (observed meang − minimum meang)/(maximum meang − minimum meang) for each gene g. These gene scores were then sorted in descending order by the Treg: Tconv log ratio that includes activated and resting cells and graphed as a Heat map in Spotfire Decision Site (not shown). The microarray data from this study has been submitted in to the GEO repository. Accession number: GSE29262. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29262.
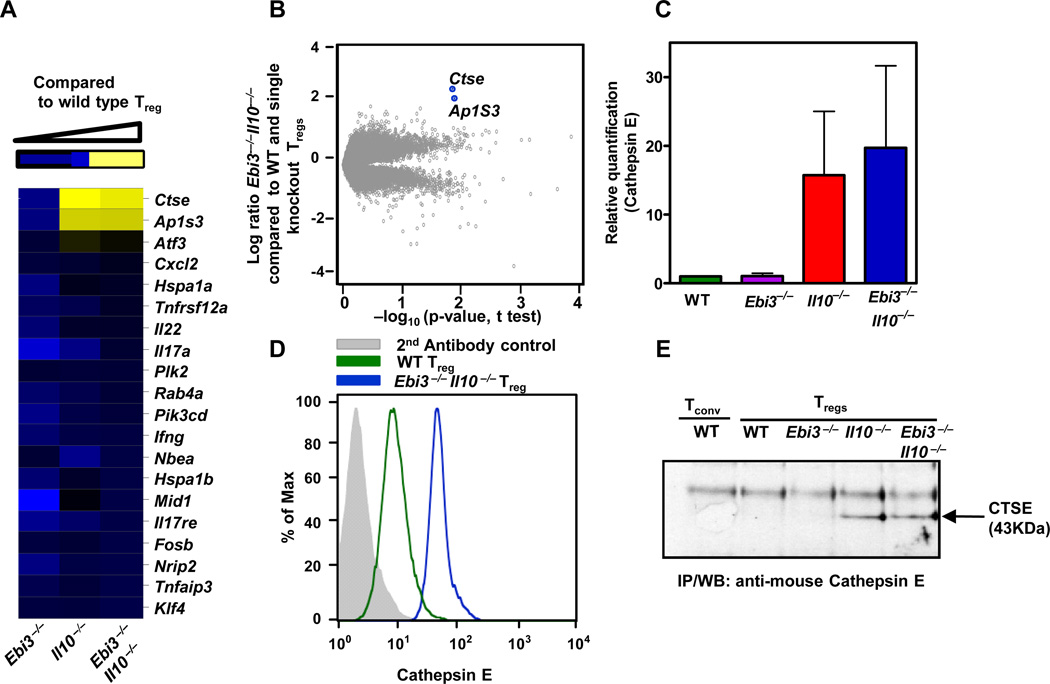
Figure 3. Up-regulation of CTSE by Ebi3−/−Il10−/− Tregs.
(A) mRNA was isolated from wild type or knock out Treg purified by FACS and used for Affymetrix analysis. Modulated genes in knock out Treg compared to wild type Tregs is depicted in a heat map. (B) Volcano plot comparing wild type and Ebi3−/− Il10−/−, Tregs. Highest modulated genes are marked. (C) mRNA was isolated from wild type or knock out Treg purified by FACS, cDNA synthesized and Ctse expression assessed by qPCR. Data are the mean of 2 independent experiments. (D) Wild type or knock out Treg were stained for intracellular CTSE [grey - 2nd antibody control, open histograms; in green - wild type Tregs and in blue; Ebi3−/− Il10−/− Tregs]. (E) Equal numbers of FACS purified wild type or knock out Treg were lysed, CTSE immunoprecipitated and analysed by SDS-PAGE/western blot. Data are representative (A, B, D and E) of three independent experiments.
Statistical analysis
Unless otherwise stated Students t test was used to determine statistical significance. All calculations were done using GraphPad software. A p-value less than 0.05 was considered significant.
RESULTS
Tregs that lack IL-10 and IL-35 maintain their suppressive activity
We first assessed the functional capacity of Tregs that lacked the ability to secrete IL-10 or IL-35 by generating Ebi3−/−Il10−/− and Il12a−/−Il10−/− mice (note that both Ebi3 and Il12a/p35 are required for IL-35 production) (16, 17). Purified wild type, Ebi3−/−, Il10−/−, Ebi3−/−Il10−/− and Il12a−/−Il10−/− Tregs were assessed in a standard Treg assay [note that these double-deficient Tregs would not be able to secrete IL-10 or IL-35, and although Ebi3 is also used by IL-27 and Il12a/p35 is also used by IL-12, these cytokines are not produced by Tregs (16)]. Surprisingly, Ebi3−/−Il10−/− and Il12a−/−Il10−/− Treg function was comparable or slightly better than wild type Tregs in suppressing their target conventional T cells (Tconv) cells (Figure 1A). We have previously shown that if Tregs are optimally stimulated by anti-CD3 and anti-CD28-coated beads and in contact with Tconv cells in the upper chamber (insert) of a Transwell™ plate, they can suppress third-party Tconv cells in the lower chamber across a semi-permeable membrane (17). Importantly, this suppression requires, and is limited to, IL-10 and IL-35. Thus, we anticipated that the loss of IL-10 and IL-35 would render Ebi3−/−Il10−/− Tregs unable to suppress across a Transwell™. Strikingly, Ebi3−/−Il10−/− Tregs suppressed Tconv cells across a Transwell™ comparable to their wild type counterparts, even though Ebi3−/− and Il10−/− Tregs were partially defective (Figure 1B). This equivalency in function was further supported by experiments with CFSE-labeled target Tconv cells and Transwell™ experiments with titrated Ebi3−/−Il10−/− Tregs in presence of fixed or unfixed Tconv cells (Figure S1 A and B). These data suggests that Ebi3−/−Il10−/− Tregs, unlike Ebi3−/− and the Il10−/− Tregs are functionally intact in in vitro suppression assays.
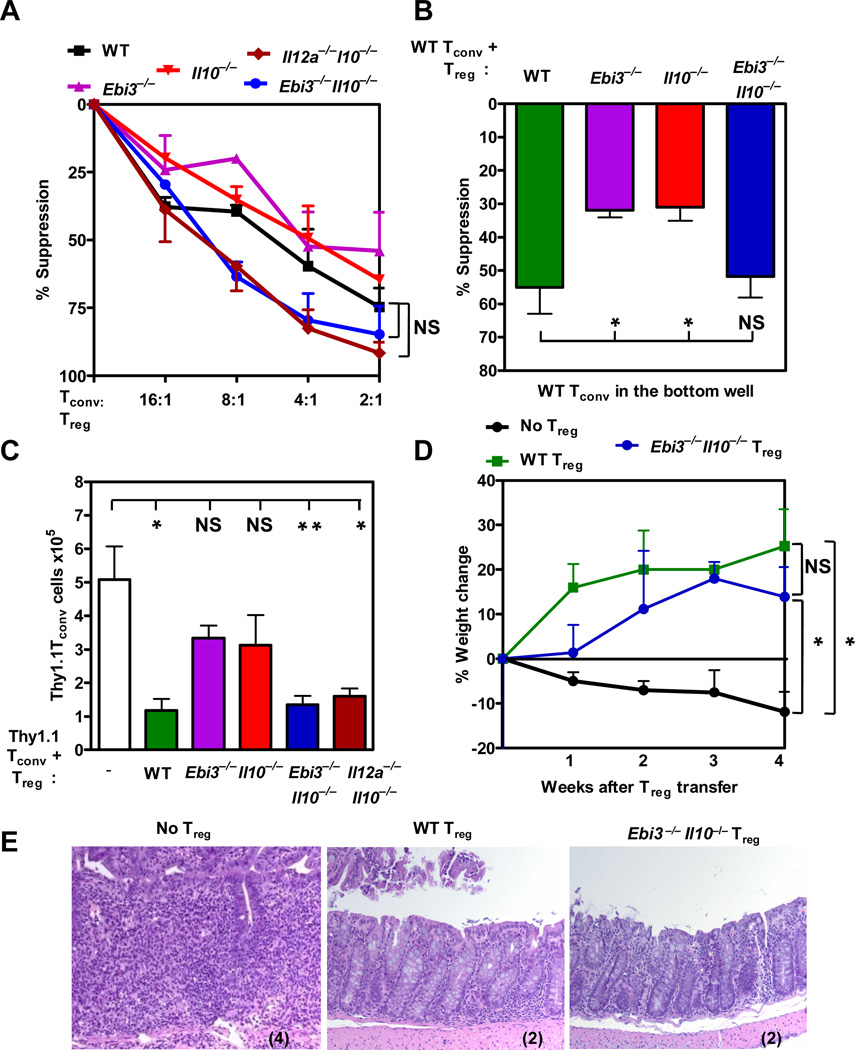
Figure 1. Ebi3−/−Il10−/− Tregs are suppressive in vitro and in vivo.
