Abstract
A clinically and pathologically heterogeneous type of frontotemporal lobar degeneration has abnormal tau pathology in neurons and glia (FTLD-tau). Familial FTLD-tau is usually due to mutations in the tau gene (MAPT). Even FTLD-tau determined by MAPT mutations ha s clinical and pathologic heterogeneity. Tauopathies are subclassified according to the predominant species of tau that accumulates, with respect to alternative splicing of MAPT, with tau proteins containing 3 (3R) or 4 repeats (4R) of ~ 32 amino acids in the microtubule binding domain. In Pick's disease (PiD), 3R tau predominates, whereas 4R tau is characteristic of corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP). Depending upon the specific mutation in MAPT, familial FTLD-tau can have 3R, 4R or a combination of 3R and 4R tau. PiD is the least common FTLD-tau characterized by neuronal Pick bodies in a stereotypic neuroanatomical distribution. PSP and CBD are more common than PiD and have extensive clinical and pathologic overlap, with no distinctive clinical syndrome or biomarker that permits their differentiation. Diagnosis rests upon postmortem examination of the brain and demonstration of globose tangles, oligodendroglial coiled bodies and tufted astrocytes in PSP or threads, pretangles and astrocytic plaques in CBD. The anatomical distribution of tau pathology determines the clinical presentation of PSP and CBD, as well as PiD. The basis for this selective cortical vulnerability in FTLD-tau is unknown.
Keywords: corticobasal degeneration, corticobasal syndrome, frontotemporal lobar degeneration – tau, Pick’s disease, progressive supranuclear palsy, Richardson syndrome
INTRODUCTION
Frontotemporal lobar degeneration (FTLD) is a term for the group of non-Alzheimer degenerative dementias with focal cortical neuronal loss and gliosis (McKhann et al., 2001). It encompasses a range of different clinical syndromes [e.g., behavioral variant frontotemporal dementia (bvFTD), progressive nonfluent aphasia (PNFA), semantic dementia (SD) and corticobasal syndrome (CBS)] and a range of different pathologies (Mackenzie et al., 2009). The most common is FTLD associated with TDP-43 pathology (FTLD-TDP), with tauopathies (FTLD-tau) considered slightly less common (Wider and Wszolek, 2008). This brief review summarizes the neuropathology of sporadic FTLD-tau. Mutations in the gene for the microtubule associated protein tau (MAPT) account for most cases of familial FTLD-tau (Hutton et al., 1998). The reader is referred to recent reviews of this topic for more details (Forman et al., 2005, van Swieten and Spillantini, 2007).
Tau protein is the major structural protein of neurofibrillary tangles in Alzheimer disease (AD) (Grundke-Iqbal et al., 1986a). It is a heat-resistant phospho-protein that promotes microtubule polymerization and stabilization. Once considered to be relatively restricted to neurons (Binder et al., 1985), it is now known that tau accumulates not only in neurons in neurofibrillary tangles, but also in glia in a wide range of neurodegenerative disorders and in the aging brain. Disorders in which tau pathology is considered the major contributing factor to neurodegeneration are referred to as “primary tauopathies.” Tau protein in the brain is heterogeneous due to alternative splice forms, as well as post-translational modifications, including phosphorylation (Grundke-Iqbal et al., 1986b). Exon 10 of MAPT is alternatively spliced to generate tau species with either three or four conserved ~32 amino acid repeats in the microtubule binding domain of tau protein (Andreadis et al., 1992), referred to as 3R and 4R tau. There is preferential accumulation of 3R or 4R tau in various tauopathies, providing a biochemical subclassification of the tauopathies. In AD, neurofibrillary pathology is composed of an equimolar ratio of 3R and 4R tau (Goedert et al., 1989) (Table 1).
Table 1.
