Abstract
Landau-Kleffner syndrome (LKS), or acquired epileptiform aphasia, is an epilepsy syndrome involving progressive neuropsychological impairment related to the appearance of paroxysmal electroencephalograph (EEG) activity. LKS appears to share a common pathophysiologic mechanism with continuous spike-wave of sleep (CSWS), acquired epileptic opercular syndrome (AEOS), and even benign childhood epilepsy with centrotemporal spikes (BECTS), with differentiating factors including age of onset, area of primary epileptogenicity, and severity of clinical presentation. This article covers the clinical, diagnostic, therapeutic, and prognostic features of LKS. In a child with autistic spectrum disorder, the presence of a fluctuating clinical course or regression should raise suspicion for the presence of associated epilepsy.
Introduction
The correlation between paroxysmal EEG discharges and language deterioration was first suggested by Landau and Kleffner (1957), who reported five children with acquired aphasia associated with a convulsive disorder 1. They reported language improvement concordant with EEG improvement and suggested a functional ablation of language areas by persistent convulsive discharges as the pathophysiology. Landau-Keffner syndrome (LKS) has been in the International Classification of Epileptic Syndromes since 1985 2. This rare syndrome raises epistemological questions regarding the pathogenesis of language, EEG abnormalities, and related epilepsy syndromes. LKS may demonstrate a direct link between epilepsy and language deficits 3.
Clinical Manifestations
Landau-Kleffner syndrome is characterized by acquired aphasia and paroxysmal, sleep-activated EEG paroxysms predominating over the temporal or parieto-occipital regions. Secondary symptoms include psychomotor or behavioral disturbances and epilepsy with a favorable outcome for seizure control. The prevalence is unclear. A male predominance exists, with an approximately 2:1 ratio. This regressive syndrome affects children after having achieved early developmental milestones, with 3–9 years being the usual age of presentation 4.
The first manifestation of the language disturbance is an apparent “word deafness,” or auditory verbal agnosia 5. Parents report a child no longer responds to their commands, even with raised voices. This auditory agnosia extends to familiar noises including bells, whistles, or a ringing phone. Audiograms and brainstem auditory evoked response (BAER) are normal. Delays and abnormalities in long-latency cortical evoked responses suggest localization to the posterior temporal regions 6. Dichotic listening tasks have shown permanent one-ear extinction contralateral to the affected temporal cortex, and a study of long-latency auditory evoked potentials in five children having recovered from LKS revealed unilateral voltage reduction involving the N1c peak, arising from associative auditory areas, versus a normal N1b peak related to primary auditory cortex 7. The findings suggest long term dysfunction of associative auditory cortex.
Word deafness can deteriorate into total unresponsiveness and impaired expressive communication. Expression is marked by a gradual increase in misarticulations and telegraphic speech; a fluent jargon, or total mutism can occur 5, 7. The children may express themselves with a crude sign system or gestures 5. The language disorder can be progressive or incremental, characterized by remissions and exacerbations. The fluctuating course of aphasia in LKS remains among its most puzzling features.
A relationship between younger age of onset and worse longterm outcome has been reported. Bishop 4, in an analysis of 119 reported cases up to 1985, argued that the association between age of onset and prognosis suggests LKS is a disorder of higher-level auditory processing. In a younger child without advanced language development, the effect is devastating, as the normal auditory route leading to acquisition of language is blocked. In the older child the result will be less severe, since language has been partially learned. An analogous scenario is profound deafness, when Wernicke's area is intact but the sensory input is defective. Profound deafness in a young child has a more profound effect on language development than in an older child. Even the relatively mild and fluctuating hearing loss of otitis media may impair language in a young child in the process of developing language 8. Children with LKS may not follow precisely an auditory processing disorder model 9. Given the usual age of presentation between 4 and 7 years, the child has not acquired sufficient reading and writing skills; the older child, however, may lose these skills. Partial retention of writing skills suggests a better prognosis in the reeducation phase 10.
The language disorder of LKS has commonalities with autism spectrum disorder. Communication deficits in autism include abnormal development of spoken language and impaired ability to initiate or sustain conversation. The autistic child's language is often stereotyped, repetitive, and idiosyncratic, with echolalia and neologisms 11. Confusing the picture is the fact that seizures may occur in autism, and EEG abnormalities are common 12. Furthermore, at least a third of autistic toddlers demonstrate neurodevelopmental regression, involving language, sociability, play, and cognition 13. LKS represents selective loss of language in association with an abnormally paroxysmal EEG, eventually characterized by electrographic status epilepticus of slow-wave sleep (ESES).