(A) Wild type or knock out Treg purified by FACS were titrated in a standard Treg assay with Tconv cells and anti-CD3 and anti-CD28 coated latex beads. Proliferation of Tconv responder cells was determined by [3H]-thymidine incorporation (p-value: wild type Tregs compared to Ebi3−/−Il10−/−, and Il12a−/−Il10−/− Tregs, Not significant (NS)). (B) Wild type or knock out Treg were cultured with anti-CD3 and anti-CD28 coated latex beads and Tconv cells in the inserts of a Transwell™ culture plate. Third party, wild type responder Tconv was activated in the bottom chamber of the plate. Proliferation of responder cells was determined by [3H]-thymidine incorporation. Proliferation ranged from 30,000–60,000 cpm. p-value: *: <0.05, NS: Not significant. (C) Congenically marked wild type Tconv cells, wild type or knock out Tregs purified by FACS were injected at 4:1 ratio into Rag1−/− mice. CD4+ cell numbers in the spleen were analyzed after 7 days by flow cytometry. p-value: * <0.05. (D) Wild type Tconv cells (0.5×106) were injected into Rag1−/− mice. The weight of the mice was monitored weekly for weight loss. Once the mice had lost 5% of its body weight wild type or knock out Tregs were injected. Mice were monitored for percent weight change calculated based on the weight at the time of Treg injection. p-value: * <0.05 and NS: Not significant. (E) Colonic tissue sections stained with H & E stain were scored in a blinded manner. Representative images of sections from 3 independent experiments are shown with histological score in parentheses. Data represent the mean ± SEM of (A) 3, (B) 3–5, (C) 4–9 mice per group, (D&E) 3 independent experiments.
We next asked if Ebi3−/−Il10−/− Tregs were functionally equivalent in several in vivo models. The adoptive transfer of Tregs into neonatal Scurfy or Foxp3−/− mice has been shown to restore normal immune homeostasis and prevent the lethal, systemic autoimmune disease that develops in these mice (19, 25, 26). Two day old neonatal Foxp3−/− mice were injected with 106 wild type, Ebi3−/−, Il10−/− or Ebi3−/−Il10−/− Tregs. Clinical symptoms, histological analysis and CD4+ T cell numbers were determined when the mice were ~4 weeks old. Although no defects were observed with the Ebi3−/− Tregs recipients, increased histological scores were observed with Il10−/− Treg recipients. In contrast, Ebi3−/−Il10−/− Tregs were clearly capable of fully restoring immune homeostasis despite the loss of these two key regulatory cytokines (Figure S2 A–C). We also assessed the ability of these Treg populations to rescue immune homeostasis in mixed bone marrow chimeras generated using a 50:50 mixture of bone marrow from Foxp3−/− mice and either wild type, Ebi3−/−, Il10−/− or Ebi3−/−Il10−/− mice transferred into Rag1−/− mice. Interestingly, significant defects were observed in the ability of Ebi3−/− and Il10−/− bone marrow to rescue the Foxp3−/− phenotype (Figure S2 D and E). In contrast, the Foxp3−/− bone marrow recipients of Ebi3−/−Il10−/− Tregs were largely intact and comparable to their wild type Treg, Foxp3−/− recipient counterparts (Figure S2 D and E).
Tregs have been shown to regulate the homeostatic expansion of Tconv cells in lymphopenic Rag1−/− mice (27–29). Purified wild type Thy1.1 Tconv cells, either alone or in presence of wild type, Ebi3−/−, Il10−/−, Ebi3−/−Il10−/− or Il12a−/−Il10−/− Thy1.2+ Treg cells, were adoptively transferred into Rag1−/− mice, and splenic Thy1.1 Tconv and Thy1.2 Treg cell numbers (data not shown) determined seven days later. In the presence of wild type, but not Ebi3−/− or Il10−/− Tregs, Tconv cell expansion was significantly reduced (Figures 1C). Surprisingly, the capacity of Ebi3−/−Il10−/− and Il12a−/−Il10−/−Treg cells to control Tconv cell expansion was comparable to wild type Tregs.
Tregs cure colitis in mice, a model for inflammatory bowel disease (IBD) in humans, in an IL-10- and IL-35-dependent manner (16, 30). Colitis in mice is induced experimentally by transferring low numbers of naïve CD4+CD45RBhiCD25− Tconv cells into Rag1−/− mice (31). Recovery from disease, marked by weight gain and decreased histopathology, is observed only in mice that receive purified Treg cells approximately four weeks after the initial Tconv cell transfer (14). We used this recovery model of colitis to assess the functional capacity of Ebi3−/−Il10−/− Tregs in vivo. Approximately 4 weeks post Tconv cell transfer, recipients developed clinical symptoms of colitis (monitored by weight loss) and were either left untreated or were treated with either wild type, Ebi3−/−, Il10−/− or Ebi3−/−Il10−/− Tregs. As expected, mice that did not receive Tregs continued to lose weight, and exhibited substantial histiocytic infiltration and goblet cell destruction during the subsequent four weeks (Figures 1D, 1E, and S2F). In contrast, the wild type Treg recipients started to gain weight within a week of transfer. Despite previous studies clearly demonstrating the inability of Ebi3−/− or Il10−/− Tregs to cure colitis, weight gain and improved histological parameters were evident in the Ebi3−/−Il10−/− Treg recipients, suggesting that these double inhibitory cytokine-deficient Tregs had regained their regulatory potential (Figures 1D, 1E and S2F).