Classification of Most Common Subtypes of FTLD-Tau compared to AD
| Disorder | Anatomy (major areas affected in typical cases) | Major clinical feature |
|---|---|---|
| 4R TAUOPATHIES
| ||
| Corticobasal degeneration | Cortex & basal ganglia | Focal cortical syndrome & parkinsonism |
| Progressive supranuclear palsy | Basal ganglia, brainstem & cerebellum | Atypical parkinsonism |
| FTDP-17T | Cortex, basal ganglia & brainstem | Focal cortical syndrome & parkinsonism |
|
| ||
| 3R TAUOPATHIES
| ||
| Pick’s disease | Cortex & limbic lobe | Dementia & focal cortical syndromes |
| FTDP-17T | Cortex, basal ganglia & brainstem | Dementia & focal cortical syndromes |
|
| ||
| 3R+4R TAUOPATHIES
| ||
| Alzheimer disease | Cortex & limbic lobe | Dementia |
| FTDP-17T | Cortex & limbic lobe | Dementia & psychosis |
FTDP-17T = frontotemporal dementia and Parkinsonism linked to chromosome 17, with MAPT mutation and associated with FTLD-tau, to be distinguished from FTDP-17U due to mutations in progranulin gene and associated with ubiquitin-positive, TDP-43 immunoreactive inclusions (Baker et al., 2006)
3R TAUOPATHIES
Pick’s disease (PiD)
PiD is a rare cause of frontal lobe dementia, accounting for less than 5% in autopsy series of dementia (Barker et al., 2002). It is classically associated with circumscribed “lobar” atrophy. The distribution of focal cortical degeneration determines the clinical presentation. Clinical presentation with bvFTD is seen in PiD with frontotemporal atrophy (Constantinidis et al., 1974), while frontoparietal atrophy presents with apraxia (Lang et al., 1994) and peri-Sylvian atrophy with PNFA (Graff-Radford et al., 1990). When amnestic symptoms prevail, clinical diagnosis is often initially AD. It is a disorder that affects men and women equally and is usually associated with a “presenile dementia” with age of onset younger than 65 years. Mutations in the tau gene (MAPT) account for most pathologically confirmed cases of familial PiD (Murrell et al., 1999, Hogg et al., 2003, Bronner et al., 2005).
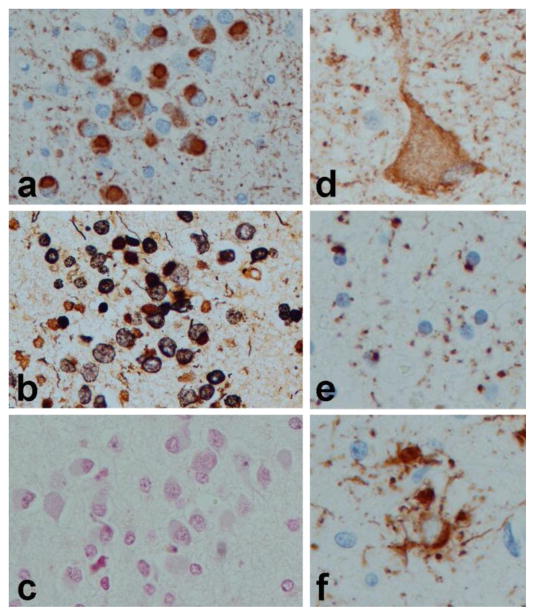
The cardinal neuropathologic features are circumscribed cortical atrophy associated with neuronal loss, gliosis and argyrophilic, round intraneuronal inclusions (Pick bodies). Pyramidal neurons in the hippocampus and granular neurons in the dentate fascia are particularly vulnerable (Fig. 1a). Pick bodies are composed of tau protein enriched in 3R tau, which can be shown with biochemical studies (Buee and Delacourte, 1999), or more recently with antibodies specific to tau isoforms (de Silva et al., 2006). They are argyrophilic on some silver stains (e.g., Bielschowsky (Fig. 1b)), but consistently negative with the Gallyas silver stain (Fig. 1c). A less specific feature of PiD is the ballooned neuron (also known as Pick cell (Fig. 1d)). Tau-immunoreactive glial inclusions, including small, round inclusions in oligodendroglia (Fig. 1e) and ramified astrocytes (Fig. 1f) are sometimes present in PiD, but they are not as frequent as in the 4R tauopathies (see below). Interestingly, glial lesions in PiD contain predominantly 4R tau (Hogg et al., 2003), which may contribute to the variability in the ratio between 3R and 4R tau observed in biochemical studies of PiD (Zhukareva et al., 2002). Involvement of deep gray matter and brainstem is common, with a predilection for the monoaminergic nuclei (Yoshimura, 1989). There is overlap between subcortical nuclei affected in PiD compared to both corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) (Feany et al., 1996).