While there is considerable overlap in the semiology of LKS and autism, some differences emerge. The great majority of children with autism who undergo language regression do so before three years of age 14, versus a mean age of language regression in LKS of 5–7 years. Only 10% of children with LKS regress before three years 4. As regression in autism occurs early, it usually entails the loss of single words, versus more drastic changes in LKS children who are typically older and have more developed vocabulary and language. LKS does not feature the behavioral profile that encompasses the core deficits of autism, i.e., abnormalities of reciprocal social relatedness and restricted stereotypical patterns of interests and behaviors. There is an intricate relationship between LKS, autism, ESES, and developmental dysphasias and the interaction between epileptiform discharges and cognitive dysfunction remains enigmatic 15. The presence of fluctuation in language and behavioral deficits, however, should raise concern regarding an accompanying diagnosis of epilepsy 13.
Focal epilepsy can interfere with language. Aphasic status epilepticus 16 and post-ictal aphasia 17 have been reported. Children with severe focal epilepsy from static brain lesions involving language cortex may have episodic ictal aphasia or status epilepticus and become permanently aphasic 3.
The early stages of LKS, with hyperkinesis and mild verbal auditory agnosia, may be confused with attention deficit hyperactivity disorder (ADHD) 10. Personality disturbances, aggressiveness, and depression are noted 18. Nonverbal developmental disorders may occur, but operational and intellectual capacities are usually preserved in LKS 9, 19, 20. The differential diagnosis also includes deafness, elective mutism, and acute psychiatric disorders.
Epileptic Manifestations
Seizures occur in approximately 70% of patients, one-third as a single seizure or episode of status, mostly at onset 21. In others, infrequent seizures occur between ages 5–10 years. After age 10, only one-fifth of patients continue with sporadic seizures; by age 15, seizures rarely persist. Seizures are often nocturnal simple partial motor, placing LKS on a spectrum that includes BECTS. Generalized tonic-clonic seizures, atypical absences, and myoclonic-astatic seizures occur less frequently 3. Complex partial seizures with psychomotor automatisms are rare 22. Tonic seizures are not characteristic 3, 22. The frequency and type of seizures have no influence on prognosis. Treatment with anticonvulsant monotherapy is generally effective for seizure control, but not for the aphasia 3, 9.
EEG Findings
Epileptiform abnormalities in LKS are variable, but striking. Bilateral independent temporal or temporoparietal spikes, bilateral 1–3 Hz slow-wave maximally temporal activity, generalized sharp- or slow-wave discharges, and multifocal or unilateral spikes are described 23. A report on spectral and topographic mapping of the EEG revealed variability in the mode of propagation of paroxymal discharges 24. Background activity is often normal or borderline. There is seldom activation by hyperventilation or photic stimulation. Epileptiform discharges are activated by sleep, especially sleep onset (Fig. 1). The significant activation of the spike-and-wave during non-REM sleep has led some to analogize to CSWS 21. Eventually in LKS, essentially all patients have bilateral spike-and-wave over 85% of non-REM sleep (ESES) 21. During a given night, however, these continuous discharges can be focal, restricted to the temporoparietal region 3. While nap recordings show the same abnormalities as those recorded during full nocturnal sleep, long-term EEG monitoring may be necessary to detect this phenomena 25.
FIGURE 1.
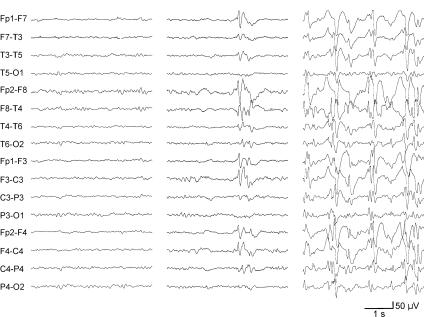
EEG in 5 year old girl with LKS. Note awake/drowsy background (left), activation of epileptiform activity in Stage 1 sleep (middle), and ESES in NREM sleep (right). Courtesy of William D. Gaillard, M.D.
An interdependent relationship between language and epileptic manifestations has been described 26, 27, though not all studies have suggested this 22. Holmes referred to the EEG abnormalities as epiphenomena of underlying pathology of cortex concerned with speech, rather than the cause of the aphasia 28. This view is supported by several observations: transient suppression of EEG discharges with intravenous benzodiazepines does not result in improvement of aphasia 21; EEG changes may not be accompanied by a change in aphasia 29; aphasia does not respond to conventional anticonvulsants despite seizure control 21, 28; aphasia persists into adulthood despite normalization of EEG 9, 20. Alternatively, improvement and worsening may coincide in the same direction, particularly in the sleep EEG 3, 9. Disappearance of continuous spike-wave may herald improvement of aphasia 25. It seems reasonable to undertake treatment to terminate continuous EEG discharges early in LKS.