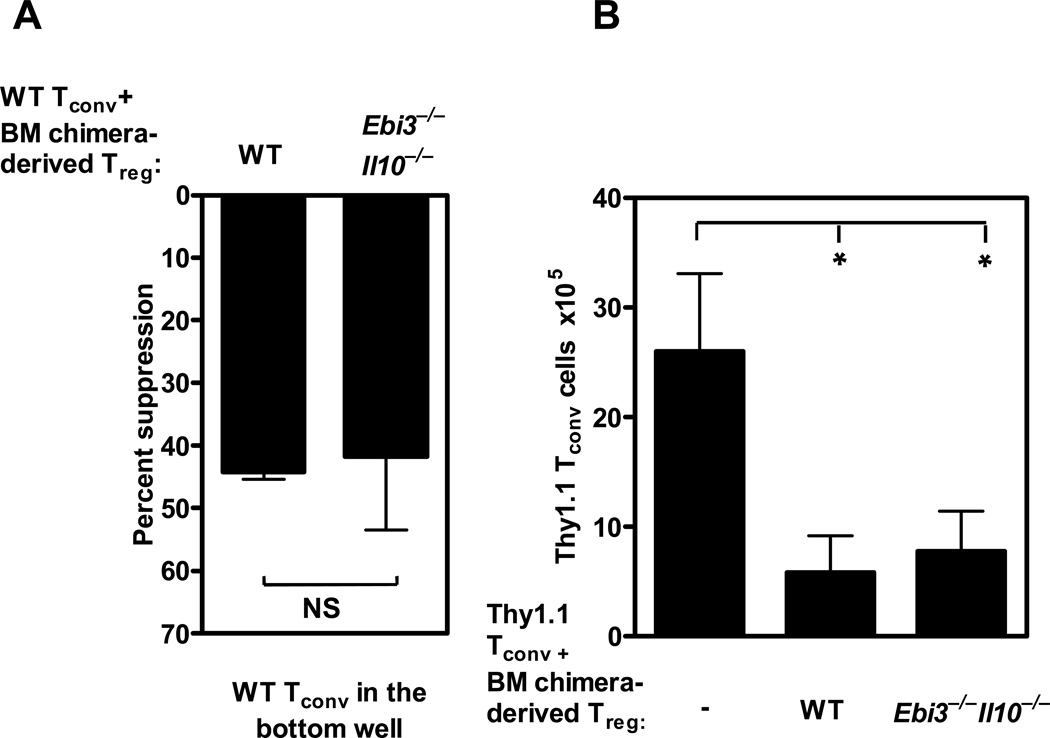
To rule out the possibility that this regulatory restoration had occurred as a consequence of their development in the absence of IL-10 and IL-35 and/or due to alternate cell-extrinsic mechanisms, we directly compared the suppressive capacity of wild type and Ebi3−/−Il10−/− Tregs that had developed in the same environment. To address this possibility, we generated mixed bone marrow chimeras with a 1:1 ratio of congenically-marked Thy1.1+ wild type bone marrow with Thy 1.2+ wild type or Ebi3−/−Il10−/− bone marrow into sublethally-irradiated Rag1−/− mice. Eight weeks post-transfer, Tregs were purified by FACS from the mixed bone marrow chimeras and assessed in in vitro Transwell™ and in vivo homeostasis assays. Chimera-derived Ebi3−/−Il10−/− Tregs and wild type Tregs suppressed third party Tconv cells comparably across a Transwell™ (Figure 2A). In contrast, similarly prepared Ebi3−/− and Il10−/− Tregs were defective (data not shown). Furthermore, Thy1.2+ Ebi3−/−Il10−/− Tregs and wild type Tregs that had developed in the bone marrow chimeras suppressed Tconv expansion comparably in homeostasis assay (Figure 2B). Taken together, these data suggest that a cell-intrinsic modification had occurred in the Ebi3−/−Il10−/− Tregs to render them functionally comparable to wild type Tregs in order to compensate for their inability to secrete IL-10 and IL-35.
Figure 2. Ebi3−/−Il10−/− Tregs that developed in a mixed bone marrow chimera can function in vitro and in vivo.
Congenically labeled wild type bone marrow and knock out bone marrow was mixed at a 1:1 ratio and injected into sub lethally irradiated Rag1−/− mice. (A) After 8 weeks Thy.1.2+ wild type Treg cells or knock out Treg cells were purified by FACS from the bone marrow chimeric mice and cultured in the inserts of a Transwell™ plate in the presence of wild type Tconv cells and anti-CD3 and anti-CD28 coated latex beads. Wild type naive Tconv cells were activated in the presence of anti-CD3 and anti-CD28 coated beads in the bottom chamber of a Transwell™ for 72 h. Proliferation was determined by [3H]-thymidine incorporation. Data represents the mean± SEM of two independent experiments. (p-value; NS: Not significant). (B) Purified wild type or Ebi3−/−Il10−/− bone marrow chimera-derived Tregs were injected into Rag1−/− mice in the presence of congenically marked naïve Tconv cells. The expansion of naïve Thy1.1 CD4+ T cells were assessed by flow cytometry. Data represents the mean ± SEM of two independent experiments with 3–4 mice per group (p-value *=0.06).
Loss of IL-10/IL-35 results in a compensatory increase in CTSE
Given that Ebi3−/−Il10−/− Tregs can suppress Tconv cells across a Transwell™, they had clearly acquired a suppressive mechanism that operated via a soluble mediator. Beyond IL-10 and IL-35, transforming growth factor-β (TGFβ) is the only other known cytokine or soluble factor that would likely function across a Transwell™ that has been suggested to play a role in Treg function [note that cAMP and adenosine are highly labile inhibitors that are only active in very close proximity] (2, 3, 9). We assessed any potential role for TGFβ by comparing the capacity of wild type and Ebi3−/−Il10−/− Tregs to suppress across a Transwell™ using third party Tconv cells from CD4-dnTGFβRII transgenic mice that are resistant to TGFβ-mediated suppression (32). The data clearly show that the suppressive capacity of wild type and Ebi3−/−Il10−/− Tregs was comparable when Tconv cells resistant to TGFβ-mediated suppression were used as target cells (Figure S1C). This suggested that the compensatory suppressive mechanism used by Ebi3−/−Il10−/− Tregs was not TGFβ.
To identify this compensatory suppressive mechanism, we compared the gene expression profile of wild type, Ebi3−/−, Il10−/− and Ebi3−/−Il10−/− Tregs using Affymetrix GeneChip arrays. We first generated a list of highly differentially expressed wild type Treg signature genes, by comparison of the array profile with wild type Tconv, to determine if there were any notable global changes in gene expression in wild type versus Ebi3−/−Il10−/− Tregs. Minimal variations were observed in the expression (up or down) of 47 highly modulated Treg signature genes (Data not shown). Indeed, global analysis revealed very few differences between wild type and Ebi3−/−Il10−/− Tregs (Figures 3A, 3B and Data not shown). The two notable exceptions were Ap1S3 (adaptor-related protein complex 1, sigma 3 subunit) and Ctse (cathepsin E) (Figures 3A, 3B). AP1S3 is the sigma subunit of the adaptor protein-1 (AP1) complex that is a component of the clathrin-coated vesicles associated with the trans-Golgi network (TGN) that mediate vesicular formation and transport (33). The significance of its up-regulation in Ebi3−/−Il10−/− Tregs is unknown and was not selected for further study here. CTSE is an intracellular aspartic protease of the endolysosomal pathway that has been primarily implicated as a component of the antigen processing machinery for the MHC class II pathway (34). Quantitative PCR, immunoprecipitation/western blot analysis and intracellular staining with purified Tregs confirmed that CTSE mRNA and protein are highly up-regulated in Il10−/− and Ebi3−/−Il10−/− Tregs compared with wild type and Ebi3−/− Tregs (Figure 3C–E and S3 A–C).