Figure 1.
Histopathology of Pick’s disease. Pick bodies in dentate fascia with tau immunohistochemistry (a), Bielschowsky silver stain (b), and Gallyas silver stain (c); Ballooned neurons (Pick cell) in cortex with tau immunohistochemistry (d); white matter oligodendroglial inclusions (e); and ramified astrocytes 9f). All images, originally (×400)
4R TAUOPATHIES
Corticobasal degeneration
CBD is a 4R tauopathy that has a range of clinical presentations because it is associated with focal cortical degeneration. Factors that determine the distribution of focal cortical degeneration, as in PiD, remains unknown. The classic clinical presentation of CBD, which is referred to as the “corticobasal syndrome (CBS),” is associated with asymmetrical rigidity and apraxia, often with dystonia and alien limb sign (Litvan et al., 2000) and accompanied by asymmetrical cortical degeneration of the superior frontal gyrus and superior parietal lobule. Atypical presentations are common, including presentations similar to bvFTD, with focal atrophy in the frontal lobes (Bergeron et al., 1996) or PNFA with focal degeneration in peri-Sylvian areas (Ikeda et al., 1996). It is increasingly recognized that CBD need not always be asymmetrical (Hassan et al., 2010), and that some of these patients have a clinical presentation indistinguishable from that of PSP (see below) (Ling et al., 2010). It is also clear that the CBS is not specific to CBD, with other pathologies presenting clinically with asymmetrical rigidity and apraxia with dystonia (Boeve et al., 1999, Wadia and Lang, 2007, Ling et al., 2010, Kouri et al., 2011).
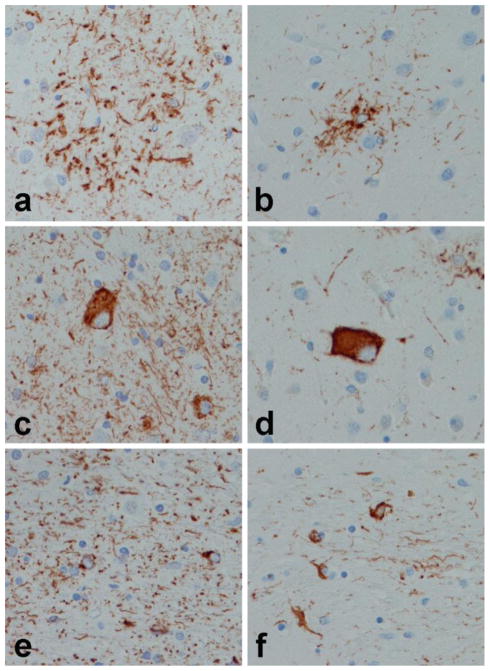
The characteristic pathology in CBD is phospho-tau accumulation in cell processes of neurons and glia in the cortex, basal ganglia, thalamus and brainstem (Dickson et al., 2002). The forebrain typically is more affected than the hindbrain, but there are atypical forms of CBD where hindbrain pathology is prominent. The most specific histopathological lesion in CBD is the astrocytic plaque (Feany and Dickson, 1995) (Fig. 2a), which is not seen in other disorders (Komori et al., 1998). Ballooned neurons, also known as swollen achromatic neurons (Rebeiz et al., 1967), similar to those in PiD (Fig. 1d), are usually numerous in affected cortical areas, but they are not specific and can be seen in a number of other disorders (Fujino et al., 2004). Neuronal inclusions in CBS are pleomorphic and often not visible with silver stains due to presence of so-called pretangle-like lesions (Fig. 2c). Neuropathologic research criteria for CBD emphasize the presence of abnormal tau-positive, thread-like processes in both gray and white matter of cortical and subcortical regions, accompanied by variable, sometimes sparse, oligodendroglial inclusions (Fig. 2e). The marked neuritic pathology in both gray and white matter has been validated as diagnostically useful for CBD in one study (Dickson et al., 2002), but not yet independently confirmed.