The placement of LKS on a spectrum with BECTS poses a challenge in the management of patients presenting with BECTS features who develop more ominous signs including neuropsychiatric dysfunction and medication refractoriness 30, 31, 32. Patients with otherwise classical seizures of rolandic epilepsy may develop atypical seizures, including generalized tonic-clonic, atonic, and atypical absences, as well as ESES and cognitive or behavioral disturbances. Attempts to distinguish EEG patterns specifically associated with LKS have identified unilateral slow wave foci, bilateral independent spike-and-wave discharges, and major activation of spike-and-wave discharges during sleep 33.
The interaction between LKS and autism poses another diagnostic dilemma. In a study of extended sleep EEGs in children with autism without epilepsy, an epileptiform EEG was identified in 14% of 155 children with regression, versus 6% of 364 without regression 34. Our study of 894 patients with autism spectrum disorder studied with overnight EEG revealed epileptiform potentials in 19% and no study revealed ESES 35. Overnight EEG appears warranted in autistic children with regression or fluctuation in their clinical course, but otherwise is not indicated in unselected patients.
Etiology
The etiology of LKS remains unknown, and may be due to diverse causes. Encephalitis has been postulated, but not verified 36. The clinical course is quite different from that of children with chronic encephalitis of the Rasmussen type. In a detailed report of two LKS cases that underwent partial temporal lobectomies, Cole et al. 37 from the Montreal Neurological Institute failed to uncover encephalitis. Pathological specimens were normal with the exception of mild subpial gliosis and occasional fibrous astrocytes throughout cortical gray matter. There was no evidence of inflammation, demyelination, hippocampal sclerosis, or dysgenesis. A report confirming encephalitic changes on cortical biopsy was given by Lou et al. 38. However, their case was atypical with elevated CSF protein and focal imaging abnormalities. Other biopsied cases did not have encephalitic changes 22, 39. Angiography has occasionally suggested isolated arteritis of small and medium-sized vessels in some studies 40, but not others 22, 37.
Other etiologies reported include a genetic predisposition 1, 24, 41, toxoplasmosis 42, neurocysticercosis 43, 44, temporal astrocytoma 45, temporal ganglioglioma 46, hemophilus inflluenzae meningitis 19, subacute sclerosing panencephalitis 47, inflammatory demyelinating disease 48, 49, and abnormal zinc metabolism 50. LKS may represent a final common pathway with multiple potential etiologies, acquired or genetic. The language disturbance, EEG abnormalities and epilepsy are likely the result of an insult to temporoparietal areas of the developing brain. As with other epilepsy syndromes, it would be useful to classify LKS into cryptogenic or symptomatic subgroups. Differences in pathogenesis, treatment, and prognosis may emerge, analogous to the infantile spasms of West syndrome.
Diagnostic Studies
Investigations have not delineated sufficient evidence to explain the pathophysiology. CSF 29, 37, 39, 41, computed tomography (CT) 20, 21, 24, 41, 52, and magnetic resonance imaging (MRI) 21, 41, 54 findings are normal. There are uncommonly mild elevations of CSF protein 36, 40, 48, white matter changes on CT/MRI, or a structural lesion 43, 44, 45, 46. Purists may argue that such cases represent other conditions mimicking LKS. Slight enlargement or asymmetry of the temporal horns have been reported 37, 54, possibly secondary to the long-term epileptic process.
Various single photon emission computed tomography (SPECT) and positron emission tomography (PET) studies on small numbers of patients have shown temporal lobe abnormalities in brain perfusion and glucose metabolism 52, 53, 55. Chugani et al. 54 studied 17 LKS children with FDG-PET, demonstrating hypometabolism in the middle temporal gyri. Decreased temporoparietal perfusion has been seen with SPECT 55. A correlation between the aphasia of LKS with temporal lobe hypometabolism is not certain, as similar findings are observed in children with epilepsy who are not aphasic.