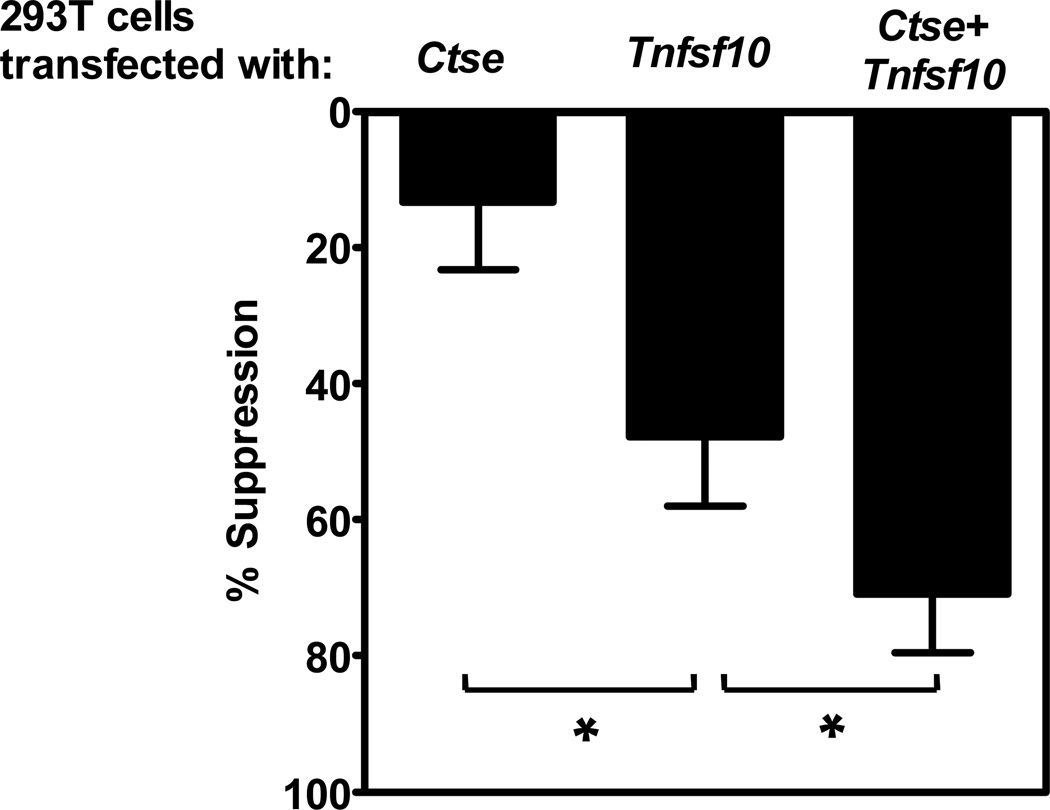
Interestingly, CTSE has been implicated in the cleavage and/or processing of TRAIL (TNF-related apoptosis-inducing ligand; Tnfsf10; tumor necrosis factor (ligand) superfamily, member 10), and its release from the cell surface (35, 36). TRAIL is a suppressive molecule of the TNF superfamily that can function in its surface bound form or as a soluble trimer (37, 38). TRAIL can mediate apoptosis, programmed necrosis (necroptosis) or suppress proliferation (37, 39). Furthermore, activated CD4+Foxp3+ Tregs and CD8+ Tregs may express and utilize TRAIL as a suppressive mechanism (40, 41). Thus, we speculated that increased CTSE in Ebi3−/−Il10−/− Tregs might result in an increase in the functional capacity of surface TRAIL and/or an increase the release of soluble TRAIL. To directly examine this possibility, 293T cells were transfected with expression plasmids encoding Ctse and/or Tnfsf10 and used to assess the ability of TRAIL to limit T cell proliferation. TRAIL transfectants limited T cell proliferation and this was further enhanced in the presence of CTSE (Figure 4). These data suggest that CTSE may play a role in enhancing the function of TRAIL by either increasing its activity via processing or increasing the generation of soluble TRAIL. This data also raised the possibility that Ebi3−/−Il10−/− Tregs are dependent on TRAIL for their suppressive activity, while wild type Tregs are not.
Figure 4. Cathepsin E enhances the suppression of Tconv cells by TRAIL.
293T cells were transfected either with Ctse and Tnfsf10 alone or together. The cells were irradiated with 3000 rads 48h post transfection and seeded at a density of 7000 cells per well in a 96 well flat bottom plate. Freshly isolated C57BL/6 Tconv cells were added to the seeded plate at 8×104 per well and stimulated with anti-CD3 and anti-CD28 coated beads for 72 hours. Proliferation of responder cells was determined by [3H]-thymidine incorporation. Tconv cell proliferation was calculated by subtracting the basal [3H]-thymidine incorporation of irradiated 293T plus T cells without anti-CD3 and anti-CD28 stimulation. Data represent the average of three independent experiments. p-value, * : <0.05.
IL-10/IL-35 deficient Tregs suppress via TRAIL
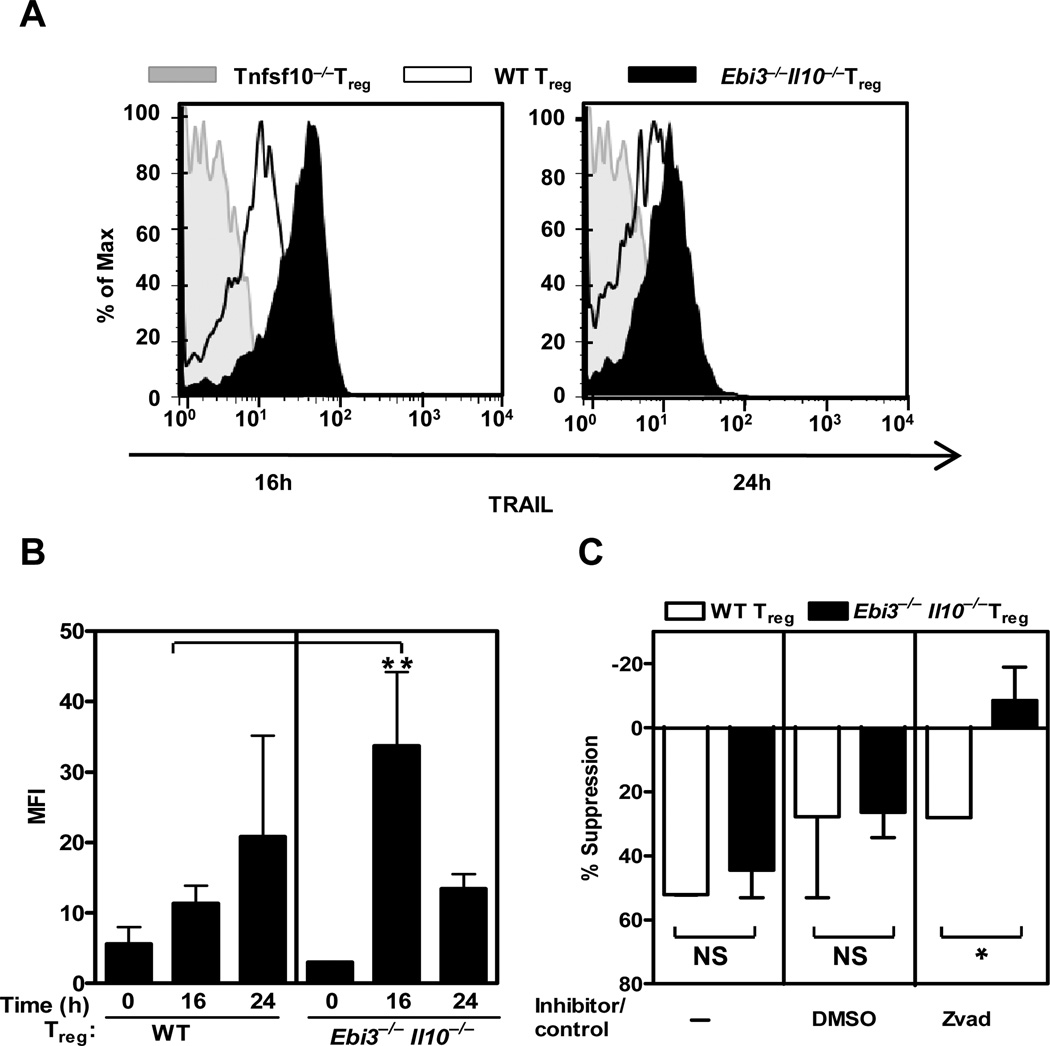
We first assessed whether there were any changes in the level or rate of TRAIL expression during activation of wild type, Ebi3−/−, Il10−/− or Ebi3−/−Il10−/− Tregs. Minimal alterations in Tnfsf10 (TRAIL) mRNA expression was observed over time or between the four Treg population (Figures S3D). While all Treg populations exhibited increased TRAIL surface expression following activation, Ebi3−/−Il10−/− Tregs expressed significantly higher levels of TRAIL after 16h but not 24h, post-activation (Figure 5A, B and S3E). This suggested that the kinetics of TRAIL expression is accelerated in Ebi3−/−Il10−/− Tregs. Interestingly, while IL10 appeared to influence CTSE expression (Figure 3C and E and S3 A–C), IL-35 may influence other parameters that influence TRAIL expression as Ebi3−/− Tregs expressed slightly higher levels of TRAIL at 16h compared with wild type or Il10−/− Tregs (Figure S3E).