Figure 2.
Tau immunohistochemistry in CBD (a, c and e) and PSP (b, d and f). Astrocytic lesions in CBD are referred to as astrocytic plaques (a) due to plaque-like arrangement of cell processes, while in PSP they are called tufted astrocytes (b) because of the tuft like arrangement of cell processes around astrocyte cell bodies. Neuronal lesion is CBD (c) are granular and irregularly dense cytoplasmic deposits consistent with so-called pretangles, while globus neurofibrillary tangles (d) are characteristic of PSP. Oligodendroglial lesions are sparse compared to the dense plexus of thread like structures in white matter of CBD (e), while oligodendroglial coiled bodies (f) are frequent in affected white matter of PSP. All images, originally (×400)
Progressive supranuclear palsy
Progressive supranuclear palsy affects men and women equally and in most cases presents as an atypical parkinsonism with axial rigidity, postural instability and unexplained falls, with most patients also developing progressive vertical gaze palsy (for which the disorder is named), dysarthria and dysphagia (Steele et al., 1964). The classic clinical presentation is referred to as Richardson syndrome (Williams et al., 2005, Williams et al., 2008) to distinguish it from other clinical variants of PSP and to specify clinical features rather than pathology (i.e. PSP pathology). Other clinical presentations of PSP include bvFTD (Bigio et al., 1999), PNFA or apraxia of speech (AOS) (Josephs et al., 2005), CBS (Tsuboi et al., 2005, Josephs et al., 2006) and pure akinesia with gait failure (PAGF) (Williams et al., 2007, Ahmed et al., 2008). As noted above, some PSP has asymmetric cortical atrophy and can clinically mimic CBS (Boeve et al., 1999, Wadia and Lang, 2007, Ling et al., 2010). In a subset of patients, the clinical features initially are similar to those in Parkinson disease, so-called “PSP-P” (Williams et al., 2005). The distribution of tau pathology determines the particular clinical presentation; some cases have severe brainstem involvement (e.g., PSP-PAGF) and others have severe cortical involvement (e.g., PSP-bvFTD, PSP-CBS and PSP-AOS).
The core neuroanatomical regions affected in all cases of PSP include the basal ganglia, subthalamic nucleus and the substantia nigra (Hauw et al., 1994). Cortical involvement is greatest in motor sn premotor cortices (Josephs et al., 2008). Pathology of the cerebellar dentate nucleus and the cerebellar outflow pathway (dentato-rubro-thalamic pathway) is usually severe and associated with profound atrophy of the superior cerebellar peduncle (Tsuboi et al., 2003), which can be used as a biologic marker of disease progression with structural imaging (Nilsson et al., 2007). Atrophy of superior cerebellar peduncle may be mild or absent in atypical cortical presentations of PSP.
The hallmark glial lesion is the tuft-shaped astrocyte (Yamada et al., 1992) or tufted astrocyte (Fig. 2b), while the most characteristic neuronal lesion is the globose neurofibrillary tangle (Fig. 2d). Tufted astrocytes are most abundant in the motor cortex and the corpus striatum. Neuronal loss and gliosis is most marked in the substantia nigra and subthalamic nucleus, where many thread-like processes and oligodendroglial coiled bodies are often found in the thalamic and lenticular fasciculi (Fig. 2f). In PSP threads and coiled bodies are found together, while coiled bodies are less common in thread-rich areas of CBD.