Treatment
The pharmacologic treatment of LKS is problematic due to several confounding observations. The benign course of the epilepsy versus devastating language impairment, fluctuating course of aphasia, lag of improvement in relation to the EEG, possibility of spontaneous remission, and rarity of the disorder render multiple barriers to controlled clinical trials. The determination of treatment efficacy is difficult. There is relatively scarce mention in the literature regarding antiepileptics of choice. Marescaux et al. 51 observed that phenobarbital, carbamazepine, and phenytoin were ineffective or even aggravating. Phenobarbital, having no effect on language, intensified behavioral problems, particularily hyperkinesis. Carbamazepine and phenytoin appeared to increase the duration of spike-wave activity in sleep. Valproate, ethosuximide, clonazepam, and clobazam were demonstrated to be partially or transiently effective. Clobazam has been reported to significantly reduce continuous spike-wave discharges in several small studies, associated with language improvement 27, 51, 56. Vigabatrin 57 and felbamate 58 have also been reported as effective.
Corticosteroids have been an efficacious treatment for both clinical and EEG abnormalities. This was reported by McKinney and McGreal 39, leading to the speculation of chronic encephalitis as the etiology of LKS. Effectiveness may be increased by early introduction 59. A recurrence of epileptiform EEG followed by an aphasic relapse has been described after tapering steroids 51. Prolonged, chronic, or intermittent therapy may be warranted if significant improvement of neuropsychological function is attained 3, 51. Another recent addition is IVIG 49, 60, 61. The rationale for its use in LKS lies in the refractory nature of the epileptiform abnormalities, and the reports of beneficial effects in other intractable childhood epilepsies.
Surgical therapy including temporal lobectomy in lesional 45, 46 and nonlesional 37 cases has been associated with improvement in language and seizure control. Multiple subpial transection (MST), designed to selectively disrupt intracortical horizontal fibers with minimal injury to vertically oriented cortical columns, has been suggested in treating epilepsies arising from eloquent or unresectable cortex. Morrell 62 reported a series of 14 LKS cases in which the epileptogenic discharges arose unilaterally, and were surgically treated with MST. Seven patients recovered age-appropriate speech; four showed marked improvement but continued in speech therapy programs; and the remaining three had no change. Other series have been small and demonstrated improvements that are often temporary 63, or in one series of five patients partial improvement but associated with a later extension of the procedure in one patient following relapse of ESES and clinical deficits 64. The most appropriate timing of this procedure and its long term ramifications are unknown. Further experience is needed to clarify the role of MST in the treatment of LKS.
Speech/language therapy is indispensable with periodic language and neuropsychological evaluations. Adverse behavioral manifestations may partly reflect frustration caused by aphasia. Introduction of an effective communication system could assist in alleviating such negative behavior. Some children with long standing verbal auditory agnosia are successfully integrated into schools for the deaf, although others continue to have marked deficits in social adaptation and communication. The patients who recover verbal language will drop the use of signs, allaying concern by some educators that there is detrimental competition between the two systems 3, 65.
Impaired comprehension secondary to background noise in adolescents and adults who had recovered from LKS 9 suggests that improvement in the acoustic environment may enhance speech recognition ability. Listening may be assisted by increasing speech volume over ambient noise. This is accomplished with low-gain output personal or classroom FM systems or acoustically modifying the classroom 66. A comprehensive linguistic study of a 26-year old, left-handed male, with onset of LKS at age five years who learned sign language at age 13, revealed that sign language was the most efficient mode of communication. Severe restrictions in comprehension and production of spoken English or lip reading, and lesser impairment of reading, persisted. Functional MRI (fMRI) revealed strong activation of auditory cortex (R>L) to heard speech, little response to silent lip-reading, and strong activation of right temporo-parieto-occipital association cortex while viewing sign language 67. Further fMRI studies may provide an understanding of the extent of cortical impairment and applicability of various therapeutic strategies.
Prognosis
Several variables may influence prognosis, including age of onset, pattern of language deficit, frequency and topography of EEG discharges, duration of epilepsy, and efficacy, and adverse effects of anticonvulsants 3. There are few longterm follow up studies and no firm conclusions regarding potential for recovery 3, 9, 20. Outcomes range from complete recovery to permanent severe aphasia, with most experiencing improvement and residual moderate language deficits 68. In a study by Soprano et al. 69, no child with persistent EEG abnormalities recovered normal or near normal language; even among the nine whose EEG normalized; only three had complete recovery. A recent long term study of 11 patients with a mean follow-up of nine years eight months revealed complete language recovery in only 18.2% of cases and mental retardation in 63.6%70. Adverse prognostic factors appear to be onset before 4 years, duration of aphasia longer than one year, and duration and continuity of ESES 4, 70.