Figure 5. TRAIL dependence and modulation in Ebi3−/− Il10−/−Tregs.
Wild type or knock out Treg purified by FACS were activated in presence of anti-CD3 and anti-CD28 coated latex beads with IL2 for 16 and 24h. (A) Cells were collected and surface TRAIL expression detected by flow cytometry using an anti-mouse TRAIL antibody. Data are representative of 3 independent experiments. (B) Mean fluorescence intensity (MFI) of surface TRAIL expression following activation from 3–4 independent experiments were plotted. Students t Test; p-value ** = <0.05. (C) Wild type or knock out Treg were cultured in the insert of a Transwell™ culture plate in the presence of wild type Tconv cells. zVAD or DMSO control was added to the Transwell™ assay. Freshly purified wild type responder Tconv cells were activated in the bottom chamber of the plate. Proliferation of responder cells was determined by [3H]-thymidine incorporation. Data represent the mean ± SEM of 2 independent experiments. p-value * = 0.07.
We then used various approaches to determine the extent to which this accelerated TRAIL expression meant that the Ebi3−/−Il10−/− Tregs were dependent on TRAIL-mediated suppression. TRAIL mediates its suppression in part via caspase-mediated apoptosis (37). Thus we asked if Ebi3−/−Il10−/− Tregs mediated suppression in a caspase-dependent fashion by performing a Transwell™ suppression assay in the presence of the general caspase inhibitor z-VAD-Fmk or a vehicle control (42). Although wild type Treg suppression was unaffected by z-VAD-Fmk or its DMSO vehicle control, Ebi3−/−Il10−/− Treg mediated suppression was blocked (Figure 5C). These data suggest that Ebi3−/−Il10−/− Tregs suppress Tconv proliferation via a caspase dependent pathway.
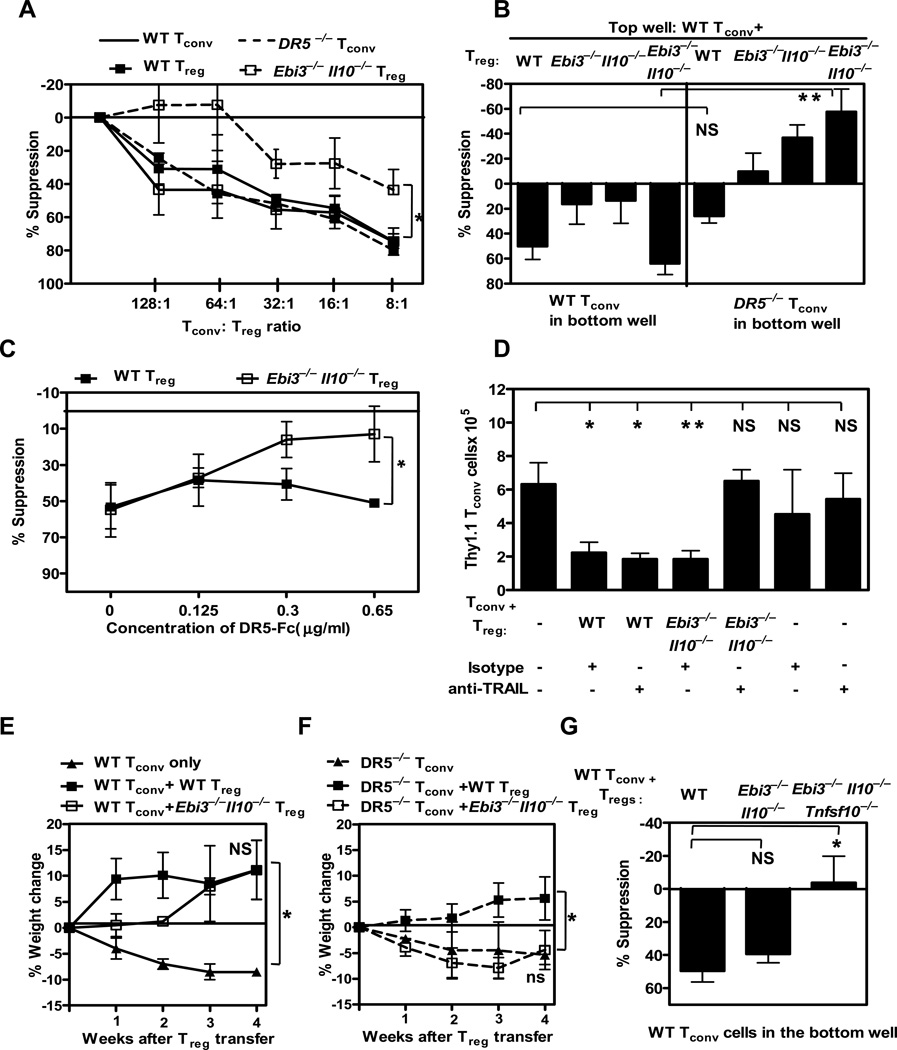
TRAIL signaling in the mouse is mediated through DR5 (death receptor 5; Tnfrsf10b; tumor necrosis factor receptor superfamily, member 10b; also known as TRAIL-R2) (43). Therefore, we first asked if the Ebi3−/−Il10−/− Tregs were able to suppress Tnfrsf10b−/− Tconv cells (hereafter referred to as DR5−/−) in conventional and Transwell™ suppression assays. As previously shown, wild type and Ebi3−/−Il10−/− Tregs suppressed wild type Tconv cells comparably (Figure 6A). Furthermore, wild type Tregs could effectively suppress DR5−/− Tconv cells. However, Ebi3−/−Il10−/− Tregs were less effective at suppressing DR5−/− Tconv cells in a standard Treg assay (Figure 6A) and completely failed to suppress across a Transwell™ (Figure 6B). Secondly, we assessed whether a DR5-Fc fusion protein or an anti-TRAIL blocking antibody were able to inhibit Ebi3−/−Il10−/− Treg–mediated suppression of wild type Tconv cells. While DR5-Fc had a minimal effect on the suppression mediated by wild type Tregs across a Transwell™, blocked suppression by Ebi3−/−Il10−/− Tregs in a dose dependent manner (Figure 6C). Similarly, anti-TRAIL, but not an isotype control antibody, reduced the suppressive capacity of Ebi3−/−Il10−/−, but not wild type, Tregs [note that this TRAIL mAb is known to block activity weakly in vitro but very effectively in vivo (44)] (Figure S4A). These results suggest that Ebi3−/−Il10−/− Tregs mediate suppression across a Transwell™ in vitro via soluble TRAIL.