Clinical and pathological overlap in 4R tauopathies
While PiD is biochemically and histopathologically a distinct disorder, almost all available evidence suggests that CBD and PSP form a 4R tauopathy disease spectrum, with clinical presentation driven more by the distribution of the pathology than the histopathologic appearance of the pathology. (The latter is also true for PiD, as noted above.) Typical cases of CBD presenting as CBS and PSP presenting as Richardson syndrome form the extreme ends of the spectrum, but there is considerable overlap in the middle (Fig. 3). As noted above, one of the most common underlying pathologies of CBS is PSP (in some autopsy series being more common than CBD (Ling et al., 2010)). There is also increasing recognition that CBD, especially symmetrical CBD, can present with a clinical syndrome similar to Richardson syndrome (Ling et al., 2010, Kouri et al., 2011). Pathologically, both CBD and PSP are associated with neuronal, oligodendroglial and astrocytic lesions that are immunoreactive for 4R tau. The morphologic features of neuronal and astrocytic lesions and the relative proportion of oligodendroglial lesions (greater in PSP than in CBD ) have been used to differentiate PSP and CBD (Komori et al., 1998, Dickson, 1999, Dickson et al., 2010), but the molecular bases of these structural differences is unknown, and there are no specific markers that permit one to differentiate tau pathology in CBD from PSP. Biochemically, both CBD and PSP have increased levels of insoluble 4R tau with few differences noted, except for evidence from a relatively small series of PSP and CBD cases of differential low molecular weight tau fragments, suggesting different proteolytic processes in PSP and CBD (Arai et al., 2004). The biological basis for this difference remains completely unknown. In terms of the little that is known about genetics of CBD and PSP, the evidence suggests that they have a common genetic underpinning. For example, a common genetic risk factor for both PSP and CBD is the MAPT H1 haplotype (Baker et al., 1999, Di Maria et al., 2000, Houlden et al., 2001) and the particular subhaplotype, H1c, recognized by the single nucleotide polymorphism, rs242557 (Pittman et al., 2005, Rademakers et al., 2005) . More recently, in a genome wide association analysis of pathologically confirmed PSP and CBD, no other gene came close to the association that MAPT had for both PSP and CBD (Schellenberg, 2010). It is worth noting that patients with mutations in MAPT may have clinical and pathologic features that overlap with both PSP (Stanford et al., 2000) and CBD (Bugiani et al., 1999).
Figure 3.
Clinicopathologic spectrum of the 4R tauopathies. Anatomical distribution of the tau pathology determines the clinical syndrome. (see text for abbreviations)
While it will remain important for pathologists to make distinctions between PSP and CBD, since pathology is still the “gold standard” with respect to diagnostic subtyping of FTLD-tau, it is less clear that efforts to distinguish PSP and CBD will pay off in significant clinical benefits. It may be more important to find commonalities between CBD and PSP that can differentiate them from PiD, a 3R tauopathy, and more importantly from FTLD-TDP, if treatments are developed specific to 4R tau. It will also be important to develop improved methods to differentiate FTLD-tau from FTLD-TDP. Understanding the biologic basis for selective cortical vulnerability is a long-term goal to understand clinical and pathological heterogeneity of FTLD in the primary tauopathies.
Acknowledgments
The authors thank Virginia Philips, Linda Rousseau and Monica Castanedes-Casey for their expert technical assistance. We appreciate the gift of CP13 from Peter Davies, Albert Einstein College of Medicine. Most of the cases used in this study were donated to the Society of Progressive Supranuclear Palsy brain bank and generous donations of family members in this endeavor are greatly appreciated. This study was supported by NIH grants P50-NS72187, P50-AG25711, P50-AG16574, P01-AG17216, R01-AG37491 and R21 AG38736, as well as The Robert E. Jacoby endowment and the Mayo Foundation for Education and Research.