Conclusion
LKS is an epilepsy syndrome characterized by acquired aphasia and epileptiform EEG abnormalities eventually characterized by ESES. The language disorder could be the result of a paroxysmal disruption of language function during the time of its greatest development and vulnerability 71. In experimental animal models, there is clear evidence that functional disruption may interfere with the process of neuronal connection and cortical function 72. Seizures during a critical period for circuit development cause the emergence and fixation of permanent aberrant connections 73, 74. LKS is a condition of unpredictable outcome and varying severity with a potentially relapsing remitting course, requiring constant adaptation and resourcefulness from parents, speech/language therapists, neuropsychologists, and neurologists.
Acknowledgments
The authors are grateful to Suzanne Reigle, B.S., and Kathleen Kelly, R.EEG T. for their assistance. This work was supported by a grant to GLH from the NINDS (NS27984) and a Mental Retardation Research Center Grant from NIH (HD18755-19).
References
- 1.Landau WM, Kleffner FR. Syndrome of acquired aphasia with convulsive disorder in children. Neurology 1957;7:523–530. [DOI] [PubMed] [Google Scholar]
- 2.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for classification of epilepsies and epileptic syndromes. Epilepsia 1985;26:268–278. [PubMed] [Google Scholar]
- 3.Deonna TW. Acquired epileptiform aphasia in children (Landau-Kleffner syndrome). J Clin Neurophysiology 1991;9:288–298. [DOI] [PubMed] [Google Scholar]
- 4.Bishop DVM. Age of onset and outcome in “acquired aphasia with convulsive disorder” (Landau Kleffner syndrome). Dev Med Child Neurol 1985;27:705–712. [DOI] [PubMed] [Google Scholar]
- 5.Rapin I, Mattis S, Rowan AJ, Golden GG. Verbal auditory agnosia and seizures in children. Dev Med Child Neurol 1977;19:192–207. [PubMed] [Google Scholar]
- 6.Zovari N, Choyakh F. Les potentiels evoques auditifs precoces, de latence moyenne et tardifs dans un cas d'aphasie acquise-epilepsie (syndrome de Landau-Kleffner). Rev Laryngol Otol Rhinol (Bord) 1997;40:299–308. [PubMed] [Google Scholar]
- 7.Wioland N, Rudolf G, Metz-Lutz MN. Electrophysiological evidence of persisting unilateral auditory cortex dysfunction in the late outcome of Landau and Kleffner syndrome. Clin Neurophysiol 2001;112:319–23. [DOI] [PubMed] [Google Scholar]
- 8.Lewis N. Otitis media and linguistic incompetence. Arch Otolaryngology 1976;102:387–390. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani JF, Landau WM. Acquired aphasia with convulsive disorder: course and prognosis. Neurology 1980;30:524–529. [DOI] [PubMed] [Google Scholar]
- 10.Beaumanoir A. The Landau-Kleffner syndrome. In: Roger J, Dravet C, Bureau M, Dreifuss FE, Wolf P. Epileptic syndromes in infancy, childhood, and adolescence. : J Libbey Eurotext, 1985: 81–191. [Google Scholar]
- 11.Dunn M, Rapin I. Communication in autistic children. In: Accardo PJ, Shapiro BK, Caputo AJ. Behavior belongs in the brain: neurobehavioral syndromes. : York Press; 1997:97–111. [Google Scholar]
- 12.Volkmar FR, Nelson DS. Seizure disorders in autism. J Am Acad Child Adolesc Psychiatry 1990;29:127–129. [DOI] [PubMed] [Google Scholar]
- 13.Rapin I. Autistic regression and disintegrative disorder: how important the role of epilepsy? Semin Pediatr Neurol 1995;2:278–285. [DOI] [PubMed] [Google Scholar]
- 14.Tuchman R, Rapin I. Progression in pervasive developmental disorders: seizures and EEG correlates. Pediatrics 1997;99:560–566. [DOI] [PubMed] [Google Scholar]
- 15.Ballaban-Gil K, Tuchman R. Epilepsy and epileptiform EEG: association with autism and language disorders. Ment Retard Dev Disabil Res Rev 2000;6:300–308. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum DH, Siegel M, Barr WB, Rowan AJ. Epileptic aphasia. Neurology 1986;36:822–825. [DOI] [PubMed] [Google Scholar]