Figure 6. Ebi3−/−Il10−/− Treg mediated suppression is TRAIL-dependent.
Wild type or knock out Tregs purified by FACS (A) were titrated in a Treg assay with wild type or DR5−/− Tconv cells and stimulated with anti-CD3 and anti-CD28 coated latex beads or (B) were cultured with wild type Tconv cells in the insert of a Transwell™ culture plate. Wild type or DR5−/− Tconv cells were activated in the bottom chamber of the plate with anti-CD3 and anti-CD28 coated latex beads. Proliferation of responder wild type or DR5−/− Tconv cells was determined by [3H]-thymidine incorporation. CPM ranged between 30,000–65,000. Results shown here are average of 4–5 independent experiments. (C) Wild type and Ebi3−/−Il10−/− Tregs were stimulated with anti-CD3 and anti-CD28 coated latex beads in the presence of Tconv cells in the insert of a Transwell™ culture plate. Freshly purified wild type responder Tconv cells were activated in the bottom wells in the presence of a titrated amount of DR5-Fc. Data is average of 2–3 independent experiments, One way ANCOVA p-value *= 0.01 (D) Congenically marked wild type Tconv cells and wild type or knock out Tregs were injected at 4:1 ratio in to Rag−/− mice. On days 1 and 3 TRAIL neutralizing mAb or isotype control were injected by i.p. CD4, Thy1.1 and Thy 1.2 T cell numbers in the spleen were analyzed after 7 days by flow cytometry. Data includes 3–6 mice per group from 3 independent experiments. (E) Wild type littermate control Tconv cells or (F) DR5−/− Tconv cells (0.5 × 106 cells) were injected into Rag1−/− mice. The weight of the mice was monitored weekly for weight loss. Percent weight change was calculated based on the weight at the time of Treg injection. (G) Wild type or knock out Tregs purified by FACS were cultured with wild type Tconv cells in the insert of a Transwell™ culture plate. Wild type Tconv cells were activated in the bottom chamber of the plate with anti-CD3 and anti-CD28-coated latex beads. Proliferation of responder Tconv cells was determined by [3H]-thymidine incorporation. CPM ranged between 30,000–70,000. Results shown here are mean ± SEM of 3 independent experiments. p-value - A–G; *: < 0.05, ** < 0.005, NS: Not significant.
We then assessed the contribution of TRAIL-mediated suppression by Ebi3−/−Il10−/− Tregs in vivo. First, congenic Thy1.1+ wild type Tconv cells were either injected into Rag−/− mice alone or in the presence of Thy1.2+ wild type or Ebi3−/−Il10−/− Tregs. Isotype control antibody or anti-TRAIL was injected on days 0 and 3, and homeostatic expansion of the Thy1.1+ Tconv cells determined 7 days later. Tconv cell expansion, wild type Treg-mediated suppression, and Treg numbers were unaffected by the anti-TRAIL treatment (Figure 6D and data not shown). In striking contrast, TRAIL inhibition blocked the ability of Ebi3−/−Il10−/− Tregs to suppress Tconv cell expansion in vivo.
Second, we assessed the extent to which the Ebi3−/−Il10−/− Tregs could cure colitis induced by DR5−/− Tconv cells. The development and severity of colitis induced by wild type or DR5−/− Tconv cells in Rag−/− mice were comparable (Figure 6E, 6F and S4B). At the onset of clinical symptoms (5% loss of body weight; ~4 weeks), mice were treated with wild type or Ebi3−/−Il10−/− Tregs. Wild type Treg recipients gained weight and recovered from the clinical symptoms of colitis regardless of whether the disease had been induced by wild type or DR5−/− Tconv cells (Figure 6E and 6F). In contrast, Ebi3−/−Il10−/− Tregs could cure colitis caused by wild type but not DR5−/− Tconv cells. Histological analysis of the colon 4 weeks post-Treg treatment confirmed that Ebi3−/−Il10−/− Tregs were unable to reverse DR5−/− Tconv cell-induced colitis (Figure S4B).
Third, if TRAIL was essential for Ebi3−/−Il10−/− Treg-mediated suppression, then its genetic deletion should abrogate their regulatory capacity. Our data suggest that although wild type and Ebi3−/−Il10−/− Treg could effectively mediate suppression of Tconv cells across a Transwell™, Ebi3−/−Il10−/−Tnfsf10−/− Tregs could not inhibit Tconv target cell proliferation (Figure 6G). Taken together these data clearly demonstrate that Ebi3−/−Il10−/− Tregs require TRAIL for maximal suppressive function, and that soluble TRAIL appears to be their only mechanism of suppression. In contrast, wild type Tregs exhibit minimal TRAIL-dependence and use IL-35 and IL-10 as their soluble mediators of suppression.