References
- Ahmed Z, Josephs KA, Gonzalez J, Delledonne A, Dickson DW. Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido-nigroluysial degeneration and axonal dystrophy. Brain. 2008;131:460–72. doi: 10.1093/brain/awm301. [DOI] [PubMed] [Google Scholar]
- Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–33. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- Arai T, Ikeda K, Akiyama H, et al. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol. 2004;55:72–9. doi: 10.1002/ana.10793. [DOI] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–5. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–12. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Bergeron C, Pollanen MS, Weyer L, Black SE, Lang AE. Unusual clinical presentations of cortical-basal ganglionic degeneration. Ann Neurol. 1996;40:893–900. doi: 10.1002/ana.410400611. [DOI] [PubMed] [Google Scholar]
- Bigio EH, Brown DF, White CL., 3rd Progressive supranuclear palsy with dementia: cortical pathology. J Neuropathol Exp Neurol. 1999;58:359–64. doi: 10.1097/00005072-199904000-00006. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–8. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53:795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- Bronner IF, Ter Meulen BC, Azmani A, et al. Hereditary Pick's disease with the G272V tau mutation shows predominant three-repeat tau pathology. Brain. 2005;128:2645–53. doi: 10.1093/brain/awh591. [DOI] [PubMed] [Google Scholar]
- Buee L, Delacourte A. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick's disease. Brain Pathol. 1999;9:681–93. doi: 10.1111/j.1750-3639.1999.tb00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani O, Murrell JR, Giaccone G, et al. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol. 1999;58:667–77. doi: 10.1097/00005072-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Constantinidis J, Richard J, Tissot R. Pick's disease. Histological and clinical correlations. Eur Neurol. 1974;11:208–17. doi: 10.1159/000114320. [DOI] [PubMed] [Google Scholar]
- De Silva R, Lashley T, Strand C, et al. An immunohistochemical study of cases of sporadic and inherited frontotemporal lobar degeneration using 3R- and 4R-specific tau monoclonal antibodies. Acta Neuropathol. 2006;111:329–40. doi: 10.1007/s00401-006-0048-x. [DOI] [PubMed] [Google Scholar]
- Di Maria E, Tabaton M, Vigo T, et al. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann Neurol. 2000;47:374–7. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl 2):II6–15. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23:394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–46. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol. 1995;146:1388–96. [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Mattiace LA, Dickson DW. Neuropathologic overlap of progressive supranuclear palsy, Pick's disease and corticobasal degeneration. J Neuropathol Exp Neurol. 1996;55:53–67. doi: 10.1097/00005072-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Forman MS, Lee VM-Y, Trojanowski JQ. Frontotemporal dementia with parkinsonism linked to chromosome 17. In: BEAL MF, LANG AE, LUDOLPH A, editors. Neurodegenerative Diseases: Neurobiology, Pathogenesis and Therapeutics. New York: Cambridge University Press; 2005. [Google Scholar]
- Fujino Y, Delucia MW, Davies P, Dickson DW. Ballooned neurones in the limbic lobe are associated with Alzheimer type pathology and lack diagnostic specificity. Neuropathol Appl Neurobiol. 2004;30:676–82. doi: 10.1111/j.1365-2990.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. Embo J. 1989;8:393–9. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford NR, Damasio AR, Hyman BT, et al. Progressive aphasia in a patient with Pick's disease: a neuropsychological, radiologic, and anatomic study. Neurology. 1990;40:620–6. doi: 10.1212/wnl.40.4.620. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986a;261:6084–9. [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986b;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A, Whitwell JL, Boeve BF, et al. Symmetric corticobasal degeneration (S-CBD) Parkinsonism Relat Disord. 2010;16:208–14. doi: 10.1016/j.parkreldis.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–9. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Hogg M, Grujic ZM, Baker M, et al. The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol. 2003;106:323–36. doi: 10.1007/s00401-003-0734-x. [DOI] [PubMed] [Google Scholar]
- Houlden H, Baker M, Morris HR, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56:1702–6. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Akiyama H, Iritani S, et al. Corticobasal degeneration with primary progressive aphasia and accentuated cortical lesion in superior temporal gyrus: case report and review. Acta Neuropathol. 1996;92:534–9. doi: 10.1007/s004010050558. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Boeve BF, Duffy JR, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–96. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Katsuse O, Beccano-Kelly DA, et al. Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol. 2006;65:396–405. doi: 10.1097/01.jnen.0000218446.38158.61. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29:280–9. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Arai N, Oda M, et al. Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 1998;96:401–8. doi: 10.1007/s004010050911. [DOI] [PubMed] [Google Scholar]
- Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW. Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol. 2011;7:263–72. doi: 10.1038/nrneurol.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Bergeron C, Pollanen MS, Ashby P. Parietal Pick's disease mimicking cortical-basal ganglionic degeneration. Neurology. 1994;44:1436–40. doi: 10.1212/wnl.44.8.1436. [DOI] [PubMed] [Google Scholar]
- Ling H, O'sullivan SS, Holton JL, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133:2045–57. doi: 10.1093/brain/awq123. [DOI] [PubMed] [Google Scholar]
- Litvan I, Grimes DA, Lang AE. Phenotypes and prognosis: clinicopathologic studies of corticobasal degeneration. Adv Neurol. 2000;82:183–96. [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–8. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Murrell JR, Spillantini MG, Zolo P, et al. Tau gene mutation G389R causes a tauopathy with abundant pick body-like inclusions and axonal deposits. J Neuropathol Exp Neurol. 1999;58:1207–26. doi: 10.1097/00005072-199912000-00002. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Markenroth Bloch K, Brockstedt S, Latt J, Widner H, Larsson EM. Tracking the neurodegeneration of parkinsonian disorders--a pilot study. Neuroradiology. 2007;49:111–9. doi: 10.1007/s00234-006-0165-1. [DOI] [PubMed] [Google Scholar]
- Pittman AM, Myers AJ, Abou-Sleiman P, et al. Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J Med Genet. 2005;42:837–46. doi: 10.1136/jmg.2005.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Melquist S, Cruts M, et al. High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet. 2005;14:3281–92. doi: 10.1093/hmg/ddi361. [DOI] [PubMed] [Google Scholar]
- Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia: a progressive disorder of late adult life. Trans Am Neurol Assoc. 1967;92:23–6. [PubMed] [Google Scholar]
- Schellenberg GD. A genome-wide association study of progressive supranuclear palsy and corticobasal degeneration: genes that modify risk. Dement Geriatr Cogn Disord. 2010;30(Suppl 1):18–19. [Google Scholar]
- Stanford PM, Halliday GM, Brooks WS, et al. Progressive supranuclear palsy pathology caused by a novel silent mutation in exon 10 of the tau gene: expansion of the disease phenotype caused by tau gene mutations. Brain. 2000;123 ( Pt 5):880–93. doi: 10.1093/brain/123.5.880. [DOI] [PubMed] [Google Scholar]
- Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. a Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum with Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Arch Neurol. 1964;10:333–59. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Josephs KA, Boeve BF, et al. Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov Disord. 2005;20:982–8. doi: 10.1002/mds.20478. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW. Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2003;60:1766–9. doi: 10.1212/01.wnl.0000068011.21396.f4. [DOI] [PubMed] [Google Scholar]
- Van Swieten J, Spillantini MG. Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol. 2007;17:63–73. doi: 10.1111/j.1750-3639.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadia PM, Lang AE. The many faces of corticobasal degeneration. Parkinsonism Relat Disord. 2007;13(Suppl 3):S336–40. doi: 10.1016/S1353-8020(08)70027-0. [DOI] [PubMed] [Google Scholar]
- Wider C, Wszolek ZK. Etiology and pathophysiology of frontotemporal dementia, Parkinson disease and Alzheimer disease: lessons from genetic studies. Neurodegener Dis. 2008;5:122–5. doi: 10.1159/000113680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, De Silva R, Paviour DC, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005;128:1247–58. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- Williams DR, Holton JL, Strand K, Revesz T, Lees AJ. Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov Disord. 2007;22:2235–41. doi: 10.1002/mds.21698. [DOI] [PubMed] [Google Scholar]
- Williams DR, Lees AJ, Wherrett JR, Steele JC. J. Clifford Richardson and 50 years of progressive supranuclear palsy. Neurology. 2008;70:566–73. doi: 10.1212/01.wnl.0000286938.39473.0e. [DOI] [PubMed] [Google Scholar]
- Yamada T, Mcgeer PL, Mcgeer EG. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett. 1992;135:99–102. doi: 10.1016/0304-3940(92)90145-w. [DOI] [PubMed] [Google Scholar]
- Yoshimura N. Topography of Pick body distribution in Pick's disease: a contribution to understanding the relationship between Pick's and Alzheimer's diseases. Clin Neuropathol. 1989;8:1–6. [PubMed] [Google Scholar]
- Zhukareva V, Mann D, Pickering-Brown S, et al. Sporadic Pick's disease: a tauopathy characterized by a spectrum of pathological tau isoforms in gray and white matter. Ann Neurol. 2002;51:730–9. doi: 10.1002/ana.10222. [DOI] [PubMed] [Google Scholar]