- 17.Deonna T, Fletcher P, Voumard C. Temporary regression during language acquisition: a linguistic analysis of a 2 1/2 year old child with epileptic aphasia. Dev Med Child Neurol 1982;24:156–163. [DOI] [PubMed] [Google Scholar]
- 18.White H, Sreenivasan J. Epilepsy aphasia syndrome in children: an unusual presentation to psychiatry. Can J Psychiatry 1987;32:599–601. [DOI] [PubMed] [Google Scholar]
- 19.Ansink BJ, Sarphatie H, VanDongen HR. The Landau-Kleffner syndrome: case report and theoretical considerations. Neuropediatrics 1989;20:132–138. [DOI] [PubMed] [Google Scholar]
- 20.Deonna T, Peter C, Ziegler HL. Adult follow-up of the acquired aphasia-epilepsy syndrome in childhood: report of seven cases. Neuropediatrics 1989;20:132–138. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch E, Marescaux C, Maquet P, Metz-Lutz MN. Landau-Kleffner syndromes: a clinical and EEG study of five cases. Epilepsia 1990;31:756–767. [DOI] [PubMed] [Google Scholar]
- 22.Dugas M, LeHeusey MF, Reginer N. Aphasie acquise de l'enfant avec epilepsie (syndrome de Landau-Kleffner): douze observations personelles. Rev Neurol 1982;138:755–780. [PubMed] [Google Scholar]
- 23.Gomez MR, Klass DW. Epilepsies in childhood: the Landau-Kleffner Syndrome. Dev Med Child Neurol 1990;32:270–274. [DOI] [PubMed] [Google Scholar]
- 24.Nakano S, Okuno T, Mikawa H. Landau-Kleffner syndrome: EEG topographic studies. Brain Dev 1989;11:43–50. [DOI] [PubMed] [Google Scholar]
- 25.Ming L, Xiao-yu H, Jiong Q, Xi-ru W. Correlation between CSWS and aphasia in Landau-Kleffner syndrome: a study of three cases. Brain Dev 1996;18:197–200. [DOI] [PubMed] [Google Scholar]
- 26.Gordon N. Acquired aphasia in childhood: the Landau-Kleffner syndrome. Dev Med Child Neurol 1990;32:270–274. [DOI] [PubMed] [Google Scholar]
- 27.Lanzi G, Veggiotti P, Conte S, Partesana E, Resi C. A correlated fluctuation of language and EEG abnormalities in a case of the Landau-Kleffner syndrome. Brain Dev 1994;16:329–334. [DOI] [PubMed] [Google Scholar]
- 28.Holmes GL, McKeever M, Saunders Z. Epileptiform activity in aphasia of childhood: an epiphenomenon? Epilepsia 1981;22:631–639. [DOI] [PubMed] [Google Scholar]
- 29.Gascon G, Victor D, Lombroso CT. Language disorder, convulsive disorder and EEG study of five cases. Epilepsia 1990;31:756–767.1700952 [Google Scholar]
- 30.Galanopoulou AS, Bojko A, Lado F, Moshe SL. The spectrum of neuropsychiatric abnormalities associated with electrical status epilepticus in sleep. Brain Dev 2000;22:279–295. [DOI] [PubMed] [Google Scholar]
- 31.Aicardi J. Atypical semiology of rolandic epilepsy in some related syndromes. Epileptic Disord 2000;1:S5–9. [PubMed] [Google Scholar]
- 32.Fejerman N, Caraballo R, Tenembaum SN. Atypical evolutions of benign localization-related epilepsies in children: are they predictable? Epilepsia 2000;41:380–390. [DOI] [PubMed] [Google Scholar]
- 33.Massa R, de Saint-Martin A, Hirsch E, Marescaux C, Motte J, Seegmuller C, Kleitz C, Metz-Lutz M. Landau-Kleffner syndrome: sleep EEG characteristics at onset. Clin Neurophysiol 2000;111Suppl 2:S87–93. [DOI] [PubMed] [Google Scholar]
- 34.Tuchman RF, Rapin I. Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics 1997;22:560–566. [DOI] [PubMed] [Google Scholar]
- 35.Pearl PL, Conry JA, Reigle S, Stahl AM, Allen E, Rich S, Mott SH, Weinstein SL, Gaillard ND. Lack of utility of EEG monitoring in autistic syndrome patients for the identification of Landau-Kleffner syndrome. Ann Neurol 2001;50:S115. [Google Scholar]
- 36.Lou HC, Brandt S, Bruhn P. Progressive aphasia and epilepsy with a self-limited course. In: Perry JK. Epilepsy: the VIII International Symposium. : Raven Press, 1977:295–303. [Google Scholar]