Differential utilization of suppressive mechanisms by genetically distinct Tregs
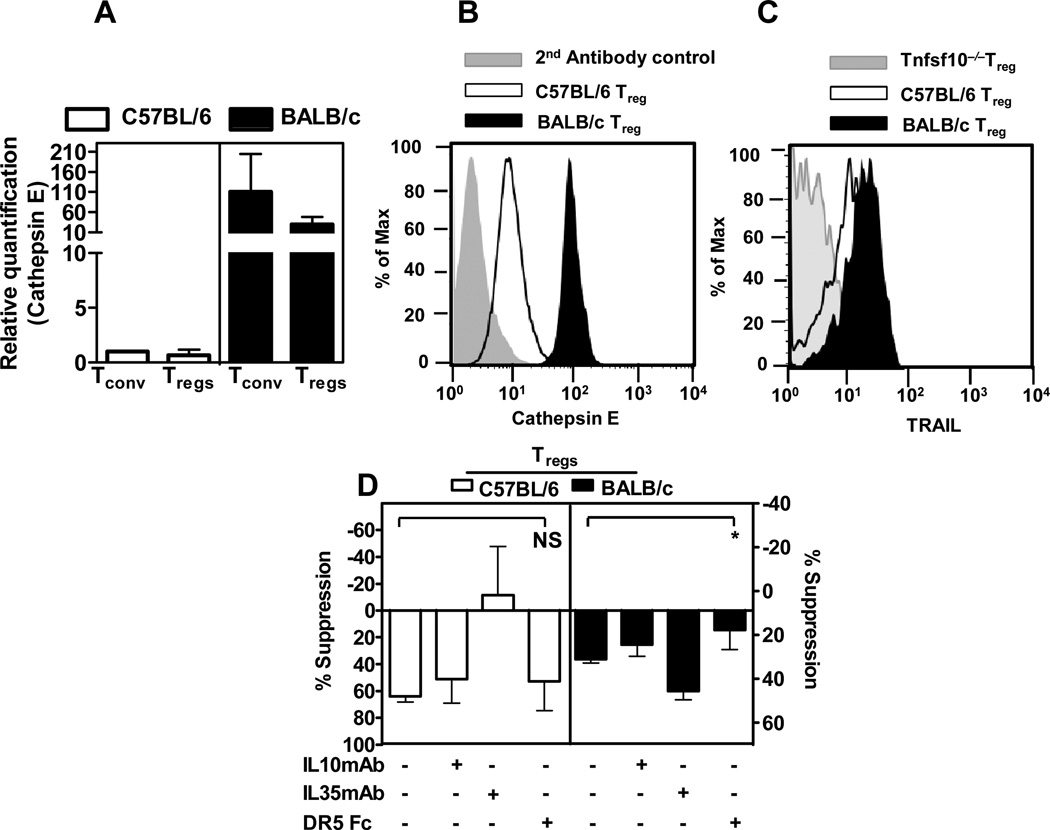
Loss of IL-10 and IL-35 production by Tregs led to increased CTSE expression and subsequent dependence on TRAIL-mediated suppression. We questioned the extent to which unmanipulated examples of this Treg functional plasticity might exist. Differential CTSE expression has been reported in different inbred mouse strains (21). In particular, C57BL/6 mice express low levels of CTSE, while expression in BALB/c and 129 mice is high. We first confirmed these observations by assessing Ctse expression by qPCR and intracellular staining (Figure 7A and 7B). The results clearly indicate that BALB/c Tregs express higher levels of CTSE, consistent with previous observations (21). Next we assessed the kinetics of TRAIL surface expression on BALB/c Tregs following activation. Interestingly, BALB/c Tregs expressed slightly higher levels of surface TRAIL than C57BL/6 Tregs, particularly at 16h post-activation (Figure 7C). Indeed, the pattern of CTSE and TRAIL expression exhibited by BALB/c Tregs was analogous to observations made with Ebi3−/−Il10−/− Tregs (compare Figures 3C, 3D and 5A with Figures 7A, 7B and 7C), and was consistent with previous suggestions (45). We then examined the suppressive capacity of BALB/c and C57BL/6 Tregs in presence or absence of reagents that block IL-10, IL-35 or TRAIL. While anti-IL-10 and the isotype control antibody had little effect on the suppression mediated by either Treg population in a Transwell™ assay, IL-35 neutralizing mAb blocked suppression mediated by C57BL/6, but not BALB/c, Tregs (Figure 7D). In contrast, DR5-Fc inhibited suppression mediated by BALB/c, but not C57BL/6, Tregs. Thus C57BL/6 Tregs seems to be more dependent on IL-35, while BALB/c Tregs are dependent on TRAIL-mediated suppression. This raises the possibility that genetic variations predispose Tregs to preferential modes of immunosuppression.
Figure 7. BALB/c Treg preferentially use TRAIL-mediated pathways compared to C57BL/6 Tregs.
(A) mRNA was isolated from freshly purified C57BL/6 or BALB/c Tconv cells and Tregs, cDNA synthesized and qPCR performed to assess Ctse expression. (B) Intracellular staining for CTSE was performed with purified C57BL/6 or BALB/c Treg [Grey filled - secondary antibody only control; open histogram - C57BL/6 Tregs and closed histogram BALB/c Tregs]. (C) TRAIL staining was performed with Tnfsf10−/−, wild type C57BL/6 or BALB/c Tregs, activated in presence of anti-CD3 and anti-CD28 coated latex beads with IL2 for 16h and surface TRAIL expression was detected by flow cytometry using a anti-mouse TRAIL antibody (MFI from three independent experiments, p-value:0.07). (D) Wild type C57BL/6 or BALB/c Tregs were mixed at 1:2 ratio with naïve wild type Tconv cells in the presence of anti-CD3 and anti-CD28 coated beads in the insert of a Transwell™ culture plate for 72h. Neutralizing antibodies against IL-10, IL-35 or a DR5-Fc protein were added to the Transwell™ assay at pre-determined concentrations as described in the methods. Freshly purified wild type responder Tconv were activated in the bottom chamber of a Transwell™ culture plate. Proliferation of the responder cells was determined by [3H]-thymidine incorporation. p-value *: < 0.05. Data represent 3–4 independent experiments.
DISCUSSION
Tregs can function in diverse anatomical locations and in a wide variety of immunological and disease settings (46). Consequently, the large array of suppressive mechanisms that Tregs are reported to possess may help them maintain immune homeostasis under diverse scenarios. Indeed, Tregs may have specialized mechanisms for controlling specific cell types as Tregs appear to require IRF-4, T-bet and STAT3 to suppress Th2, Th1 and Th17 cells, respectively (10–12). However, this may have a greater influence on their migratory behavior than the mechanisms they use to mediate suppression. Importantly, the relative importance of specific mechanisms of Treg function and whether Tregs possess mechanistic flexibility has not been elucidated. Previous studies have reported that deficiency of IL-10 or IL-35 alone results in defective Treg function (16, 18). Thus our finding that Tregs lacking IL-35 and IL-10 are fully functional, instead relying on TRAIL-mediated suppression as a primary mechanism of action, was very surprising. This implies that Tregs can exhibit remarkable functional plasticity and possess control mechanisms to compensate for the loss of key regulatory tools.
There is a reciprocal relationship in the expression of IL-10 and CTSE (47). Our data clearly show that Ebi3−/−Il10−/− Tregs are dependent on TRAIL for their regulatory function in vitro and in vivo. Furthermore, our studies suggest that increased expression of CTSE enhances the rate and extent of TRAIL surface expression and TRAIL function in mediating T cell suppression. It is possible that CTSE may ‘process’ full length TRAIL to enhance its ligand binding and/or may mediate the cleavage of cell surface TRAIL to generate a soluble version. Soluble TRAIL is thought to be either secreted into microvesicles (48) or cleaved from the cell surface (49). While the precise mechanism by which CTSE enhances TRAIL function requires further elucidation, consistent with our results, previous studies have shown that proteolytic cleavage of TRAIL from the cell surface can be mediated by CTSE (35, 36). Thus in Ebi3−/−Il10−/− Tregs, CTSE up-regulation may play a role in the generation of soluble TRAIL. In contrast, expression of IL-10 by wild type Tregs may suppress CTSE expression and thus reduce the contribution of TRAIL-mediated killing. These data also support the capacity of activated Tregs to utilize TRAIL (40, 41), and further highlight the complex inter-regulatory pathways modulated by inhibitory cytokines. However, TRAIL is clearly not utilized by Il10−/− Tregs, emphasizing that loss of IL-35 expression also contributes to the ability of Ebi3−/−Il10−/− Tregs to mediate suppression via TRAIL. While the contribution of IL35 in minimizing TRAIL-mediated suppression remains to be defined, it is noteworthy that Ebi3−/− Tregs exhibit accelerated TRAIL expression following activation, raising the possibility that IL-35 may suppress a distinct component of the TRAIL processing machinery.
An important question is the extent of the physiological impact of the Treg functional plasticity revealed in our study has applicability. As shown here and in previous studies, substantial differences in CTSE expression occur in different mouse strains with, BALB/c mice expressing high levels of CTSE while C57BL/6 mice expressing low levels (21, 45). Interestingly, BALB/c Tregs appeared to phenocopy Ebi3−/−Il10−/− Tregs in terms of their pattern of CTSE and TRAIL expression and thus their dominant dependence on TRAIL-mediated suppression. Although there are certainly multiple genetic factors that might underlie differences in the function of Tregs from distinct genetic backgrounds, our data suggest differential CTSE expression may be one contributing factor. Whether this is related to the necessity of Tregs to adapt to the different T helper cell bias exhibited in different mouse strains remains to be determined (50, 51). Given that previous studies have shown that Tregs can utilize different transcription factors to tackle different Th environments (10–12), it is possible that these may underlie the differential utilization of Treg suppressive mechanisms observed here. This remarkable Treg functional plasticity may also be important in providing a backup mechanism in scenarios where IL-10 and IL-35 production and/or signaling may be perturbed and thus may empower Tregs with the ability to adjust to different environmental settings. Lastly, the possibility that TRAIL may be a legitimate target for the treatment of diseases impacted by excessive Treg function, such as cancer, requires further study.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Doug Green and Benny Chain for advice, Rick Blumberg, Doug Green, Sasha Rudensky, Terry Geiger and Jim Ihle for mice, Amanda Burton and Kate Vignali for technical assistance, Creg Workman for help with Affymetrix analysis, Karen Forbes, Amy Krause and Ashley Castellaw for mouse colony maintenance and breeding, Richard Cross, Greig Lennon and Stephanie Morgan for FACS, the staff of the Shared Animal Resource Center at St. Jude for the animal husbandry, and the Hartwell Center for Biotechnology and Bioinformatics at St Jude for real-time PCR primer/probe synthesis.
This work was supported by the National Institutes of Health (R01 AI39480 and AI091977 to D.A.A.V.; R01 EY06765, EY015570 and P30 EY02687 to T.A.F.; R01 CA109446 to T.G.; F32 AI072816 to L.W.C.), Research to Prevent Blindness, New York (to T.A.F.), NCI Comprehensive Cancer Center Support CORE grant (CA21765, to D.A.A.V.), and the American Lebanese Syrian Associated Charities (ALSAC, to D.A.A.V.)
Abbreviations
- CTSE
Cathepsin E
- TRAIL
TNF-related apoptosis-inducing ligand
- Tnfsf10
tumor necrosis factor (ligand) superfamily, member 10
- DR5
death receptor 5
- TRAIL-R2; Tnfrsf10b
tumor necrosis factor receptor superfamily, member 10b
- IBD
inflammatory bowel disease
REFERENCES
- 1.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 2.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park Y, Oh SJ, Chung DH. CD4(+)CD25(+) regulatory T cells attenuate Hypersensitivity Pneumonitis by suppressing IFN-gamma production by CD4(+) and CD8(+) T cells. J Leukoc Biol. 2009;86:1427–1437. doi: 10.1189/jlb.0908542. [DOI] [PubMed] [Google Scholar]
- 6.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaiss MM, Axmann R, Zwerina J, Polzer K, Guckel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–4112. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 9.Vignali D. How many mechanisms do regulatory T cells need? European Journal of Immunology. 2008;38:908–911. doi: 10.1002/eji.200738114. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 14.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 15.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 17.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 20.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 21.Tulone C, Tsang J, Prokopowicz Z, Grosvenor N, Chain B. Natural cathepsin E deficiency in the immune system of C57BL/6J mice. Immunogenetics. 2007;59:927–935. doi: 10.1007/s00251-007-0256-0. [DOI] [PubMed] [Google Scholar]
- 22.Collison LW, Vignali DA. In vitro Treg suppression assays. Methods Mol Biol. 2011;707:21–37. doi: 10.1007/978-1-61737-979-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Workman CJ, Collison LW, Bettini M, Pillai MR, Rehg JE, Vignali DA. In vivo Treg suppression assays. Methods Mol Biol. 2011;707:119–156. doi: 10.1007/978-1-61737-979-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocke DM, Durbin B. Approximate variance-stabilizing transformations for gene-expression microarray data. Bioinformatics. 2003;19:966–972. doi: 10.1093/bioinformatics/btg107. [DOI] [PubMed] [Google Scholar]
- 25.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 26.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGFbeta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 28.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 29.Bell EB, Sparshott SM, Drayson MT, Ford WL. The stable and permanent expansion of functional T lymphocytes in athymic nude rats after a single injection of mature T cells. J Immunol. 1987;139:1379–1384. [PubMed] [Google Scholar]
- 30.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 32.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 33.Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 34.Zaidi N, Kalbacher H. Cathepsin E: a mini review. Biochem Biophys Res Commun. 2008;367:517–522. doi: 10.1016/j.bbrc.2007.12.163. [DOI] [PubMed] [Google Scholar]
- 35.Yasukochi A, Kawakubo T, Nakamura S, Yamamoto K. Cathepsin E enhances anticancer activity of doxorubicin on human prostate cancer cells showing resistance to TRAIL-mediated apoptosis. Biol Chem. 2010;391:947–958. doi: 10.1515/BC.2010.087. [DOI] [PubMed] [Google Scholar]
- 36.Kawakubo T, Okamoto K, Iwata J, Shin M, Okamoto Y, Yasukochi A, Nakayama KI, Kadowaki T, Tsukuba T, Yamamoto K. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res. 2007;67:10869–10878. doi: 10.1158/0008-5472.CAN-07-2048. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann O, Zipp F, Weber JR. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) in central nervous system inflammation. J Mol Med. 2009;87:753–763. doi: 10.1007/s00109-009-0484-x. [DOI] [PubMed] [Google Scholar]
- 39.Kimberley FC, Screaton GR. Following a TRAIL: update on a ligand and its five receptors. Cell Res. 2004;14:359–372. doi: 10.1038/sj.cr.7290236. [DOI] [PubMed] [Google Scholar]
- 40.Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death Differ. 2007;14:2076–2084. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- 41.Griffith TS, Brincks EL, Gurung P, Kucaba TA, Ferguson TA. Systemic immunological tolerance to ocular antigens is mediated by TRAIL-expressing CD8+ T cells. J Immunol. 2010;186:791–798. doi: 10.4049/jimmunol.1002678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregoli PA, Bondurant MC. Function of caspases in regulating apoptosis caused by erythropoietin deprivation in erythroid progenitors. J Cell Physiol. 1999;178:133–143. doi: 10.1002/(SICI)1097-4652(199902)178:2<133::AID-JCP2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 44.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang XR, Zhang LY, Devadas S, Li L, Keegan AD, Shi YF. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death Differ. 2003;10:203–210. doi: 10.1038/sj.cdd.4401138. [DOI] [PubMed] [Google Scholar]
- 46.Wohlfert E, Belkaid Y. Plasticity of T reg at infected sites. Mucosal Immunol. 2010;3:213–215. doi: 10.1038/mi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Backus GS, Howden R, Fostel J, Bauer AK, Cho HY, Marzec J, Peden DB, Kleeberger SR. Protective role of interleukin-10 in ozone-induced pulmonary inflammation. Environ Health Perspect. 2010;118:1721–1727. doi: 10.1289/ehp.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, Pineiro A, Larrad L, Alava MA, Naval J, Anel A. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736–6744. doi: 10.4049/jimmunol.167.12.6736. [DOI] [PubMed] [Google Scholar]
- 49.Mariani SM, Krammer PH. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur J Immunol. 1998;28:973–982. doi: 10.1002/(SICI)1521-4141(199803)28:03<973::AID-IMMU973>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 50.Gorham JD, Guler ML, Steen RG, Mackey AJ, Daly MJ, Frederick K, Dietrich WF, Murphy KM. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc Natl Acad Sci U S A. 1996;93:12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okamoto M, Van Stry M, Chung L, Koyanagi M, Sun X, Suzuki Y, Ohara O, Kitamura H, Hijikata A, Kubo M, Bix M. Mina, an Il4 repressor, controls T helper type 2 bias. Nat Immunol. 2009;10:872–879. doi: 10.1038/ni.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.