- 37.Cole AJ, Andermann F, Taylor L, Olivier A, Rasmussen T, Robitaille Y, Spire JP. The Landau-Kleffner syndrome of acquired epileptic aphasia: unusual clinical outcome, surgical experience, and absence of encephalitis. Neurology 1988;38:31–38. [DOI] [PubMed] [Google Scholar]
- 38.Lou HC, Brandt S, Bruhn P. Aphasia and epilepsy in children. Acta Neurol Scand 1977;56:46–54. [DOI] [PubMed] [Google Scholar]
- 39.McKinney W, McGreal DA. An aphasic syndrome in children. Can Med Assoc J 1974;110:637–639. [PMC free article] [PubMed] [Google Scholar]
- 40.Pascual-Castroviejo I, Lopez-Martin V, Martinez-Bermejo A, Perez-Hinojosa A. Is cerebral arteritis the cause of the Landau-Kleffner syndrome? Four cases in childhood with angiographic study. Can J Neurol Sci 1992;19:46–52. [PubMed] [Google Scholar]
- 41.Feekery CJ, Parry-Fielder B, Hopkins JJ. Landau-Kleffner syndrome: six patients including discordant monozygotic twins. Pediatr Neurol 1993;9:49–53. [DOI] [PubMed] [Google Scholar]
- 42.Maichalowicz R, Jozwiak S, Szwabowska-Orzeszko E, Ignatowicz L, Ignatowicz R. [The Landau-Kleffner syndrome]. Zespol Laudau-Kleffnera. Wiad Lek 1989;42:256–259. [PubMed] [Google Scholar]
- 43.Otero E, Cordova S, Diaz F, Garcia-Terul I, Del Brutto OH. Acquired epileptic aphasia due to neurocysticercosis. Epilepsia 1989;30:569–572. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia MS, Shome S, Chadda RK, Savrabh H. Landau-Kleffner syndrome in cerebral neurocysticercosis. Indian Pediatr 1994;31:584–587. [PubMed] [Google Scholar]
- 45.Solomon GE, Parson D, Pavlakis S, Fraser R, Labar D. Intracranial EEG monitoring in Landau-Kleffner syndrome associated with a left temporal lobe astrocytoma. Epilepsia 1993;34:557–560. [DOI] [PubMed] [Google Scholar]
- 46.Nass R, Heier L, Walker R. Landau-Kleffner syndrome: temporal lobe tumor resection results in good outcome. Pediatr Neurol 1993;9:303–305. [DOI] [PubMed] [Google Scholar]
- 47.Bicknese AR, Preston J, Ettinger AB, Brook S. Epileptic Aphasia (Landau-Kleffner syndrome) secondary to progressive encephalitis. Ann Neurol 1996;40:306–307. [Google Scholar]
- 48.Perniola T, Margari L, Buttiglione M, Andreula C, Simone IL, Santostasi R. A case of Landau-Kleffner syndrome secondary to inflammatory demyelinating disease. Epilepsia 1993;34:551–556. [DOI] [PubMed] [Google Scholar]
- 49.Fayad M, Choveiri R, Mikati M. Landau-Kleffner syndrome: consistent response to repeated intravenous gamma-globulin doses: a case report. Epilepsia 1997;38:489–494. [DOI] [PubMed] [Google Scholar]
- 50.Lerman-Sagie T, Statter M, Lerman P. Low erythrocyte zinc content in acquired aphasia with convulsive disorder (the Landau-Kleffner syndrome). J Child Neurol 1987;2:28–30. [DOI] [PubMed] [Google Scholar]
- 51.Marescaux C, Hirsch E, Finck P, Maquet P, Schlumberger E, Sellal F, Metz-Lutz MN, Alembik Y, Salmon E, Franck G. Landau-Kleffner syndrome: a pharmacologic study of five cases. Epilepsia 1990;31:768–777. [DOI] [PubMed] [Google Scholar]
- 52.Guerreiro MM, Camargo EE, Kato M, Menezes Netto JR, Silva EA, Scotoni AE, Silveira DC, Guerreiro CA. Brain single photon emission computed tomography imaging in Landau-Kleffner syndrome. Epilepsia 1996;37:60–67. [DOI] [PubMed] [Google Scholar]
- 53.Rintahaka PJ, Chugani HT, Sankar R. Landau-Kleffner syndrome with continuous spikes and waves during slow-wave sleep. J Child Neurol 1995;10:127–133. [DOI] [PubMed] [Google Scholar]
- 54.Da Silva EA, Chugani DC, Muzik O, Chugani HT. The Landau-Kleffner syndrome: metabolic abnormalities in temporal lobe are a common feature. J Child Neurol 1997;12:489–495. [DOI] [PubMed] [Google Scholar]
- 55.O'Tuama LA, Urion DK, Janicek MJ, Treves ST, Bjornson B, Moriarity JM. Regional cerebral perfusion in Landau-Kleffner syndrome and related childhood aphasias. J Nucl Med 1992;33:1758–1765. [PubMed] [Google Scholar]
- 56.Genton P, Maton B, Ogihara M, Samoggia G, Guerrini R, Medina MT, Dravet C, Roger J. Continuous focal spikes during REM sleep in a case of acquired aphasia (Landau-Kleffner syndrome). Sleep 1992;15:454–460. [DOI] [PubMed] [Google Scholar]
- 57.Appleton R, Hughes A, Beirae M, Acomb B. Vigabatrin in the Landau-Kleffner syndrome. Dev Med Child Neurol 1993;35:457–458. [PubMed] [Google Scholar]
- 58.Glauser TA, Olberding LS, Titanic MK, Picirillo DM. Felbamate in the treatment of acquired epileptic aphasia. Epil Res 1995;20:85–89. [DOI] [PubMed] [Google Scholar]
- 59.Lerman P, Lerman-Sagie T, Kivitz S. Effect of early corticosteroid therapy for Landau-Kleffner syndrome. Dev Med Child Neurol 1991;33:257–266. [DOI] [PubMed] [Google Scholar]
- 60.Lagae LG, Silberstein J, Gilliss PL, Casaer PJ. Successful use of intravenous immunoglobulins in Landau-Kleffner syndrome. Pediatr Neurol 1998;18:165–168. [DOI] [PubMed] [Google Scholar]
- 61.Mikati MA, Saab R. Succeessful use of intravenous immunoglobulin as initial monotherapy in Landau-Kleffner syndrome. Epilepsia 2000; 41:880–886. [DOI] [PubMed] [Google Scholar]
- 62.Morrell F, Whisler WM, Smith MC, Hoeppner TJ, Pierre-Louis SJ, Kanner AM, Buelow JM, Ristanovic R, Bergen D. Landau-Kleffner syndrome: treatment with subpial intracortical transection. Brain 1995;118:1529–1546. [DOI] [PubMed] [Google Scholar]
- 63.Nass R, Gross A, Wisoff J, Devinsky O. Outcome of multiple subpial transactions for autistic epileptiform regression. Pediatr Neurol 1999;21:464–470. [DOI] [PubMed] [Google Scholar]
- 64.Irwin K, Birch V, Lees J, Polkey C, Alarcon G, Binnie C, Smedley M, Baird G, Robinson RO. Multiple subpial transaction in Landau-Kleffner syndrome. Dev Med Child Neurol 2001;43:248–252. [DOI] [PubMed] [Google Scholar]
- 65.Tharpe AM, Johnson GD, Glasscock ME. Diagnostic and management considerations of acquired epileptic aphasia or Landau-Kleffner syndrome. Am J Otol 1991;12:210–214. [PubMed] [Google Scholar]
- 66.Tharpe AM, Olsen BJ. Landau-Kleffner syndrome: acquired epileptic aphasia in children. J Am Acad Audiol 1994;5:146–150. [PubMed] [Google Scholar]
- 67.Sieratzki JS, Calvert GA, Brammer M, David A, Woll B. Accessability of spoken, written, and sign language in Landau-Kleffner syndrome: a linguistic and functional MRI study. Epileptic Disord 2001;3:79–89. [PubMed] [Google Scholar]
- 68.Mouridsen SE. The Landau-Kleffner syndrome: a review. Eur Adolesc Psychiatry 1995;4:223–228. [DOI] [PubMed] [Google Scholar]
- 69.Soprano AM, Garcia EF, Caraballo R, Fejerman N. Acquired epileptiform aphasia: neuropsychologic follow-up of 12 patients. Pediatr Neurol 1994;11:230–235. [DOI] [PubMed] [Google Scholar]
- 70.Rossi PG, Parmeggiani A, Posar A, Scaduto MC, Chiodo S, Vatti G. Landau-Kleffner syndrome (LKS): long-term follow-up and links with electrical status epilepticus during sleep (ESES). Brain Dev 1999;21:90–98. [DOI] [PubMed] [Google Scholar]
- 71.DeNegri M. Electrical status epilepticus during sleep (ESES). Different clinical syndromes: towards a unifying view? Brain Dev 1997;19:447–451. [PubMed] [Google Scholar]
- 72.Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia 1993;34:S37–S53. [DOI] [PubMed] [Google Scholar]
- 73.Holmes GL, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. Mossy fiber sprouting following recurrent seizures during early development in rats. Journal of Comparative Neurology 1999;404:537–553. [DOI] [PubMed] [Google Scholar]
- 74.Holmes GL, Gairsa J-L, Chevassus-Au-Louis N, Yang Y, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Annals of Neurology 1998;44:845–857. [DOI] [PubMed] [Google Scholar]


